Abstract
The synthesis of two optical isomers of N,N′-bis(1-phenylethyl)-2,6-pyridinedicarboxamide and the constant circularly polarized luminescence (CPL) activity of their acetonitrile trivalent europium complex solutions over a long period of time open new perspectives for performing accurate routine CPL calibration tests at low cost.
Generally speaking, circularly polarized luminescence (CPL) spectroscopy is the emission analog to circular dichroism (CD) spectroscopy. CD allows one to detect the differential absorption of left and right circularly polarized light, while CPL measures the difference in the emission intensity of left circularly polarized light versus right circularly polarized light. CPL has primarily been focused on studies aimed at investigating the chiral structures and solution dynamics of luminescent lanthanide complexes. A recent review has summarized the various types of applications of CPL aimed at getting quantitative and/or qualitative chiral structural information of selected systems that do not racemize on the emission timescale.1 Some of the more important applications of CPL are briefly discussed below.
CPL is becoming increasingly useful as a probe for luminescent lanthanide complexes as sensory systems for anion binding in aqueous media or the existence of chiral lanthanide structures (i.e. to establish the predominant isomer in solution or if the solution of a complex containing an achiral ligand is indeed a racemic mixture), and as an indicator of changes in chiral structure (i.e. the importance of the helical wrapping of the ligand strand contribution and, therefore, the influence of this latter on the diastereomeric induction). In addition, information concerning metal-ion environments and the associated chiral structures of metal-containing biological systems can be obtained through the measurement of CPL.
It is common to report the degree of CPL in terms of the luminescence dissymmetry factor, glum(λ), which is defined as follows: glum(λ) = 2ΔI/I = 2(IL − IR)/(IL + IR), where IL and IR refer, respectively to the intensity of left and right circularly polarized emissions. The transitions studied in CPL are generally the magnetic dipole allowed transitions that are electric dipole forbidden, where one predicts the CPL would be large. For Eu(III), the 5D0 → 7F1 transition is particularly well-suited for CPL measurements since it satisfies the magnetic-dipole selection rule, ΔJ = 0, ±1(except 0 ↔ 0), and therefore would be the transition to be considered for calibration purposes. It should be noted that a value of 0 for glum corresponds to no circular polarization, while the absolute maximum value is 2. For example, absolute glum values of 0.25 and 0.29 have been reported for Eu(III) complexes with chiral DOTA- (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraaceticacid) and 2-hydroxyisophthalamide-based ligand derivatives.2,3 It is often the case that rigid lanthanide systems exhibit large glum values, while racemic or other types of mixtures give glum values in the range of ∼10−2–10−3.1 As a result, an accurate determination of the sign and magnitude of the CPL signal needs to be accomplished.
Unlike CD, the measurement of CPL is mainly dependent on the use of home-built instruments that have been designed, developed and improved by a limited number of research groups around the world over the last three decades.1,4-6 However, the growing interest in developing chiral luminescent probes, and in particular lanthanide-based systems, has recently resulted in the commercialization of the first CPL spectrometer.7 Although most of the CPL studies published in the literature have been done with custom-made instruments operating in a differential photon-counting mode,1 the detection of CPL can now be done with a high degree of sensitivity (∼ 1 part in 104–105) and reliability, depending on the total light intensity. The availability of a relatively simple CPL standard would be a necessary etalon due to the absence of comparative results between the currently home-built instruments used and commercial spectrometers available. In addition, all CPL instruments need to be regularly tested and calibrated for the detection of small degrees of circular polarization in the total emitted light intensity accurately. To date, the commercially available (Aldrich) NMR shift reagent tris(3-trifluoroacetyl-(+)-camphorato)europium(III) in DMSO, Eu((+)-facam)3, is used as a CPL standard,1,8 even though Schippers in 1982 suggested that the use of this “system/DMSO as a CPL calibration standard is questionable”.4 The magnitude of Schippers' measured glum values (−0.25, −0.78, and +0.072 at 588.2, 595.2 and 613.5 nm) did not match those reported by Brittain8 in 1976 (−0.84, −1.98, and +0.30). In 1999, Maupin also showed that the CPL activity of Eu(facam)3 complexes in DMSO is relatively sensitive to the presence of small amounts of water.9 In addition to the water sensitivity, the high cost of Eu((−)-facam)3 with the other enantiomeric form of facam limits its use as an effective and reliable CPL standard for routine tests.
In this communication, we report on the use of a more suitable CPL calibrating agent based on optical isomers of N,N′-bis(1-phenylethyl)-2,6-pyridinedicarboxamide (1) coordinated to europium(III) ions in a Eu : 1 ratio of 1 : 3. In particular, the simple preparation in high yield of these new optical isomers, (R,R)-1 and (S,S)-1, and the constant CPL activity of their MeCN europium(III) complex solutions over a long period of time are interesting properties to be considered for performing routine CPL calibrations. The isomer (R,S)-1 (or meso-1) has also been prepared as a control. It should be added that our ligand of interest is based on an analog of the N,N′-dibenzyl-2,6-pyridinedicarboxamide ligand,10 where the dibenzyl substituents have been replaced by chiral phenylethyl groups.
The three diamide stereoisomers were synthesized (Scheme 1) from 2,6-pyridinedicarboxylic acid chloride with a slight excess of (S)-(−), (R)-(+), or (±)-(α)-methylbenzylamine (2) in a one-step procedure using modified Schotten–Baumann conditions11 (see ESI†). In these conditions, a two-phase medium is provided by the organic solvent, CH2Cl2, and the aqueous base, sodium bicarbonate. Ligands (R,R)-1 and (S,S)-1 were obtained in 90% yield, while (R,S)-1 was isolated in 28% yield and 97.8% purity after four recrystallizations, as confirmed by NMR.12 The opposite optical rotation values of (R,R)-1 and (S,S)-1 (−2213.4° and +213.6°, respectively) confirm the chirality arising from the asymmetric carbons and the preparation of each enantiomeric form of 1, while an optical rotation value of −0.8° for (R,S)-1 corroborates the synthesis of the meso ligand with high purity.
Scheme 1.
The Eu-containing complex solutions were prepared in situ from stock solutions of Eu(III), Eu(OTf)3 (OTf = trifluoromethanesulfonate) and each optical isomer of 1 in MeCN. The Eu(III) concentration was 6.67 × 10−3 M and the Eu: 1 ratio was 1 : 5 to ensure complete formation of the tris complex. These experimental conditions were determined based on the identification of the various Eu-containing species using the 5D0 ← 7F0 excitation spectroscopy. For instance, the 5D0 ← 7F0 excitation spectra of Eu–(R,R)-1 solutions at various Eu : 1 ratios confirm the successive formation of 1 : n (n = 1–3) species (Fig. 1). At the lowest Eu : 1 ratio, the 1 : 1 complex (579.2 nm) is the major complex in solution; the other species in solution is the 1 : 2 complex (579.8 nm) while a third species corresponding to the tris complex (580.4 nm) is observed at higher ligand concentration. Finally, the high resolution excitation spectra show that the Eu(III) ions are in the form of the 1 : 3 species in a 6.67 × 10−3 MMeCN solution with a stoichiometric 1 : 5 ratio.13 It should be noted that 1 forms stable tris complexes, as demonstrated by the measurement of a constant CPL activity over an extended period, and the observation of only two components for the 5D0 → 7F1 transition is compatible with trigonal symmetry of the complex. A complete study of the stability, structural, chiroptical and photophysical properties of these compounds of interest will be described in a forthcoming publication. Furthermore, our solution stability results are consistent with those published by Le Borgne et al.10 The authors showed the slow formation of stable triple-helical complexes with the N,N′-dibenzyl-2,6-pyridinedicarboxamide ligand (solutions equilibrated for one week), and also that the existence of several inert conformational isomers, due to the blocked rotations around the OC–N bonds, prevents complete characterization in solution. The slow complexation processes are due to the stabilization of this ligand in its syn,syn,Z,Z conformation.
Fig. 1.
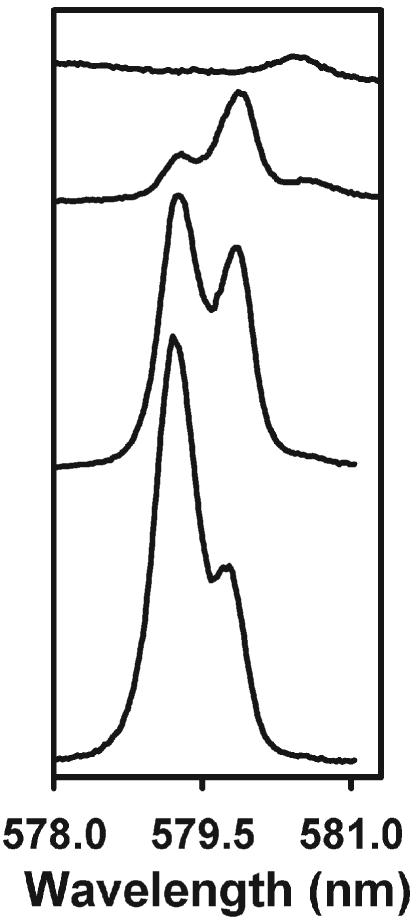
5D0 ← 7F0 excitation spectra of the Eu–(R,R)-1 solutions at various Eu : 1 ratios (1 : 1, 1 : 2, 1 : 3 and 1 : 5 from bottom to top) in MeCN at 295 K. The Eu(III) concentration was 6.67 × 10−3 M. The luminescence was monitored at 615.6 nm.
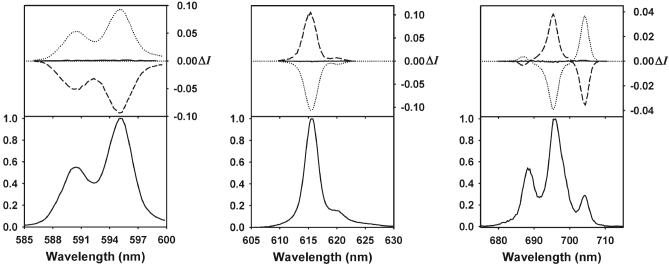
The CPL spectra for the 5D0 → 7F1, 7F2, and 7F4 transitions of each of the complex solutions with a Eu : 1 ratio of 1 : 513 were measured following excitation at 308 nm in MeCN at 295 K. As shown in Fig. 2, the detection of a CPL signal confirms the presence of stable chiral emitting species on the luminescence timescale. In addition, the observation of mirror image CPL spectra for the Eu(III) complexes with both enantiomers, (R,R)-1 and (S,S)-1, shows that the emitted light is polarized in a direction determined by the helicity of the Eu(III) ion, which in turn is controlled by the absolute configuration at the carbon centers in the amide substituents. This result is also corroborated by the absence of detectable CPL activity for the Eu(III) compound with (R,S)-1. The CPL results are summarized in Table 1.
Fig. 2.
Circularly polarized luminescence (upper curves) and total luminescence (lower curves) spectra of the Eu-containing compounds in a Eu : 1 ratio of 1 : 5 with the three optical isomers of N,N′-bis(1-phenylethyl)-2,6-pyridinedicarboxamide, (R,R)-1 (dashed lines), (S,S)-1 (dotted lines), and (R,S)-1 (solid lines), in 6.67 × 10−3 M MeCN at 295 K, following excitation at 308 nm. Left: 5D0 → 7F1 transition; middle: 5D0 → 7F2 transition; right: 5D0 → 7F4 transition.
Table 1.
Summary of CPL results for the Eu-containing compounds in a Eu : 1 ratio of 1 : 5 with the two optical isomers of N,N′-bis(1-phenylethyl)-2,6-pyridinedicarboxamide, (R,R)-1 and (S,S)-1 in 6.67 × 10−3 M MeCN at 295 K, following excitation at 308 nm
| glum ± σda |
|||
|---|---|---|---|
| Electronic transition |
Wavelength/nm | (R,R)-1 | (S,S)-1 |
| 5D0 → 7F1 | 590.5 | −0.19 ± 0.01 | +0.19 ± 0.01 |
| 595.3 | −0.18 ± 0.01 | +0.18 ± 0.01 | |
| 5D0 → 7F2 | 615.6 | +0.21 ± 0.01 | −0.21 ± 0.01 |
| 5D0 → 7F3b | 649.6 | −0.22 ± 0.01 | +0.23 ± 0.01 |
| 5D0 → 7F4 | 688.8 | +0.001 ± 0.01 | −0.001 ± 0.01 |
| 696.0 | +0.07 ± 0.01 | −0.07 ± 0.01 | |
| 704.1 | −0.24 ± 0.01 | +0.25 ± 0.01 | |
The most important feature of these systems is the stability of their complex solutions resulting in a CPL activity independent of the “age” of the solution. For instance, the glum values amounted to −0.18 and −0.18 at 595.3 nm (5D0 → 7F1)for a 1 : 5 Eu–(R,R)-1 solution left on the shelf and measured seven months apart. In addition, these solutions have been exposed to a series of UV excitations (λexc = 308 nm) for several consecutive days without showing photochemical degradation.
In conclusion, we present here new Eu-containing compounds that can be used for performing accurate routine CPL tests at low cost. The advantages of these systems are (i) the ease of the synthesis of the optical isomers of 1, which can be performed by undergraduate students, (ii) their complex solution stability (i.e. several months), once they have reached their thermodynamic equilibrium, and (iii) the lack of noticeable photochemical degradation under continuous UV excitation (i.e. three days). The combination of these properties opens new perspectives for the design of lanthanide(III) complexes acting as probes for chiral recognition.
Supplementary Material
Acknowledgments
We thank the National Institute of Health Minority Biomedical Research Support(2S06GM008192-24A1) and Research Corporation Cottrell Science Award (CC6624) for the financial support of this research. We are also grateful to San José State University and San José State University Research Foundation for an Award for Research, Scholarship or Creativity Activity for G. M.
Footnotes
Electronic supplementary information (ESI) available: Synthetic procedures and characterization data for all isolated compounds.
Notes and references
- 1.Riehl JP, Muller G. In: Handbook on the Physics and Chemistry of Rare Earths. Gschneidner KA Jr., Bünzli J-CG, Pecharsky VK, editors. Vol. 34. North-Holland Publishing Company; Amsterdam: 2005. pp. 289–357. ch. 220. and references therein. [Google Scholar]
- 2.Parker D, Dickins RS, Puschmann H, Crossland C, Howard JAK. Chem. Rev. 2002;102:1977. doi: 10.1021/cr010452+. [DOI] [PubMed] [Google Scholar]
- 3.Petoud S, Muller G, Moore EG, Xu J, Sokolnicki J, Riehl JP, Le UN, Cohen SM, Raymond KN. J. Am. Chem. Soc. 2007;129:77. doi: 10.1021/ja064902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schippers PH. University of Leiden; The Netherlands: 1982. PhD Dissertation. [Google Scholar]
- 5.Riehl JP, Richardson FS. Chem. Rev. 1986;86:1. and references therein. [Google Scholar]
- 6.Brittain HG. Appl. Spectrosc. Rev. 2000;35:175. and references therein. [Google Scholar]
- 7.JASCO CPL-200 instrument consisting essentially of two CD spectrometers with the second one used as the emission spectrometer.
- 8.Brittain HG, Richardson FS. J. Am. Chem. Soc. 1976;98:5858–5863. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- 9.Maupin CL. Michigan Technological University; USA: 1999. PhD Dissertation. [Google Scholar]
- 10.Le Borgne T, Bénech J-M, Floquet S, Bernardinelli G, Aliprandini C, Bettens P, Piguet C. Dalton Trans. 2003:3856. [Google Scholar]
- 11.Hart DJ, Magomedov NA. J. Am. Chem. Soc. 2001;123:5892. doi: 10.1021/ja010066+. [DOI] [PubMed] [Google Scholar]
- 12.1H NMR in C6D6 is useful for determining the ratio of (S,S)-1 and/or (R,R)-1 versus (R,S)-1: the former isomers exhibit a doublet at δ 1.26 ppm that is nicely resolved from (R,S)-1 at δ 1.32 ppm.
- 13.Although the 5D0 ← 7F0 excitation study indicates that the 1 : 3 complex is probably the main species in solution at a 1 : 3.5 ratio, a 1 : 5 ratio has been used as a precaution. Preliminary results suggest that the glum value is not affected by the excess of ligand, once the 1 : 3 complex has been completely formed in solution (ratios going from 1 : 3.5 to 1 : 5).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.