Abstract
PURPOSE
To determine macular pigment (MP) optical density (OD) in patients with ABCA4-associated retinal degenerations (ABCA4-RD) and the response of MP and vision to supplementation with lutein.
METHODS
Stargardt disease or cone-rod dystrophy patients with foveal fixation and with known or suspected disease-causing mutations in the ABCA4 gene were included. MPOD profiles were measured with heterochromatic flicker photometry. Serum carotenoids, visual acuity, foveal sensitivity and retinal thickness were quantified. Changes in MPOD and central vision were determined in a subset of patients receiving oral supplementation with lutein for 6 months.
RESULTS
MPOD in patients ranged from normal to markedly abnormal. As a group, ABCA4-RD patients had reduced foveal MPOD and there was strong correlation with retinal thickness. Average foveal tissue concentration of MP, estimated by dividing MPOD by retinal thickness, was normal in patients whereas serum concentration of lutein and zeaxanthin was significantly lower than normal. After oral lutein supplementation for 6 months, 91% of the patients showed significant increases in serum lutein and 63% of the patient eyes showed a significant augmentation in MPOD. The retinal responders tended to be female, and have lower serum lutein and zeaxanthin, lower MPOD and greater retinal thickness at baseline. Responding eyes had significantly lower baseline MP concentration compared to non-responding eyes. Central vision was unchanged after the period of supplementation.
CONCLUSIONS
MP is strongly affected by the stage of ABCA4 disease leading to abnormal foveal architecture. MP could be augmented by supplemental lutein in some patients. There was no change in central vision after 6 months of lutein supplementation. Long-term influences on the natural history of this supplement on macular degenerations require further study.
Keywords: carotenoids, lutein, macular degeneration, Stargardt disease, zeaxanthin
INTRODUCTION
The ABCA4 gene encodes the ABCR protein which localizes to the rims of rod and cone outer segments1,2 and accelerates removal of all-trans-retinal from light-exposed photoreceptors by transporting A2-PE, a retinoid adduct formed by all-trans-retinal and phosphatidylethanolamine3–5. Mutations in the ABCA4 gene cause a major proportion of autosomal recessive retinal degenerations (RD) with macular involvement6–12. Pathophysiology of ABCA4-RD involves trapping of A2-PE3,13,14 within disc membranes of the photoreceptor outer segments (POS). Phagocytosis of the shed POS by adjacent retinal pigment epithelial (RPE) cells in ABCA4 deficient retinas results in excessive intracellular accumulation of lipofuscin, an aggregate of lipids, proteins and fluorescent retinoids, including cytotoxic bis-retinoid A2E derived from the trapped A2-PE3,15. In extra-macular retina of patients with known ABCA4 mutations, we have provided evidence supporting abnormal increase in lipofuscin autofluorescence in the RPE preceding dysfunction and degeneration of the overlying retina11. We have also shown that parapapillary retina is relatively spared retinal degeneration12. Details of the macular disease sequence remain to be studied.
Macular degenerations, including those caused by ABCA4 mutations, commonly show a counterintuitive stage when foveal vision and structure are relatively preserved compared to the surrounding parafoveal region9,10,16–18. It has been hypothesized that macular pigment (MP), a yellowish carotenoid mainly composed of lutein and zeaxanthin concentrated at the fovea, may contribute to relative preservation of this retinal region in macular degenerations19. The mechanism of MP protection may involve passive absorption of shorter wavelengths of light20–25. Exposure to light not only causes A2E accumulation but also increases the potential toxicity of the accumulated A2E via photooxidation26,27. Further, it has been recently proposed that lutein and zeaxanthin specifically protect A2-PE in photoreceptors and A2E in RPE cells from photooxidation and thus MP may have a particularly important role in ABCA4 disease28.
To understand better the preserved foveas in ABCA4-RD, we explored the relationship between MP and systemic, ocular and retinal features. Seeking ways to prevent loss of this remaining foveal vision in ABCA4-RD, we also performed a short-term open-label pilot study asking whether retinal MP could be modified with oral lutein supplementation.
METHODS
Subjects
Stargardt disease or cone-rod dystrophy patients (n=17), most of whom had known ABCA4 gene mutations10,11,29, were included in this study (Table 1). All participants were in general good health. The subjects, all with central retinal disease, were selected for participation because of their relatively spared foveal function in at least one eye. All subjects had a routine ocular examination and best-corrected visual acuity (VA) determined with the ETDRS chart. Eyes included in the study had stable foveal fixation, as documented by the correspondence of the center of the anatomical fovea to fixation with optical coherence tomography (OCT). When two eyes were eligible only the one with full MP profile and/or highest peak MP density was included in the analyses related to baseline parameters. This choice was made to be consistent with de facto inclusion of the “better” eye in patients with only one eye with foveal vision. Normal data from a group of subjects (n=29) without ocular disease that participated in our previous study30 were reanalyzed according to the current methods. A subset of the subjects (11 patients and 8 controls) underwent a pilot trial of supplementation with oral lutein for six months. In patients with interocular differences in disease severity, we anticipated possible interocular differences in response to lutein supplementation. Therefore, data from individual eyes are presented for the lutein supplementation section of the manuscript. Informed consent was given by all subjects in compliance with the Declaration of Helsinki, and institutional review board approval was obtained.
TABLE 1.
Clinical and Molecular Characteristics of the Patients
| Patient | Age (y)/Gender | ABCA4 Mutation | Visual Acuity* | Refraction† | Kinetic Visual Field Extent (V-4e)‡ | Lutein Trial Participant? | |||
|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | RE | LE | ||||
| 1 | 14/M | G863A/R943Q | 20/32 | 20/32 | −0.50 | −0.50 | 109 | 105 | Y |
| 2 | 17/F | E1087K/G1961E | 20/25 | 20/25 | −1.00 | −1.25 | 103 | 104 | N |
| 3 | 18/M | IVS48+21C>T | 20/20 | 20/125 | −1.00 | −1.00 | 126 | 105 | N |
| 4§ | 19/F | R1129L/L1940P | 20/40 | 20/50 | +0.25 | +0.25 | 90 | 93 | Y |
| 5 | 21/M | P1511del1ccgC/R1705Q | 20/25 | 20/25 | −0.75 | −0.25 | 103 | 107 | Y |
| 6 | 24/M | T1019M/G1961E | 20/50 | 20/200 | −1.25 | −1.50 | 112 | 105 | Y |
| 7§ | 26/M | ∥ | 20/40 | 20/32 | +1.00 | +0.75 | 86 | 88 | Y |
| 8 | 30/F | ∥ | 20/50 | 20/40 | +2.25 | +1.75 | 105 | 110 | Y |
| 9 | 30/M | R1108C/R152Q | 20/20 | 20/32 | −2.25 | −3.50 | 99 | 93 | Y |
| 10 | 32/F | V935A/IVS40+5G>A | 20/32 | 20/40 | −0.75 | −1.25 | 103 | 92 | N |
| 11 | 34/F | R681X/R1300Q | 20/20 | 20/20 | −1.50 | −1.75 | 110 | 96 | N |
| 12 | 37/M | C54Y/G1961E | 20/32 | 20/25 | −3.00 | −2.00 | 99 | 105 | Y |
| 13# | 38/F | V256V/G1961E | 20/25 | 20/25 | −1.00 | −1.25 | 106 | 101 | Y |
| 14# | 42/F | V256V/G1961E | 20/25 | 20/32 | −0.50 | −0.75 | 107 | 94 | Y |
| 15 | 47/F | R1300Q/R2107H | 20/32 | 20/20 | +0.75 | +0.25 | 108 | 103 | N |
| 16§ | 49/M | ∥ | 20/32 | 20/32 | −4.50 | −4.50 | 84 | 79 | Y |
| 17 | 56/M | G1977S | 20/25 | 20/25 | −5.50 | −5.50 | 99 | 109 | N |
Best corrected visual acuity.
Spherical equivalent.
Expressed as a percentage of normal mean of V-4e target; 2 SD below normal equals 90%.
Clinical diagnosis of cone-rod dystrophy; remaining patients had a clinical diagnosis of Stargardt disease.
Mutation unknown.
Patients are siblings.
Evaluation of the Macula: Macular Pigment, Central Retinal Function and Structure
Macular pigment optical density (MPOD) was measured by heterochromatic flicker photometry (HFP) using an LED-based MP densitometer (Macular Metrics Corp., Rehoboth, MA). This psychophysical technique compares flicker photometric sensitivity measured at and near the fovea to that obtained at a more peripheral retinal location22,31. Sensitivity is determined by alternating a short wavelength test light that is maximally absorbed by MP in counterphase with a longer wavelength reference light that is not absorbed by MP. The intensity of the test light is adjusted until perception of flicker is minimized or eliminated, at which point the two lights are equated in apparent brightness. The peripheral to foveal sensitivity ratio is used to determine the peak density of MP. Details of the methodology in patients with hereditary retinal degenerations have been provided30,32. In brief, flickering stimuli (460 nm, test; 570 nm, reference, 1.7 log td) were centered on a 6° diameter background field (1.5 log td, 470 nm) while the patients fixated centrally on a 5’ spot. Four different stimuli were used and consisted of two discs (0.34° and 1° diameter) and two annuli (2° and 4° diameter, 0.4° wide). We will assume that flicker perception is dominated by the edges33, although other work has suggested that flicker may not be detected at the edge but perceived by more central retinal eccentricities when using discoid stimuli34. Using the former assumption, these stimuli represent eccentricities of 0.17° and 0.5° (henceforth referred to as “foveal”) and 1° and 2° (henceforth referred to as “parafoveal”); 0.17° eccentric stimulus will be referred to as 0.2°. Peripheral sensitivities were determined with a 2° diameter disc centered on the background while subjects fixated on a small red LED situated 7° to the nasal side of the background field; the radiance setting of the 460nm test light needed for a flicker null at this eccentricity was not significantly different in patients compared to normal subjects (171±61 vs. 157±18 counts; P=0.17).
Foveal visual function was measured using a modified35 automated perimeter (Zeiss Humphrey Instruments, Dublin, CA) and a red (650 nm) target (1.7° diameter, 200 ms duration) in the dark-adapted state. Macular structure was quantified by OCT (Zeiss Humphrey Instruments, Dublin, CA). The principles of OCT36 and our methodology10,37 have been published. Horizontal scans crossing the anatomical fovea were obtained in all subjects. Retinal thickness at the center of the fovea and at 0.5° of eccentricity was measured37. Serum carotenoids (lutein, zeaxanthin, beta-carotene) were measured using high-performance liquid chromatography (Craft Technologies, Inc., Wilson, NC). Dietary information was provided through the Health Habits and History questionnaire (HHHQ) developed by the National Cancer Institute38; data were analyzed using the HHHQ Diet System Analysis Software39.
Supplementation with Lutein
A subset of patients (n=11) participated in an open-label 6-month pilot trial of oral lutein supplementation (Table 1). There was no placebo control group. Following two baseline visits (separated by no more than one month; except P4, who had a single baseline visit), subjects supplemented their diet with a commercially-available form of lutein at 20 mg per day (TWIN Laboratories Inc., NY). Subjects were instructed to take the lutein supplement with a meal with the most fat of the day, presuming this would enhance absorption of the supplement40. A further visit occurred 6 months after starting the supplement. Baseline and follow-up visits included a clinical examination, fasting (overnight) venous blood sample for serum carotenoids, and measurements of MPOD and absolute dark-adapted sensitivity at the fovea with a 650 nm target30,32.
Data Analysis
Statistical software (SAS, ver. 9.1; SAS, Cary, NC) was used to analyze data. Mean values from the two baseline visits were used to describe the study groups and calculate change after lutein supplementation. T-tests were performed to compare means and significance levels for correlation coefficients. Inter-session variability was assessed with signed and absolute differences of measurements between the first and second baseline visit. Means of inter-session differences and person-specific variables were compared with independent t-tests. Proportions were compared using chi-square tests with exact computation of the P values.
RESULTS
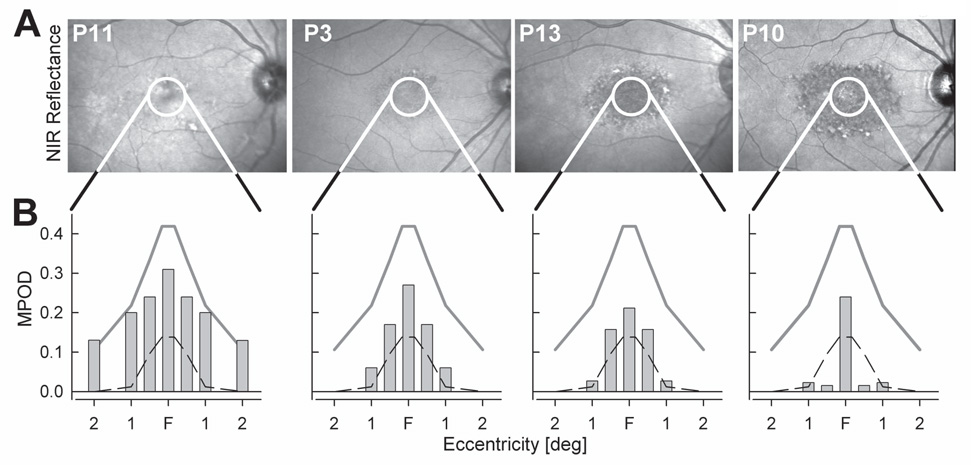
Patients had a clinical diagnosis of either Stargardt disease (n=14) or cone-rod dystrophy (n=3) and all but three had known mutations in the ABCA4 gene (Table 1). All patients had macular disease with foveal sparing in at least one eye. The macular appearance on infrared reflectance imaging ranged from a mottled pattern with scattered dark and light lesions, to dark areas surrounding a lighter foveal center and bordered by a granular-appearing annulus (Fig.1A). Kinetic visual fields were full in peripheral extent in all but two patients (Table 1); small scotomas around fixation were frequently detected. The four examples of MPOD at different eccentricities displayed below the fundus images (Fig.1B) indicate that diseased eyes could have results that were within normal limits or show abnormalities.
Figure 1. Patients with ABCA4-RD representing the spectrum of fundus appearance and MPOD.
(A) Near-infrared (NIR) reflectance images of the central retina in four patients. Overlaid white circles delimit the central 5° diameter region within which MPOD measurements were performed.(B) MPOD in patients shown in Panel A. Foveal center (F) corresponds to MPOD determined with a 0.2° radius stimulus; MPOD determined with circular stimuli of 0.5°, 1°, 2° radius are plotted with symmetric duplicatation from F. Gray lines represent mean normal MPOD and dashed lines represent lower limit (mean-2SD) of normal.
Low macular pigment in ABCA4-RD
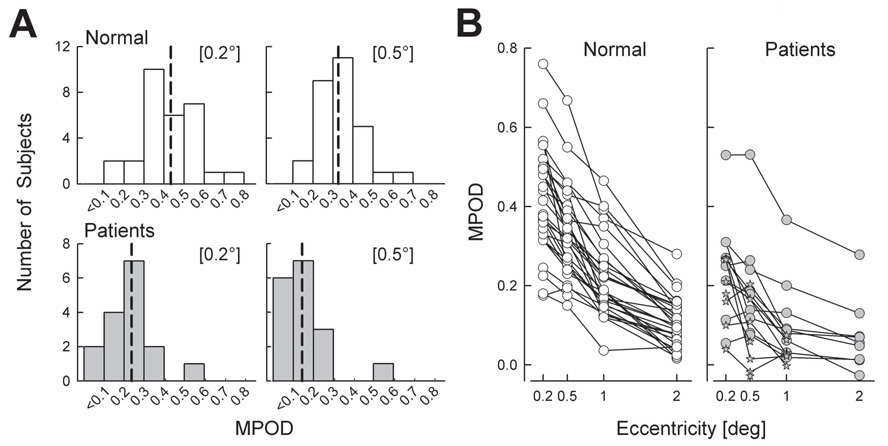
Patients had significantly lower MPOD compared to normal eyes (Table 2).The distribution of MPOD in patient eyes was shifted toward lower values compared to the normal eyes (Fig.2A). At 0.5° eccentricity, 76% of the patients showed MPOD below 0.2, whereas only 7% of normal subjects had such low values.
TABLE 2.
Baseline Group Summary Statistics*
| Patients [n=17] |
Normal [n=29] |
P† | |
|---|---|---|---|
| General | |||
| Age [years] | 32±12 | 30±12 | N.S. |
| BMI [kg.m−2] | 24±5 | 23±3 | N.S. |
| Female Gender [%] | 47 | 55 | N.S. |
| Smoker [%] | 18 | 14 | N.S. |
| Caucasian Race [%] | 82 | 90 | N.S. |
| Light Irides [%] | 44 | 48 | N.S. |
| Diet | |||
| Lutein [mg/day] | 2.6±1.7 | 2.8±2.1 | N.S. |
| Fat [g/day] | 89±51 | 66±41 | N.S. |
| Serum | |||
| Lutein [µmol/L] | 0.20±0.10 | 0.31±0.14 | 0.003 |
| Zeaxanthin [µmol/L] | 0.07±0.04 | 0.13±0.06 | 0.002 |
| β-Carotene [µmol/L] | 0.47±0.61 | 0.59±0.39 | N.S. |
| MPOD | |||
| 0.2° | 0.22±0.12 | 0.42±0.14 | <0.001 |
| 0.5° | 0.15±0.13 | 0.33±0.12 | <0.001 |
| Retinal Thickness | |||
| 0° [µm] | 103±50 | 198±14 | <0.001 |
| 0.5° [µm] | 116±53 | 205±14 | <0.001 |
| MP Concentration | |||
| 0.2° [µm−1] | 0.23±0.12 | 0.22±0.07 | N.S. |
| 0.5° [µm−1] | 0.12±0.09 | 0.16±0.06 | 0.030 |
| MPOD Profile | |||
| 0.2° | 0.26±0.13‡ | 0.42±0.14 | 0.002 |
| 0.5° | 0.20±0.14‡ | 0.33±0.11 | 0.004 |
| 1° | 0.12±0.11‡ | 0.22±0.10 | 0.012 |
| 2° | 0.07±0.09‡ | 0.11±0.06 | N.S. |
| Half-width at half-peak [deg] | 0.98±0.63‡ | 1.28±0.33 | 0.045 |
Non-categorical variables are specified as mean ± s.d.
Not significant (N.S.) values correspond to P>0.05.
Determined in subset of 9 patients with full spatial profiles.
Figure 2. MPOD in patients compared to normal subjects.
(A) Frequency distribution histograms of MPOD measured at foveal locations (0.2° and 0.5°). Vertical dashed lines indicate median value of each distribution. (B) All individual MPOD values measured in patients and normals. Full profiles are shown with circles. Stars represent patient eyes in which MPOD could only be determined in a subset of the four eccentricities.
MPOD normally peaks near the center of the fovea and declines with eccentricity 41,42. The spatial distribution of MP was studied in a subset of 11 patient eyes in which MPOD could be determined at all four eccentricities (Fig.2B). On average, MPOD in these eyes was lower than normal at each eccentricity (Table 2, MPOD Profile). The distribution of MPOD profiles as estimated from the half-width at half-peak were narrower in these 11 patients compared to normals (Table 2). The remaining 6 patients could not perform HFP at the parafoveal locations due to a lack of perception of the flicker. These eyes with an indeterminate spatial MPOD distribution corresponded to some of the lowest foveal MPOD (Fig.2B, stars).
MP levels have been related to dietary 22,43–46, demographic42,46–48, lifestyle49, systemic45,50–54 and ocular55 characteristics in studies of normal populations. Examination of some of these factors (Table 2) showed that patients and normals included in this study were well matched in terms of age, body mass index (BMI), gender, smoking status, race, and color of irides. Mean dietary fat intake was higher in patients compared with our normals but the groups were not significantly different and the patient values were similar to those reported in other studies in normals 45,56,57. Dietary lutein was similar in patients and normal subjects. The effect of factors contributing to low MPOD were further explored in patients by comparing subgroups in the first (MPOD<=0.08) and fourth (MPOD>=0.19) quartiles of the distribution of MP values for the conventional 0.5° eccentric stimulus. Females (60% vs. 40%, P=0.36) and subjects with light-colored irides (60% vs. 20%, P=0.52) were more commonly observed in the low MPOD compared to the high MPOD group of patients; the only smoker in these two subsets was in the low MPOD group. These results are in agreement with our previous observations in patients with other hereditary retinal degenerations30,32 and in reports of normal subjects43,46,49, 55.
Low serum lutein in ABCA4-RD
Serum levels of lutein and zeaxanthin were significantly lower in patients compared to our group of normals (Table 2). Serum levels of xanthophylls in most patients also fell within the lower end of normal values reported by other investigators in large population-based studies56–59. When considering the subset of subjects with low serum lutein (<=0.19 µmol/L, lowest quartile of our normal population), patients had significantly lower zeaxanthin levels compared to normals but could not be otherwise distinguished from them by other variables. Low serum xanthophyll levels were not associated with any one category of patients. For example, there were no significant differences between serum xanthophyll levels of patients based on gender, smoking status and color of irides (data not shown). Examination of concurrent use of dietary supplements revealed that 8/17 patients were using multivitamins containing low doses of carotenoids prior to admission to the study. But there were no differences between those who had used supplements and the rest of the patients in any of the variables measured (data not shown).
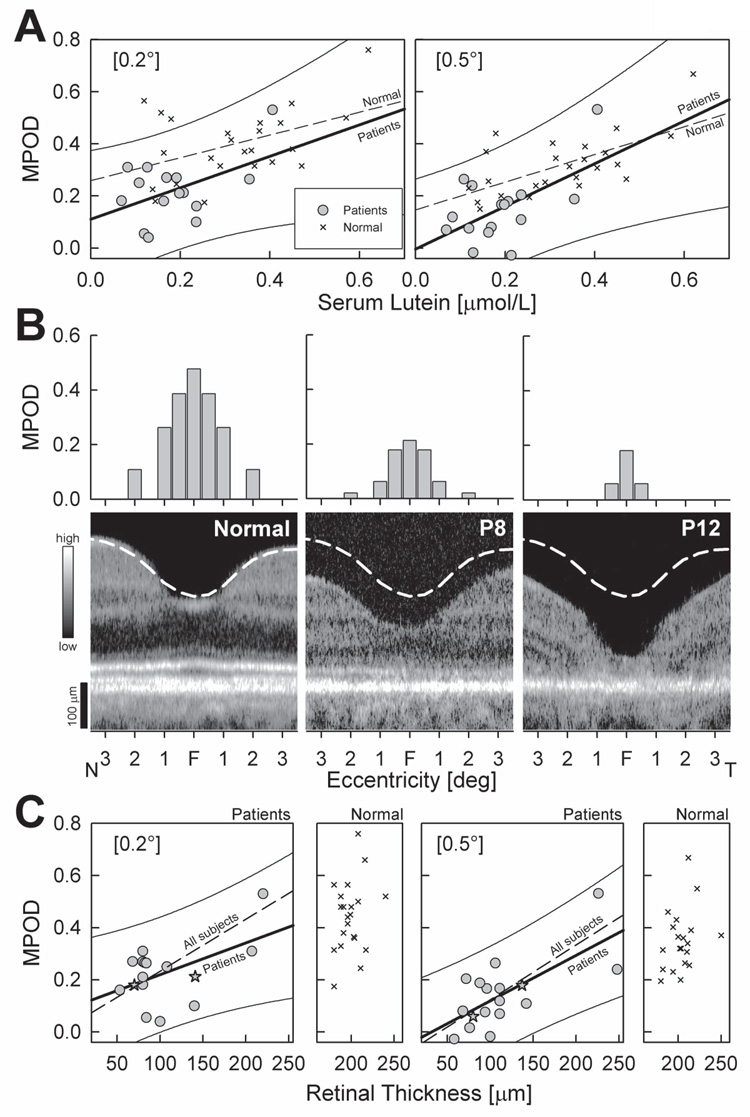
Serum lutein was related to MPOD in patients and in normals (Fig.3A). Linear correlation coefficients between these variables were similar in patients (r=0.46; P=0.03) and normal subjects (r=0.44; P=0.002) at 0.2° eccentricity. This relationship improved notably for the more eccentric 0.5° location in normal subjects (r=0.63; P<0.001), but remained unchanged in patients (r=0.47; P=0.02).
Figure 3. Systemic and ocular factors in relationship to MPOD.
(A) Foveal MPOD (0.2° and 0.5°) as a function of serum lutein concentrations in patients (gray circles) compared to normal subjects (crosses). Linear regressions for patients (thick line) and normals (dashed line) are shown; for clarity the 95% prediction interval (thin lines) is shown for the patient data only. (B) MPOD as a function of eccentricity (top panels; plotted as in Fig.1B) and corresponding foveal microstructure by OCT (bottom panels) in two patients (P8 and P12) compared to a normal subject. OCT scans are crossing the anatomic foveal center (F) horizontally, from 3.5° temporal (T) to 3.5° nasal (N). Images are displayed with logarithm of reflectivity mapped to a gray scale (left). Dashed white lines on OCTs represent mean normal location of the vitreo-retinal boundary. (C) Foveal MPOD (0.2° and 0.5°) as a function of retinal thickness in patients (gray symbols) compared to normal subjects (crosses; smaller panels). Linear regressions for patients (thick line) and for all subjects (patients + normal subjects; dashed lines) are shown; for clarity the 95% prediction interval (thin lines) are shown for the patient data only. Stars specify the patients P8 and P12 shown in Panel B.
Relationship of foveal structure to MPOD
Cross-sectional images through the fovea in two patients (Fig.3B, bottom panels) illustrate the types of abnormalities encountered. Patient 8 shows some photoreceptor layer thinning and localized disruption of the signal originating from the photoreceptor inner/outer segment interface11,37. Patient 12, with more advanced disease, shows severe central retinal thinning and an adjacent (~1° to 3° eccentric) region with loss of the photoreceptor layer. MPOD in Patient 8 was reduced but measurable at all eccentricities, but in Patient 12 MPOD was only measurable at the foveal locations (Fig 3B, top panels).
Average foveal thickness in patients was reduced to approximately half the normal values, and this was highly significant (Table 2). The relationship between foveal MPOD and retinal thickness was examined in the patients compared with normals (Fig.3C). Patients showed a positive correlation between MPOD at 0.2° and retinal thickness at the foveal center (Fig.3C, r=0.43, P=0.03) and a robust relationship (r=0.66, P<0.001) at the 0.5° locus, consistent with previous observations30,32,60. The relationship between retinal thickness and MPOD was much stronger when patients and normal subjects were considered as a single group under the assumption that patient foveas have normal thickness before the onset of retinal degeneration. The regression lines considering all subjects (Fig. 3C) had robust correlation coefficients (0.2°=0.69 and 0.5°=0.76, P<0.0001); the intercepts were not significantly different than zero (0.2°=0.10, P=0.58; 0.5°=−0.06, P=0.10).
To a first approximation retinal tissue concentration of MP can be estimated by dividing MPOD by retinal thickness. Foveal MP concentration in patients was not different than normals at 0.2° but it was lower at 0.5° (Table 2). Serum lutein concentration in patients was not significantly correlated to MP concentrations at 0.2° (r=0.26, P=0.44) and 0.5° (r=0.36, P=0.12) unlike the stronger relationship observed in normal subjects (r=0.28, P=0.03 and r=0.65, P<0.001, for 0.2° and 0.5°, respectively).
The relationship between central visual function and MPOD was probed with visual acuity and dark-adapted sensitivity to a 650 nm stimulus. MPOD at 0.5° was not significantly correlated with visual acuity (r=0.36, P=0.07) and dark-adapted foveal sensitivity (r=0.33, P=0.11).
Effects of Lutein Supplementation
Subset of patients (n=11) and normals (n=8) that took part in the 6-month pilot trial of lutein supplementation (Table 1) were well matched in terms of age, BMI, gender, smoking status, race, and color of irides (Table 3). All but one of the 11 patients responded with an increase in serum lutein. As a group, the change in serum lutein with supplementation (post-supplementation value minus mean of two baseline values) was significantly greater than the mean absolute difference between the two baseline values (0.74±0.44 vs. −0.01±0.03 µmol/L; P<0.001). Post-supplementation, there was no significant difference in serum lutein remaining between patients and normal subjects (Table 3). Serum zeaxanthin levels also showed an increase in both groups with supplementation as expected from the small amounts of zeaxanthin contained in the marigold extracts, the main component of the supplementation capsule53. However, serum zeaxanthin in patients remained significantly lower than normals post-supplementation (Table 3). There were no changes measured in serum beta-carotene levels in either group (Table 3).
TABLE 3.
Supplemented Group Summary Statistics*
| Patients [n=11] |
Normal [n=8] |
P† | |
|---|---|---|---|
| General | |||
| Age [years] | 30±11 | 27±8 | N.S. |
| BMI [kg.m−2] | 23±4 | 24±4 | N.S. |
| Female Gender [%] | 36 | 50 | N.S. |
| Smoker [%] | 27 | 13 | 0.042 |
| Caucasian Race [%] | 82 | 75 | N.S. |
| Light Irides [%] | 55 | 38 | N.S. |
| Serum (pre-supplementation) | |||
| Lutein [µmol/L] | 0.18±0.08 | 0.34±0.11 | 0.002 |
| Zeaxanthin [µmol/L] | 0.07±0.03 | 0.14±0.05 | 0.001 |
| β-Carotene [µmol/L] | 0.40±0.51 | 0.44±0.24 | N.S. |
| Serum (post-supplementation) | |||
| Lutein [µmol/L] | 0.84±0.50 | 1.06±0.41 | N.S. |
| Zeaxanthin [µmol/L] | 0.12±0.04 | 0.21±0.07 | 0.001 |
| β-Carotene [µmol/L] | 0.40±0.48 | 0.44±0.18 | N.S. |
Non-categorical variables are specified as mean ± s.d.
Not significant (N.S.) values correspond to P>0.05.
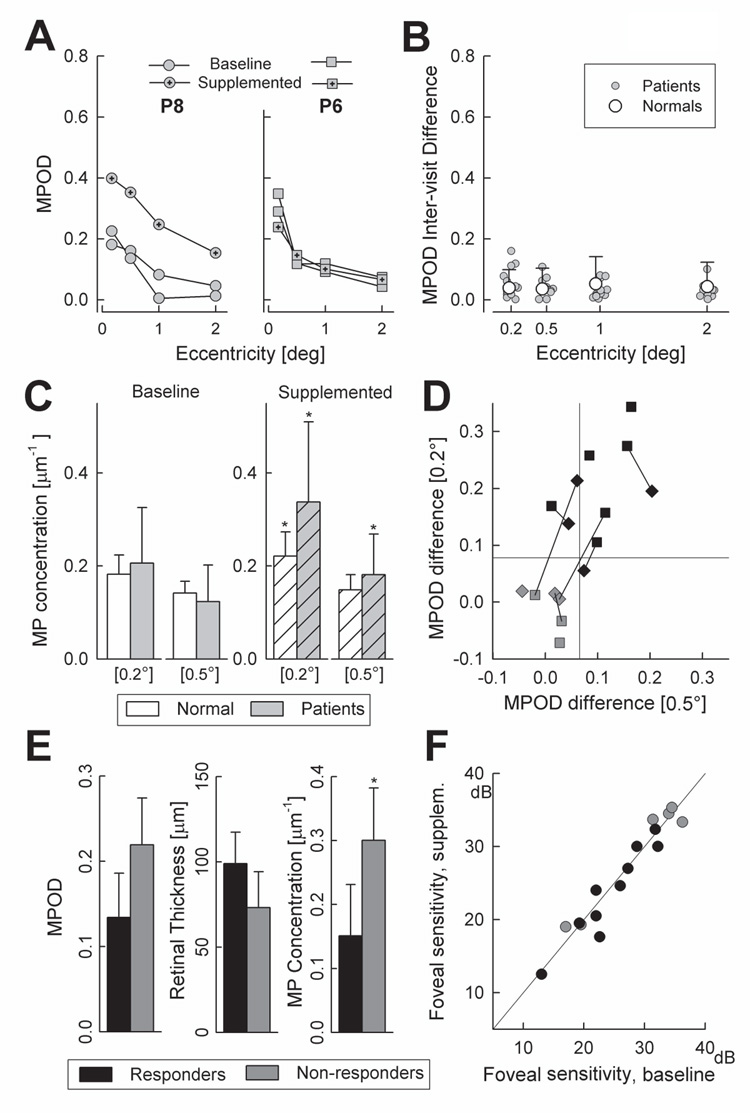
MPOD measurements before and after oral lutein supplementation are illustrated using the data from two patients (Fig.4A). Both patients had increases in serum lutein (Patient 8: from 0.21 to 0.51 µmol/L; Patient 6: from 0.08 to 0.64 µmol/L) post-supplementation, but showed different retinal responses by MPOD. Patient 8 had increases in MPOD at each eccentricity when compared to the baselines; in contrast, the post-supplementation MPOD profile of Patient 6 was very similar to the baseline profiles. The apparent differences in response to supplementation were not due to a lack of reliability in MPOD estimates. Inter-visit reproducibility of the MPOD profiles was measured in two visits in 11 patients (13 eyes). Absolute differences between the MPODs obtained at each visit in patients were not significantly different than those in normal subjects (Fig.4B). Previously published inter-visit density differences21,41,47,61–64 also showed similar ranges suggesting that MPOD could be reproducibly obtained using HFP in maculopathy eyes with foveal fixation.
Figure 4. Effects of lutein supplementation.
(A) MPOD profiles at two baseline visits and following supplementation in two patients. (B) Inter-visit absolute MPOD differences for each eccentricity in individual patients (gray circles) compared to normal mean+2SD (white circles and bar). Some of the patient symbols are laterally shifted for better visibility. (C) MP concentrations (MPOD divided by retinal thickness) for foveal locations (0.2° and 0.5°) in patients (gray bars) compared to normal subjects (white bars) at baseline (uniform filled) and 6-month post-supplementation (hatched). Error bars define 1SD from mean; asterisk denote significant (P<0.05) change compared to baseline. (D) Change in MPOD after supplementation at 0.2° plotted against 0.5° eccentricity. Baseline intersession variability (95% confidence limits) is defined by the horizontal and vertical lines. Black symbols: retinal “responders”; gray symbols: retinal “non-responders”; squares: right eyes; diamonds: left eyes. Lines connect the symbols for the two eyes of the same patient. (E) Baseline variables at 0.2° eccentricity in retinal “responders” (black fill) and “non-responders” (gray fill); asterisk denotes statistically significant difference. (F) Foveal sensitivity (650nm, 1.7° diameter, dark-adapted) in patients at baseline and after 6 months of lutein supplementation. Diagonal line represents no change.
Sixteen eyes of 10 patients (6 bilateral, 4 unilateral measurements) were used for summary statistics of the MPOD response to lutein supplementation. Patient 4 showed no serum response to lutein supplementation and was not included in this analysis (she had no MPOD change). Mean foveal MPOD increased with supplementation: at 0.2°, from 0.17±0.09 to 0.28±0.14 (paired t-test, P<0.001); at 0.5°, from 0.11±0.06 to 0.18±0.10 (P<0.001). Parafoveal increases were not significant. Magnitude of MPOD changes with supplementation were larger in patients compared to normals: at 0.2°, 0.12±0.12 vs. 0.07±0.06 (P=0.28); at 0.5°, 0.06±0.07 vs. 0.01±0.04 (P=0.02); these two foveal locations were used to assess changes in MP concentration with supplementation. In normal subjects, oral lutein led to significant increases in MP concentration at the foveal center but not at 0.5°. In patients, the retinal MP concentration increased at both foveal locations (Fig.4C).
Retinal MPOD responders to lutein supplementation were then compared to non-responders in order to seek explanations for their difference. For this analysis, responding was defined by the 95th percentile for differences between the two baseline MPODs (Fig.4D). Over half of the eyes responded with a significant increase in MPOD (Fig.4D, black symbols). In the majority, the response occurred at both eccentricities but in some eyes the response was limited to one retinal location. Baseline serum values in responders were on the average lower for lutein and zeaxanthin compared to non-responders, suggesting that changes in the retina were related to initial levels of serum xanthophylls. Accepting the small number of subjects, we asked if there was an association between the MPOD response and some of the general characteristics examined earlier. Interestingly, the 3 female patients (5/5 eyes) who supplemented responded with an increase in MPOD whereas responding in male participants was less frequent (4/7 male patients; 5/11 eyes). Retinal responders and non-responders did not differ significantly in age (36±7 vs. 27±12 yrs), frequency of lighter irides (60% vs. 50%) or smokers (both groups, 20%).
Were there eye-specific variables that could be related to the MPOD response? Responders tended to have lower MPOD at 0.2° and thicker foveas but these differences did not reach statistical significance at this central foveal location (Fig. 4E) or at 0.5°. Baseline mean MP concentration on the other hand, was significantly lower in responders compared to non-responders (Fig.4E) suggesting that MPOD changed in those with the lowest initial MP concentrations. In terms of central visual function, responders and non-responders showed no differences in baseline visual acuity (0.19±0.12 vs. 0.21±0.10 log MAR) and foveal sensitivity (25.5±4.5 vs. 28.8±8.3 dB) (Fig.4E) group. An estimate of interocular differences in MPOD response to lutein supplementation was obtained in 6 patients with bilateral measurements. Three patients responded bilaterally whereas one patient did not respond in either eye (Fig. 4D). Two patients showed unilateral responding; in both cases, eyes that responded had thicker retinas compared to the eyes that did not respond.
Foveal absolute sensitivity as a measure of central visual function was little changed after supplementation from the mean baseline value of 26.2±6.3 dB to 26.0±6.7 dB. Pre- and post-supplementation results were highly correlated (r=0.94, P<0.001) (Fig.4F). The mean change in foveal sensitivity (−1.20±2.5 dB) in eyes that responded with an increase in MPOD (black symbols, Fig. 4F) was not different that the mean change in non-responding eyes (1.21±2.7 dB; P>0.05) (Fig.4F, gray symbols). Similarly, the mean change in LogMAR acuity in responding eyes (−0.02±0.03) was not different than the mean change in non-responding eyes (−0.02±0.06) (P>0.05).
DISCUSSION
The macular pigments, lutein and zeaxanthin, are highly concentrated at the fovea and are hypothesized to improve normal vision and protect photoreceptors and the RPE from oxidative damage65. MPs originate from dietary consumption of lutein, and lutein-free diets in non-human primates can result in abnormalities of foveal photoreceptors and RPE66,67,68. Epidemiological studies have shown an association between lower dietary and serum levels of lutein and higher risk of age-related maculopathy69,70, but questions about causality remain65 and await experimental clarification. Recently one such specific antioxidant mechanism has been proposed: lutein and zeaxanthin appear to protect visual cycle byproducts A2-PE and A2E from photooxidation28. In order to extend the understanding of this new mechanism, we evaluated a cohort of patients with a shared prototypical lipofuscinopathy due to ABCA4 mutations, and at a similar disease stage with relative preservation of the fovea compared to surrounding parafoveal retina. Pathogenesis of human ABCA4 disease involves a dramatic increase in A2-PE and A2E3,13,14. Consistent with earlier reports71,72, we found foveal MPOD to be significantly lower than normal in these patients. Unexpectedly, we found MP concentration to be normal at the preserved foveal center in these patients reemphasizing the importance of measuring foveal structure when interpreting MPOD abnormalities30,32. Our results lend support to a role of MP in ABCA4-RD and do not contradict the long-held hypothesis that protection afforded by MP contributes to foveal sparing19. Future longitudinal studies could directly test whether the rate of foveal disease progression is related to MPOD and/or MP concentration.
Microdensitometry studies in primate retinas have shown that MP concentration is not uniform across the foveal depth; there is a major peak at the Henle fiber layer and relatively uniform lower concentration along photoreceptor nuclei and inner and outer segments 73. Our estimate of MP concentration derived by dividing the total MPOD by the total retinal thickness would thus not represent the true MP concentration in any given retinal layer. The estimate, however, may be a useful approximation to the average foveal MP concentration under the assumption that cone outer segments and nuclei as well as cone axons in the Henle fiber layer thin proportionally in retinal degenerative disease. Future studies combining polarization-sensitive OCT-based74 delineation of Henle fiber layer thickness and MP imaging42 may allow a better estimate of the maximal tissue concentration of MP and its relationship to foveal sparing in disease.
The psychophysical HFP technique used in the current work to estimate MPOD is the most common method75; alternatives include retinal reflectance71,76, lipofuscin fluorescence77, Raman spectroscopy78 and other psychophysical methods79,80. All psychophysical methods, including HFP, require stable foveal fixation, and patient eyes were selected accordingly. HFP-based MPOD values in our patients were highly repeatable with an inter-session variability that was comparable to normal subjects from this study and from other published work21,41,47,61–64. Repeatability does not necessarily imply validity and assumptions implicit in the use of HFP technique to estimate MPOD were not explicitly proven in our study. It is assumed, for example, that the difference in L/M-cone mediated sensitivity to the blue and green stimuli is invariant across the measured central retinal locations22,81. Theoretically, outer retinal degeneration could affect the relative abundance and/or photopigment density of L and M cones differentially across the regions tested. Our use of a molecularly homogeneous population of ABCA4-lipofuscinopathy patients would be expected to minimize the potential for spatially variant degeneration of L and M cones. Further, reduced L/M-cone photopigment density previously reported in patients with retinal degenerations and/or maculopathies81–85 would be expected to diminish the spatial differences in cone pigment optical density and reduce the extent of MP density measurement error.
Serum lutein and zeaxanthin levels of the patients at baseline as a group were about half of that observed in our normal subjects even though estimates of dietary lutein intake were not different between the two groups. Serum carotenoids in our patients were also at the low end of the distribution of values from large population-based studies56–59. Patients with the lowest serum levels of lutein were not different from the rest of the patients or normal subjects in variables such as age, gender, diet or BMI, although some showed concomitant low levels of zeaxanthin, possibly reflecting an overall low carotenoid intake/uptake not revealed by the dietary questionnaire. Analysis of patients with and without previous history of multivitamin supplementation disclosed no differences in serum lutein and zeaxanthin concentrations arguing against possible interactions with other carotenoids present in those preparations86. It is tempting to consider a causal relationship between the serum lutein concentration and maculopathy. One possibility is that lower serum lutein levels predisposed this cohort of patients to more severe maculopathy such as hypothesized in age-related macular degeneration69. Support for such a hypothesis is lacking, however, since the patients included in this study had relatively mild disease within the severity spectrum of ABCA4-lipofuscinopathy11,12. An alternative hypothesis would be to consider the involvement of a systemic regulatory mechanism for lowering serum xanthophyll in response to reduced demand from degeneration of photoreceptors and RPE. Means of systemic signaling from the retina has been previously proposed to explain reduced blood levels of docosahexaenoic acid observed in many hereditary retinal degenerations87.
Augmentation of retinal MP concentration has been proposed not only to prevent age-related multifactorial degenerative diseases but also to prevent or delay retinal degeneration in Mendelian hereditary conditions88,89. We supplemented our ABCA4 patients with lutein for six months to evaluate short-term effects as a prelude to longer term studies. Serum lutein increased significantly in supplemented patients consistent with previous observations30,32,64,90,91. Retinal MPOD and MP concentration also increased significantly in more than half of the “responding” eyes of patients, consistent with previous studies30,32,64,90. Responders had a tendency to be female and, at baseline, have lower serum lutein, greater retinal thickness and lower retinal MP concentrations. This suggests that low levels of baseline xanthophylls in serum and retina may help predict MPOD increase upon supplementation. Whether other techniques of measuring MPOD would detect higher percentages of responders to supplementation or capable of detecting accumulation at cellular compartments such as the RPE92 not probed by the HFP method, awaits further study.
Increasing knowledge about the molecular basis of genetic retinal degenerations has provided an opportunity to consider gene- or mechanism-specific therapeutic interventions in otherwise incurable diseases such as ABCA4-RD. The present study builds upon available knowledge and uses clinically-feasible techniques to evaluate molecularly-identified patients at a specific disease stage. In this disease subset, we tried to understand whether or not there is vulnerability to a recently-described antioxidant mechanism28. The data were then used to perform a pilot trial of nutrient supplementation that could theoretically decrease the vulnerability. Such strategic approaches in molecularly-clarified retinopathies with specific consideration of baseline parameters that have high predictive value could reduce the variability of results and the length of clinical trials of these slowly-progressive degenerative disorders.
ACKNOWLEDGEMENTS
Thanks are due to Andy Cheung, Michelle Doobrajh, Elaine Smilko, Alejandro Román, and Marisa Román for their critical help.
Supported by grants from the National Institutes of Health (EY13203); Foundation Fighting Blindness, Inc.; Macula Vision Research Foundation; The Macular Disease Foundation; The Chatlos Foundation, Inc., Howard Hughes Medical Institute, Ruth and Milton Steinbach Award, Alcon Research Institute, and The Paul and Evanina Bell Mackall Foundation Trust.
References
- 1.Papermaster DS, Reilly P, Schneider BG. Cone lamellae and red and green rod outer segment disks contain a large intrinsic membrane protein on their margins: an ultrastructural immunocytochemical study of frog retinas. Vision Res. 1982;22:1417–1428. doi: 10.1016/0042-6989(82)90204-8. [DOI] [PubMed] [Google Scholar]
- 2.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 3.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in ABCR knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATPbinding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 5.Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- 6.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Mir A, Paloma E, Allikmets R, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 8.Cremers FPM, van de Pol DJR, van Driel M, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum Mol Genet. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 9.Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockney RR. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol. 1999;117:504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 10.Fishman GA, Stone EM, Eliason DA, Taylor CM, Lindeman M, Derlacki DJ. ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol. 2003;121:851–855. doi: 10.1001/archopht.121.6.851. [DOI] [PubMed] [Google Scholar]
- 11.Cideciyan AV, Aleman TS, Swider M, Schwartz SB, Steinberg JD, Brucker AJ, Maguire AM, Bennett J, Stone EM, Jacobson SG. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet. 2004;13:525–534. doi: 10.1093/hmg/ddh048. [DOI] [PubMed] [Google Scholar]
- 12.Cideciyan AV, Swider M, Aleman TS, Sumaroka A, Schwartz SB, Roman MI, Milam AH, Bennett J, Stone EM, Jacobson SG. ABCA4-associated retinal degenerations spare structure and function of the human parapapillary retina. Invest. Ophthalmol. Vis. Sci. 2005;46:4739–4746. doi: 10.1167/iovs.05-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, Itagaki Y, Nakanishi K. A2E, a byproduct of the visual cycle. Vision Res. 2003;43:2983–2990. doi: 10.1016/s0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- 15.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA. 2000;97:7154–7259. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinckers A, Cruysberg JR, aan de Kerk AL. Main types of bull's eye maculopathy. Functional classification. Doc Ophthalmol. 1984;58:257–267. doi: 10.1007/BF00153630. [DOI] [PubMed] [Google Scholar]
- 17.Wajima R, Katsumi O, Mehta MC, Itabashi R, Hirose T. Pattern-reversal visual-evoked response in bull's eye maculopathy associated with Stargardt's disease. Ophthalmic Res. 1995;27:234–242. doi: 10.1159/000267711. [DOI] [PubMed] [Google Scholar]
- 18.Kurz-Levin MM, Halfyard AS, Bunce C, Bird AC, Holder GE. Clinical variations in assessment of bull's-eye maculopathy. Arch Ophthalmol. 2002;120:567–575. doi: 10.1001/archopht.120.5.567. [DOI] [PubMed] [Google Scholar]
- 19.Weiter JJ, Delori F, Dorey CK. Central sparing in annular macular degeneration. Am J Ophthalmol. 1988;106:286–292. doi: 10.1016/0002-9394(88)90363-7. [DOI] [PubMed] [Google Scholar]
- 20.Landrum JT, Bone RA, Kilburn MD. The macular pigment: a possible role in protection from age-related macular degeneration. Adv Pharmacol. 1997;38:537–556. doi: 10.1016/s1054-3589(08)60998-9. [DOI] [PubMed] [Google Scholar]
- 21.Hammond BR, Jr, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Invest Ophthalmol Vis Sci. 1998;39:397–406. [PubMed] [Google Scholar]
- 22.Snodderly DM, Hammond BR., Jr . In vivo psychophysical assessment of nutritional and environmental influences on human ocular tissues: lens and macular pigment. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Boca Raton, FL: CRC Press; 1999. pp. 251–273. [Google Scholar]
- 23.Junghans A, Sies H, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys. 2001;391:160–164. doi: 10.1006/abbi.2001.2411. [DOI] [PubMed] [Google Scholar]
- 24.Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest. Ophthalmol. Vis. Sci. 2002;43:3538–3549. [PubMed] [Google Scholar]
- 25.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 26.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JE, Kukielczak BM, Hu DN, Miller DS, Bilski P, Sik RH, Motten AG, Chignell CF. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells, Photochem. Photobiol. 2002;75:184–190. doi: 10.1562/0031-8655(2002)075<0184:troaip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Webster AR, Heon E, Lotery AJ, Vandenburgh K, Casavant TL, Oh KT, Beck G, Fishman GA, Lam BL, Levin A, Heckenlively JR, Jacobson SG, Weleber RG, Sheffield VC, Stone EM. An analysis of allelic variation in the ABCA4 gene. Invest Ophthalmol Vis Sci. 2001;42:1179–1189. [PubMed] [Google Scholar]
- 30.Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, Steinberg JD, Cideciyan AV, Maguire MA, Jacobson SG. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest. Ophthalmol. Vis. Sci. 2001;42:1873–1881. [PubMed] [Google Scholar]
- 31.Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–2489. [PubMed] [Google Scholar]
- 32.Duncan JL, Aleman TS, Gardner LM, de Castro E, Marks DA, Emmons JM, Bieber ML, Steinberg JD, Bennett J, Stone EM, MacDonald IM, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in choroideremia. Exp. Eye Res. 2002;74:371–381. doi: 10.1006/exer.2001.1126. [DOI] [PubMed] [Google Scholar]
- 33.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultz and Ewald Hering. Vision Res. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- 34.Bone RA, Landrum JT, Gilbert J. Macular pigment and the edge hypothesis of flicker photometry. Vision Res. 2004;44:3045–3051. doi: 10.1016/j.visres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson SG, Voigt WJ, Parel JM, Apathy PP, Nghiem-Phu L, Myers SW, Patella VM. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93:1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- 36.Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Puliafito CA, Fujimoto JG. Optical coherence tomography of the human retina. Arch. Ophthalmol. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, Traboulsi EI, Heon E, Pittler SJ, Milam AH, Maguire AM, Palczewski K, Stone EM, Bennett J. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 39.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 40.Khachik F, Beecher GR, Smith JC., Jr Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem. 1995;22:236–246. doi: 10.1002/jcb.240590830. [DOI] [PubMed] [Google Scholar]
- 41.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A. 1997;14:1187–1196. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- 42.Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am A. 2006;23:521–538. doi: 10.1364/josaa.23.000521. [DOI] [PubMed] [Google Scholar]
- 43.Schalch W, Dayhaw-Barker P, Barker FM., II . The carotenoids of the human retina. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Boca Raton: CRC Press; 1999. pp. 215–250. [Google Scholar]
- 44.Johnson EJ, Hammond BR, Yeum K-J, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 45.Nolan J, O'donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S. Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci. 2004;45:3940–3950. doi: 10.1167/iovs.04-0273. [DOI] [PubMed] [Google Scholar]
- 46.Hammond BR, Jr, Curran-Celentano J, Judd S, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36:2001–2012. doi: 10.1016/0042-6989(95)00290-1. [DOI] [PubMed] [Google Scholar]
- 47.Snodderly DM, Mares JA, Wooten BR, Oxton L, Gruber M, Ficek T CAREDS Macular Pigment Study Group. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the carotenoids and age-related eye disease study. Invest Ophthalmol Vis Sci. 2004;45:531–538. doi: 10.1167/iovs.03-0762. [DOI] [PubMed] [Google Scholar]
- 48.Ciulla TA, Hammond BR., Jr Macular pigment density and aging, assessed in the normal elderly and those with cataracts and age-related macular degeneration. Am J Ophthalmol. 2004;138:582–587. doi: 10.1016/j.ajo.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 49.Hammond BR, Jr, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res. 1996;36:3003–3009. doi: 10.1016/0042-6989(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 50.Johnson EJ, Hammond BR, Yeum K-J, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 51.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 52.Broekmans WMR, Berendschot TTJM, Klöpping-Ketelaars IAA, de Vries AJ, Goldbohm RA, Tijburg LBM, Kardinaal AFM, van Poppel G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr. 2002;76:595–603. doi: 10.1093/ajcn/76.3.595. [DOI] [PubMed] [Google Scholar]
- 53.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J. Nutr. 2003;133:992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- 54.Beatty S, Nolan J, Kavanagh H, O'Donovan O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch Biochem Biophys. 2004;430:70–76. doi: 10.1016/j.abb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Hammond BR, Jr, Fuld K, Snodderly DM. Iris color and macular pigment optical density. Exp Eye Res. 1996;62:293–297. doi: 10.1006/exer.1996.0035. [DOI] [PubMed] [Google Scholar]
- 56.Ford ES. Variations in serum carotenoid concentrations among United States adults by ethnicity and sex. Ethn Dis. 2000;10:208–217. [PubMed] [Google Scholar]
- 57.Curran-Celentano J, Hammond BR, Jr, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802. doi: 10.1093/ajcn/74.6.796. [DOI] [PubMed] [Google Scholar]
- 58.Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based casecontrol study. Arch Ophthalmol. 1995;113:1518–1523. doi: 10.1001/archopht.1995.01100120048007. [DOI] [PubMed] [Google Scholar]
- 59.Gruber M, Chappell R, Millen A, LaRowe T, Moeller SM, Iannaccone A, Kritchevsky SB, Mares J. Correlates of serum lutein + zeaxanthin: findings from the third national health and nutrition examination survey. J Nutr. 2004;134:2387–2394. doi: 10.1093/jn/134.9.2387. [DOI] [PubMed] [Google Scholar]
- 60.Liew SH, Gilbert CE, Spector TD, Mellerio J, Kuijk FJ, Beatty S, Fitzke F, Marshall J, Hammond CJ. Central retinal thickness is positively correlated with macular pigment optical density. Exp Eye Res. 2006;82:915–920. doi: 10.1016/j.exer.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Hammond BR, Jr, Fuld K. Interocular differences in macular pigment density. Invest Ophthalmol Vis Sci. 1992;33:350–355. [PubMed] [Google Scholar]
- 62.Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res. 2004;79:21–27. doi: 10.1016/j.exer.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Nolan JM, Stack J, Mellerio J, Godhinio M, O'donovan O, Neelam K, Beatty S. Monthly consistency of macular pigment optical density and serum concentrations of lutein and zeaxanthin. Curr Eye Res. 2006;31:199–213. doi: 10.1080/02713680500514677. [DOI] [PubMed] [Google Scholar]
- 64.Hammond BR, Jr, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- 65.Davies NP, Morland AB. Macular pigments: their characteristics and putative role. Prog Retin Eye Res. 2004;23:533–559. doi: 10.1016/j.preteyeres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863. [PubMed] [Google Scholar]
- 67.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:3244–3256. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- 68.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Max Snodderly D. Nutritional manipulation of primate retinas. IV. Effects of n--3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp Eye Res. 2005;81:513–529. doi: 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 70.Beatty S, Boulton M, Henson D, Koh H-H, Murray IJ. Macular pigment and age related macular degeneration. Br J Ophthalmol. 1993;83:867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Hargitai J, Tammur J, Hutchinson A, Allikmets R, Chang S, Gouras P. Macular pigment and visual acuity in Stargardt macular dystrophy. Graefe’s Arch Clin Exp Ophthalmol. 2002;240:802–809. doi: 10.1007/s00417-002-0554-z. [DOI] [PubMed] [Google Scholar]
- 72.Zhao D-Y, Wintch SW, Ermakov IV, Gellermann W, Bernstein PS. Resonance Raman measurement of macular carotenoids in retinal, choroidal, and macular dystrophies. Arch Ophthalmol. 2003;121:967–972. doi: 10.1001/archopht.121.7.967. [DOI] [PubMed] [Google Scholar]
- 73.Snodderly DM, Auran JD, Delori FC. The macular pigment. II: spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 74.Cense B, Chen TC, Park BH, Pierce MC, de Boer JF. Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2004;45:2606–2612. doi: 10.1167/iovs.03-1160. [DOI] [PubMed] [Google Scholar]
- 75.Hammond BR, Jr, Wooten BR, Smollon B. Assessment of the validity of in vivo methods of measuring human macular pigment optical density. Optom Vis Sci. 2005;82:387–404. doi: 10.1097/01.OPX.0000162652.85875.D2. [DOI] [PubMed] [Google Scholar]
- 76.Kilbride PE, Alexander KR, Fishman M, Fishman GA. Human macular pigment assessed by imaging fundus reflectometry. Vision Res. 1989;29:663–674. doi: 10.1016/0042-6989(89)90028-x. [DOI] [PubMed] [Google Scholar]
- 77.Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A. 2001;18:1212–1230. doi: 10.1364/josaa.18.001212. [DOI] [PubMed] [Google Scholar]
- 78.Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci. 1998;39:2003–2011. [PubMed] [Google Scholar]
- 79.Moreland JD, Robson AG, Soto-Leon N, Kulikowski JJ. Macular pigment and the colour-specificity of visual evoked potentials. Vision Res. 1998;38:3241–3245. doi: 10.1016/s0042-6989(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 80.Davies NP, Morland AB. Color matching in diabetes: optical density of the crystalline lens and macular pigments. Invest Ophthalmol Vis Sci. 2002;43:281–289. [PubMed] [Google Scholar]
- 81.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A. 2000;17:1918–1932. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morse PH, Smith VC, Pokorny JM, Burch JV. Fundus flavimaculatus with cystoid macular changes and abnormal Stiles-Crawford effect. Am J Ophthalmol. 1981;91:190–196. doi: 10.1016/0002-9394(81)90172-0. [DOI] [PubMed] [Google Scholar]
- 83.van Meel GJ, van Norren D. Foveal densitometry as a diagnostic technique in Stargardt's disease. Am J Ophthalmol. 1986;102:353–362. doi: 10.1016/0002-9394(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 84.Kilbride PE, Fishman M, Fishman GA, Hutman LP. Foveal cone pigment density difference and reflectance in retinitis pigmentosa. Arch Ophthalmol. 1986;104:220–224. doi: 10.1001/archopht.1986.01050140074023. [DOI] [PubMed] [Google Scholar]
- 85.Elsner AE, Burns SA, Weiter JJ. Cone photopigment in older subjects: decreased optical density in early age-related macular degeneration. J Opt Soc Am A. 2002;19:215–222. doi: 10.1364/josaa.19.000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and β-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62:604–610. doi: 10.1093/ajcn/62.3.604. [DOI] [PubMed] [Google Scholar]
- 87.Anderson RE, Maude MB, Alvarez RA, Acland G, Aguirre GD. A hypothesis to explain the reduced blood levels of docosahexaenoic acid in inherited retinal degenerations caused by mutations in genes encoding retina-specific proteins. Lipids. 1999;34 Suppl:S235–S237. doi: 10.1007/BF02562304. [DOI] [PubMed] [Google Scholar]
- 88.Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry. 2000;71:147–164. [PubMed] [Google Scholar]
- 89.Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial. BMC Ophthalmol. 2006;6:23. doi: 10.1186/1471-2415-6-23. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hininger IA, Meyer-Wenger A, Moser U, Wright A, Southon S, Thurnham D, Chopra M, Van Den Berg H, Olmedilla B, Favier AE, Roussel AM. No significant effects of lutein, lycopene or beta-carotene supplementation on biological markers of oxidative stress and LDL oxidizability in healthy adult subjects. J Am Coll Nutr. 2001;20:232–238. doi: 10.1080/07315724.2001.10719037. [DOI] [PubMed] [Google Scholar]
- 91.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 92.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]