Abstract
Background
Quantitative data from gene expression experiments are often normalized by transcription levels of reference or housekeeping genes. An inherent assumption for their use is that the expression of these genes is highly uniform in living organisms during various phases of development, in different cell types and under diverse environmental conditions. To date, the validation of reference genes in plants has received very little attention and suitable reference genes have not been defined for a great number of crop species including Coffea arabica. The aim of the research reported herein was to compare the relative expression of a set of potential reference genes across different types of tissue/organ samples of coffee. We also validated the expression profiles of the selected reference genes at various stages of development and under a specific biotic stress.
Results
The expression levels of five frequently used housekeeping genes (reference genes), namely alcohol dehydrogenase (adh), 14-3-3, polyubiquitin (poly), β-actin (actin) and glyceraldehyde-3-phosphate dehydrogenase (gapdh) was assessed by quantitative real-time RT-PCR over a set of five tissue/organ samples (root, stem, leaf, flower, and fruits) of Coffea arabica plants. In addition to these commonly used internal controls, three other genes encoding a cysteine proteinase (cys), a caffeine synthase (ccs) and the 60S ribosomal protein L7 (rpl7) were also tested. Their stability and suitability as reference genes were validated by geNorm, NormFinder and BestKeeper programs. The obtained results revealed significantly variable expression levels of all reference genes analyzed, with the exception of gapdh, which showed no significant changes in expression among the investigated experimental conditions.
Conclusion
Our data suggests that the expression of housekeeping genes is not completely stable in coffee. Based on our results, gapdh, followed by 14-3-3 and rpl7 were found to be homogeneously expressed and are therefore adequate for normalization purposes, showing equivalent transcript levels in different tissue/organ samples. Gapdh is therefore the recommended reference gene for measuring gene expression in Coffea arabica. Its use will enable more accurate and reliable normalization of tissue/organ-specific gene expression studies in this important cherry crop plant.
Background
The study of biological regulations is very often correlated to quantification assays. In order to detect differential expression of a gene(s) in distinct biological samples, such as tissue types or under different experimental conditions, the invention of quantitative PCR (qPCR) has transformed the field of gene expression analysis in living organisms [1]. In comparison to classical reverse transcription-polymerase chain reaction (RT-PCR), the main advantages of qPCR are higher sensitivity, specificity and broad quantification range of up to seven orders of magnitude [2-6]. Regardless of being an extremely powerful technique, qPCR has its pitfalls, the most important one being the need of appropriate data normalization with a reference gene [6-14].
According to Andersen et al. [8], accurate data normalization is an absolute requirement for correct measurement of gene expression. Expression of the reference gene used to normalize qPCR analyses should be unaffected throughout many biological contexts; otherwise, it may lead to erroneous results [6-9,15-18]. Until recently, several such genes (β-actin, rRNA, β-tubulin, alcohol dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, 14-3-3 and polyubiquitin) have been used as internal controls for gene expression analyses under the assumption of stable expression [19-26]. However, several reports have demonstrated that the expression levels of these so-called reference genes differ among different tissue/organ types [10,16,27-33]. Consequently, these genes are unsuitable as transcriptional inner controls, and their use to normalize qPCR data in different tissues may induce significant experimental errors that could result in inappropriate biological data interpretation [9,14,17,21-25,34-39].
Recognizing the importance of reference gene(s) in normalization of qPCR data, various housekeeping genes have been evaluated for stable expression under specific conditions in various organisms. In plants, only a few of them have been investigated in some detail in rice [15,40,41], poplar [36], potato [39], soybean [42,43] and Arabidopsis thaliana [30]. So far, suitable internal controls for gene expression studies have not been defined for Coffea arabica.
Coffee is an agricultural crop of significant economic importance. Coffea arabica L. (arabica type coffee) is typical of the highland growing regions and is responsible for almost 75% of world production [44]. In this study, we report the validation of housekeeping genes to identify the most suitable internal reference gene(s) for normalization of qPCR data obtained among five different tissues/organs (root, stem, leaf, flower, and fruits) of C. arabica. To further validate our results, we evaluated the expression levels of our best reference genes at different developmental stages of flowers and cherries and under a specific biotic stress. Following the current literature, five candidate reference genes, namely alcohol dehydrogenase (adh), polyubiquitin (poly), 14-3-3, β-actin (actin) and glyceraldehyde-3-phosphate dehydrogenase (gapdh), were selected. In addition to these commonly used internal controls, three other genes coding for a cysteine proteinase (cys), a caffeine synthase (ccs) and the 60S ribosomal protein L7 (rpl7), respectively, were included in this analysis. These potential reference genes were ranked according to their expression profiles and stability.
Results and discussion
The expression profile of eight candidate reference genes (actin, adh, 14-3-3, ccs, gapdh, poly, rpl7, or cys) was firstly assessed by qPCR over a panel of five coffee tissue/organ samples (root, stem, leaf, flower, or fruit).
Descriptive analysis of the reference candidate genes
Descriptive statistics of the derived crossing points (CP), based on BestKeeper program [45], were calculated to investigate the variation level of each candidate gene following Pfaffl et al. [46]. According to this analysis (see Table 1), the gene with lowest expression level was actin, for which CP values were obtained around cycles 31–34; while the highest was gapdh, whose CP values were obtained around cycles 21–23. The expression levels of 14-3-3, ccs, gapdh, and rpl7 presented fluctuations of approximately ± 0.6 x-fold (0.52 x-fold < SD < 0.82 x-fold), whereas poly expression showed higher ranges of CP variation (SD = ± 1.39 cycles) as well as up- down-regulation (± 2.09 x-fold). The coefficient of variation (CV) of the assay was 3.57% (total essay variability), which is within the range (from 3.4% to 11.6%) of previously reported values for qPCR [46].
Table 1.
Descriptive statistics and expression level analyses of the tested candidate reference genes based on their crossing point (CP) values
| Factor | actin | adh | 14-3-3 | ccs | gapdh | poly | rpl7 | cys |
| N | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| GM [CP] | 32.72 | 29.7 | 29.63 | 26.67 | 22.86 | 28.46 | 30.76 | 26.67 |
| AM [CP] | 32.74 | 29.71 | 29.64 | 26.67 | 22.88 | 28.49 | 30.77 | 26.7 |
| Min [CP] | 31.4 | 28.64 | 28.94 | 25.92 | 21.74 | 26.79 | 30.13 | 25.24 |
| Max [CP] | 34.35 | 31.12 | 31.21 | 27.37 | 23.77 | 30.07 | 31.93 | 28.44 |
| SD [± CP] | 1.23 | 0.99 | 0.92 | 0.58 | 0.94 | 1.39 | 0.69 | 1.31 |
| CV [%CP] | 3.75 | 3.35 | 3.09 | 2.19 | 4.12 | 4.89 | 2.24 | 4.89 |
| Min [x-fold] | 0.41 | 0.43 | 0.66 | 0.53 | 0.54 | 0.18 | 0.68 | 0.41 |
| Max [x-fold] | 3.04 | 3.1 | 2.62 | 1.83 | 1.64 | 5.22 | 2.02 | 3.04 |
| SD [± x-fold] | 1.07 | 1.08 | 0.82 | 0.52 | 0.52 | 2.09 | 0.53 | 1.08 |
Abbreviations: N: number of samples; CP: crossing-point; GM [CP]: geometric CP mean; AM [CP]: arithmetic CP mean; Min [CP] and Max [CP]: CP threshold values; SD [± CP]: CP standard deviation; CV [%CP]: variance coefficient expressed as percentage of CP level; Min [x-fold] and Max [x-fold]: threshold expression levels expressed as absolute x-fold over- or under-regulation coefficient; SD [± x-fold]: standard deviation of absolute regulation coefficient.
Descriptive statistics for the expression analyses of each reference candidate gene in the five distinct coffee plant tissue/organ types was also obtained (see Additional File 1). According to this analysis, the gene that exhibit the minor gene expression variation among the analyzed tissues was gapdh [SD (± x-fold) = 0.04], while the gene with major variation was ccs [SD (± x-fold) = 0.59]. In addition, ccs also presented the highest expression variation in flowers and fruits tissue samples, and therefore it cannot be used as a reference gene. Numerous studies have shown that the expression of housekeeping genes can vary under given situations [28]. This may partly be explained by the fact that housekeeping genes are not only implicated in the basal cell metabolism but also participate in other cellular functions [47,48].
A model-based approach for estimation of expression variation
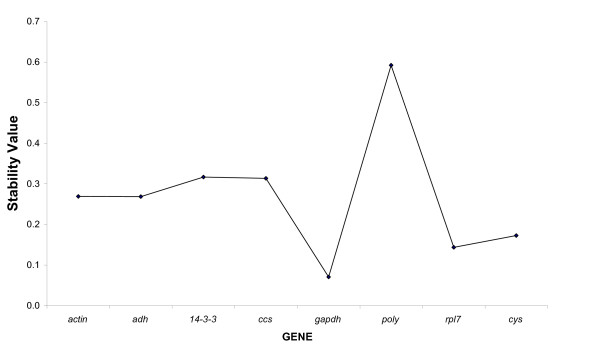
The model-based variance estimation approach, a Visual Basic application for Microsoft Excel (termed NormFinder) [8,49], was used to evaluate expression stability of reference candidate genes. This analysis allowed the ranking of candidate genes since the estimated variation directly indicates the introduced error associated with their use. According to this method, the gene with minimal estimated intra- and intertissue variation was gapdh (expression stability = 0.071) while the gene with the maximal variation was poly (expression stability = 0.592) (see Figure 1), thus corroborating the results obtained in descriptive analysis.
Figure 1.
Stability of the investigated candidate reference genes. Stability values of the eight candidate reference genes according to the model-based approach. A lower value of average expression stability indicates more stable expression.
Ranking the candidate reference genes
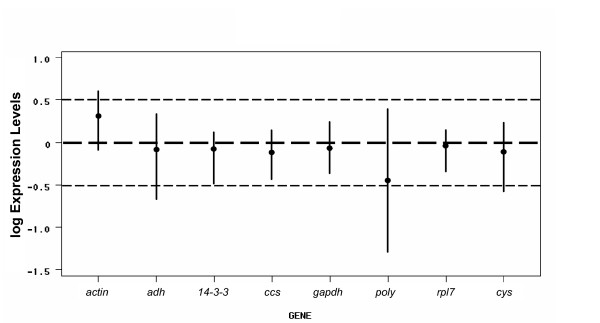
The relationship between the stability value and the intra- and intertissue expression variations is present in Figure 2. This figure clearly demonstrates the distinct specificities of the investigated genes. According to this analysis, the best candidate gene should present the minimal combined inter- and intra-tissue expression variation. Consistent with the descriptive analysis (see Table 1 and Additional File 1) and the model-based variance estimation approach (see Figure 1), gapdh showed not only the highest expression levels but was also the most stable gene studied. In Figure 2, it can be observed that almost all genes presented average of log expression levels near 0 (the thick dashed line). Log difference >0 (as observed for actin) or <0 (as observed for poly) implies that variability in expression levels is significant, so the gene could be incorrectly used as reference gene for normalization. In this context, the poly gene presents the highest intertissue expression variation (SD = ± 0.65; see Figure 2). Thus, among the tested genes, gapdh, followed by rpl7 and 14-3-3, showed the most stable expression over the investigated panel of five distinct coffee tissue/organ types.
Figure 2.
Gene expression differences among the candidate reference genes. The log-transformed gene expression levels are represented by black circles. The intertissue variation is indicated by vertical bars that give a confidence interval for the difference. The two thin dashed lines represent the maximal standard deviation of the reference candidate genes, with a log expression levels difference between 0.5 and -0.5.
The candidate genes were also ranked according to their M values using the geNorm program. The average expression stabilities (M values) of all tested genes were lower than 1.5, with 14-3-3 and actin showing the most stable expression (data not shown). Although actin gene has shown highest stability following geNorm analysis, this gene presented the lowest expression profile according the BestKeeper analysis. Corroborating the previous analysis, poly remained the least stable gene.
As a whole, our analysis indicates that housekeeping genes are differently regulated in different tissues/organs of coffee plants and may exhibit variable expression patterns. The observed differences in gene expression ratios along a comprehensive panel of tissues/organs are consistent with the data presented by Barber et al. [1], Iskandar et al. [50] and Jain et al. [15]. Our results also provide evidences that normalizations to the expression level of a single gene in samples from distinct tissue types may induce to errors, thus corroborating previous studies [29,51,52].
Comparison of the identified best reference genes to published data
In order to validate our potential candidate reference genes (gapdh, rpl7 and 14-3-3), the expression stability of these genes under the influence of a specific biotic stress was investigated. In this case, the obtained results were compared to those dealing with similar coffee gene expression analysis but using ubiquitin as a reference gene for normalization [53-56].
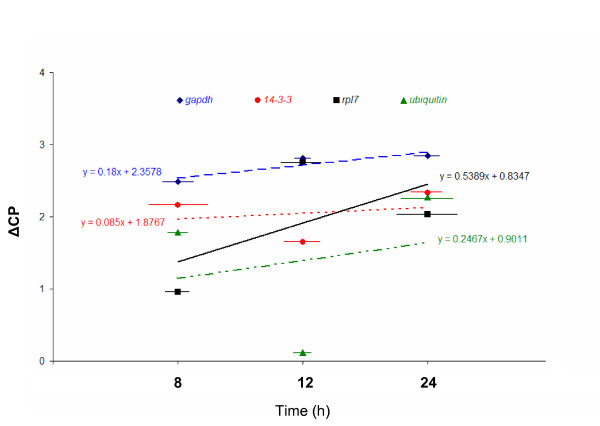
The comparison was conduced by linear regressions analyzes of the CP difference (ΔCP) obtained from the assayed expression levels of the tested genes in leaves of C. arabica inoculated, or not (control plants), with Hemileia vastatrix. The average CP (N = 3) was calculated for each gene and the ΔCP (CPinoculated leaf - CPnon-inoculated leaf) was determined for each time-point (8, 12 and 24 h after fungus inoculation).
As it can be observed in Figure 3, the regression lines for 14-3-3 and gapdh have slopes close to zero (14-3-3 = 0.085; gapdh = 0.18), indicating similar expression levels in inoculated and non-inoculated leaves, and reinforcing their use as effective normalization genes [1,57,58]. In contrast, the slope value for rpl7 was significantly different from zero and higher than the one obtained for ubiquitin (ubiquitin = 0.2467; rpl7 = 0.5389), thus limiting its use as a normalization factor under biotic stress condition.
Figure 3.
Evaluation of the expression of selected reference genes during fungus infection. The expression of selected reference genes (gapdh, rpl7 and 14-3-3) and of a commonly used coffee normalization gene (ubiquitin) was monitored in leaves of C. arabica var. Mundo Novo inoculated with Hemileia vastatrix. The crossing point (CP) difference (ΔCP = CPinoculated leaf - CPnon-inoculated leaf) was calculated for each time-point (8, 12 and 24 h after challenge by the rust fungus) to investigate the expression levels of each reference gene. The standard error of the triplicates for each time-point is indicated by horizontal bars.
Validation of data results in different developmental stages of flowers and coffee cherries
An additional validation step of the expression levels of gapdh, 14-3-3, rpl7 and ubiquitin was performed using unpooled tissue samples from flower and cherry developmental series. The employed sample set is given in the Additional File 2.
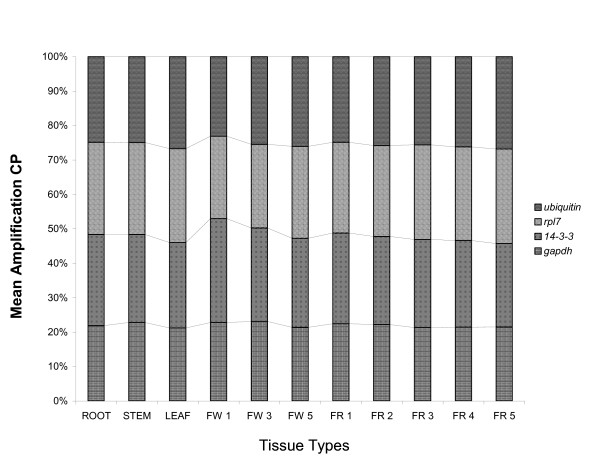
In this assay, the highly expressed gene was gapdh ( = 21 cycles) followed by ubiquitin ( = 24 cycles), while 14-3-3 and rpl7 presented the same mean CP (25 cycles). The comparison of gene contributions is present in Figure 4. As already observed, gapdh showed the greatest stability in expression among all coffee tissue/organ samples analyzed, while expression of rpl7 and 14-3-3 varied the most, especially in flowers at stage 1 of development (see Figure 4 and Additional File 2). As mentioned earlier, ubiquitin was used as a standard reference gene for comparison.
Figure 4.
Validation of the selected reference genes in samples from flowers and cherries at different developmental stages. Comparison of gene contributions, by mean amplification crossing points (CP) represented in percentage, in each coffee tissue/organ type. The investigated tissue/organ sample set was: root, stem, leaf, three different stages of flower development (FW 1, FW 3 and FW 5) and five different kinds of coffee cherries (FR 1, FR 2, FR 3, FR 4 and FR 5).
General remarks about the selected reference genes
According to these results, the gene encoding glyceraldeyde-3-phosphate dehydrogenase (GAPDH), an enzyme of glycolysis [59], outperformed all other reference genes tested and should therefore be considered a suitable reference gene for expression studies in arabica coffee plants. This observation corroborates the quantification of gapdh expression in different tissues of sugarcane [50] and Eucalyptus [20]. In contrast, several reports in human and animal systems have suggested that this reference gene has limitations for its use as internal control due to its marked variability of expression among tissue types [1,16,51,60].
The assayed 14-3-3 gene also showed a stable expression (see Figure 2) that was supported by the descriptive analysis (see Table 1 and also Additional File 1) and confirmed in the biotic stress assay (see Figure 3). However, its expression presented some variation among different stages of coffee flower development (see Figure 4). Papini-Terzi et al. [19] recommended 14-3-3 as a suitable reference gene for expression normalization in a wide range of tissue samples of sugarcane.
The gene encoding the transcription regulator and structural constituent of the 60S subunit of the cytosolic ribosome (rpl7) could also be used as an internal control in gene expression studies in C. arabica, due to its stability (see Figure 2) and acceptable variation among tissue/organ types (see Table 1). Our results are in agreement with previous published data for this gene since small variation among tissue types was detected by descriptive analysis (see Additional File 1). Nevertheless, in leaves of C. arabica inoculated with H. vastatrix, it was observed that the expression ratio of rpl7 (expressed by ΔCP) was not constant and the absolute value of rpl7 linear regression slope was superior to that observed for a commonly used coffee normalization gene (ubiquitin) (see Figure 3). In addition, this gene, like 14-3-3, presented a variable expression level among different stages of coffee flower development (see Figure 4). In sugarcane, the relative expression of rpl35-4, a gene coding for the ribosomal L35-4 60S protein, was also reported to be stable [61]. These authors estimated the sugarcane leaf transcriptome using Serial Analysis of Gene Expression (SAGE) and reported that tag associated with the rpl35-4 transcript presented minimum variation among the analyzed SAGE libraries.
The remaining tested genes showed to be unsuitable as internal controls for normalization purposes in C. arabica.
Conclusion
This study provides the most extensive collection of arabica coffee tissue/organ mRNA expression data for eight reference candidate genes. Our analysis evidenced stable levels of gapdh, 14-3-3 and rpl7 mRNA in different Coffea arabica tissue/organ types. Consequently, these genes can be used for accurate and reliable normalization in future gene expression studies in coffee (e.g., they can be used as a reference for a target gene in a specific tissue or experimental condition). In this respect, we suggest gapdh as the most relevant reference gene for accurate normalization purposes in C. arabica, showing almost constant expression levels in the investigated experimental set-up.
Moreover, we have shown that depending on the reference inner control gene, the within-tissue variation of mRNA expression levels is generally small, whereas among tissues/organs the variation can be substantial. This indicates that normalizations to a single gene across different tissue types are unwise. Since the variation observed between normal tissues of different types may in part be due to the different metabolic demands of those tissues, comparisons within a tissue type between normal and diseased states are similarly unwise.
Methods
Plant material and growth conditions
Freshly harvested roots, stems, and leaves were obtained from ten 4 month-old coffee plants (Coffea arabica var. Mundo Novo IAC 388-17-1) grown under greenhouse conditions (28°C, 60% RH) in Campinas, São Paulo, Brazil. Flower and fruit samples, at different developmental stages, were collected from 4–5 year-old plants of var. Mundo Novo grown under field conditions at Botucatu and Campinas, São Paulo, Brazil (see Additional File 2). After harvesting, fresh tissue samples were frozen immediately in liquid nitrogen until RNA extraction.
Biotic stress assay
For the biotic stress assay, equally-aged sets of Coffea arabica var. Mundo Novo plants were kept in growth chamber (16 h/8 h light/dark; 23°C; 70% RH) for at least one week, before being inoculated with the coffee leaf-rust fungus Hemileia vastatrix Berk and Br. race II, that elicits a compatible reaction in coffee. The urediniospores (100 mg) were harvested in a C. arabica field in Campinas, São Paulo, Brazil, and diluted in 10 ml of sterile water under dark conditions.
Leaves from the second pair of plagiotropic shoots from the apex of 4 month-old coffee plants were inoculated with an aqueous suspension of fresh urediniospores (10 mg/ml). To allow spore germination, the inoculated leaves were covered with a wet black plastic film for 24 h. Inoculated leaves were not detached from the plants. Mock-inoculated controls as well as non-inoculated controls were performed. The biological samples were obtained from three independent experiments.
Leaves were randomly sampled at different time-points after inoculation: 0, 8, 12, and 24 h, and immediately deep-frozen. To confirm the infection by the leaf-rust fungus, some inoculated leaves were maintained in plants.
RNA isolation and quality controls
Tissue samples of 2.0 to 2.5 mg were weighed and ground to fine powder in liquid nitrogen using a pre-cooled mortar and pestle. Total RNA from the majority of the samples was extracted using TRIzol reagent (Invitrogen) according to manufacturer's instructions. Alternatively, total RNA from seeds was isolated by lithium chloride (LiCl) method, according to Mason and Schmidt [62]. Only RNA samples with 260/280 ratio between 1.9 and 2.1 and 260/230 ratio greater than 2.0 were used for subsequent analyses. The integrity of the RNA samples was also assessed on 1.0% agarose/formaldehyde gel electrophoresis.
Reverse transcription
Five micrograms of total RNA were treated with DNAse I (Promega) and an aliquot of 500 ng of the treated RNA was reverse-transcribed using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). Both were used following the manufacturer's instructions. The cDNA sample concentration was determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies).
Primer design
Primers were designed for Coffea orthologs of commonly used housekeeping genes representing distinct functional classes, identified by BLAST searches in the Brazilian Coffee EST database [63,64] as well as in the public coffee EST database (at the SOL site hosted by Cornell University [65]). For primer design, the Primer Express 2.0 software (PE Applied Biosystems, USA) with default parameters was employed. The accession numbers, gene description, primers sequences and amplicon lengths are shown in Table 2. All primer pairs produced a single product and amplified the target transcript with equal efficiency over a 1000-fold range of input material.
Table 2.
Candidate reference genes and primer sequences used for quantitative PCR analysis
| Gene name | Source gene a | Gene description | Primer sequence b | Amplicon length (bp) |
| poly | SGN-U347154 | hexameric polyubiquitin | 5' CGCTGACTACAATATCCAAAAGGA 3' | 67 |
| 5' CTGCATTCCACCCCTCAGA 3' | ||||
| adh | SGN-U350348 | alcohol dehydrogenase class III | 5' CCTCAAGCCGGCGAAGT 3' | 55 |
| 5' CTGTATGGCAGAGGGCAGTGT 3' | ||||
| actin | SGN-U353034 | actin 7 | 5' AATTGTCCGTGACATCAAGGAA 3' | 82 |
| 5' TGAGCTGCTCTTGGCTGTTTC 3' | ||||
| gapdh | SGN-U347734 | glyceraldehyde-3-phosphate dehydrogenase | 5' TTGAAGGGCGGTGCAAA 3' | 59 |
| 5' AACATGGGTGCATCCTTGCT 3' | ||||
| rpl7 | SGN-U351477 | 60S ribosomal protein L7 | 5' CATTCGAGGTATCAATGCTATGCA 3' | 66 |
| 5' TGTCTCAGGCGCAGAAGCT 3' | ||||
| ccs | SGN-U350284 | caffeine synthase 1 | 5' CAATGCCCGGCTCTTTCTAC 3' | 68 |
| 5' GTAACAAGAGTGTAAAAAATGCATGGA 3' | ||||
| cys | SGN-U352616 | cysteine proteinase | 5' GCGATCGCTACCGTCCAA 3' | 63 |
| 5' CTTTTTCTCTCCAGTCAATGGAGTT 3' | ||||
| 14-3-3 | SGN-U356404 | 14-3-3 protein | 5' TGTGCTCTTTAGCTTCCAAACG 3' | 75 |
| 5' CTTCACGAGACATATTGTCTTACTCAAA 3' |
a Unigene accession number according to the SOL Genomics Network [65]
b Forward (upper line) and reverse (lower line) primer sequences
Quantitative PCR
The PCR mixture contained 5 μl of a 1:10 dilution of the synthesized cDNA, primers to a final concentration of 700 nM each, 17.5 μl of the SYBR Green PCR Master Mix (Applied Biosystems, USA) and PCR-grade water up to a total volume of 35 μl. The mixes were homogenized and split in three samples of 10 μl, thus each gene reaction was performed in triplicate. PCR reactions in the absence of template were also performed as negative controls for each primer pair. An equimolar pool of cDNA samples of five coffee tissue/organ types (root, stem, leaf, flower, and fruit) was prepared to be used as a common reference for all qPCR. The quantitative PCRs were performed employing the ABI Prism 7300 Sequence Detection System (PE Applied Biosystems, USA). All PCR reactions were performed under the following conditions: 2 min at 50°C, 10 min at 95°C, and 45 cycles of 15 s at 95°C and 1 min at 65°C in 96-well optical reaction plates (Applied Biosystems, USA). Confirmation of amplicon specificity was based on the dissociation curve at the end of each run and by product visualization after electrophoresis on an 8% polyacrylamide gel.
Analysis of candidate reference genes
To estimate the expression variation level of the eight candidate genes (actin, adh, ccs, 14-3-3, gapdh, poly, rpl7, or cys) over a five coffee tissue/organ sample set (root, stem, leaf, flower, or fruit), the BestKeeper descriptive statistical method [45,66] was applied.
To access the levels of gene expression for each gene in the different coffee tissue types, the method described by Ramakers et al. [67] with modifications was used. Optic data were exported from 7300 Real-Time PCR System (PE Applied Biosystems, USA) into MS Excel. Four cycles at the exponential phase, near and including the crossing point (CP), were used. The fluorescence data were logarithmically transformed, and pasted into statistical software package (SAS version 8e, SAS Institute, Cary, NC, USA) for linear regression analysis, including determination of intercepts, slopes (x), PCR efficiency (E = 10slope) and their respective standard errors and correlation coefficients (R2). The gene expression values (x-fold) were obtained according to a mathematical model proposed by Pfaffl [68]: x-fold = Ereference gene ΔCP, where ΔCP = CPpool of tissues - CPtissue sample.
Knowing the expression levels, the stability value was assessed utilizing NormFinder [8,49] and geNorm [17,69] programs.
Abbreviations
qPCR: Quantitative PCR; poly: hexameric polyubiquitin; adh: alcohol dehydrogenase class III; actin: actin 7; rpl7: 60S ribosomal protein L7; ccs: caffeine synthase 1; gapdh: glyceraldehyde-3-phosphate dehydrogenase; cys: cysteine proteinase; CP: crossing point.
Authors' contributions
CFB-C and IGM conceived and designed this study. FES performed the biological assays. CFB-C and FES carried out the molecular genetic studies, participated in the qPCR experiments (acquisition, analysis and interpretation of data). MPM and IGM contributed with reagents/materials/analysis tools. IGM coordinated the study. CFB-C wrote the manuscript. All authors contributed, read, corrected and approved the final manuscript. The authors declare no conflict of interest in this work.
Supplementary Material
Results from Bestkeeper descriptive statistical analysis. The data provided represent the descriptive statistics, based on crossing point (CP) values, for the expression analyses of the candidate reference genes in the five distinct coffee plant tissue/organ set.
Coffee tissue/organ sample set (Coffea arabica var. Mundo Novo – IAC 388-17-1) used in the present study. Freshly harvested roots, stems, and leaves were obtained from 4 month-old coffee plants grown under greenhouse conditions (28°C, 60% RH) in Campinas, São Paulo, Brazil. Flower and fruit samples, at different developmental stages, were collected from 4–5 year-old field grown plants in Botucatu and in Campinas, São Paulo, Brazil. After harvesting, fresh tissue samples were frozen immediately in liquid nitrogen until RNA extraction.
Acknowledgments
Acknowledgements
The authors thank Adalgisa Soares de Oliveira, Carla Cristina da Silva and Marcos Brandalise (EMBRAPA/IAC) for providing part of the plant materials used in this study. CFB-C was recipient of a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. FES was recipient of a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. This research was sponsored by Consórcio Brasileiro de Pesquisa e Desenvolvimento do Café (CBP&D-Café), Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Contributor Information
Carla F Barsalobres-Cavallari, Email: barsalobres@gmail.com.
Fábio E Severino, Email: fabio.bjj@gmail.com.
Mirian P Maluf, Email: maluf@iac.sp.gov.br.
Ivan G Maia, Email: igmaia@ibb.unesp.br.
References
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Gachon C, Mingam A, Charrier B. Real-time PCR: what relevance to plant studies? J Exp Bot. 2004;55:1445–1454. doi: 10.1093/jxb/erh181. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Real-time, fluorescence-based quantitative PCR: a snapshot of current procedures and preferences. Expert Rev Mol Diagn. 2005;5:493–498. doi: 10.1586/14737159.5.4.493. [DOI] [PubMed] [Google Scholar]
- Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/S0301-472X(02)00806-8. [DOI] [PubMed] [Google Scholar]
- Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonak J, Lind K, Sindelka R, Sjoback R, Sjogreen B, Strombom L. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Gorzelniak K, Janke J, Engeli S, Sharma AM. Validation of endogenous controls for gene expression studies in human adipocytes and preadipocytes. Horm Metab Res. 2001;33:625–627. doi: 10.1055/s-2001-17911. [DOI] [PubMed] [Google Scholar]
- von Smolinski D, Leverkoehne I, von Samson-Himmelstjerna G, Gruber AD. Impact of formalin-fixation and paraffin-embedding on the ratio between mRNA copy numbers of differently expressed genes. Histochem Cell Biol. 2005;124:177–188. doi: 10.1007/s00418-005-0013-0. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR-a perspective. J Mol Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, Van Wuytswinkel O. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J. 2008 doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research Communications. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. Biotechniques. 2000;29:332–337. doi: 10.2144/00292rv02. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of Real-Time quantitative RT-PCR by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/S0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Papini-Terzi FS, Rocha FR, Vêncio RZ, Oliveira KC, Felix Jde M, Vicentini R, Rocha Cde S, Simões AC, Ulian EC, di Mauro SM, da Silva AM, Pereira CA, Menossi M, Souza GM. Transcription profiling of signal transduction-related genes in sugarcane tissues. DNA Res. 2005;12:27–38. doi: 10.1093/dnares/12.1.27. [DOI] [PubMed] [Google Scholar]
- Vicentini R, Sassaki FT, Gimenes MA, Maia IG, Menossi M. In silico evaluation of the Eucalyptus transcriptome. Genetics and Molecular Biology. 2005;28:487–495. doi: 10.1590/S1415-47572005000400002. [DOI] [Google Scholar]
- Ohl F, Jung M, Radonic A, Sachs M, Loening SA, Jung K. Identification and validation of suitable endogenous reference genes for gene expression studies of human bladder cancer. J Urol. 2006;175:1915–1920. doi: 10.1016/S0022-5347(05)00919-5. [DOI] [PubMed] [Google Scholar]
- Pombo-Suarez M, Calaza M, Gomez-Reino JJ, Gonzalez A. Reference genes for normalization of gene expression studies in human osteoarthritic articular cartilage. BMC Mol Biol. 2008;9:17. doi: 10.1186/1471-2199-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante C, Matsuoka M, Asensio E, Canavate J, Reith M, Manchado M. Selection of housekeeping genes for gene expression studies in larvae from flatfish using real-time PCR. BMC Mol Biol. 2008;9:28. doi: 10.1186/1471-2199-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld B, Elfving B, Wegener G. Reference genes for normalization: A study of rat brain tissue. Synapse. 2008;62:302–309. doi: 10.1002/syn.20496. [DOI] [PubMed] [Google Scholar]
- Langnaese K, John R, Schweizer H, Ebmeyer U, Keilhoff G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol Biol. 2008;9:53. doi: 10.1186/1471-2199-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Royo L, Astudillo A, Escudero D, Alvarez F, Rodriguez A, Gomez E, Otero J. Identifying the most suitable endogenous control for determining gene expression in hearts from organ donors. BMC Molecular Biology. 2007;8:114. doi: 10.1186/1471-2199-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss DL, Baarsch MJ, Murtaugh MP. Regulation of hypoxanthine phosphoribosyltransferase, glyceraldehyde-3-phosphate dehydrogenase and beta-actin mRNA expression in porcine immune cells and tissues. Anim Biotechnol. 1998;9:67–78. doi: 10.1080/10495399809525893. [DOI] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291–295. doi: 10.1016/S0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Lee PD, Sladek R, Greenwood CM, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002;12:292–297. doi: 10.1101/gr.217802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Naka H, Katagiri T, Hirono I. Cloning and characterization of glyceraldehyde-3-phosphate dehydrogenase cDNA of Japanese flounder Paralichthys olivaceus. Fisheries Sci. 2000;66:737–742. doi: 10.1046/j.1444-2906.2000.00120.x. [DOI] [Google Scholar]
- Olsvik PA, Lie KK, Jordal AEO, Nilsen TO, Hordvik I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol. 2005;6:21. doi: 10.1186/1471-2199-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hu J. Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci. 2007;95:356–368. doi: 10.1093/toxsci/kfl161. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett J, Chang J, Kim L, Bustin S, Johnson M, Rook G, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem. 2005;344:141–143. doi: 10.1016/j.ab.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Sturzenbaum SR, Kille P. Control genes in quantitative molecular biological techniques: the variability of invariance. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:281–289. doi: 10.1016/S1096-4959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Nicot N, Hausman J-F, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Ding J, Jia J, Yang L, Wen H, Zhang C, Liu W, Zhang D. Validation of a rice specific gene, sucrose phosphate synthase, used as the endogenous reference gene for qualitative and real-time quantitative PCR detection of transgenes. J Agric Food Chem. 2004;52:3372–3377. doi: 10.1021/jf049915d. [DOI] [PubMed] [Google Scholar]
- Kim BR, Nam HY, Kim SU, Kim SI, Chang YJ. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett. 2003;25:1869–1872. doi: 10.1023/A:1026298032009. [DOI] [PubMed] [Google Scholar]
- Jian B, Liu B, Bi Y, Hou W, Wu C, Han T. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Molecular Biology. 2008;9:59. doi: 10.1186/1471-2199-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Thibivilliers S, Bilgin D, Radwan O, Benitez M, Clough S, Stacey G. Identification of four soybean reference genes for gene expression normalization. The Plant Genome. 2008;1:44–54. doi: 10.3835/plantgenome2008.02.0091. [DOI] [Google Scholar]
- De Los Santos-Briones C, Hernández-Sotomayor SMT. Coffee biotechnology. Brazilian Journal of Plant Physiology. 2006;18:217–227. doi: 10.1590/S1677-04202006000100015. [DOI] [Google Scholar]
- BestKeeper Software http://www.gene-quantification.de/bestkeeper.html
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Singh R, Green MR. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259:365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Ishitani R, Sunaga K, Hirano A, Saunders P, Katsube N, Chuang DM. Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J Neurochem. 1996;66:928–935. doi: 10.1046/j.1471-4159.1996.66030928.x. [DOI] [PubMed] [Google Scholar]
- NormFinder Software http://www.mdl.dk/publicationsnormfinder.htm
- Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM. Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression. Plant Mol Biol Rep. 2004;22:325–337. doi: 10.1007/BF02772676. [DOI] [Google Scholar]
- Glare EM, Divjak M, Bailey MJ, Walters EH. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax. 2002;57:765–770. doi: 10.1136/thorax.57.9.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savli H, Karadenizli A, Kolayli F, Gundes S, Ozbek U, Vahaboglu H. Expression stability of six housekeeping genes: A proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J Med Microbiol. 2003;52:403–408. doi: 10.1099/jmm.0.05132-0. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Santos P, Agostini C, Bon M-C, Petitot A-S, C Silva M, Guerra-Guimaraes L, Ribeiro ANA, Argout X, Nicole M. Coffee (Coffea arabica L.) genes early expressed during infection by the rust fungus (Hemileia vastatrix) Molecular Plant Pathology. 2004;5:527–536. doi: 10.1111/j.1364-3703.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Ganesh D, Petitot A-S, Silva MC, Alary R, Lecouls A-C, Fernandez D. Monitoring of the early molecular resistance responses of coffee (Coffea arabica L.) to the rust fungus (Hemileia vastatrix) using real-time quantitative RT-PCR. Plant Science. 2006;170:1045–1051. doi: 10.1016/j.plantsci.2005.12.009. [DOI] [Google Scholar]
- Silva MdC, Várzea V, Guerra-Guimarães L, Azinheira HG, Fernandez D, Petitot A-S, Bertrand B, Lashermes P, Nicole M. Coffee resistance to the main diseases: leaf rust and coffee berry disease. Brazilian Journal of Plant Physiology. 2006;18:119–147. doi: 10.1590/S1677-04202006000100010. [DOI] [Google Scholar]
- Petitot A-S, Lecouls A-C, Fernandez D. Sub-genomic origin and regulation patterns of a duplicated WRKY gene in the allotetraploid species Coffea arabica. Tree Genetics & Genomes. 2007;4:379–390. doi: 10.1007/s11295-007-0117-x. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem. 2002;302:52–59. doi: 10.1006/abio.2001.5530. [DOI] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real time quantitative PCR: Applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Ke L, Chen Z, Yung W. A reliability test of standard-based quantitative PCR: Exogenous vs. endogenous standards. Mol Cell Probes. 2000;14:127–135. doi: 10.1006/mcpr.2000.0288. [DOI] [PubMed] [Google Scholar]
- Calsa T, Jr, Figueira A. Serial analysis of gene expression in sugarcane (Saccharum spp.) leaves revealed alternative C4 metabolism and putative antisense transcripts. Plant Mol Biol. 2007;63:745–762. doi: 10.1007/s11103-006-9121-z. [DOI] [PubMed] [Google Scholar]
- Mason MG, Schmidt S. Rapid isolation of total RNA and genomic DNA from Hakea actities. Funct Plant Biol. 2002;29:1013–1016. doi: 10.1071/PP01151. [DOI] [PubMed] [Google Scholar]
- Vieira LGE, Andrade AC, Colombo CA, Moraes AHdA, Metha Â, Oliveira ACd, Labate CA, Marino CL, Monteiro-Vitorello CdB, Monte DdC, Giglioti E, Kimura ET, Romano E, Kuramae EE, Lemos EGM, Almeida ERP, Jorge EC, Albuquerque EVS, Silva FR, Vinecky F, Sawazaki HE, Dorry HFA, Carrer H, Abreu IN, Batista JAN, Teixeira JB, Kitajima JP, Xavier KG, Lima LM, Camargo LEA, Pereira LFP, Coutinho LL, Lemos MVF, Romano MR, Machado MA, Costa MMC, Grossi-de-Sá MF, Goldman MHS, Ferro MIT, Tinoco MLP, Oliveira MC, Van Sluys M-A, Shimizu MM, Maluf MP, Eira MTS, Guerreiro Filho O, Arruda P, Mazzafera P, Mariani PDSC, Oliveira RLBC, Harakava R, Balbao SF, Tsai SM, Mauro SMZ, Santos SN, Siqueira WJ, Costa GGL, Formighieri EF, Carazzolle MF, Pereira GAG. Brazilian coffee genome project: an EST-based genomic resource. Brazilian Journal of Plant Physiology. 2006;18:95–108. [Google Scholar]
- Coffee Genome Project http://www.lge.ibi.unicamp.br/cafe/
- Solanaceae Genome Project (SOL) http://www.sgn.cornell.edu/content/coffee.pl
- Pfaffl MW. Quantification strategies in real-time PCR. The real-time PCR encyclopaedia A-Z of quantitative PCR. 2004. pp. 87–120.
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- geNorm Software http://medgen.ugent.be/~jvdesomp/genorm/
- Majerowicz N, Söndahl M. Induction and differentiation of reproductive buds in Coffea arabica L. Brazilian Journal of Plant Physiology. 2005;17:247–254. doi: 10.1590/S1677-04202005000200008. [DOI] [Google Scholar]
- Pezzopane JRM, Pedro Júnior MJ, Thomaziello RA, Camargo MBPd. Escala para avaliação de estádios fenológicos do cafeeiro arábica. Bragantia. 2003;62:499–505. [Google Scholar]
- Morais H, Caramori PH, Koguishi MS, Ribeiro AMdA. Escala fenológica detalhada da fase reprodutiva de Cooffea arabica. Bragantia. 2008;67:257–260. [Google Scholar]
- De Castro RD, Marraccini P. Cytology, biochemistry and molecular changes during coffee fruit development. Brazilian Journal of Plant Physiology. 2006;18:175–199. doi: 10.1590/S1677-04202006000100013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results from Bestkeeper descriptive statistical analysis. The data provided represent the descriptive statistics, based on crossing point (CP) values, for the expression analyses of the candidate reference genes in the five distinct coffee plant tissue/organ set.
Coffee tissue/organ sample set (Coffea arabica var. Mundo Novo – IAC 388-17-1) used in the present study. Freshly harvested roots, stems, and leaves were obtained from 4 month-old coffee plants grown under greenhouse conditions (28°C, 60% RH) in Campinas, São Paulo, Brazil. Flower and fruit samples, at different developmental stages, were collected from 4–5 year-old field grown plants in Botucatu and in Campinas, São Paulo, Brazil. After harvesting, fresh tissue samples were frozen immediately in liquid nitrogen until RNA extraction.