Abstract
Background/ Aim
Weight loss in response to sibutramine is highly variable. We assessed the association of specific markers of polymorphisms of candidate a2A adrenoreceptor, 5-HT transporter and GNβ3 genes and weight loss with sibutramine.
Methods
We conducted a randomized, double-blind, pharmacogenetic study of behavioral therapy and sibutramine (10 or 15 mg daily) or placebo for 12 weeks in 181 overweight or obese participants. We measured body weight, BMI, body composition, gastric emptying and genetic variation (α2A C1291G, 5-HTTLPR, and GNβ3 C825T genotypes). ANCOVA was used to assess treatment effects on, and associations of the specific markers of candidate genes with weight loss and body composition.
Results
Sibutramine, 10 and 15 mg, caused significant weight loss (p = 0.009); there was a statistically significant gene by dose interaction for GNβ3 genotype. For each candidate gene, significant treatment effects at 12 weeks were observed (p<0.017) for all specific genotype variants (delta weight loss in the 2 sibutramine doses versus placebo): α2A CC genotype ( Δ ~5kg), GNβ3 TC/TT genotype (Δ ~6kg), and 5-HTTLPR LS/SS (Δ ~4.5kg). Gene pairs resulted in significantly greater sibutramine treatment effects on weight (both p<0.002): in participants with 5-HTTLPR LS/SS with GNβ3 TC/TT, Δ ~6kg and those with a2A CC with GNβ3 TC/TT, Δ ~8kg; however, effects were not synergistic. Treatment with sibutramine also resulted in significantly greater reduction of body fat for specific α2A CC and GNβ3 TC/TT genotype variants individually (both p<0.02).
Conclusions
Selection of patients with obesity based on candidate genes may enhance response to multidimensional sibutramine and behavioral therapy.
Keywords: Adrenergic, norepinephrine, serotonin, transporter, SLC6A4
INTRODUCTION
Sibutramine, a noradrenergic and serotonergic reuptake inhibitor approved for the long term treatment of obesity, induces satiety, prevents decline in metabolic rate associated with hypocaloric diets (1), and causes weight loss especially when combined with behavioral therapy (2–4). However, there are large differences in weight loss among individuals treated with sibutramine.
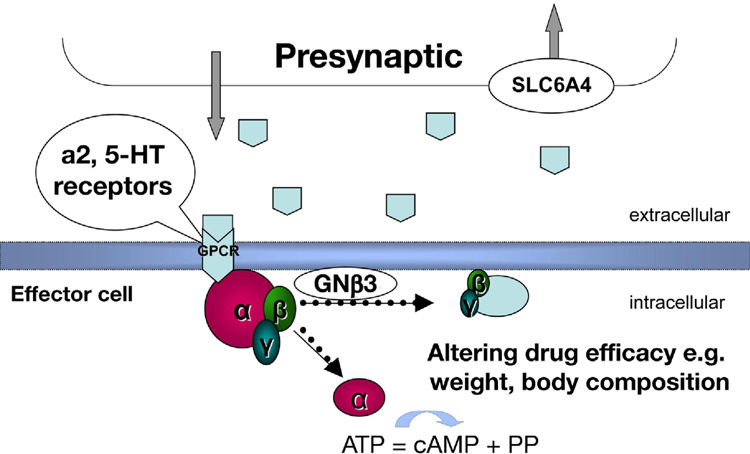
Genetic variations that potentially affect serotonergic and adrenergic functions may influence appetite, glucose and fat metabolism, and responses to psychotropic medication (5–8). This study explored whether genetic variations may account for the differences in weight loss with sibutramine treatment by examining polymorphisms of specific markers of candidate genes that may affect sibutramine’s pharmacological actions (Figure 1): α2A adrenoreceptors; the serotonin transporter protein, SERT [or solute carrier 6A4 (SLC6A4)]; and the downstream effects of ligand-receptor interaction mediated through guanine nucleotide binding (G) proteins. G proteins are composed of three different α, β, and γ subunit isoforms which are involved in transduction of ~80% of ligand-receptor interactions; the βγ subunits form a functional monomer. On receptor activation, α and βγ subunits dissociate and modulate several intracellular effector systems.
Figure 1. Conceptual diagram.
shows the potential interaction between α2A adrenergic mechanisms, G protein translation and serotonin reuptake through SLC6A4 in mediating the effects of the norepinephrine and serotonin reuptake inhibitor, sibutramine.
The specific variations in the three candidate genes were selected because of associations with differences in biological functions. The C-1291G single nucleotide polymorphism (SNP) of the α2A-adrenoreceptor gene is located in the promoter region and may alter gene expression and receptor density. With the 5-HTTLPR variation located in the promoter of the SLC6A4 gene, the short allele is associated with reduced function of SERT (5), variation in response to SSRI in generalized social anxiety disorder (9) and in tolerability of serotonergic antidepressants (10).
The Gβ3 protein is encoded by the GNβ3 gene, in which there is a common C825T SNP located on exon 10. The 825T allele results in alternative splicing, formation of a truncated splice variant, Gβ3s, enhanced G-protein activation, association with affective and metabolic disorders (11,12), and altered response to α2-adrenergic activation (13).
Neither of these genetic variants is conclusively associated with obesity per se, though α2A-adrenoceptor gene is associated with storage of fat in the abdominal area (14), 5HTTLPR with change in BMI from adolescence to young adulthood (15), and with obesity /overweight in an adult male population (16). In preliminary pharmacogenetic studies, GNβ3 CC genotype [111 patients (17)] and SLC6A4 LS/SS (heterozygous/short) genotype [48 patients evaluated in our laboratory (18)] were associated with greater weight loss with sibutramine treatment than placebo. In our prior study (18), sibutramine-induced weight loss was also associated with delayed gastric emptying of solids measured by radioscintigraphy.
The aim of this study was to examine, in overweight and obese individuals, the influence of specific markers of candidate genes controlling serotonergic and adrenergic mechanisms (specifically, α2A-receptor, 5-HTTLPR and GNβ3) on weight loss and body composition change in response to sibutramine or placebo treatment. Secondary aims were to evaluate whether weight at 4 weeks of treatment predicted weight achieved at 12 weeks of treatment, and whether the candidate genes also influenced treatment effects on weight loss at 4 weeks.
METHODS
Study Design and Participants
This was a randomized, double-blind, placebo-controlled, parallel-group study of two-doses (10 and 15 mg) sibutramine in combination with behavioral therapy and its effects on weight loss; we explored the influence on weight and body composition of specific markers indicative of variation in three candidate genes. Although the study was conducted at a tertiary referral center,,the 181 otherwise healthy overweight and obese participants were recruited from among people in southeastern Minnesota. The study was approved by the Mayo Clinic Institutional Review Board. All participants provided signed informed consent.
Eligibility Criteria
Overweight and obese males and females, aged 18–65 years with a BMI of 25–50 Kg/m2, who were not currently on appetite suppressants, orlistat or MAOI inhibitors. Volunteers who were not on treatment for cardiac, pulmonary, gastrointestinal, hepatic, renal, hematological, neurological, or endocrine conditions were eligible for participation. Individuals with unstable psychiatric disease, weight >300 pounds, blood pressure >140 mmHg systolic and >90 mmHg diastolic, or >105 mm Hg diastolic after a 30 minute rest period were excluded.
Randomization and Interventions
Participants were recruited between July 2006 and September 2007. Participants received placebo or sibutramine, 10 or 15 mg daily, for 12 weeks in a 1:1:1 randomized, parallel-group design. Patient randomization to each treatment group was balanced on gender, 5HTTLPR variation, and BMI category (overweight versus obese) to ensure balanced randomization in the treatment groups as these are potentially influential covariates, based on our preliminary study results (18). Treatment allocation was concealed to the participants and investigators. Participants received standardized, structured behavioral therapy for weight management at entry, 4, 8 and 12 weeks later.
Behavioral Therapy
To standardize for potential differences in the behavioral aspects of weight reduction therapy, all participants were given a standard behavioral weight management text, the “LEARN” Manual (19) and were to read one of the 12 sessions, in chronological order, each week. Participants met for 15 minutes with a master’s or doctoral level psychologist with training in obesity treatment at study entry and at weeks 4, 8 and 12. The psychologists followed a structured study session outline and documented the content of their visit to ensure adherence to the behavior therapy protocol.
Characterization of Participants at Baseline
Motivational level for exercise
At time of entry to the study, the motivational level for physical activity was assessed using the Stage of Change for Physical Activity Questionnaire (20) to identify the proportion in each treatment group performing regular physical activity.
Psychological assessment of participants
In order to screen for significant psychiatric or psychological dysfunction, we used the Hospital Anxiety and Depression Scale [HADS (21)], the self-administered alcoholism screening test [SAAST, substance abuse (22)], the Questionnaire on Eating and Weight Patterns-Revised (binge eating disorder and bulimia), and the Weight Efficacy Life-Style Questionnaire (WEL). bipotential participants with HADS score of ≥8 on a subscale, or a total score of ≥11, or difficulties with substance or eating disorders were excluded and referred to their primary care doctor for further health care.
Detection of Variations of Candidate Genes
Genomic DNA was isolated using the Gentra Puregene Blood Kit (Qiagen Inc., Valencia, CA). Venous blood DNA was analyzed for 5HTTLPR (by PCR followed by gel electrophoresis), α2A C1291G (restriction site MspI, rs1800544), and GNβ3 C825T (rs5443 by TAQMAN®, Applied Biosystems, Foster City, CA) genotypes. We have published elsewhere (18,23,24) the methods and frequency distribution of alleles in controls. Assay details and GenBank numbers for each polymorphism are included in the Appendix.
Weight, Blood Pressure and Compliance Measurements
Automated measurements of body weight and blood pressure were recorded in the Clinical Research Unit at the start, monthly, and at week 12, and fasting blood glucose at baseline and week 12. Participants were evaluated by the study coordinator or physician (for ~10 minutes) every 4 weeks for waist, hip and thigh measurements, medical monitoring (symptoms of increased blood pressure and potential medication side effects), adherence to study protocol and medications, and use of prescription or over-the-counter medications. At the end of each 4 weeks’ of treatment, compliance to medication intake was assessed by pill count.
Body Composition
Total body fat was measured by dual energy x-ray absorptiometry (Lunar Corp., Madison, WI) in the Mayo Clinical Research Unit (25) at baseline and at week 12.
Gastric Emptying
Prior to treatment and in the last week of the treatment phase, participants underwent a stable isotope breath test to measure gastric emptying (26). The test meal consisted of 100 mg [13C]-Spirulina platensis, 27 g freeze dried egg mix, 6 saltine crackers, and 6 oz water and had a caloric content of 238 kcal. Breath samples obtained at 45, 90, 120,150, 180, and 240 minutes post-meal were collected and stored in Vacutainers (Becton Dickinson, Franklin Lakes, NJ) and submitted for measurement of 13CO2 enrichment by an external reference laboratory. The use of this test was conducted under IND #67,129 (held by ABDiagnostics, LLC, Brentwood, TN). The method for calculating gastric emptying t1/2 followed prior studies in our laboratory (26).
Statistical Analyses
Sample size considerations are included in the Appendix.
All analyses used the intent-to-treat principle. The primary endpoint was weight (kg) measured at 12 weeks post-start of treatment; secondary endpoints were body fat composition and gastric emptying of solids. Unadjusted p values are provided; thus, p<0.017 suggests significant associations for the three candidate variations with weight loss.
ANCOVA models were used to assess associations of each candidate gene (wildtype versus non-wildtype) with weight loss, that is, to compare the placebo and two sibutramine groups at the end of the treatment period, using as covariates corresponding baseline weight, BMI category, gender, and genetic marker status (wildtype versus non-wildtype). A summary of the actual weight loss (pre-treatment baseline weight and the 12-week post start of treatment weight) was also compiled for each treatment group in subjects who completed the study (“per protocol subset”). In order to adhere to the intent-to-treat paradigm, subjects with missing values had their post-treatment weight imputed using the overall (patients) mean value with a corresponding adjustment in the residual error degrees of freedom of the ANCOVA (i.e., subtracting one degree of freedom for each missing value imputed). A similar analysis was used to assess treatment effects on the 4-week post-start of treatment weight values. In addition, a multiple linear regression model was used to predict the 12-week post-treatment weights using baseline weight, BMI group, gender treatment group, and weight at week 4 post-treatment. The partial r-square values in this analysis provided an estimate of the proportion of variation in week 12 weight values accounted for by week 4 weight values‥
Separate analyses with gender, each individual genetic marker, and treatment group by genetic marker interaction term were included as covariates in these separate ANCOVA models for post-treatment weight. The corresponding F-tests in the ANCOVA for these interaction terms provided tests of the hypothesis that genetic marker status influences response (e.g., weight) to treatment.
Since sufficient numbers of patients in specific pairwise combinations of the genetic markers were observed, similar additional ANCOVA models examined whether pairwise combinations of genetic markers affect the weight change response to treatment.
χ2 tests were used to explore whether adverse events were associated with genetic variations.
Finally, we assessed gastric emptying T1/2 and compared the three treatment groups by ANCOVA using baseline T1/2 values as covariate.
RESULTS
Demographics, Genotype Distributions and Study Flow
At baseline, the three groups had similar demographic, obesity (weight, BMI, waist), fasting blood glucose and genotype distributions (Table IA and IB). The trial flow is summarized in the supplemental figure at the end of the paper. Adherence with study medication for all participants over the three months of treatment was 97±0.3% (mean use at months 1, 2 and 3 being 97.7, 96.6 and 96.7% respectively). Thirteen subjects withdrew from the two sibutramine groups, 1 due to hypertension (10mg dose). Ten subjects in the placebo group withdrew from the study, 1 due to hypertension.
Table I.
| Table IA. Demographics and Psychological Characteristics at Baseline (mean ± SE unless otherwise noted) | |||
|---|---|---|---|
| Placebo (N=62) |
Sibutramine, 10 mg (N=58) |
Sibutramine, 15 mg (N=61) |
|
| # obese / # overweight | 51/11 | 48/10 | 50/11 |
| Age, y | 41.3 ± 1.4 | 42.2 ± 1.4 | 44.8 ± 1.3 |
| Gender (%Female) | 87% | 91% | 89% |
| Waist circumference, cm | 105.1 ± 1.6 | 102.3 ± 1.5 | 101.9 ± 1.5 |
| Weight, kg | 95.9 ± 2.1 | 95.6 ± 2.2 | 93.1 ± 2.2 |
| BMI, kg/m2 | 34.5 ± 0.6 | 34.8 ± 0.7 | 33.9 ± 0.6 |
| Fasting blood glucose, pre | 94.6 ± 1.3 | 95.6 ± 2.2 | 93.2 ± 2.1 |
| Anxiety (HAD score) | 4.4 ± 0.3 | 5.0 ± 0.4 | 4.5 ± 0.4 |
| Gastric emptying, T ½, min | 77.9 ± 2.5 | 82.1 ± 3.1 | 82.4 ± 2.5 |
| Depression (HAD score) | 3.1 ± 2.3 | 3.3 ± 0.3 | 3.0 ± 0.3 |
| SAAST score | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 |
| Hunger | 5.7 ± 0.4 | 5.4 ± 0.5 | 5.4 ± 0.4 |
| Disinhibition | 8.9 ± 0.5 | 8.8 ± 0.5 | 8.8 ± 0.4 |
| Cognitive Restraint | 9.8 ± 0.6 | 9.1 ± 0.6 | 10.6 ± 0.7 |
| % of participants performing regular physical activity | 40 | 31 | 41 |
| Table IB. Distribution of Genotypes (single and combined) by Treatment Groups | ||||
|---|---|---|---|---|
| Note that randomization was balanced on 5HTTLPR genotype. The randomization also resulted in similar distributions of single and combined genotypes, particularly for α2A and 5HTTLPR. | ||||
|
Genotypes: |
Placebo (n=62) |
Sibutramine, 10 mg (n=58) |
Sibutramine, 15 mg (n=61) |
P values§ |
| α2A | 0.62 | |||
| α2A (CC), n (%) | 36 (58) | 29 (50) | 31 (51) | |
| α2A (GC/GG), n (%) | 26 (42) | 29 (50) | 30 (49) | |
| GN β3 | 0.08 | |||
| GNβ3 (CC), n (%) | 22 (35) | 31 (53) | 32 (52) | |
| GNβ3 (TC/TT), n (%) | 40 (65) | 27 (47) | 29 (48) | |
| 5HTTLPR | 0.92 | |||
| 5HTTLPR (LL), n (%) | 20 (32) | 17 (29) | 18 (29) | |
| 5HTTLPR (LS/SS), n (%) | 42 (68) | 41 (71) | 43 (71) | |
| α2A and GN β3 | 0.25 | |||
| α2A (CC) and GN β3 (TC/TT), n(%) | 28(45) | 15(26) | 16(26) | |
| α2A (CC) and GN β3 (CC), n(%) | 8(13) | 14(24) | 15(25) | |
| α2A (CG/GG) and GN β3 (TC/TT),n(%) | 12(19) | 12(21) | 13(21) | |
| α2A (CG/GG) and GN β3 (CC),n(%) | 14(23) | 17(29) | 17(28) | |
| 5HTTLPR and GN β3 | 0.31 | |||
| 5HTTLPR (LS/SS) and GN β3 (TC/TT),n(%) | 28(45) | 17(29) | 18(30) | |
| 5HTTLPR (LS/SS) and GN β3 (CC),n(%) | 14(23) | 24(41) | 25(41) | |
| 5HTTLPR (LL) and GN β3 (TC/TT),n(%) | 12(19) | 10(17) | 11(18) | |
| 5HTTLPR (LL) and GN β3 (CC),n(%) | 8(13) | 7(12) | 7(11) | |
| α2A and 5HTTLPR | 0.41 | |||
| α2A (CC) and 5HTTLPR (LS/SS), n(%) | 21(34) | 19(38) | 24(31) | |
| α2A (CC) and 5HTTLPR (LL), n(%) | 15(24) | 10(17) | 7(11) | |
| α2A (CG/GG) and 5HTTLPR (LS/SS), n(%) | 21(34) | 22(38) | 19(31) | |
| α2A (CG/GG) and 5HTTLPR (LL), n(%) | 5(8) | 7(12) | 11(18) | |
Three-Factor Eating Questionnaire was used to measure hunger, disinhibition, cognitive restraint; SAAST score is derived from responses to self-administered alcoholism screening test, based on 35 questions. As would be expected based on the randomization, there were no significant differences in the three groups.
Chi-square test for association between genotype (or genotype combination) and treatment group assignment.
Baseline Psychological Assessment
The three study groups were similar in eating behaviors (scores on the Three Factor Eating Inventory of hunger, cognitive restraint and disinhibition - Table 1A) and in the self-report of symptoms of anxiety or depression (by the HADS), difficulties with alcohol usage (SAAST) and motivational level for physical activity.
Weight, BMI, Body Composition and Gastric Emptying after 12 Weeks Treatment
There were statistically significant effects of sibutramine on body weight, BMI and percent fat (based on analysis of body composition). Sibutramine, 10 and 15 mg doses, resulted in significantly lower values for weight, BMI and proportion of body fat (Table IIA, which shows ITT analysis) compared to placebo (p<0.01, p<0.001, and p=0.05, respectively). Table IIA summarizes the least squares mean values that are based on the post-treatment observations (e.g., weight) corrected for baseline differences. There was no significant overall treatment effect of sibutramine on gastric emptying of solids.
Table II.
| Table IIA. Effect of Sibutramine Treatment on Weight, BMI, and Body Fat Composition and Influence of Genotype on Effect of Sibutramine Treatment on Proportion of Fat [values are least squares means ± SEM (adjusted for covariates) from ANCOVA analyses] | ||||
|---|---|---|---|---|
| Placebo | Sibutramine, 10 mg | Sibutramine, 15 mg | p † | |
| Weight, kg * | 94.3±1.2 | 90.5±1.3 | 91.3±1.2 | 0.009 |
| BMI, kg/m2 | 34.4±0.4 | 33.0±0.4 | 33.0±0.4 | 0.001 |
| Gastric emptying T 1/2, min | 87.6±4.6 | 84.9±4.8 | 79.5±4.6 | ns |
| Dexa body composition, lean | 43.6±0.8 | 43.1±0.8 | 42.7±0.7 | ns |
| Dexa body composition, % fat | 51.2±0.8 | 49.7±0.8 | 50.0±0.8 | 0.051 |
| Dexa % fat by genotype: α2A CC | 51.6±0.9 | 49.8±0.9 | 49.1±0.9 | 0.016 |
| Dexa % fat by genotype: α2A CG/GG | 50.9±0.9 | 49.8±0.9 | 51.0±0.9 | ns |
| Dexa % fat by genotype: 5HTTLPR LL | 51.3±1.0 | 49.4±1.1 | 50.9±1.1 | ns |
| Dexa % fat by genotype:5HTTLPR LS/SS | 51.3±0.9 | 50.0±0.9 | 49.7±0.9 | ns |
| Dexa % fat by genotype: GNβ3 CC | 51.1±1.0 | 50.4±1.0 | 50.8±0.9 | ns |
| Dexa % fat by genotype: GNβ3 TC/TT | 51.5±0.9 | 49.3±0.9 | 49.3±1.0 | 0.014 |
| Table IIB. Per Protocol Observations of Weight, BMI and Gastric Emptying in the Therapeutic Trial | |||
|---|---|---|---|
| Placebo | Sibutramine, 10 mg | Sibutramine, 15 mg | |
| N= | 52 | 52 | 54 |
| Weight, kg, at baseline | 97.4±2.3 | 95.9±2.3 | 94.4±2.2 |
| Weight, kg, at end of 12 weeks’ treatment | 96.1±2.4 | 91.4±2.4 | 89.6±2.3 |
| BMI, kg/m2 at baseline | 35.3±0.7 | 35.0±0.8 | 34.3±0.6 |
| BMI, kg/m2 at end of 12 weeks’ treatment | 34.8±0.7 | 33.4±0.8 | 32.5±0.6 |
| Gastric emptying T1/2, min at baseline | 77.8±3.0 | 81.2±3.4 | 82.8±2.8 |
| Gastric emptying T1/2, min at end of 12 weeks’ treatment | 87.1±5.1 | 87.2±5.1 | 81.3±3.0 |
| Table IIC. Proportion (%) ± SE (%) of Patients who Lost >5kg Weight in 12 Weeks of Treatment with Placebo (PLA) or Sibutramine (SIB) Based on Genotype of Interest (per protocol results, “collapsing” over other genotype) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
α2A CC |
α2A CG/GG |
GNβ3 TC/TT |
GNβ3 CC |
5HTTLPR LS/SS |
5HTTLPR LL |
α2A CC + GNβ3 TC/TT |
α2A CG/GG + GNβ3 CC |
|
| PLA | 13% ± 6% | 18% ± 8% | 9% ± 5% | 26% ± 10% | 14% ± 6% | 19% ± 10% | 4% ± 4% | 14% ± 10% |
| SIB † | 49% ± 7% | 26% ± 6% | 56% ± 7% | 22% ± 6% | 40% ± 6% | 36% ± 6% | 63% ± 9% | 11% ± 6% |
From ITT analyses based on ANCOVA models (genotype specific values unadjusted for multiple gene specific comparisons)
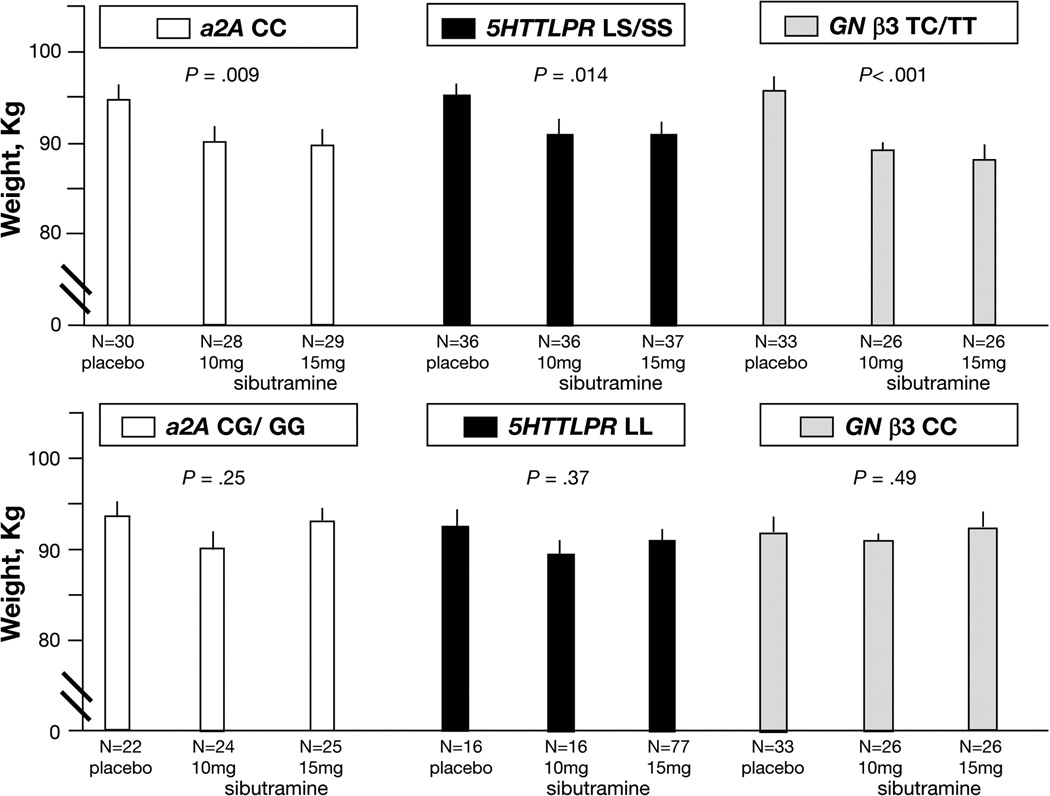
Effect of genotype on treatment-induced weight loss is shown in figure 2. Individual candidate genes were associated with ~5kg weight loss, and two pairwise combinations of these candidate genes were associated with ~7kg during three months’ treatment with sibutramine (see figure 2).
(summarized across 2 doses)
The per protocol data (participants completing the therapies) are summarized in Table IIB.
Influence of Individual Candidate Genes on Weight Following Treatment with Sibutramine
There was a statistically significant gene by treatment (placebo, sibutramine 10mg, and sibutramine 15mg doses) interaction for GNβ3 genotype, reflecting differential treatment effects in one GNβ3 genotype (TC/TT) versus the other (CC) genotype (p=0.018); this is reflected in the different patterns of the effects of sibutramine compared to placebo in association with the two GNβ3 genotypes (TC/TT in contrast to CC; see Figure 2, right side). Such statistical interaction of treatment group and genotype was not observed with α2A CC versus CG/GG (p=0.33) or with 5HTTLPR LS/SS versus LL (p=0.64) genotypes. Thus, the patterns of post-treatment weights with the 2 sibutramine doses and placebo were not sufficiently different for the two variants of α2A and 5HTTLPR.
Figure 2. Relationship between 12-Week Post-Treatment Body Weight and Sibutramine Dose Based on Single Genotypes.
P-values indicate overall treatment effects for specific genotype. Least squares (adjusted for baseline weight and other covariates) means ± SE are plotted, unadjusted p-values from ITT analyses based on ANCOVA models. N refers to number of patients with complete data in each group. Upper panel identifies significant effects of sibutramine on post treatment weight in patients with the single genetic makeup indicated. Lower panel shows the corresponding treatment group values of post-treatment weight in patients with the alternative genetic variations for these single genes, and in which no significant treatment effects were detected.
However, for each candidate gene, significant overall treatment effects were observed in one genotype variant (specifically α2A CC [p=0.009], GNβ3 TC/TT [p<0.001], and 5HTTLPR LS/SS [p=0.014] genotypes), but not in the corresponding alternative (α2A CG/GG, GNβ3 CC, and 5HTTLPR LL) genotype. The post-treatment mean weights for specific genotypes and the corresponding p-values from the ANCOVA analyses for treatment effects in these specific subgroups (based on ITT) are shown in Figure 2.
Weight loss on sibutramine (averaged over 10 and 15 mg doses) versus placebo was observed for α2A CC (~5kg loss), GNβ3 TC/TT (~6kg loss), and 5HTTLPR LS/SS genotypes (~4.5kg). The proportions of participants receiving placebo or sibutramine who lost >5kg weight in association with the genotypes of interest are shown in Table IIC. From the results for sibutramine, the sensitivity and specificity for each individual genotype and the combination of α2A CC with GNβ3 TC/TT genotypes to predict a >5kg weight response to sibutramine can be computed: α2A CC (sensitivity 49%, specificity 74%), GNβ3 TC/TT (sensitivity 56%, specificity 78%), 5HTTLPR LS/SS (sensitivity 40%, specificity 64%), and the combination of α2A CC with GNβ3 TC/TT (sensitivity 63%, specificity 89%).
Influence of Individual Candidate Genes on Body Composition Following Treatment with Sibutramine
For two of the three candidate genes, significant reduction in the proportion of fat (on body composition) was observed in response to sibutramine compared to placebo for one genotype polymorphism: α2A CC and GNβ3 TC/TT (both p<0.02, ITT results in Table II).
Gene-Gene Combinations and Weight Following Treatment with Sibutramine
Given the significant treatment by GNβ3 interaction noted above, we explored three way interactions between treatment, 5HTTLPR, and GNβ3 (p=0.31), or between treatment, α2A, and GNβ3 (p=0.84); however neither of these three way interactions were significant.
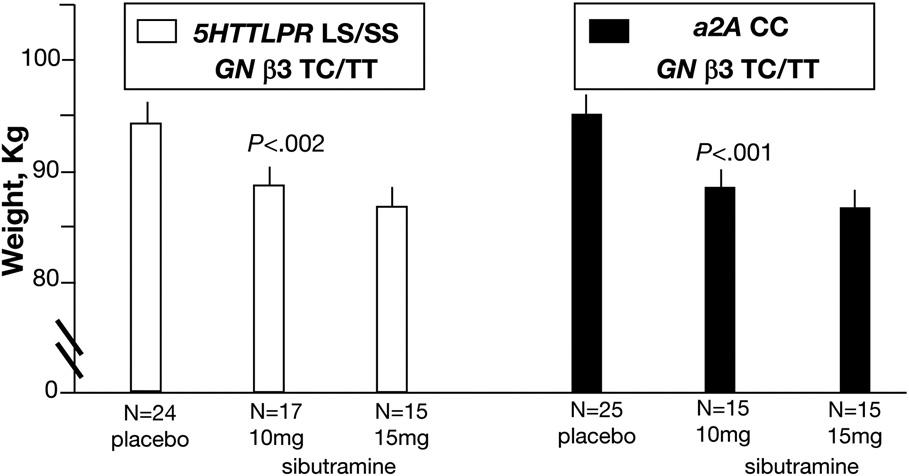
However, there were significant treatment effects (ITT results in Table II and Figure 3) demonstrated for specific combinations of genotypes in which lower post-treatment weights were observed on sibutramine compared to placebo. The three combinations of interest showed the following:
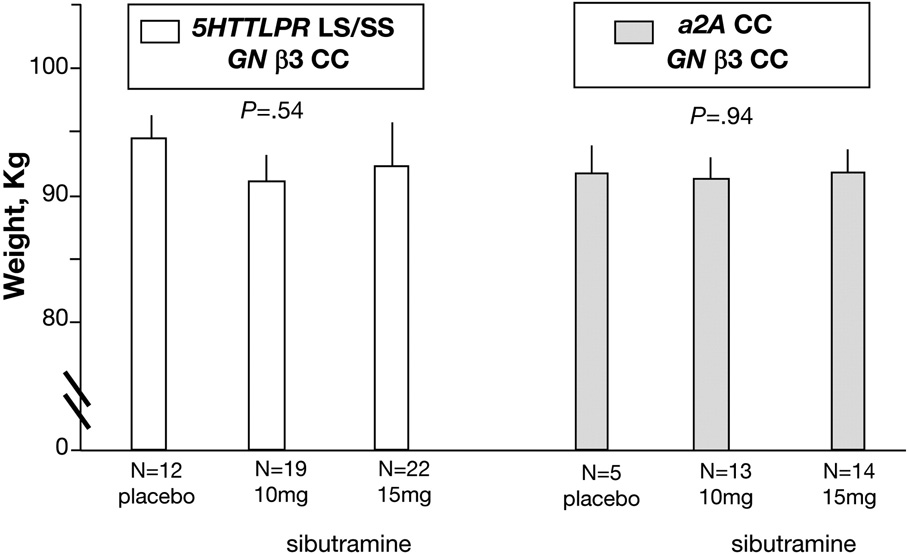
For combination of 5HTTLPR LS/SS with GNβ3 TC/TT (treatment effects, p<0.002, unadjusted, Figure 3, left panel), post-treatment differences were ~6kg, averaged over two doses of sibutramine. In contrast, in patients with the combination 5HTTLPR LS/SS and GNβ3 CC, treatment effects were not observed (p=0.54, Figure 4, left panel).
For combination of α2A CC with GNβ3 TC/TT (treatment effects, p<0.001, unadjusted, Figure 3, right panel), post-treatment differences were of ~8kg, averaged over two doses of sibutramine. In contrast, in patients with the combination of α2A CC and GNβ3 CC, no treatment effects were observed (p=0.94, Figure 4, right panel).
For combination of α2A CC and 5HTTLPR LS/SS, the unadjusted p value for treatment effects was 0.032 (therefore deemed nonsignificant), while treatment effects in the combination of α2A CC/CG and 5HTTLPR LS/SS were not observed (p=0.24, data not shown).
Figure 3. Relationship between 12-Week Post-treatment Body Weight and Sibutramine Dose Based on Gene Combinations.
P-values indicate overall treatment effects for specific genotype combination. Least squares (adjusted for baseline weight and other covariates) means ± SE are plotted, unadjusted p-values from ITT analyses based on ANCOVA models. N refers to number of patients with complete data in each group. There were significant effects of sibutramine on post treatment weight in patients with the specified genetic combination indicated.
Figure 4. Relationship between 12-Week Post-treatment Body Weight and Sibutramine Dose Based on Gene Combinations with GNβ3 Variations.
P-values indicate overall treatment effects for specific genotype combination. Least squares (adjusted for baseline weight and other covariates) means ± SE are plotted, unadjusted p-values from ITT analyses based on ANCOVA models. N refers to number of patients with complete data in each group. Treatment effects were not detected in these combinations. The lack of effects of these gene combinations with GNβ3 CC genotype (data in Figure 4) should be contrasted the significant effects of 5HTTLPR LS/SS or α2A CC with GNβ3 TC/TT (data provided in Figure 3).
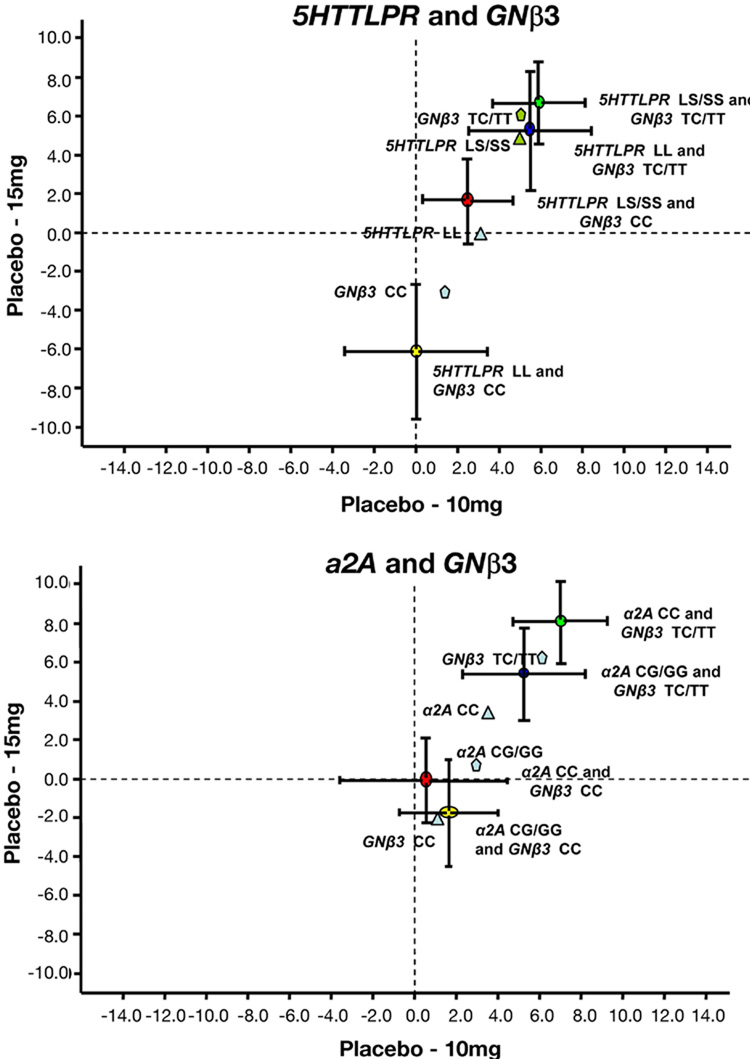
We also assessed whether the weight loss in response to sibutramine was greater for the combinations of two genes than the sum of the weight loss observed with each one of the two genes. Separate estimates of the effect of 15 mg and 10 mg sibutramine versus placebo are shown for gene combinations and individual genes of interest in Figure 5. In summary, there was no evidence that the combined genes were synergistic in response to sibutramine. The predominant effects in the combinations was attributable to the GNβ3 TC/TT genotype.
Figure 5. Estimates of treatment effect of sibutramine, 15 mg, vs. placebo (Y-axis) and sibutramine, 10 mg, vs. placebo (X-axis) in association with combined versus individual genotype variation.
Note the differences in the responses to each dose of drug relative to placebo are not significantly greater for the different combinations of two genotype variations (means+SEM) relative to weight loss with individual genotype variations (mean data shown for clarity).
Weight Loss at 4 Weeks’ Treatment, Influence of Candidate Genes, and Prediction of 12 Week Weight Loss
Overall treatment effects were observed at 4 weeks (p=0.002) with both 10 mg and 15 mg, differing from placebo (p<0.05, adjusted for two tests). Weight loss at 4 weeks was a significant predictor of weight at 12 weeks, even after adjusting for baseline weight, gender, BMI group and treatment group (partial r-square=0.77, implying that 77% of the variation in weight values at 12 weeks could be explained by the weight values at 4 weeks).
Overall sibutramine treatment effect results for the same, specific gene variants at 4 weeks were similar to those observed at 12 weeks for patients with GNβ3 TC/TT (p<0.001), 5HTTLPR LS/SS (p=0.002), and α2A CC (p<0.002). However, the GNβ3 by treatment dose interaction, which was significant at 12 weeks, was not significant at 4 weeks (p=0.27).
Adverse Events and Influence of Candidate Genes
Table III lists adverse events which were consistent with known adverse effects of sibutramine. A significantly higher proportion of participants on sibutramine reported dry mouth and constipation at any time during the trial. There were no significant associations of any of the adverse events in Table III and candidate genes.
Table III. Adverse Events Noted at Any Time during Therapeutic Trial in More than 5% Participants for Any Treatment Group (values listed are percentages of patients randomized in each treatment group) and Participants with Elevated Blood Pressure.
There were no significant associations between candidate genes and adverse events.
| Placebo | Sibutramine 10 mg | Sibutramine 15 mg | p-value§ | |
|---|---|---|---|---|
| Dry Mouth | 24 | 59 | 70 | <0.001 |
| Constipation | 8 | 24 | 34 | 0.006 |
| Insomnia | 8 | 24 | 25 | 0.068 |
| Headache | 11 | 5 | 13 | 0.28 |
| Anxiety/Restlessness | 2 | 7 | 0 | 0.061† |
| Palpitations | 0 | 7 | 8 | 0.82 |
| Elevated BP* | 0 | 2 | 2 | 1.0† |
In addition, 1 participant randomized to 10 mg sibutramine withdrew because of elevated BP.
Chi-square test for association with treatment group
Fisher’s Exact Test
DISCUSSION
In this double-blind trial, sibutramine in combination with behavioral therapy resulted in the anticipated degree of weight reduction, but it did not retard gastric emptying of solids. Sibutramine reduced the maximum calorie ingestion in a challenge meal in humans (18) and fundic accommodation in dogs (27), suggesting its effects on weight loss may be mediated centrally (e.g., at the appetite center) or peripherally by changing satiation.
The degree of weight loss at 4 weeks predicted weight loss at 12 weeks, consistent with. analysis of pooled data from seven studies of sibutramine-induced weight loss showing that 2-kg weight loss at 1 month has high sensitivity but low specificity as an indicator of long-term success among non-diabetic subjects (28). Our study provides insights on the pharmacogenetics of sibutramine in combination with behavioral therapy in obesity. In addition to the magnitude of weight loss at 4 or 12 weeks (28), which is included in the recommendation for sibutramine prescription, candidate gene variations provide useful markers of response to sibutramine. They may help select obese patients likely to experience improved outcome with this multimodal treatment since the different markers are present in almost 50% of patients..
Effects of Single Gene Variations
In support of the primary aim of the study, variations in the candidate α2A receptor, 5HTTLPR and GNβ3 genes were individually associated with the degree of weight loss with sibutramine at 12 weeks (the primary goal of the study), as well as at 4 weeks.
These results confirm our preliminary observation (18) that participants with 5HTTLPR LS/SS (but not LL) genotype had greater weight loss with sibutramine compared to placebo. The LS/SS genotype reduces reuptake of 5-HT (5), potentially complementing the pharmacological effect of the serotonin reuptake inhibition by sibutramine. Pharmacogenetic effects of 5HTTLPR genotypes are documented in psychiatric disease (9, 10, 29) and irritable bowel syndrome (30).
However, results in our current study do not confirm the observation of Hauner et al. that GNβ3 CC genotype was associated with greater weight loss on sibutramine versus placebo (17). Our study shows that the GNβ3 TT/TC genotype was associated with greater weight loss with sibutramine than with placebo. The 825T allele is associated with a functionally active splice variant of the G-protein β3 subunit (Gβ3-s) and enhanced intracellular signal transduction. Our hypothesis was that the T allele is associated with greater weight loss since sibutramine inhibits presynaptic reuptake of the neurotransmitters allowing for greater pharmacological action through enhanced intracellular signal transduction.
Effect of Combinations of Gene Variations
Combinations of gene variations were associated with significantly greater weight loss in response to sibutramine than placebo. However, there was no evidence of synergism between combinations of two genotypes on response to sibutramine therapy compared to the effect on weight loss associated with individual genotypes (Figure 5).
Gene-to-gene interactions involving adrenergic genotype variation (chiefly β3 and α2B adrenoceptor genes) were previously associated with obesity phenotype (31–33), but not with pharmacogenetics of obesity therapy.
Potential Effects of Genetic Variation on Adrenergic Mechanisms in Obesity
In animal studies, norepinephrine secretion is strongly associated with eating: α2-adrenoceptors increase and α1-adrenoceptors suppress eating through effects on paraventricular nucleus of the hypothalamus (34). The hypophagic effects of sibutramine and its metabolite are attributed in part to their α1-adrenergic effects (35). Hindbrain adrenergic neurons orchestrate multiple concurrent glucoregulatory responses involved in feeding and appetite control (36), Apart from the central effects, α2A adrenoceptors are involved in the inhibition of subcutaneous fat lipolysis, which favors weight gain (37).
Several lines of evidence suggest that α2A C-1291G polymorphism is functionally important through its significant associations with measures of receptor function (38); attention deficit hyperactivity disorder (ADHD); improvement of inattention with methylphenidate treatment in ADHD (39); higher weight gain in schizophrenic patients treated with clozapine and olanzapine (40,41); and personality traits in adolescents (42). The influence of this candidate gene on loss of weight with sibutramine appears unrelated to the gene’s association with psychiatric or personality traits since all treatment groups in this study were balanced on all domains.
This study suggests that α2A C-1291C genetic variation may result in reduced presynaptic uptake of norepinephrine at α2A adrenoceptors, which may also complement the reuptake inhibition by sibutramine and contribute to the greater weight loss.
Study Strengths and Limitations
The strengths include study design (placebo-controlled, randomized, dose-response study), standardized behavioral treatment, single-center conduct and team to ensure consistency, racial homogeneity of the study population, use of a candidate gene approach to avoid spurious results from multiple comparisons, selection of biomarkers to assess each candidate gene based on biological relevance, and a priori sufficient statistical power to assess each gene biomarker of interest based on frequency of the minor allele of each gene and the sample size that was feasible in a single-center study. Moreover, given the preliminary data from the prior study (18), participant randomization was based on gender, BMI and 5HTTLPR genotype.
There are some limitations of this study. First, the effects on cellular functions of each genotype considered as candidates modulating weight loss in response to sibutramine were not examined. Second, the genotype variations studied may not be the causes but may be in linkage disequilibrium with the causative factor of the altered pharmacogenetics of sibutramine. Third, we have not evaluated each variant separately and had to combine the less prevalent groups for each SNP [SS 5HTTLPR 20%, GG α2A 7% and TT GNβ3 3% in controls studied from the same geographical region (18,23,24)]. However, there is evidence of a biological effect for at least one of the genotypes, e.g. the reduced serotonin transporter function in a lymphoblastoid cell line associated with the short allele in 5HTTLPR (5). Fourth, the sample size was too small to appraise three-way statistical interactions that assess the magnitude of effect in association with variations in three genes. Fifth, we elected to study candidate genes and cannot exclude other potential genetic factors that may impact the response to sibutramine. Sixth, we studied specific markers (e.g. SNPs) of the genes of interest, and further studies with alternative markers (or Tag SNPs) may enhance the predictive accuracy in the response to sibutramine. Finally, the sample size was too small to assess the influence on response to sibutramine of genetic variation in 5-HT2A and 5-HT2C receptors, since prevalence of the minor allele in controls from the same communities was 15% for 5-HT2A 1438G/A and 35% for 5-HT2C cys23ser (23,24,43). These mechanisms are of interest in view of associations between 5-HT2A (1438 G/A) and eating disorders and anorexia nervosa (44) and with food and alcohol intake in obese people (45), and between 5-HT2C receptor cys23ser substitution in the gene’s coding region with weight loss (46).
CONCLUSION
We conclude that the selected markers of the candidate genes are individually significant predictors of weight loss in response to multidimensional sibutramine and behavioral therapy.
Supplementary Material
Acknowledgments
Dr. Camilleri is funded in part by grants RO1 DK 67071 and K24 DK 02638 from National Institutes of Health. The studies were conducted in the Mayo Clinical Research Unit which is supported by NIH grant RR024150. DEXA measurements for this study were supported by Public Health Service Grant DK50456 which funds the Minnesota Obesity Center. We thank the Nursing Core of the Mayo Clinic CTSA Clinical Research Unit and Mrs. Cindy Stanislav for secretarial assistance.
APPENDIX
Sample Size Considerations
a. Weight loss in response to sibutramine
Our previous studies (14) with sibutramine suggested the residual pooled standard deviation for change in weight would be 4.0 kg. A two-sample t-test (two-sided α = 0.025) with 60 subjects per group provided 80% power to detect an overall (main effect) difference between an active treatment group and placebo of ~ 2.25 kg. Thus, an entire sample size of 180 patients was planned.
b. Effect of genetic variation on weight response to sibutramine
A stratified randomization procedure was used to assign subjects to treatment groups with balanced proportions of subjects on gender, and 5HTTLPR polymorphism in each treatment group. Assessing the influence of polymorphisms on treatment differences (interactions) is also a comparison of differences, i.e., sibutramine vs. placebo in those with the genetic polymorphism vs. those without (wildtype). Assuming a similar marginal distribution for 5HTTLPR as previously observed (35% LL vs. 65% LS/SS), we estimated there would be ~60 patients in the LL strata and 120 patients in the LS/SS strata in the 180 patients planned for the study. Consequently, we anticipated 20 patients per treatment group in the LL strata and 40 patients per treatment group in the LS/SS strata for 5HTTLPR genotype. Using a pooled estimate of the standard deviation across all strata and treatment groups implies a standard deviation of √2*4.0 for a test of differential treatment effects (e.g. placebo vs. an active treatment group in the LL strata vs. the LS/SS strata). Thus, there would have been ~80% power (at a 2-sided α level of 0.025) to detect a differential treatment effect (interaction) between two treatment groups of 4.9 kg. The anticipated analyses (ANCOVA, see below) would have provided similar power for somewhat smaller overall main effects and gene by treatment interactions. Since the minor allele frequency of the other two candidate gene [G allele 43% in α2A C1291G (21) and T allele 49% in GNβ3 C825T (22)] were greater than that of the S allele in 5HTTLPR polymorphism, there was at least equivalent power to detect an interaction for the other two genes of interest.
Detection of Polymorphisms of Candidate Genes
Analysis of the α2A -1291 (C–G) Genbank M23533, rs1800544 was examined using restriction fragment length polymorphism (RFLP) at the MspI site, and validated by direct sequence. Primers to amplify the MspI region (in the promoter) as follows: sense 5’TCACACCGGAGGTTACTTCCCTCG3’; antisense 5’TCCGACGACA GCGCGAGTT 3’.
Analysis of the α2C adrenoreceptor polymorphism Del 322–325, (Genbank #J03853) (a 12 base pair deletion from nucleotide 964 in the region coding for the third intracellular loop of the receptor) used the restriction fragment patterns of NciI, and confirmed by direct sequencing with the primers: sense 5’CCACCATCGTCGCCGTGTGGCTCATCT 3’; and antisense 5’AGGCCTCGCGGCAGATGCCGTACA 3’.
5HTTLPR Polymorphisms (Genbank #X76753) was identified by PCR-based fragment length polymorphisms and confirmed by direct sequencing. We used oligonucleotide primers flanking the 5HTTLPR long polymorphic region corresponding to nucleotide positions 1671 (sense 5'GCCGCTCTGAATGCCAGCAC 3'), and 2219 (antisense 5' GGAGGAACTGACCCCTGAAAACTG 3') to generate 528 and/or 572 base pair PCR amplified fragments.
GNβ3 (Genbank #AY631782, rs5443 ) - was determined by Taqman® SNP Genotyping Assay C_2184734_10 (Applied Biosystems, Foster City, CA). The cycling conditions were 95°C 10 minutes, then 40 cycles of 95°C 15 sec, 60°C 1 min. The SNP analysis is done using the Sequence Detection software v1.3.1 (Applied Biosystems, Forest City, CA) The sense and antisense primers used for validation by direct sequence of the C825T (rs5443) polymorphisms were 5’ TGACCCACTTGCCACCCGTGC 3’ and 5’GCAGCAGCCAGGGCTGGC 3’.
All polymorphisms were confirmed by random direct sequencing. Samples were sequenced by Mayo Clinic’s DNA Sequencing Core Facility using Applied Biosystems BigDye® terminator v1.1 cycle sequencing chemistry and analyzed on Applied Biosystems 3730XL DNA Analyzer. Where there was not a restriction site available, as in the case of the SERT-transporter, polymorphisms were confirmed by direct sequence alone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registration Number of Clinical trial at Clinical Trials.gov: NCT 00433641
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Contributor Information
April B.M. Grudell, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Seth Sweetser, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Deborah J. Eckert, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Maria I. Vazquez-Roque, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Paula J. Carlson, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Duane D. Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Autumn E. Braddock, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
Matthew M. Clark, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
Karen M. Graszer, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
Sarah A. Kalsy, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
Alan R. Zinsmeister, Department of Health Sciences Research, Division of Biostatistics, Mayo Clinic, Rochester, Minnesota
REFERENCES
- 1.Hainer V, Kabrnova K, Aldhoon B, Kunesova M, Wagenknecht M. Serotonin and norepinephrine reuptake inhibition and eating behavior. Ann NY Acad Sci. 2006;1083:252–269. doi: 10.1196/annals.1367.017. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 4.Hansen DL, Astrup A, Toubro S, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi-centre STORM trial. Intl J Obesity. 2001;25:496–501. doi: 10.1038/sj.ijo.0801481. [DOI] [PubMed] [Google Scholar]
- 5.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 6.Rosmond R, Bouchard C, Bjorntorp P. A C-1291G polymorphism in the alpha2A adrenergic receptor gene (ADRA2A) promoter is associated with cortisol escape from dexamethasone and elevated glucose levels. J Intern Med. 2002;251:252–257. doi: 10.1046/j.1365-2796.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Serretti A, Lilli R, Lorenzi C, Lattuada E, Cusin C, Smeraldi E. Serotonin transporter gene (5-HTTLPR) and major psychoses. Mol Psychiatr. 2002;7:95–99. doi: 10.1038/sj.mp.4000936. [DOI] [PubMed] [Google Scholar]
- 8.Tsai SJ, Wang YC, Younger WYY, Lin CH, Yang KH, Hong CJ. Association analysis of polymorphisms in the promoter region of the α2a-adrenoceptor gene with schizophrenia and clozapine response. Schizophr Res. 2001;49:53–58. doi: 10.1016/s0920-9964(00)00127-4. [DOI] [PubMed] [Google Scholar]
- 9.Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology (Berl) 2006;187:68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- 10.Murphy GM, Jr, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61:1163–1169. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 11.Avissar S, Schreiber G. Ziskind-Somerfeld Research Award. The involvement of guanine nucleotide binding proteins in the pathogenesis and treatment of affective disorders. Biol Psychiatr. 1992;31:435–459. doi: 10.1016/0006-3223(92)90257-z. [DOI] [PubMed] [Google Scholar]
- 12.Andersen G, Overgaard J, Albrechtsen A, Glümer C, Borch-Johnsen K, Jørgensen T, et al. Studies of the association of the GNB3 825C>T polymorphism with components of the metabolic syndrome in white Danes. Diabetologia. 2006;49:75–82. doi: 10.1007/s00125-005-0049-7. [DOI] [PubMed] [Google Scholar]
- 13.Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R, Heusch G, Siffert W. G protein beta3 subunit 825T allele and enhanced coronary vasoconstriction on alpha(2)-adrenoceptor activation. Circ Res. 1999;85:965–969. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 14.Garenc C, Pérusse L, Chagnon YC, Rankinen T, Gagnon J, Borecki IB, et al. The alpha 2-adrenergic receptor gene and body fat content and distribution: the HERITAGE Family Study. Mol Med. 2002;8:88–94. [PMC free article] [PubMed] [Google Scholar]
- 15.Fuemmeler BF, Agurs-Collins TD, McClernon FJ, Kollins SH, Kail ME, Bergen AW, Ashley-Koch AE. Genes implicated in serotonergic and dopaminergic functioning predict BMI categories. Obesity. 2008;16:348–355. doi: 10.1038/oby.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sookoian S, Gianotti TF, Gemma C, Burgueño A, Pirola CJ. Contribution of the functional 5-HTTLPR variant of the SLC6A4 gene to obesity risk in male adults. Obesity. 2008;16:488–491. doi: 10.1038/oby.2007.64. [DOI] [PubMed] [Google Scholar]
- 17.Hauner H, Meier M, Jöckel KH, Frey UH, Siffert W. Prediction of successful weight reduction under sibutramine therapy through genotyping of the G-protein beta3 subunit gene (GNB3) C825T polymorphism. Pharmacogenetics. 2003;13:453–459. doi: 10.1097/00008571-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez Roque MI, Camilleri M, Clark MM, Tepoel DA, Jensen MD, Graszer KM, et al. Alteration of gastric functions and candidate genes associated with weight reduction in response to sibutramine. Clin Gastroenterol Hepatol. 2007;5:829–837. doi: 10.1016/j.cgh.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 19.Brownell K. The LEARN Program for Weight Management. 10th ed. Dallas: American Health Publishing Company; 2004. [Google Scholar]
- 20.Marcus BH, Rakowski W, Rossi JS. Assessing motivational readiness and decision making for exercise. Health Psychol. 1992;11:257–261. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Swenson WM, Morse RM. The use of a self-administered alcoholism screening test (SAAST) in a medical center. Mayo Clin Proc. 1975;50:204–208. [PubMed] [Google Scholar]
- 23.Kim HJ, Camilleri M, Carlson PJ, Cremonini F, Ferber I, Stephens D, et al. Association of distinct alpha (2) adrenoceptors and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andresen V, Camilleri M, Kim HJ, Stephens DA, Carlson PJ, Talley NJ, et al. Is there an association between GNβ3 C825T genotype and lower functional gastrointestinal disorders? Gastroenterology. 2006;130:1985–1994. doi: 10.1053/j.gastro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 26.Szarka LA, Camilleri M, Vella A, Burton D, Baxter K, Simonson J, Zinsmeister AR. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2008.01.009. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Chen JD. Peripheral mechanisms of sibutramine involving proximal gastric motility in dogs. Obesity. 2006;14:1363–1370. doi: 10.1038/oby.2006.154. [DOI] [PubMed] [Google Scholar]
- 28.Finer N, Ryan DH, Renz CL, Hewkin AC. Prediction of response to sibutramine therapy in obese non-diabetic and diabetic patients. Diabetes Obes Metab. 2006;8:206–213. doi: 10.1111/j.1463-1326.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 29.Serretti A, Benedetti F, Zanardi R, Smeraldi E. The influence of serotonin transporter promoter polymorphism (SERTPR) and other polymorphisms of the serotonin pathway on the efficacy of antidepressant treatments. Prog Neuropsychopharmacol Biol Psychiatr. 2005;29:1074–1084. doi: 10.1016/j.pnpbp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, et al. Serotonin transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 31.Ukkola O, Rankinen T, Weisnagel SJ, Sun G, Pérusse L, Chagnon YC, et al. Interactions among the alpha2-, beta2-, and beta3-adrenergic receptor genes and obesity-related phenotypes in the Quebec Family Study. Metabolism. 2000;49:1063–1070. doi: 10.1053/meta.2000.7708. [DOI] [PubMed] [Google Scholar]
- 32.Dionne IJ, Turner AN, Tchernof A, Pollin TI, Avrithi D, Gray D, et al. Identification of an interactive effect of beta3- and alpha2b-adrenoceptor gene polymorphisms on fat mass in Caucasian women. Diabetes. 2001;50:91–95. doi: 10.2337/diabetes.50.1.91. [DOI] [PubMed] [Google Scholar]
- 33.Lima JJ, Feng H, Duckworth L, Wang J, Sylvester JE, Kissoon N, Garg H. Association analyses of adrenergic receptor polymorphisms with obesity and metabolic alterations. Metabolism. 2007;56:757–765. doi: 10.1016/j.metabol.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 35.Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol. 1997;121:1613–1618. doi: 10.1038/sj.bjp.0701311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Deupree JD, Smith SD, Kratochvil CJ, Bohac D, Ellis CR, Polaha J, et al. Possible involvement of alpha-2A adrenergic receptors in attention deficit hyperactivity disorder: radioligand binding and polymorphism studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:877–884. doi: 10.1002/ajmg.b.30371. [DOI] [PubMed] [Google Scholar]
- 39.Polanczyk G, Zeni C, Genro JP, Guimarães AP, Roman T, Hutz MH, Rohde LA. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatr. 2007;64:218–224. doi: 10.1001/archpsyc.64.2.218. [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Bai YM, Chen JY, Lin CC, Lai IC, Liou YJ. Polymorphism of the adrenergic receptor alpha 2a -1291C>G genetic variation and clozapine-induced weight gain. J Neural Transm. 2005;112:1463–1468. doi: 10.1007/s00702-005-0291-7. [DOI] [PubMed] [Google Scholar]
- 41.Park YM, Chung YC, Lee SH, Lee KJ, Kim H, Byun YC, et al. Weight gain associated with the alpha2a-adrenergic receptor −1,291 C/G polymorphism and olanzapine treatment. Am J Med Genet B Neuropsychiatr Genet. 2006;141:394–397. doi: 10.1002/ajmg.b.30311. [DOI] [PubMed] [Google Scholar]
- 42.Mäestu J, Allik J, Merenäkk L, Eensoo D, Parik J, Veidebaum T, Harro J. Associations between an alpha 2a adrenergic receptor gene polymorphism and adolescent personality. Am J Med Genet B Neuropsychiatr Genet. 2007 Sep 25; doi: 10.1002/ajmg.b.30621. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri CE, Carlson PJ, Camilleri M, Castillo EJ, Locke GR, III, Geno DM, Stephens DA, Zinsmeister AR, Urrutia R. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterology. 2006;101:581–592. doi: 10.1111/j.1572-0241.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 44.Ricca V, Nacmias B, Cellini E, Di Bernardo M, Rotella CM, Sorbi S. 5-HT2A receptor gene polymorphism and eating disorders. Neuroscience Letters. 2002;323:105–108. doi: 10.1016/s0304-3940(02)00088-5. [DOI] [PubMed] [Google Scholar]
- 45.Aubert R, Betoulle D, Herbeth B, Siest G, Fumeron F. 5-HT2A receptor gene polymorphism is associated with food and alcohol intake in obese people. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24:920–924. doi: 10.1038/sj.ijo.0801253. [DOI] [PubMed] [Google Scholar]
- 46.Westberg L, Bah J, Rastam M, Gillberg C, Wentz E, Melke J, Hellstrand M, Eriksson E. Association between a polymorphism of the 5-HT2C receptor and weight loss in teenage girls. Neuropsychopharmacology. 2002;26:789–793. doi: 10.1016/S0893-133X(01)00417-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.