Abstract
Major signaling cascades have been shown to play a role in the regulation of intracellular transport of organelles. In Xenopus melanophores, aggregation and dispersion of pigment granules is regulated by the second messenger cAMP through the protein kinase A (PKA) signaling pathway. PKA is bound to pigment granules where it forms complexes with molecular motors involved in pigment transport. Association of PKA with pigment granules occurs via binding to A-kinase anchoring proteins (AKAPs), whose identity remains largely unknown. Here we used mass-spectrometry to examine a 80 kDa AKAP detected in preparations of purified pigment granules. We found that tryptic digests of granule protein fractions enriched in the 80 kDa AKAP contained peptides that corresponded to the actin-binding protein moesin, which has been shown to function as an AKAP in mammalian cells. We also found that recombinant Xenopus moesin interacted with PKA in vitro, co-purified with pigment granules, and bound to pigment granules in cells. Overexpression in melanophores of a mutant moesin lacking conserved PKA binding domain did not affect aggregation of pigment granules, but partially inhibited their dispersion. We conclude that Xenopus moesin is an AKAP whose PKA scaffolding activity plays a role in the regulation of pigment dispersion in Xenopus melanophores.
Keywords: moesin, PKA, AKAP, pigment granule, melanophore
Intracellular transport of organelles and particles is ubiquitous in animal cells and has fundamental importance for such diverse processes as secretion (1), neuronal signaling (2, 3), organization of endomembranes (4), and cell division (5, 6). The driving force for intracellular transport is provided by molecular motors bound to the surface of cargo organelles and moving along microtubules and actin filaments (AFs) (7-11). Because several types of motors are simultaneously bound to the same cargo and the same organelles move along both types of cytoskeletal tracks (12), transport events have to be precisely regulated to ensure the delivery of organelles and particles to specific cellular destinations. While there is evidence that major signaling cascades may be involved in this regulation (13), the exact regulatory mechanisms remain largely unknown.
An excellent model system to examine the mechanisms of regulation of intracellular transport by signaling events is Xenopus melanophores. The major function of these cells is fast and synchronous movement of thousands of membrane-bounded pigment granules, which aggregate in the cell center or redisperse uniformly throughout the cytoplasm by movement along the microtubules and AFs mediated by the molecular motors cytoplasmic dynein, kinesin-2, and myosin V (14). Switching between these motors is regulated by a second messenger, cAMP, and involves the cAMP effector PKA (14). PKA is bound to pigment granules in cells and co-purifies with isolated pigment granules (15). Binding to the surface of pigment granules places PKA near molecular motors whose activity is regulated, and therefore is likely important for the pigment transport regulation. However, the mechanisms of PKA anchoring to pigment granules are not fully understood.
PKA targeting to discrete locations in the cytoplasm is commonly achieved through the interaction with AKAPs, - a family of structurally diverse but functionally related proteins (16-19). Molecules of all known AKAPs contain a PKA-anchoring domain that shares structural similarity among the AKAP family members, and a targeting domain, which is unique for each AKAP type (16-19). PKA anchoring domains of AKAPs bind PKA, and targeting domains scaffold PKA on the cytoplasmic surface of the plasma membrane (20-22) or endomembrane compartments, such as membrane organelles (23-25). Experimental evidence indicates that AKAP-based targeting brings PKA into close proximity to its substrates, and therefore directs and amplifies the cAMP signal transduction (16-19).
Similar to other membrane organelles, pigment granules carry AKAPs. A standard approach for the detection of AKAPs involves a blot overlay assay with recombinant PKA regulatory subunit RIIα, which directly interacts with PKA anchoring domains of AKAPs (26). Using this assay, we found that preparations of pigment granules isolated from Xenopus melanophores contained two protein bands with molecular masses of approximately 80 kDa and 160 kDa that bound PKA in blot overlayş (15). It has also been shown that a Ras-like GTPase Rab32, which has been demonstrated to function as an AKAP in fibroblasts (25), co-purified with pigment granules isolated from melanophores (27). Therefore, experimental evidence indicates that pigment granules bind several AKAPs that anchor PKA and therefore establish multiple entry sites for the cAMP signal on the surface of pigment granules. Because in other experimental systems AKAPs play a major role in the structural organization of complexes of PKA with its substrates, to fully understand PKA-based signal transduction in melanophores it is necessary to identify pigment-granule bound AKAPs and their binding partners.
Here we identify a 80 kDa AKAP in the preparations of pigment granules from Xenopus melanophores using mass-spectrometry. We developed an experimental protocol for the enrichment of the 80 kDa AKAP, which involved limited proteolysis of preparations of pigment granules with the highly selective protease factor Xa. We found that while most proteins remained bound to pigment granules, 80 kDa AKAP was released in the soluble fraction after the factor Xa treatment. Analysis of proteins in the soluble fraction with mass-spectrometry demonstrated the presence of the actin-binding protein moesin, which has a molecular mass similar to the 80 kDa AKAP, and is known to function as an AKAP in mammalian cells (28). We found that recombinant Xenopus moesin interacted with PKA in blot overlays, was present in preparations of pigment granules, and bound to pigment granules in vivo. Overexpression of a moesin construct that lacked a conserved PKA binding domain partially inhibited pigment dispersion, but did not affect pigment aggregation. We conclude that Xenopus moesin is an AKAP whose PKA scaffolding activity plays a role in the regulation of pigment dispersion in Xenopus melanophores.
Results and Discussion
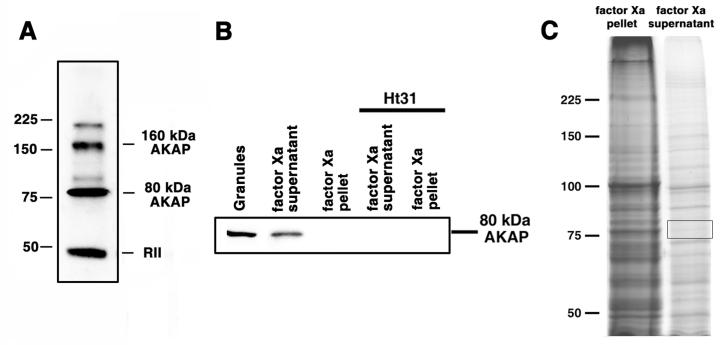
Preparations of pigment granules contain two protein bands of approximately 80 kDa and 160 kDa that bind the RIIα subunit of PKA in blot overlays in a manner sensitive to Ht31, a synthetic peptide that has been shown to prevent PKÃ-AKAP interactions, but not control inactive peptide Ht31P (15). The highly specific RIIα binding of the 80 kDa and 160 kDa bands indicates that these proteins are AKAPs. To identify the 80 kDa AKAP, we examined proteins with molecular masses close to 80 kDa using mass-spectrometry. To simplify the analysis of mass-spectrometry data, we developed an experimental protocol for enrichment of the 80 kDa AKAP, which involved treatment of pigment granule preparations with the highly specific protease factor Xa. Blot-overlays with recombinant RIIα of supernatants and pellets obtained by centrifugation of the protease Xa-treated preparations of pigment granules showed that proteolysis released the entire pool of the pigment granule bound 80 kDa AKAP into the solution. Remarkably, the mobilities in SDS gels of the solubilized and the pigment granule-bound bands were similar, which suggests that protease Xa instead of cleaving the 80 kDa AKAP itself digested a protein that tethered this AKAP to pigment granules. Proteins in the supernatants of proteolyzed pigment granules containing 80 kDa AKAP were separated by SDS-gel electrophoresis, and protein bands in the 75-85 kDa molecular weight range were dissected from gels (Fig 1C) and treated with trypsin. The sources of tryptic peptides were then examined using mass-spectrometry.
Figure 1. 80 kDa AKAP is released in solution after treatment of pigment granule preparations with the highly selective protease factor Xa.
A, blot overlays of pigment granule preparations with recombinant RIIα; RIIα binds two polypeptides in the pigment granule preparations with approximate molecular masses of 80 kDa and 160 kDa, as well as to itself. B, blot overlays of the indicated samples with recombinant RIIα in the absence (left three lanes) or presence (right two lanes) of Ht31. Treatment of pigment granules with factor Xa releases the 80 kDa RIIα-binding protein into solution. C, Coomassie stained SDS-PAGE gels of a pellet (left) and a supernatant (right) obtained by centrifugation of a pigment granule preparation treated with factor Xa; most of the pigment granule proteins remained bound to pigment granules after the protease treatment; boxed region on the right lane shows the gel area used for the mass-spectrometry analysis.
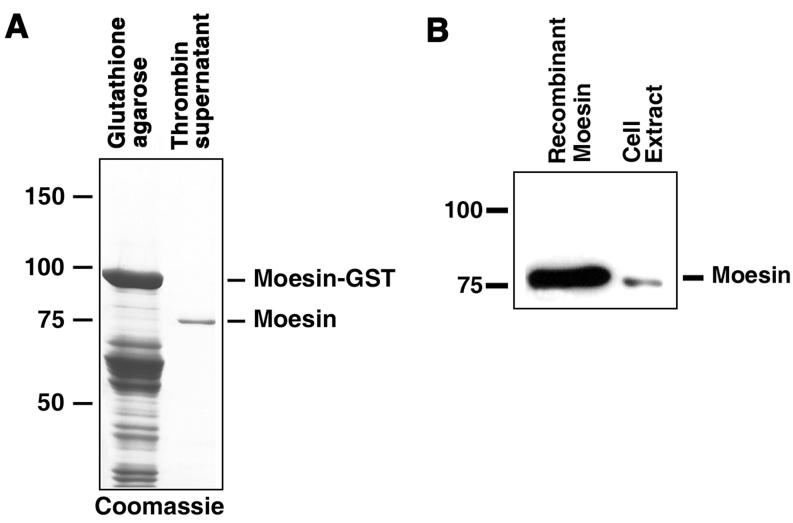
The mass-spectrometry data indicated that the most abundant tryptic peptides were derived from the Xenopus actin-binding protein moesin, - a member of the FERM family of actin-binding proteins, which also includes band 4.1, ezrin, radixin and merlin (29-31). Because mammalian FERM proteins, including human moesin, have been shown to bind PKA in vitro (28), and the amino acid sequences of FERM proteins are conserved in vertebrates (29-31), it was highly likely that Xenopus moesin also bound PKA. To test this possibility directly, we cloned Xenopus moesin DNA using RT PCR, expressed recombinant moesin in bacteria as a GST fusion, and purified the expressed fusion protein by chromatography on glutathione-agarose (Fig. 2A). Blot overlays showed that Xenopus moesin indeed bound RIIα (Fig. 2B and C), and that this binding was abolished in the presence of Ht31, and therefore was specific (32). We conclude that Xenopus moesin is an AKAP.
Figure 2. Recombinant moesin binds RIIα in a blot overlay assay.
A, Coomassie stained SDS-PAGE gels of preparations of recombinant moesin expressed in E.coli; left lane, pellet of GST agarose beads after incubation with bacterial lysates; right lane, supernatant of GST-agarose beads after the treatment with thrombin, which cleaves moesin off the GST. Recombinant moesin was purified by GST-agarose affinity chromatography and thrombin treatment to near homogeneity. B, blot overlay of recombinant moesin (left), and extract of melanophores (right) with recombinant RIIα subunit of PKA. Recombinant moesin binds RIIα
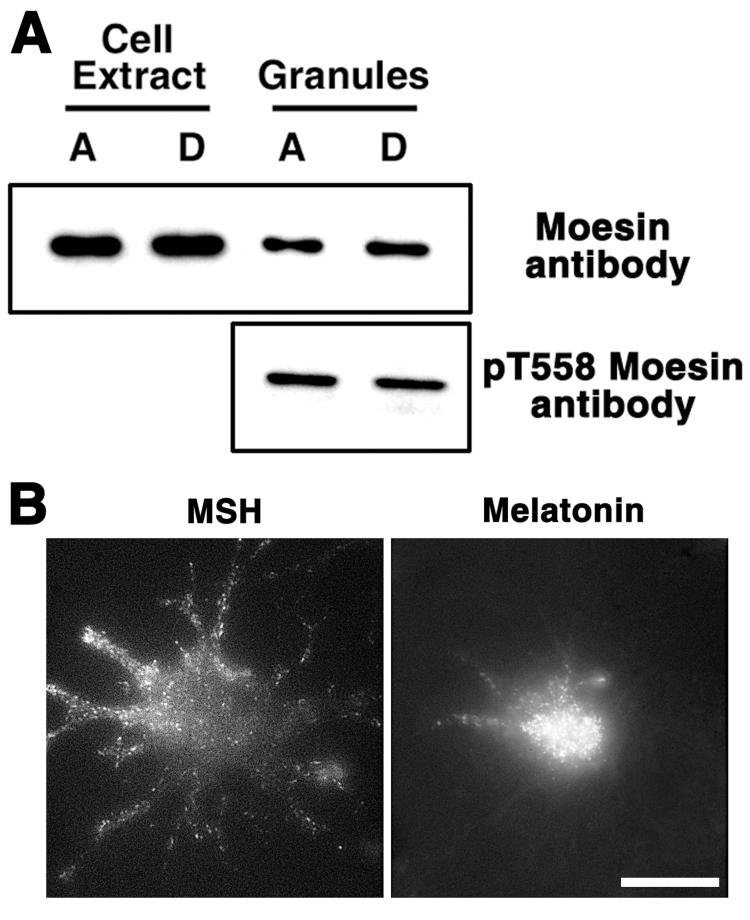
We found that moesin bound RIIα in blot overlays, and the mobility of moesin in SDS gels was similar to the mobility of the 80 kDa AKAP. Therefore it was highly likely that moesin was identical to the 80 kDa AKAP. To further examine this possibility, we tested whether moesin is associated with pigment granules as would be predicted for a pigment granule AKAP. To test whether moesin is bound to pigment granules in vivo and in vitro, we probed preparations of isolated pigment granules with an anti-moesin antibody, and transfected Xenopus melanophores, which had been treated to deplete pigment granules of melanin (33), with moesin-GFP fusion DNA. We found that intracellular moesin co-purified with pigment granules, as evidenced from the comparison of immunoblotting signals between whole cell extracts and pigment granule preparations from cells with aggregated or dispersed pigment (Fig. 3A). Comparison of the amounts of total protein and moesin in preparation of pigment granules and cell extracts showed that the fractions of total protein and moesin were ∼4.4% and 14.6 ± 3.2% (mean ± SEM, n=4), an indication that moesin was enriched in pigment granule preparations. We also found that in transfected cells, moesin-GFP was localized in fluorescent dots, which were distributed homogeneously in the cytoplasm or accumulated in the cell center in response to hormones that caused pigment dispersion (MSH) or aggregation (melatonin), and therefore behaved similarly to pigment granules (Fig. 3B). We conclude that moesin is bound to pigment granules in vivo and in vitro.
Figure 3. Moesin binds pigment granules in vivo and in vitro.
A, Top, immunoblotting with anti-moesin antibody of cell extracts or pigment granules prepared from melanophores with aggregated (A) or dispersed (D) pigment granules; bottom, immunoblotting with pT558 antibody, which recognizes the activated form of moesin, of pigment granule preparations isolated from cells with aggregated or dispersed pigment granules. Moesin in the activated form is bound to pigment granules. B, Fluorescence images of the same cell transfected with moesin-GFP treated with MSH or melatonin to induce aggregation or dispersion of pigment granules. Moesin is localized to fluorescent dots whose distribution and behavior in response to hormones is similar to pigment granules. The scale bar is 20 μm.
To determine whether binding of moesin to pigment granules was specific, we tested whether moesin in pigment granule preparations was present in a dormant form or in an active form capable for interacting with ligands such as actin or membrane-bound proteins (29-31). Activation of moesin involves unfolding of the moesin molecule via the phosphorylation of Thr-558, which inhibits intramolecular interaction between the N-terminal FERM domain, and the C-terminal actin-binding domain (34). To determine whether pigment granules bound the active form of moesin, we probed preparations of pigment granules isolated from cells with aggregated or dispersed pigment with a highly specific phosphopeptide antibody that recognizes phosphorylated Thr-558. We found that the anti-phosphoThr-558 antibody bound to a protein band whose electrophoretic mobility was identical to the mobility of moesin, and that the amounts of this band were similar in the pigment granule preparations isolated from cells with dispersed and aggregated pigment (Fig. 3A). Therefore, moesin in an active phosphorylated form was constitutively bound to pigment granules, which indicates that binding of moesin to pigment granules was specific. Taken together, these results indicate that Xenopus moesin is the 80 kDa AKAP found in preparations of pigment granules.
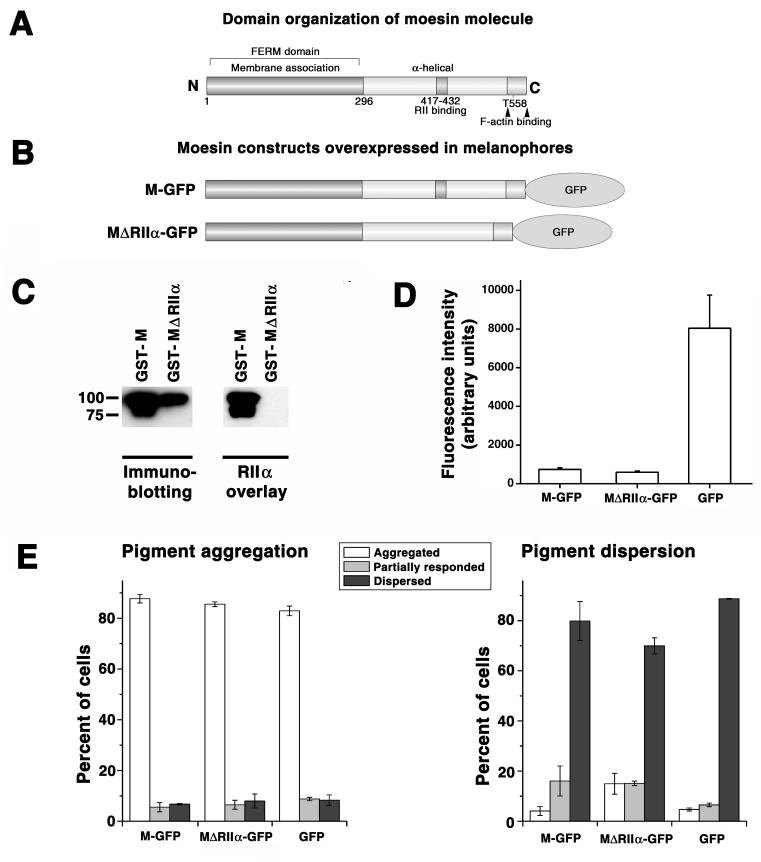
Our data indicated that moesin was a pigment granule-bound AKAP, and the results of other studies showed that PKA targeting by AKAPs caused local amplification of cAMP signal (16-19). Therefore it was possible that targeting of PKA to pigment granules by moesin was involved in cAMP signal transduction. To test this possibility, we studied the effect of inhibition of PKA binding to moesin on pigment transport by examining aggregation or dispersion of pigment granules in cells overexpressing GFP-tagged mutant moesin that bound to pigment granules (not shown) but lacked a conserved RIIα-binding domain (28) (Fig. 4, B) and therefore was incapable of RIIα binding of (Fig. 4C). Control experiments involved overexpression of the full-size moesin-GFP, or GFP alone. We found that overexpression of a mutant moesin-GFP did not affect pigment aggregation (Fig. 4C, left), but partially inhibited pigment dispersion, as evidenced from the approximately three-fold increase in the fraction of cells whose response to a dispersion signal was completely inhibited, and the two-fold increase in the fraction of cells showing partial inhibition (Fig. 4C, right). Overexpression of the full-size moesin-GFP increased the amount of partially dispersed cells, but did not inhibit dispersion completely and therefore had a much weaker inhibitory effect than the mutant moesin-GFP (Fig. 4C, right). Stronger inhibition of dispersion could not be explained by greater expression of the mutant moesin-GFP, because quantification of the GFP fluorescence in the transfected cells indicated that the cytoplasmic concentration of the mutant protein was similar to that of full-size moesin-GFP (Fig. 4D). We conclude that targeting of PKA to pigment granules by moesin is important for the regulation of pigment dispersion in melanophores.
Figure 4. Overexpression of moesin lacking the RIIα-binding domain partially inhibits pigment dispersion.
A, domain organization of the Xenopus moesin molecule; the conserved RIIα binding domain (28) is located between amino acid residues 417 and 432. B, domain organization of the GFP-tagged Xenopus moesin constructs used for transfection of melanophores. C, blot overlay of the full-size recombinant GST-moesin and a mutant recombinant GST-moesin lacking the RIIα-binding domain with recombinant RIIα subunit of PKA; left panel, immunoblotting of purified recombinant proteins with moesin antibody; right panel, blot overlay with recombinant RIIα ; left lanes, full-size GST-moesin (GST-M); right lanes, GST-moesin lacking RIIα-binding domain (GST-MΔRIIα). Full-size recombinant moesin but not recombinant moesin lacking the RIIα-binding domain binds RIIα subunit of PKA. D, comparison of the levels of expression of GFP and moesin-GFP constructs shown in panel B in melanophores. Full-size moesin-GFP and a mutant moesin lacking RIIα-binding domain are expressed at approximately the same levels, whereas GFP is expressed at significantly higher levels compared to moesin constructs. E, Quantification of responses to melatonin or MSH, applied to induce pigment aggregation or dispersion, of melanophores overexpressing full-size moesin-GFP (left set of bars; M-GFP), moesin-GFP lacking conserved RIIa-binding domain (middle sets of bars; MΔRIIα-GFP), or GFP (right sets of bars; GFP); data are expressed as the percentage of cells with aggregated (white bars), partially dispersed (gray bars), or dispersed (black bars) pigment; left panel, stimulation of pigment aggregation with melatonin; right panel, stimulation of pigment dispersion with MSH. Overexpression of moesin constructs does not affect aggregation but partially inhibits dispersion, and inhibition is significantly more prominent in cells overexpressing moesin lacking the RIIα- binding domain (MΔRIIα-GFP) compared to full-size moesin (M-GFP).
In this study, we identified moesin as the 80 kDa AKAP found in the preparations of pigment granules isolated from Xenopus melanophores. This conclusion is based on several lines of experimental evidence. First, mass-spectrometry identified tryptic peptides that corresponded to moesin in pigment granule preparations. Second, immunoblotting of preparations of pigment granules with a moesin antibody and live imaging of melanophores expressing moesin-GFP showed that moesin is bound to pigment granules in vitro and in vivo. Finally, blot overlays demonstrated the binding of the RIIα subunit of PKA to recombinant Xenopus moesin in vitro, and therefore confirmed that, like mammalian ERM proteins (28), Xenopus moesin is an AKAP. Taken together, these results show that Xenopus moesin is an AKAP bound to pigment granules in melanophores.
It is known that targeting of PKA to distinct subcellular locations by AKAPs directs and amplifies the biological effects of cAMP signaling. This conclusion is based on numerous observations that show that antagonists of PKA-AKAP interactions reduce cellular responses to elevated cAMP levels, such as activation of ion channels (22), regulation of receptor activity (20, 35), condensation of chromosomes (36), and secretion (37). We found that in Xenopus melanophores, overexpression of a dominant negative moesin construct lacking the amino acid sequence responsible for the binding of PKA, and therefore incapable for PKA targeting to pigment granules, partially inhibits pigment dispersion. Because pigment dispersion is activated by an increase in the cAMP levels (14), this result indicates that the recruitment of PKA by pigment granule-bound moesin is important for cAMP-mediated signal transduction.
While our work shows that moesin is a pigment granule-bound PKA-anchoring protein, experimental evidence indicates that it is not the only AKAP that targets PKA to the surface of pigment granules. Our RIIα blot overlay assays indicate that another protein in preparations of pigment granules with a molecular mass ∼160 kDa binds PKA in a specific, Ht31-dependent manner. In addition, a PKA-binding GTPase Rab32 has been shown to localize to the surface of pigment granules (27). While the significance of multiple AKAPs bound to pigment granules is not yet understood, it is possible that each type targets PKA to a distinct subset of protein substrates. Our past work indicates that PKA forms two separate complexes with molecular motors, one with cytoplasmic dynein and another with kinesin-2 and myosin V, which we named aggregation and dispersion regulated motor units (15). It will be important to determine whether targeting of PKA to aggregation and dispersion regulated motor units involves distinct AKAPs.
AKAPs are known to bind not only PKA but also other signaling enzymes (38). By targeting various kinases and phosphatases that belong to multiple signaling pathways to the same substrates, AKAPs create focal points for signal transduction (38). In melanophores, regulation of pigment transport involves in addition to PKA at least other enzymes: the phosphatase PP2A whose activity opposes the activity of PKA (39), protein kinase C that regulates pigment dispersion through a poorly understood cAMP/PKA-independent signaling pathway (39, 40), and the protein kinase ERK that seems to be responsible for the global control of pigment transport (41). Because concurrent binding of several types of signaling enzymes is a common property of AKAPs (38), it is possible that in melanophores, pigment granule-bound moesin is involved in the scaffolding of several signaling enzymes near the molecular motors whose activities they regulate.
Moesin and other ERM proteins are known to link the plasma membrane to the cortical actin cytoskeleton (29-31). The formation of moesin links involves simultaneous interaction of moesin molecules with membrane-bound proteins through the N-terminal FERM domain, and with AFs through the C-terminal actin-binding domain. It is likely that in melanophores moesin binds pigment granules through the FERM domain, and therefore the actin-binding domain is exposed on the surface of pigment granules. Interaction of the actin-binding domain of the granule-bound moesin with AFs, which has been demonstrated for ERM proteins bound to other membrane organelles (42-44), might facilitate myosin-based transport of pigment granules. Moesin links should keep pigment granules near the AFs and therefore increase the probability of binding to AFs of pigment-granule bound myosin V motors. This should improve the movement of pigment granules along AFs in the dispersed state, and enhance the transfer onto AFs of pigment granules moving along the cytoplasmic microtubules during pigment dispersion.
Based on our results, we propose a model for the role of moesin in pigment transport in Xenopus melanophores (Fig. 5). We suggest that moesin is involved in pigment dispersion and plays dual roles in this process. First, moesin anchors PKA on the surface of pigment granules and therefore targets PKA to molecular motors whose activity it regulates. Because overexpression of the dominant negative moesin lacking a PKA binding domain inhibits dispersion but not aggregation of pigment granules, we suggest that moesin directly or indirectly interacts with the dispersion regulated motor unit. This interaction targets PKA to molecular motors involved in pigment dispersion (Fig. 5), and assists in the regulation of their activities by cAMP. Second, moesin participates in pigment dispersion by linking pigment granules to the surrounding AFs (Fig. 5). Retention of pigment granules near the surface of AFs promotes binding to AFs of the pigment granule-bound myosin V, and therefore enhances actin-based transport of pigment granules. Analysis of the proposed roles of moesin in pigment transport is an exciting new line of future investigation.
Figure 5. Hypothesis about functions of moesin during pigment dispersion.
Moesin plays a dual role in pigment dispersion. First, moesin directly or indirectly interacts with the dispersion regulated motor unit, which includes kinesin-2, myosin V and anchoring proteins, and therefore targets PKA in close proximity to molecular motors involved in pigment dispersion. Second, moesin links pigment granules to AFs, which increases the probability of interaction with pigment granules of myosin V, and therefore facilitates actin-dependent transport of pigment granules.
Materials and Methods
Cell culture
Xenopus melanophores (15) were cultured in Xenopus tissue culture medium (70% L15 medium supplemented with antibiotics, 20% fetal bovine serum, and 5 μg/ml insulin). To induce pigment aggregation or dispersion, cells were placed in serum-free media 1 hr before the hormone addition. Aggregation or dispersion was induced by 10−8 M melatonin or melanocyte-stimulating hormone (MSH), respectively.
To quantify aggregation or dispersion responses, melanophores transfected with wild-type or mutant moesin-GFP fusion proteins were treated with melatonin or MSH for 10 min and 15 min, respectively, and fixed with formaldehyde. The amounts of cells with aggregated, partially aggregated, or dispersed pigment were determined by counting cells in each category under a phase-contrast microscope, as described previously (15).
Cells with a reduced amount of melanin in their pigment granules were obtained as described by Rogers et al. (33).
Purification of pigment granules and limited proteolysis of pigment granule proteins
Pigment granules were purified (15), and subjected to proteolysis with factor Xa. For the proteolysis, 6 U (3 μl) of factor Xa solution (Novagen, EMD Chemicals Inc., Gibbstown, NJ) was added to 100 μl of a suspension of pigment granules, and incubated for 2 h at 30°C. Pigment granules were separated from soluble proteins by centrifugation at 35,000×g for 15 min. Supernatant was collected and used for the detection of PKA binding proteins in blot overlays.
Immunoblotting
Immunoblotting was performed as described in (45), using a monoclonal anti-moesin antibody (Transduction Laboratories, BD Biosciences, Franklin Lakes, NJ), or an anti-moesin (phosphor T588) antibody (Abcam, Cambridge, MA). Immunoreactive bands were detected with SuperSignal West Femto maximum sensitivity substrate (Pierce Biotechnology, Inc., Rockford, IL).
To estimate the fraction of pigment granule-bound moesin, cell lysates were centrifuged at 2,000×g for 5 min to remove cell debris, and the supernatants were further centrifuged at 14,000×g for 10 min to separate pigment granules from the cell extract. The pellets of pigment granules were then extracted with SDS-electrophoresis sample buffer, and the volumes of the cell and pigment granule extracts were normalized and loaded onto SDS gels for quantitative immunoblotting. Protein bands that cross-reacted with a monoclonal moesin antibody were revealed by staining with IRDye800 - conjugated affinity purified anti-mouse IgG (Rockland Immunochemicals, Gilbertsville, PA), and the intensity of the infra-red signal was quantified with the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Blot overlay assays
Blot overlays with recombinant RIIα were performed as described previously (26). Proteins were separated using SDS-gel electrophoresis, transferred onto nitrocellulose membrane, and incubated overnight in the blocking solution (PBS with 0.05% Tween 80, and 5% non-fat dry milk), supplemented with purified recombinant RIIα (0.025 mg/ml). The membrane was washed in blocking solution, and protein bands that bound RIIα were identified by immunostaining with anti-RIIα antibody (BD Biosciences, Franklin Lakes, NJ). Binding specificity was confirmed by performing blot overlays in the presence of Ht31 (13 μM), a synthetic peptide that inhibits RIIα-AKAP interactions, or a similar peptide Ht31P with two substitutions that abolish the inhibitory activity (32).
Mass-spectrometry
Mass-spectrometry was performed at the Midwest Bio Services mass-spectrometry facility. Proteins in the gel slices were digested with trypsin, and tryptic peptides were extracted and loaded onto a microcapillary reversed-phase column coupled to the nanospray ionization source of the DECA XP plus ion trap mass spectrometer (ThermoFinnigan). Peptides were eluted with acetonitrile gradient and electrosprayed into the mass spectrometer. Full MS and tandem MS/MS spectra were recorded and Sequest software was used to match MS/MS spectra to the Xenopus laevis protein database.
Cloning of Xenopus moesin DNA and construction of the moesin mutant lacking the PKA binding domain
Xenopus moesin DNA was amplified by PCR using as a template cDNA synthesized with SuperScript II reverse transcriptase (Invitrogen Corp., Carlsbad, CA) from total RNA isolated from Xenopus melanophores. The following forward and reverse primers were used for the amplification: 5′-TCCCCCGGGGGATGCCAAAAACTATCAGTGT ACGT-3′, and 5′-TTTTCCTTTTGCGGCCGCTTACATGGATTCAA ATTCATCTATAC-3′. The PCR product was purified and cloned into pCR 2.1 TOPO vector using TOPO TA cloning kit (Invitrogen Corp., Carlsbad, CA). The nucleotide sequence of the cloned PCR product was identical to the published sequence of Xenopus moesin (PubMed access number U29763).
Moesin DNA lacking the conserved PKA binding domain (28) was constructed by PCR amplification of moesin DNA fragments on the N-terminal (amino acids 1-416) or the C-terminal (amino acids 433-580) sides of the PKA binding domain using the following pairs of primers: CCGCTCGAGCATGCCAAAAACTATCAGTGTA CG and CGGGGTACCCCTGCTCCTGGTTCTTCATCTG (for the N-terminal fragment), and CGGGGTACCCCTTTGCTCGACAGAAGAAGGA GG and TCCCCCCGGGCATGGATTCAAATTCATCTATA CG (for the C-terminal fragment). PCR products were digested with XhoI and KpnI (the N-terminal fragment), or KpnI and XmaI (the C-terminal fragment), and ligated into an expression vector digested with XhoI and XmaI.
For bacterial expression of recombinant proteins and for expression of GFP-tagged proteins in Xenopus melanophores, full-size and mutant moesin DNAs were subcloned into pGEX (GE Healthcare, Piscataway, NJ) or pEGFP-N2 vectors (Clontech Labs., Mountain View, CA), respectively.
Expression and purification of recombinant moesin
Recombinant moesin was expressed in E.coli and purified from bacterial lysates by affinity chromatography on GST agarose according to instructions provided by manufacturer (GE Healthcare, Piscataway, NJ). To remove the GST tag and release moesin in solution, agarose beads with bound GST-moesin were treated with thrombin.
Transfection
Transfection of Xenopus melanophores was performed using Lipofectamine 2000 reagent (Invitrogen Corp., Carlsbad, CA).
Image acquisition and analysis
Images of cells expressing moesin-GFP were obtained using a Nikon Eclipse 300 inverted microscope equipped with a 100x, 1.25 N.A. objective lens. Images were collected with a back-illuminated cooled EMCCD camera (iXonEM; Andor Technology, South Windsor, CT).
For quantification of expression levels of GFP and GFP-tagged moesin constructs, images of cells were acquired using a fluorescein filter set, and the values for average gray levels within the cell outlines were determined using the Region Measurements Tool of Metamorph software (Molecular Devices, Downingtown, PA).
Acknowledgments
We thank Drs. James Watras and Laurinda Jaffe for critical reading the manuscript. This work was supported by NIH grant GM62290.
References
- 1.Lane J, Allan V. Microtubule-based membrane movement. Biochim Biophys Acta. 1998;1376:27–55. doi: 10.1016/s0304-4157(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 2.Hirokawa N, Takemura R. Molecular motors in neuronal development, intracellular transport and diseases. Curr Opin Neurobiol. 2004;14:564–573. doi: 10.1016/j.conb.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Guzik BW, Goldstein LS. Microtubule-dependent transport in neurons: steps towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol. 2004;16:443–450. doi: 10.1016/j.ceb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Allan VJ, Schroer TA. Membrane motors. Curr Opin Cell Biol. 1999;11:476–482. doi: 10.1016/s0955-0674(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 5.Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 6.Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 7.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 8.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 9.Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- 10.Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 11.Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. doi: 10.1034/j.1600-0854.2000.010103.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown SS. Cooperation between microtubule-and actin-based motor proteins. Annu Rev Cell Dev Biol. 1999;15:63–80. doi: 10.1146/annurev.cellbio.15.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Reilein AR, Rogers SL, Tuma MC, Gelfand VI. Regulation of molecular motor proteins. Int Rev Cytol. 2001;204:179–238. doi: 10.1016/s0074-7696(01)04005-0. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento AA, Roland JT, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol. 2003;19:469–491. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- 15.Kashina AS, Semenova IV, Ivanov PA, Potekhina ES, Zaliapin I, Rodionov VI. Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr Biol. 2004;14:1877–1881. doi: 10.1016/j.cub.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Smith FD, Langeberg LK, Scott JD. The where’s and when’s of kinase anchoring. Trends Biochem Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol. 2001;308:99–114. doi: 10.1006/jmbi.2001.4585. [DOI] [PubMed] [Google Scholar]
- 18.Michel JJ, Scott JD. AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- 19.McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 21.Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 23.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 24.Schillace RV, Scott JD. Association of the type 1 protein phosphatase PP1 with the A-kinase anchoring protein AKAP220. Curr Biol. 1999;9:321–324. doi: 10.1016/s0960-9822(99)80141-9. [DOI] [PubMed] [Google Scholar]
- 25.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausken ZE, Coghlan VM, Scott JD. Overlay, ligand blotting, and band-shift techniques to study kinase anchoring. Methods Mol Biol. 1998;88:47–64. doi: 10.1385/0-89603-487-9:47. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Serpinskaya AS, Papalopulu N, Gelfand VI. Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase a recruitment. Curr Biol. 2007;17:2030–2034. doi: 10.1016/j.cub.2007.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. Embo J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773:653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 31.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 32.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 33.Rogers SL, Karcher RL, Roland JT, Minin AA, Steffen W, Gelfand VI. Regulation of melanosome movement in the cell cycle by reversible association with myosin V. J Cell Biol. 1999;146:1265–1276. doi: 10.1083/jcb.146.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270:31377–31385. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- 35.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 36.Steen RL, Cubizolles F, Le Guellec K, Collas P. A kinase-anchoring protein (AKAP)95 recruits human chromosome-associated protein (hCAP)-D2/Eg7 for chromosome condensation in mitotic extract. J Cell Biol. 2000;149:531–536. doi: 10.1083/jcb.149.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci USA. 1997;94:14942–14947. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 39.Reilein AR, Tint IS, Peunova NI, Enikolopov GN, Gelfand VI. Regulation of organelle movement in melanophores by protein kinase A (PKA), protein kinase C (PKC), and protein phosphatase 2A (PP2A) J Cell Biol. 1998;142:803–813. doi: 10.1083/jcb.142.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugden D, Rowe SJ. Protein kinase C activation antagonizes melatonin-induced pigment aggregation in Xenopus laevis melanophores. J Cell Biol. 1992;119:1515–1521. doi: 10.1083/jcb.119.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deacon SW, Nascimento A, Serpinskaya AS, Gelfand VI. Regulation of bidirectional melanosome transport by organelle bound MAP kinase. Curr Biol. 2005;15:459–463. doi: 10.1016/j.cub.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 42.Deretic D, Traverso V, Parkins N, Jackson F, de Turco EB Rodriguez, Ransom N. Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell. 2004;15:359–370. doi: 10.1091/mbc.E03-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, Mangeat P, Voelter W, Marriott G, Pfannstiel J, Faulstich H, Griffiths G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. Embo J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defacque H, Bos E, Garvalov B, Barret C, Roy C, Mangeat P, Shin HW, Rybin V, Griffiths G. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol Biol Cell. 2002;13:1190–1202. doi: 10.1091/mbc.01-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]