Abstract
We previously demonstrated that defects in lipoprotein metabolism alter the distribution of oxygenated polyunsaturated fatty acids (PUFAs) in lipoprotein particles. If these oxidation products are released by lipoprotein lipase (LpL), then their delivery to peripheral tissues with bulk lipids could influence cellular function. Using 26 week old normolipidemic and hyperlipidemic Zucker rats, we measured PUFA alcohols, epoxides, diols, ketones and triols (i.e. oxylipins) in esterified and non-esterified fractions of whole plasma, VLDL, and LpL-generated VLDL-lipolysates. Whole plasma, VLDL, and lipolysate oxylipin profiles were distinct and altered by hyperlipidemia. While >90% of the whole plasma oxylipins were esterified, the fraction of each oxylipin class in the VLDL varied: 46% of alcohols, 30% of epoxides, 19% of diols, <10% of ketones, <1% triols. Whole plasma was dominated by arachidonate alcohols, while the linoleate alcohols, epoxides and ketones showed an increased prevalence in VLDL. LpL-mediated VLDL lipolysis of PUFA alcohols, diols and ketones was detected and the relative abundance of oxygenated linoleates was enhanced in the lipolysates, relative to their corresponding VLDL. In summary esterified oxylipins were seen to be LpL substrates with heterogeneous distributions among lipoprotein classes. Moreover, oxylipin distributions are changes within the context of obesity-associated dyslipidemia. These results support the notion that the VLDL-LpL axis may facilitate the delivery of plasma oxylipins to the periphery. The physiological implication of these findings are yet to be elucidated, however these molecules are plausible indicators of systemic oxidative stress, and could report this status to the peripheral tissues.
Keywords: oxylipin, oxylipid, eicosanoid, octadecanoid, VLDL, LpL, Lipoprotein lipase, hyperlipidemia, metabolic profiling
INTRODUCTION
The addition of oxygen to polyunsaturated fatty acids produces an array of bioactive compounds referred to here as oxylipins. Many of these compounds are mediators of vascular homeostasis and inflammation when produced within the proper physiological and anatomical context. While these compounds appear in the circulation as both free and esterified products [1], little is known about their availability to tissues in vivo, and they are generally believed to have only autocrine and paracrine effects.
Previously we have demonstrated that in rats, oxylipins are distributed among lipoproteins and that the pattern of distribution is disrupted in proteinuric hyperlipidemia [2]. In fasting, normolipidemic Sprague-Dawley rats, the fraction of plasma oxylipins in high density lipoproteins (HDL) is greater than in low density lipoproteins (LDL), and the very low density lipoproteins (VLDL) oxylipins are almost nonexistent. In proteinuric hyperlipidemia (e.g. the nephrotic syndrome), both VLDL and HDL oxylipins are greatly increased, ~20 fold and ~4 fold respectively, but LDL oxylipins declined, despite increased LDL lipids. This seems counterintuitive given the well known link between LDL, oxidized LDL and vascular disease risk. The expansion of both VLDL and HDL included specific increases in linoleate and arachidonate regioisomers, and was not simply a function of the increased abundance of a particular lipoprotein lipid class or protein. Furthermore, the shift in oxylipin distribution across lipoprotein density fractions was consistent with the known disruption of lipoprotein metabolism in this model [3, 4]. Results from other studies also suggest lipases may mediate the transport, delivery, and excretion of esterified oxylipins [5, 6], leading us to speculate that lipase/lipoprotein interactions may mediate an unappreciated role for circulatory oxylipins.
Here we explore the potential role for lipoproteins and lipoprotein lipase (LpL) in the plasma transport of the oxylipin classes described by Figure 1. We hypothesize that if lipoproteins are involved in the delivery of oxylipins to peripheral tissues three elements must be satisfied: 1) lipoproteins must carry a substantial fraction of plasma oxylipins; 2) oxylipins must occur in acylated forms; 3) lipolytic enzymes must release oxylipins from lipoproteins. In addition, two more elements would be supportive: 4) whole plasma and lipoprotein density fraction oxylipin profiles should be distinct; 5) dysoxylipinemia should be concurrent with dyslipidemia. To best achieve a demonstration of these phenomena, we chose a model of obese hyperlipidemia, the Zucker obese rat, as a source of lipoproteins. Hyperlipidemia in this model is a consequence of both increased VLDL synthesis and reduced clearance, assuring an abundant source of oxylipins without regard to the underlying cause for their abundance.
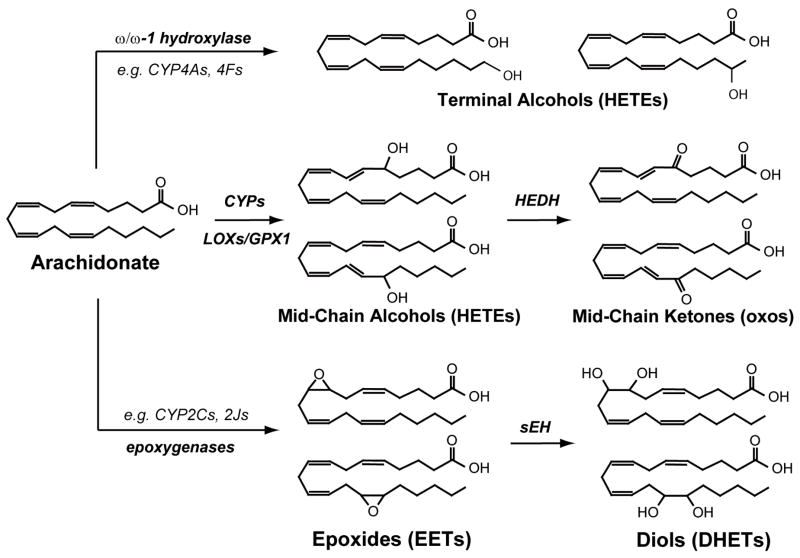
Figure 1. Representative members of the quantified oxylipin classes and their enzymatic routes of synthesis.
These include alcohol, ketones, epoxides and diols. Arachidonate metabolites are used here to represent the structures of each chemical class however all unsaturated lipids are oxidized by these routes and linoleic, γ-linolenic and arachidonic metabolites were determined in the present study. Abbreviations employed in this figure are as follows: CYP = cytochrome P450; LOX = lipoxygenase; GPX1 = glutathione peroxidase 1; sEH = soluble epoxide hydrolase; HEDH = hydroxyeicosanoid dehydrogenase; HETEs = hydroxyeicosatetraenoic acids; EETs = epoxyeicosatrienoic acids; DHETs = dihydroxyeicosatrienoic acids.
In this proof of principle investigation the availability of oxylipins to a lipid trafficking enzyme, LpL is studied. Specifically, we measured the extent to which whole plasma oxylipins are located in VLDL (i.e. the endogenous LpL-substrate), and whether LpL is able to release these VLDL oxylipins. These results were further used to compare the fractional distribution of oxygenated lipid sub-classes (Figure 1) between whole plasma, VLDL, and VLDL-lipolysates. As a secondary aim, we also test the impact of lipemia on oxylipin distributions to document the potential role of these agents in lipemia-related pathologies.
MATERIALS AND METHODS
Animals
Studies were approved by the Animal Review Committee University of California Davis. Four obese and four lean female Zucker rat littermates, all retired breeders, were obtained from the colony of Dr. Judith Stern at UC Davis. They were kept in 12 hour light/dark rooms and fed ad libitum until 26 weeks of age. Urinary albumin excretion was measured 1 day prior to exsanguination. Animals were placed in metabolic cages for 24 hours, urine was collected and UAE was assessed as previously reported [2]. Animals were anesthetized with an intraperitoneal injection of 0.75 g/kg sodium pentobarbital and exsanguinated by aortic puncture into a syringe containing 0.2 mL 0.5M EDTA. The collected blood was placed in an ice bath and plasma was separated within 1 hour. Aliquots were reserved for whole plasma analysis and the remaining plasma was used for lipoprotein (VLDL) isolation. Enzymatic kits were used for the determination of triglyceride (kit #2780-400H, Thermo DMA, Chatsworth CA), cholesterol (kit #TR12351, Thermo DMA, Chatsworth CA), phospholipids (kit# 990-54009, WAKO chemicals, Richmond VA), and non-esterified fatty acids (kit# 994-75409, WAKO chemicals, Richmond VA). Protein was measured using a bicinchoninic acid (BCA) assay (kit # 23225, Pierce chemicals, Milwaukee, WI).
Lipoprotein isolation and composition
VLDL fractions were isolated using the flotation method described by Schumaker and Puppione [7] using helium purged buffers. VLDL fractions were dialyzed in PBS containing 1mM EDTA and 1mM sodium azide. The VLDL lipid and protein content was measured as described above, and the remaining VLDL was used within 24 hours of isolation for LpL-lipolysis reactions. LpL-lipolysates and remaining VLDL fractions were stored at −20°C until analysis. Lipoprotein oxylipin content was analyzed as previously reported [2] using high performance liquid chromatography with negative mode electrospray ionization (ESI) tandem quadrupole mass spectral (MS/MS) detection (Quattro Ultima; Micromass, Manchester UK). Prostaglandins and thromboxane values are not discussed due to alkaline hydrolysis in non-enzymatic controls.
LpL Assays
Our goal in this experiment was to measure the potential for LpL to release VLDL oxylipins under physiologic conditions. Using a modification of our LpL assay [3], we maintained physiologic pH (i.e. 7.4) and controlled for triglyceride concentration by diluting obese VLDL-triglycerides to 100mg/dL using 20mM Tris-buffered saline. Since the VLDL of lean animals did not reach these levels, it was not modified. In all cases, 250μL samples were placed in glass tubes on ice with a final concentration of 5U/mL heparin, 5μg/mL LpL (SigmaAldrich.com), 4μg/mL BHT, and 1mg/mL free fatty acid free albumin (SigmaAldrich.com). To initiate lipolysis, samples were removed from ice and placed in a 37°C water bath for 30 minutes. Lipolysis was halted by re-emersion in ice and oxylipin extraction. To account for unesterified oxylipins in the plasma, sample without lipase were analyzed as background controls. To account for LpL-independent lipolysis in these reactions, a heat-treated LpL (HT-LpL) was used as a negative control. While 1M NaCl effectively inhibits LpL, the inclusion of such high salt levels in the incubations reduced oxylipin analytical performance to an unacceptable level, precluding the use of this more common means of complete LpL inhibition. The release of oxylipins by LpL was compared to those present in the HT-LpL incubations, the background control (no lipase), and the total esterified pool (i.e. released by mineral base hydrolysis) in an ANOVA model adjusting for the random effect of individual animals. Simply put, a significant increase in oxylipid release upon incubation with LpL, which was significantly reduced by heat treatment of the LpL, was considered an indication of lipolytic release. Assays of lean lipolysates produced significantly larger variance, likely due to more variable and lower initial TG concentrations, and thus were not included in the oxylipin lipolysis assessments.
Data analysis, statistics and calculations
Differences in whole plasma means were assessed using the Mann-Whitney Rank Sum Test. For multiple comparisons, ANOVA was performed as specified using JMP v 7.0.2 for Windows (SAS Institutes, Inc, Cary, NC, USA), with the application of Tukey’s honest significant difference post hoc test. Prior to data evaluations with parametric statistics, test assumptions were verified and when required, transformed by the natural logarithm to yield a normal distribution with equal variance. When specified, the false discovery rate corrections described by Benjamini and Hochberg [8] was used. Oxylipin results below the limit of detection (LOD) produced missing values in the final data set. Missing values were replaced with the LOD/2 prior to data analysis.
For some lipid class comparisons, whole plasma oxylipins were calculated with Equation 1:
where BH is base-hydrolyzed and class is defined by Figure 1. In some cases, due to different variances, linoleate alcohols (HODEs) were considered as a separate class from arachidonate alcohols (HETEs).
The free oxylipin fraction was calculated with Equation 2:
The oxylipin fraction of VLDL in whole plasma was calculated with Equation 3:
The base hydrolysable fraction was assumed to be equivalent to the esterified oxylipins in each pool, and the fraction in VLDL was estimated with Equation 4:
The oxylipin fractional abundance was calculated with Equation 5:
where fraction is whole plasma, VLDL or lipolysate and the extraction method used is a function of the fraction (e.g. BH for whole plasma and VLDL, nBH · LpL for lipolysate).
Regioisomer profiles were calculated with Equation 6:
RESULTS
Animals
Obese animals had elevated urinary albumin excretion (4.7 ± 2.5 vs. 0.63 ± 0.03 mg/day/100g BW, P< 0.029), reduced plasma albumin (2.84 ± 0.08 vs. 3.60 ± 0.02 g/dL in control, P< 0.0001), increased plasma triglycerides (169 ± 32 vs. 23 ± 8 mg/dL in control, P< 0.001), plasma cholesterol (60 ± 7 vs. 45 ± 4 mg/dL in control, P< 0.02), increased plasma phospholipids (143 ± 15 vs. 87 ± 5 mg/dL, P<0.002), and the VLDL of obese animals had elevated triglyceride/cholesterol ratios (27.6 ± 7.5 vs. 4.6 ± 4.3, P<0.002).
Plasma oxylipin compartmentalization
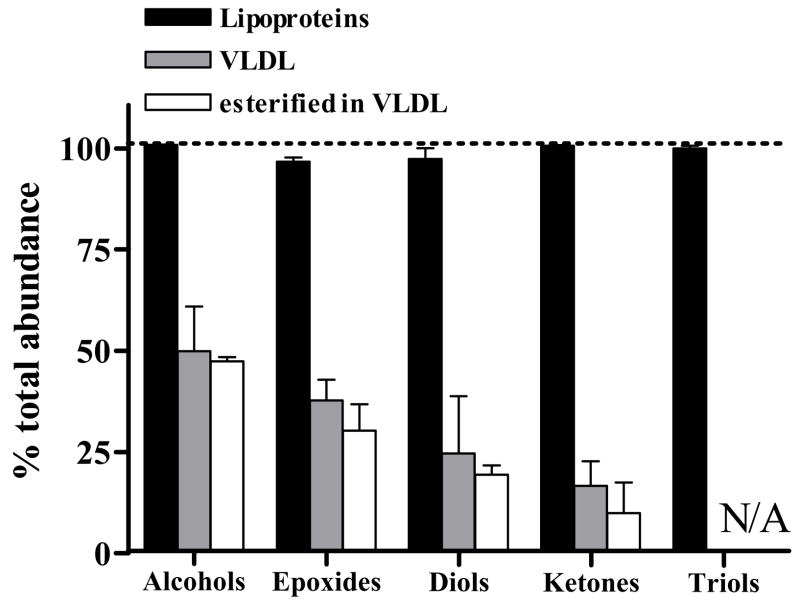
Plasma concentrations are reported for each regioisomer (Table 1). To understand the role lipoproteins, especially VLDL, have in transporting plasma oxylipins we measured the portion of whole plasma oxylipins within all lipoproteins (i.e. the δ < 1.25 g/mL density fraction), in the VLDL sub-fraction, and the esterified VLDL pool (Figure 2). Oxidized lipids derived from linoleate, arachidonate, and eicosapentaenoate were observed in both whole plasma and VLDL fractions, however only subsets were routinely found above the method detection limits and are discussed. Nearly 100% of whole plasma oxylipins were isolated within lipoproteins, regardless of oxylipin class, however, the portion in VLDL changed for each oxylipin structural class. For example, VLDL carried 50% of the whole plasma alcohols, ~10% of the ketones and no fatty acid triols. With the exception of some linoleate-derived compounds, oxylipins were apparently more than 90% esterified, since their concentrations were greatly elevated after sample saponification with sodium hydroxide.
Table 1.
Plasma oxylipins by regioisomer (nM)a
| Oxylipin | Normolipidemic | Hyperlipidemic | p-value | Fold Difference |
|---|---|---|---|---|
|
Mid-chain Alcohols | ||||
| 9-HODE | 581 (320, 840) | 1170 (780, 1600) | 0.03 | 2.0 |
| 13-HODE | 627 (300, 950) | 1270 (1000, 1500) | 0.03 | 2.0 |
| 5-HETE | 2660 (1900, 3400) | 4630 (3500, 5800) | 0.03 | 1.7 |
| 8-HETE | 808 (390, 1200) | 1440 (840, 2000) | 0.03 | 1.8 |
| 9-HETE | 1570 (800, 2300) | 2570 (1500, 3600) | 0.06 | -- |
| 11-HETE | 956 (500, 1400) | 1610 (1000, 2200) | 0.03 | 1.7 |
| 12-HETE | 1690 (830, 2600) | 2880 (2400, 3400) | 0.03 | 1.7 |
| 15-HETE | 1510 (720, 2300) | 2880 (2200, 3600) | 0.03 | 1.9 |
| 15-HETrE | 30 (20, 40) | 126 (74, 180) | 0.03 | 4.2 |
|
| ||||
|
Ketones | ||||
| 9-oxo-ODE | 133 (87, 180) | 265 (210, 320) | 0.03 | 2.0 |
| 13-oxo-ODE | 161 (120, 210) | 461 (340, 580) | 0.03 | 2.9 |
| 5-oxo-ETE | 244 (190, 290) | 607 (210, 1000) | 0.03 | 2.5 |
| 15-oxo-ETE | 89.3 (56, 120) | 303 (140, 470) | 0.03 | 3.4 |
|
| ||||
|
Epoxides | ||||
| 9(10)-EpOME | 27.2 (16, 38) | 47.4 (15, 80) | 0.2 | -- |
| 12(13)-EpOME | 34.3 (21, 47) | 76.3 (53, 100) | 0.03 | 2.2 |
| 5(6)-EET | 144 (67, 220) | 340 (120, 560) | 0.03 | 2.4 |
| 8(9)-EET | 89.3 (44, 130) | 205 (86, 320) | 0.03 | 2.3 |
| 11(12)-EET | 48.5 (28, 69) | 87.1 (33, 140) | 0.1 | -- |
| 14(15)-EET | 50.1 (21, 79) | 98.9 (19, 180) | 0.1 | -- |
|
| ||||
|
Diols | ||||
| 9,10-DHOME | 24.8 (9.3, 40) | 63.9 (13, 120) | 0.06 | -- |
| 12,13-DHOME | 35.8 (18, 53) | 114 (7.7, 220) | 0.03 | 3.2 |
| 5,6-DHET | 35 (27, 43) | 46.8 (28, 66) | 0.2 | -- |
| 8,9-DHET | -- | -- | -- | -- |
| 11,12-DHET | 26 (−42, 94) | 7.42 (3.7, 11) | 0.5 | -- |
| 14,15-DHET | 32.4 (−52, 120) | 10.4 (4.3, 17) | 0.5 | -- |
|
| ||||
|
ω ω-1 Alcohols | ||||
| 19-HETE | 27.7 (17, 39) | 35.5 (17, 54) | 0.2 | -- |
| 20-HETE | 27.9 (7.2, 49) | 34.4 (16, 53) | 0.5 | -- |
|
| ||||
|
Triols | ||||
| 9,10,13-TriHOME | 5.32 (1.7, 9) | 8.16 (−0.36, 17) | 0.5 | -- |
| 9,12,13-TriHOME | 25.5 (11, 40) | 40.8 (3.8, 78) | 0.7 | -- |
| 11,12,15-THETrE | 213 (110, 320) | 270 (140, 400) | 0.3 | -- |
All results are shown as the least-squares mean (95% CI).
Significance of 2-tailed t-Tests were evaluated after false discovery rate adjustments with a q = 0.1 (*), and 0.05(**) by the methods of Benjamini and Hochberg [8]. Dashed lines (--) indicate insignificant changes.
Figure 2. Alkaline-stable plasma oxylipins are localized in the lipoprotein density fraction as esters.
The distribution of oxylipins by class as a percent of whole plasma are shown in: all lipoproteins, VLDL, and VLDL-esters. The data are presented for obese Zucker rats (n=4).
LpL-dependent oxylipin ester-hydrolysis
The LpL-dependent release of regioisomers per 100 mg/dL VLDL TG is reported in Table 2. Each mid-chain alcohol measured was released by LpL, and HT-LpL showed reduced efficacy. The most abundant regioisomer was 5-HETE in whole plasma and VLDL, however in the lipolysate, both HODEs were more abundant. This was due to more robust release of linoleate derived HODEs (40–50% of total) as compared to HETEs (3% – 30% of total). The largest epoxy fatty acid releases were a 14-fold increase in 5(6)-EET and a 5-fold increase in 14(15)-EET. Among linoleate-derived epoxides, the 12(13)-EpOME met the criterion for LpL-dependent release, however background levels for both linoleate metabolites were high, with ~50% of the total EpOMEs appearing in the free form. The 12,13-DHOME and 14,15-DHET showed differences between HT-LpL and LpL (p ≤ 0.002). Heat treatment eliminated LpL-dependent epoxide release, however, residual release of alcohols (27 ± 5% of LpL; ~5-fold baseline) and 14,15 DHET was observed in incubations with the heat-treated enzyme (data not shown). The release of ketone-containing PUFAs by LpL incubation was not reduced by heat treatment.
Table 2. LpL-mediated lipolysis of oxylipin isomers from hyperlipidemic VLDL (n=4) a.
VLDL (TG =100 mg/dL) from 4 obese animals was incubated with 5μg/mL of active LpL, inactivated LpL (HT-LpL), or no lipase in the presence of heparin, FFA-free albumin, and BHT at pH 7.4 in PBS for 30 minutes. We tested whether the concentration of free (non-base hydrolyzed, nBH) oxylipins after incubation was greater in the presence of active LpL or HT-LpL compared to the no lipase addition. For simplicity, the concentration without LpL addition is not shown.
| LpL vs. HT-LpL | |||||
|---|---|---|---|---|---|
| Oxylipin | Totalb | LpLc | HT-LpLc | p-vald | FDR |
|
Mid-chain Alcohols (nM) | |||||
| 9-HODEe | 1150 (710, 1900) | 452 (280, 730) | 151 (93, 250) | <0.001 | ** |
| 13-HODEe | 959 (560, 1700) | 479 (280, 830) | 150 (87, 260) | <0.001 | ** |
| 5-HETEe | 2730 (1600, 4600) | 124 (74, 210) | 32.6 (19, 55) | <0.001 | ** |
| 8-HETEe | 748 (260, 2200) | 21.8 (7.5, 63) | 3.21 (1.1, | <0.001 | ** |
| 9-HETEe | 761 (360, 1600) | 56.2 (26, 120) | 5.17 (2.4, 11) | 0.002 | ** |
| 11-HETEe | 622 (310, 1200) | 70.3 (35, 140) | 14.4 (7.2, 29) | <0.001 | ** |
| 12-HETEe | 737 (390, 1400) | 98.6 (53, 180) | 31.6 (17, 59) | <0.001 | ** |
| 15-HETEe,f | 952 (550, 1600) | 101 (59, 170) | 32.6 (19, 56) | -- | -- |
| 15-HETrEe | 50.9 (34, 77) | 14.6 (9.6, 22) | 5.55 (3.7, | <0.001 | ** |
|
| |||||
|
Ketones (nM) | |||||
| 9-oxo-ODE | 89.5 (58, 140) | 29.6 (19, 45) | 43.5 (28, 67) | 0.11 | -- |
| 13-oxo-ODE | 119 (80, 180) | 71.6 (48, 110) | 48.1 (32, 72) | 0.07 | -- |
| 5-oxo-ETE | 27.7 (17, 46) | 7.54 (4.5, 13) | 5.89 (3.5, | 0.5 | -- |
| 15-oxo-ETE | 10.9 (6.5, 19) | 1.79 (1.1, 3) | 2.42 (1.4, | 0.4 | -- |
|
| |||||
|
Epoxides (nM) | |||||
| 9(10)-EpOME | 42 (32, 55) | 29.7 (22, 39) | 24.1 (18, 32) | 0.03 | * |
| 12(13)-EpOME | 45.9 (31, 68) | 50.7 (34, 75) | 30.8 (21, 46) | 0.007 | ** |
| 5(6)-EET | 158 (80, 310) | 48.5 (25, 96) | 6.15 (3.1, 12) | <0.001 | ** |
| 8(9)-EET | 86.5 (45, 170) | 4.51 (2.4, 8.6) | 4.16 (2.2, 8) | 0.9 | -- |
| 11(12)-EET | 25.7 (17, 39) | 3.51 (2.3, 5.4) | 2.22 (1.5, | 0.1 | -- |
| 14(15)-EET | 32.5 (13, 83) | 5.7 (2.2, 14) | 1.24 (0.49, | 0.01 | ** |
|
| |||||
|
Diols (nM) | |||||
| 9,10-DHOME | 6.64 (4.8, 9.3) | 3.7 (2.6, 5.2) | 2.72 (1.9, | 0.05 | * |
| 12,13-DHOMEe | 14.5 (11, 20) | 8.83 (6.4, 12) | 5.72 (4.2, | 0.001 | ** |
| 5,6-DHET | 19.8 (14, 28) | 1.23 (0.87, 1.7) | 1 (0.71, 1.4) | 0.4 | -- |
| 8,9-DHETg | -- | -- | -- | -- | |
| 11,12-DHET | 1.6 (0.86, 3) | 0.658 (0.35, | 0.365 (0.2, | 0.1 | -- |
| 14,15-DHET | 2.8 (1.8, 4.3) | 0.945 (0.61, | 0.274 (0.18, | 0.002 | ** |
|
| |||||
|
ω, ω-1 Alcohols (nM) | |||||
| 19-HETE | 6.14 (2.2, 17) | 1.62 (0.57, 4.6) | 1.06 (0.37, 3) | 0.4 | -- |
| 20-HETEh | -- | -- | -- | -- | -- |
All results are shown as the adjusted mean (95% CI).
concentration in nM with base hydrolysis.
concentration in nM without base hydrolysis.
Significance of natural-log transformed data using randomized block design ANOVA after adjustment for random effects of animals. False discovery rate adjustments with a q =0.1 (*), and 0.05(**) were made by the methods of Benjamini and Hochberg [8]. With FDR correction, we expect 3 false discoveries at q=0.10; and 1 at q=0.05.
HT-LpL was significantly increased from no lipase fractions.
failed F-test for normal distribution.
failed quality control due to peak interference
below limit of detection
Hyperlipidemia alters whole plasma, VLDL, and lipolysate oxylipin profiles
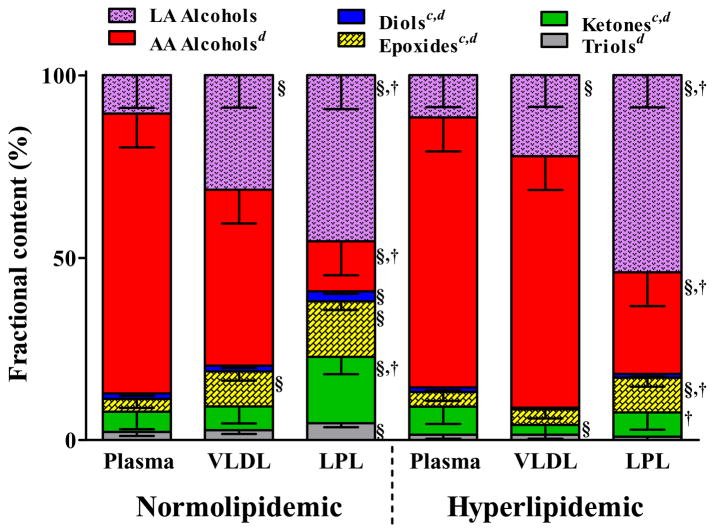
To evaluate the potential impact of hyperlipidemia on VLDL-associated oxylipin exposure of the vascular endothelium, we compared the profiles of LpL-releasable oxylipins derived from normolipidemic and hyperlipidemic rats to plasma and VLDL. These results are summarized by class in Figure 3. In each fraction, the most abundant oxylipins were mid-chain alcohols. Lipemia did not change whole plasma oxylipin profiles (p > 0.2), but did influence those of VLDL and lipolysates. Hyperlipidemic whole plasma and VLDL composition was identical, but differed from lipolysates (p = 0.04), which had twice the epoxide abundance. Conversely, in normolipidemic animals epoxides, ketones and triols were each sequentially enriched in VLDL and more so in lipolysates (Figure 3). This is reflected in shifts in the range of epoxide:diol ratios between the plasma 3.3:1 (95% CI = 2.6, 4.2) compared to either VLDL ratios of 7.3:1 (95% CI = 5.7, 9.3) and lipolysate ratios 7.4:1 (95% CI = 5.8, 9.5) after adjusting for the effect of lipemia (p < 0.0001). The epoxide:diol ratio in hyperlipidemic animals was elevated by 1.4-fold over lean animals after adjusting for the effect of fraction (p= 0.048).
Figure 3. Plasma, VLDL, and VLDL-lipolysate are unique.
The distribution of oxylipins in each compartment and the changes induced in obesity-associated hyperlipidemia are shown as mean (95% CI) of the percent of total. We measured the fractional abundance by class to determine whether plasma, VLDL and lipolysate were unique and whether there was any effect of hyperlipidemia using ANOVA after adjustment for the random effect of individual animals. An interaction of (animal status × oxylipin class) of less than 0.1 was considered was considered strong enough that it should not be ignored, and this was the case for all analyses. Significant post-hoc differences at p< 0.05 after Tukey’s adjustment are indicated within class (b vs. lean plasma, c vs. lean VLDL, d vs. lean lipolysate); and within animal (§ vs. plasma, † vs. VLDL ‡ vs. lipolysate).
Next, we tested whether the profile of regioisomers in whole plasma, VLDL, and lipolysate were different within each oxylipin class and between normolipidemic and hyperlipidemic animals. The hyperlipidemic animal data set provided a complete and complex oxylipin data matrix, however missing values and values near the LOD in the lipolysate of normolipidemic VLDL were present. For this reason, only the means and 95% confidence interval adjusted for the random effect of individual animals are given.
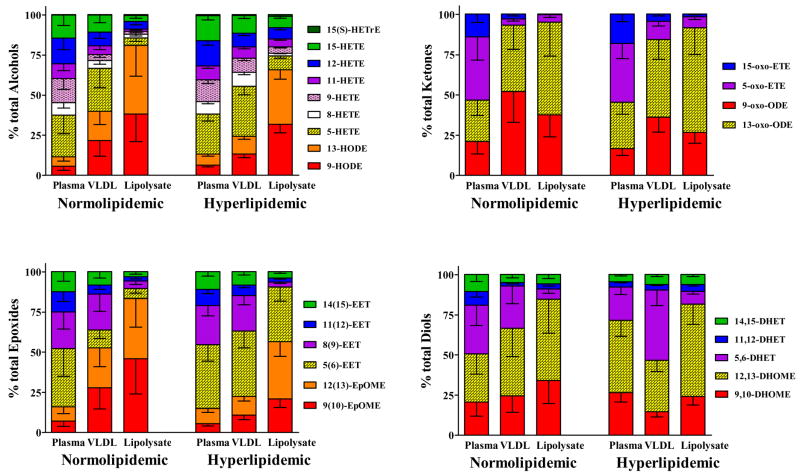
In both normal and hyperlipidemic animals, oxygenated linoleates dominated the VLDL and lipolysates. The fractional abundance of linoleate alcohols increased from whole plasma to VLDL to lipolysate, while the autooxidative marker 9-HETE and other arachidonate metabolites were relatively more abundant in the whole plasma (Figure 4). The 5-HETE remained a prominent portion of VLDL alcohols but was only a small component of lipolysates. Linoleate ketones were especially more abundant in VLDL and lipolysate, as compared to plasma. In plasma, the most abundant ketone was 5-oxo-ETE, however in VLDL and lipolysates, the 13- and 9-oxo-ODEs were most abundant Among epoxides, the arachidonate 5(6)-EET was the most abundant both in plasma and VLDL, however it was superseded by epoxy linoleates in the lipolysate. Diols were also dominated by linoleates, but no clear pattern emerged.
Figure 4. Regioisomers comprising each oxylipin class are unique in whole plasma VLDL and lipolysates of normolipidemic and hyperlipidemic animals.
The distribution of oxylipin regioisomers within each oxylipin class in are shown as mean (95% CI) of the percent of class total. The levels of many regioisomers in lean lipolysate approached the limit of detection, limiting the analyses. Therefore, the data are presented as the mean (95% CI) without post-hoc statistical analysis from 4 lean and 4 obese Zucker females.
Between normolipidemic and hyperlipidemic groups whole plasma profiles were identical, except for the arachidonate diols, which appeared to make up a greater fraction of diols in whole plasma of lean animals. This was not true of VLDL or lipolysate among alcohols and epoxides. Hyperlipidemia increased the fractional abundance of VLDL linoleates alcohols, and this effect was enhanced in lipolysates. Likewise, oxy-linoleates comprised a large fraction of all of the normolipidemic VLDL epoxides, whereas 5(6)-EET and 8(9)-EET dominated the hyperlipidemic VLDL, and 5(6)-EET constituted a substantial fraction of lipolysate epoxides.
DISCUSSION
A large body of research supports the generally held opinion that oxylipins in mammalian systems have autocrine and paracrine, but not endocrine effects. However, these studies have largely focused on prostaglandins and thromboxanes, and specific classes of circulating ester-linked oxylipids including cholesterol and phospholipids esters of HETEs and HODEs exhibit potent cellular effects [9–14]. This study demonstrates each of the five points necessary to support a plausible mechanism for the VLDL/LpL-mediated delivery of a unique subset of plasma oxylipins to peripheral tissues. It secondly demonstrates that the content of our test substrate, hyperlipidemic VLDL, is different from normolipidemic animals, providing a context for the mechanism’s potential impact on pathology.
The direct analysis of lipoprotein density fractions showed that the alkaline stable oxylipins measured here circulate almost entirely in association with lipoproteins. Moreover, their absence in lipoprotein free plasma and release by mineral base treatment show these lipids to be tightly associated, if not completely esterified. In this setting, VLDL are a major repository of acylated plasma alcohols and epoxides, and to a lesser extent diols and ketones, however they do not carry substantial amounts of triols. Since oxylipin concentrations in the δ >1.25 fraction were exceptionally low, the non-VLDL whole plasma oxylipins must be distributed within the IDL, LDL and HDL fractions.
Previous studies focused on plasma arachidonate epoxide distributions found VLDL phospholipids to contain a significant epoxy-lipid load [15]. In this study, we have found that LpL can release oxygenated VLDL lipids, suggesting that the triglyceride fraction also contains oxylipins. While lipolytic release was observed regardless of the parent fatty acid, linoleate products were the most abundant compounds liberated by LpL. These enhanced levels mirrored the relative abundance of these substrates in the VLDL fraction. While 70°C heating reduced lypolytic efficiency of LpL, it did not eliminate the release of oxylipins. The retention of esterase activity by HT-LpL has been reported by others [16]. Despite residual activity, the difference between LpL and HT-LpL was sufficient to allow us to clearly identify lipolysis of oxylipins in all classes but ketones. Triol release by LpL could not be assessed due to the low natural abundance of these compounds in VLDL. While significant release of 8(9)- or 11(12)-EET could not be detected, these metabolites appeared at concentrations near the detection limits so that these results should not be interpreted as a lack enzymatic preference. It should also be noted, that the recovery of 5(6)-EET in these procedures is estimated at ~25% due to spontaneous rearrangement to the corresponding delta-lactone [17]. Under the conditions used here, the rearrangement does not progress to diols [17], so the diol measurements are not artifactually increased.
The results presented here have implications for the targeted delivery of bioactive oxylipins to tissues. With the exception of isoprostanes [18] and related compounds [19] formed in phospholipid membranes, cyclized eicosanoids in the plasma circulate in albumin bound states [20–23] with little apolipoprotein interaction [24]. Therefore, the exposure of tissues to these plasma components is a function of their concentration and protein binding affinity (i.e. [plasma] x Kbinding). A similar situation would exist if such compounds were dissolved into the hydrophobic core of a lipoprotein particle as free fatty acids. However, this is not the case where the oxylipins exist acylated into a structural (e.g. phospholipids) or insoluble (e.g. triglycerides) compartment. In fact with respect to VLDL lipids, particle clearance resulting from lipolysis versus whole particle uptake would results in different tissue exposures, since the profiles of plasma, VLDL and lipolysate are distinct. Lipolytic clearance of VLDL dominates in the body, and in lipolyticaly active tissues, lipolysate profiles would drive tissue responses. It must be considered, however, that whole particle uptake of VLDL1 accounts for 20–30% of clearance in normolipidemic humans [25] and those tissues clearing whole VLDL1 would be exposed to the whole particle profile and subjected to further regulated release by intracellular processes. The plasma and intracellular platelet activating factor acetyl hydrolase-dependent release of isoprostanes is one recent example of this line of thought [26]. Our findings demonstrate that the exposure to oxylipins in tissues primarily undergoing lipolysis would be distinct from that in tissues primarily undergoing VLDL1 uptake. For instance, lipolytic tissues would take up more epoxides and ketones relative to alcohols than tissues taking up VLDL. Furthermore, this effect would be greater in normolipidemic versus hyperlipidemic animals. It is also exemplified by the changing epoxide:diol ratio, a putative marker of the systemic soluble epoxide hydrolase activity [27]. Further complicating the matter, the regioisomer content of each class changes across plasma, VLDL and lipolysate. For example, hydroxylated PUFA exposure to tissues undergoing whole particle uptake would be dominated by 5-HETE, while those undergoing lipolysis would be exposed to 13-HODE (Figure 4). 5-HETE is formed by 5-lipoxygenase activity and is associated with foam cell formation and insulin resistance [28]. More generally, tissues undergoing lipolysis would be exposed to more epoxides, and ketones. In each class, oxylipins are potent modulators of biological processes: as anti-inflammatory agents [29, 30], in autooxidation and coronary disease risk [31], modulation of insulin activity [32], prevention and reversal of cardiac hypertrophy [33], protection from ischemia/reperfusion injury [34], chemotaxis [35, 36], hypertensive renal injury [37], activation of PPAR-γ[38], and vascular collapse in sepsis [27]. Assuming the current understanding of lipid kinetics to be true, these results suggest the delivery of different profiles of bioactive signals by clearance mode and by lipemic status.
In summary, we have demonstrated that lipoproteins are the primary plasma transporters of oxylipins, with a substantial fraction transported in an LpL-labile VLDL compartment. We have further demonstrated that obesity induced hypertriglyceridemia alters the distribution of oxylipins in a manner likely to produce downstream effects on VLDL-target tissues, the specifics of which are currently unknown. The distribution of oxylipins within the lipid sub-compartments (i.e. triglycerides, phospholipids, cholesterylesters, etc.) was not determined in this study. However given that LpL-mediated release argues for triglyceryl oxylipins, and previous reports describe these compounds within phospholipids and cholesterol esters of oxidized LDL [12, 39], a more complete lipidomic evaluation of hyperlipidemia on lipoprotein structure and function appears warranted.
Acknowledgments
The authors would like to acknowledge Dr. George Kaysen for the use of facilities and resources instrumental to the completion of this research. This research was supported in part by the research service of the Department of Veteran’s Affairs (USA), and USDA, ARS Project # 5306-51530-016-00D. Additional support was provided by the NIEHS (R37 ES02710), NIEHS Superfund Basic Research Program (P42 ES04699) and the NIH/NIDDK UC Davis Clinical Nutrition Research Unit (P30 DK35747). GCS and JWN contributed equally to this research.
Abbreviations & Terms
- Oxylipids
oxidized unsaturated lipids including octadecanoid, eicosanoid, or docosanoid produced by either enzymatic or autooxidative processes
- Oxylipins
oxylipids with known bioactive properties
- AA
arachidonic acid
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- HT-LpL
heat treated lipoprotein lipase
- LA
Linoleic acid
- γLA
γ-Linolenic acid
- EET
epoxyeicosatrienoic acid
- EpOME
epoxyoctadecamonoenoic acid
- DHET
dihydroxyeicosatrienoic acid
- DHOME
dihydroxyoctadeca(mono)enoic acid
- oxo-ETE
oxo-eicosatetraenoic acid
- oxo-ODE
oxo-octadecadienoic acid
- VLDL
very low density lipoprotein
- IDL
intermediate density lipoprotein
- LDL
low density lipoprotein
- LpL
lipoprotein lipase
- HDL
high density lipoprotein
- PUFA
polyunsaturated fatty acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy RC, Khaselev N, Nakamura T, Hall LM. Oxidation of glycerophospholipids from biological membranes by reactive oxygen species: liquid chromatographic-mass spectrometric analysis of eicosanoid products. J Chromatogr B Biomed Sci Appl. 1999;731:59–71. doi: 10.1016/s0378-4347(99)00207-8. [DOI] [PubMed] [Google Scholar]
- 2.Newman JW, Kaysen GA, Hammock BD, Shearer GC. Proteinuria increases oxylipid concentrations in VLDL and HDL, but not LDL particles in the rat. J Lipid Res. 2007 doi: 10.1194/jlr.M700146-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Shearer GC, Stevenson FT, Atkinson DN, Jones H, Staprans I, Kaysen GA. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int. 2001;59:179–189. doi: 10.1046/j.1523-1755.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 4.Shearer GC, Kaysen GA. Endothelial bound lipoprotein lipase (LpL) depletion in hypoalbuminemia results from decreased endothelial binding, not decreased secretion. Kidney Int. 2006;70:647–653. doi: 10.1038/sj.ki.5000318. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed W, Orasanu G, Nehra V, et al. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziouzenkova O, Plutzky J. Lipolytic PPAR activation: new insights into the intersection of triglycerides and inflammation? Curr Opin Clin Nutr Metab Care. 2004;7:369–375. doi: 10.1097/01.mco.0000134358.46159.61. [DOI] [PubMed] [Google Scholar]
- 7.Schumaker VN, Puppione DL. Sequential flotation ultracentrifugation. Methods Enzymol. 1986;128:155–170. doi: 10.1016/0076-6879(86)28066-0. [DOI] [PubMed] [Google Scholar]
- 8.Benjaminit Y, Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 9.Akiba S, Ii H, Yoneda Y, Sato T. Translocation of phospholipase A2 to membranes by oxidized LDL and hydroxyoctadecadienoic acid to contribute to cholesteryl ester formation. Biochim Biophys Acta. 2004;1686:77–84. doi: 10.1016/j.bbalip.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 11.Muller C, Friedrichs B, Wingler K, Brigelius-Flohe R. Perturbation of lipid metabolism by linoleic acid hydroperoxide in CaCo-2 cells. Biol Chem. 2002;383:637–648. doi: 10.1515/BC.2002.066. [DOI] [PubMed] [Google Scholar]
- 12.Folcik VA, Cathcart MK. Predominance of esterified hydroperoxy-linoleic acid in human monocyte-oxidized LDL. J Lipid Res. 1994;35:1570–1582. [PubMed] [Google Scholar]
- 13.Ku G, Thomas CE, Akeson AL, Jackson RL. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. J Biol Chem. 1992;267:14183–14188. [PubMed] [Google Scholar]
- 14.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karara A, Wei S, Spady D, Swift L, Capdevila JH, Falck JR. Arachidonic acid epoxygenase: structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem Biophys Res Commun. 1992;182:1320–1325. doi: 10.1016/0006-291x(92)91877-s. [DOI] [PubMed] [Google Scholar]
- 16.Keiper T, Schneider JG, Dugi KA. Novel site in lipoprotein lipase (LPL415;-438) essential for substrate interaction and dimer stability. J Lipid Res. 2001;42:1180–1186. [PubMed] [Google Scholar]
- 17.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by high-performance liquid chromatography - tandem mass spectroscopy. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arneson KO, Roberts LJ., 2nd Measurement of products of docosahexaenoic acid peroxidation, neuroprostanes, and neurofurans. Methods Enzymol. 2007;433:127–143. doi: 10.1016/S0076-6879(07)33007-3. [DOI] [PubMed] [Google Scholar]
- 20.Raz A. Interaction of prostaglandins with blood plasma proteins. I. Binding of prostaglandin E 2 to human plasma proteins and its effect on the physiological activity of prostaglandin E 2 in vitro and in vivo. Biochim Biophys Acta. 1972;280:602–613. [PubMed] [Google Scholar]
- 21.Raz A. Interaction of prostaglandins with blood plasma proteins. Comparative binding of prostaglandins A 2, F 2 and E 2 to human plasma proteins. Biochem J. 1972;130:631–636. doi: 10.1042/bj1300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz A. Interaction of prostaglandins with blood plasma proteins. 3. Rate of disappearance and metabolites formation after intravenous administration of free or albumin-bound prostaglandins F 2 and A 2. Life Sci II. 1972;11:965–974. [PubMed] [Google Scholar]
- 23.Maclouf J, Kindahl H, Granstrom E, Samuelsson B. Interactions of prostaglandin H2 and thromboxane A2 with human serum albumin. Eur J Biochem. 1980;109:561–566. doi: 10.1111/j.1432-1033.1980.tb04828.x. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien WF, Torres C, Benoit R, Knuppel RA. The association between apolipoprotein A–I and prostacyclin binding in human serum. Prostaglandins. 1989;38:45–51. doi: 10.1016/0090-6980(89)90015-4. [DOI] [PubMed] [Google Scholar]
- 25.Pietzsch J, Wiedemann B, Julius U, et al. Increased clearance of low density lipoprotein precursors in patients with heterozygous familial defective apolipoprotein B-100: a stable isotope approach. J Lipid Res. 1996;37:2074–2087. [PubMed] [Google Scholar]
- 26.Stafforini DM, Sheller JR, Blackwell TS, et al. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 27.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shashkin PN, Jain N, Miller YI, et al. Insulin and glucose play a role in foam cell formation and function. Cardiovasc Diabetol. 2006;5:13. doi: 10.1186/1475-2840-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasson S, Eckel J. Disparate effects of 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid in vascular endothelial and smooth muscle cells and in cardiomyocytes. Arch Physiol Biochem. 2006;112:119–129. doi: 10.1080/13813450600712035. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 31.Shishehbor MH, Zhang R, Medina H, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. 2006;41:1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci. 2007;28:32–38. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, Li N, He Y, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seubert J, Yang B, Bradbury JA, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 35.Stamatiou P, Hamid Q, Taha R, et al. 5-oxo-ETE induces pulmonary eosinophilia in an integrin-dependent manner in Brown Norway rats. J Clin Invest. 1998;102:2165–2172. doi: 10.1172/JCI1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muro S, Hamid Q, Olivenstein R, Taha R, Rokach J, Powell WS. 5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin. J Allergy Clin Immunol. 2003;112:768–774. doi: 10.1016/s0091-6749(03)01888-8. [DOI] [PubMed] [Google Scholar]
- 37.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 38.Altmann R, Hausmann M, Spottl T, et al. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol. 2007;74:612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Milne GL, Seal JR, Havrilla CM, Wijtmans M, Porter NA. Identification and analysis of products formed from phospholipids in the free radical oxidation of human low density lipoproteins. J Lipid Res. 2005;46:307–319. doi: 10.1194/jlr.M400311-JLR200. [DOI] [PubMed] [Google Scholar]