Abstract
Objective:
To investigate whether exposure to high maternal concentrations of 25(OH)-vitamin D in pregnancy poses any risk to the child.
Design:
Prospective study.
Setting:
Princess Anne Maternity Hospital, Southampton, UK.
Subjects:
596 pregnant women were recruited. 466 (78%) children were examined at birth, 440 (74%) at age 9 months and 178 (30%) at age 9 years.
Methods:
Maternal (OH)-vitamin D concentrations were measured in late pregnancy. Anthropometry of the child was recorded at birth, 9 months and 9 years. At 9 months, atopic eczema was assessed. At 9 years, children had an echocardiogram and a DXA scan, blood pressure, arterial compliance and carotid intima-media thickness were measured and intelligence and psychological function assessed.
Results:
There were no associations between maternal 25(OH)-vitamin D concentrations and the child's body size or measures of the child's intelligence, psychological health or cardiovascular system. Children whose mothers' concentration of 25(OH)-vitamin D in pregnancy was >75 nmol/l had an increased risk of eczema on examination at 9 months (OR 3.26, 95% CI 1.15-9.29, p=0.025) and asthma at age 9 years (OR 5.40, 95% CI, 1.09-26.65, p=0.038) compared to children whose mothers' concentration was <30 nmol/l.
Conclusion:
Exposure to maternal concentrations of 25(OH)-vitamin D in pregnancy in excess of 75 nmol/l does not appear to influence the child's intelligence, psychological health or cardiovascular system; there could be an increased risk of atopic disorders, but this needs confirmation in other studies.
Keywords: pregnancy, diet, vitamin D, infant, child
Introduction
There is growing evidence from studies in both northern and southern latitudes that many women are deficient in vitamin D, both before and during pregnancy.(Alfaham et al. 1995a; Nesby-O'Dell et al. 2002a; Datta et al. 2002b; Dawodu et al. 2003; Sachan et al. 2005a; Schroth et al. 2005b; Meddeb et al. 2005b) Although vitamin D is obtainable from a few foods, such as oily fish and fortified margarines, the major source is the skin's synthesis of the vitamin through exposure to solar ultraviolet light.(Holick 2004a) Season, latitude, time of day, use of sunscreen, skin pigmentation and clothing all influence the number of ultraviolet B photons that penetrate the skin and hence the production of vitamin D.(Holick 2004b) As a consequence, vitamin D deficiency is a worldwide problem.
The importance of vitamin D for skeletal development has long been recognised. Prolonged deficiency of vitamin D in infancy and childhood results in poorer rates of skeletal growth and in rickets. A woman's serum concentration of 25(OH)-vitamin D during pregnancy is strongly predictive of her child's 25(OH)-vitamin D concentration at birth,(Hollis & Pittard, III 1984) and infants of women who are deficient in vitamin D experience depleted vitamin D concentrations in utero and are born with low stores.(Zeghoud et al. 1997) Results from a small study of 50 newborns suggest that such children have a lower bone mass at birth than those born with an adequate vitamin D status.(Weiler et al. 2005) Evidence from a recent longitudinal study shows that a woman's vitamin D status during pregnancy may have effects on her child's skeletal development that persist long after infancy.(Javaid et al. 2006) At the age of 9 years, children whose mothers had deficient or insufficient concentrations of 25(OH)-vitamin D in late pregnancy had a reduced bone size and bone-mineral content. Future research will need to examine whether this childhood deficit in bone-mineral accrual increases the risk of fragility fractures in later life.
Randomised controlled trials of vitamin D supplementation are needed to verify the observational data suggesting that an adequate maternal vitamin D status in pregnancy is necessary for optimal skeletal development in the child. Some trials of vitamin D supplementation in pregnancy have been carried out,(Mahomed & Gulmezoglu 1999) but none has examined the effect of supplementation on the child's bone mass and other health outcomes. Concerns for the potential impact of maternal vitamin D deficiency on child skeletal health has already led some countries to make recommendations on supplementation. In the UK, for example, women who are pregnant or breastfeeding are advised to take 10 micrograms of vitamin D per day.(Department of Health 1998a) Some researchers believe that higher doses of vitamin D are needed to meet the needs of the mother and her child.(Hollis & Wagner 2004) Whether exposure to high maternal concentrations of vitamin D during fetal development poses any risks to the child is uncertain.
We used longitudinal data on a UK population-based cohort of children(Javaid et al. 2006) whose mothers had taken part in a nutritional survey during pregnancy to explore the relations between maternal 25(OH)-vitamin D concentrations and outcomes in the child at birth, at age 9 months and at age 9 years.
Subjects and methods
The mothers of the children in this cohort had all taken part in a study of nutrition during pregnancy, full details of which have been published.(Godfrey et al. 1996a) In 1991-2, 596 Caucasian women aged 16 years or more with singleton pregnancies of less than 17 weeks' gestation were invited to participate in the study during their first visit to the midwives' antenatal booking clinic at the Princess Anne Maternity Hospital in Southampton, UK. A questionnaire was used to collect information about the women's occupation, educational qualifications, smoking habits and weight before pregnancy. Height and weight were measured. During late pregnancy (median 32.6 weeks;interquartile range 32.0 to 33.4; range 28 to 42 weeks), a serum sample was taken from the mothers and samples were stored at −40°C for 5 years prior to measurement of total 25(OH)-vitamin D by radioimmunoassay (IDS Diagnostics Ltd, Boldon, Tyne & Wear, UK; intra-assay and inter-assay coefficient of variation <10%). The assay was robust. Data on 25(OH)-vitamin D concentrations were available for 466 women. . Estimated exposure to ultraviolet B radiation (UV-B) was derived from the hours of sunshine recorded at a local meteorological station (Leckford, Hampshire, UK) during weeks 28 to 31 of gestation. This period was chosen, rather than the period during which serum samples were collected, because serum 25-OH vitamin D has a half-life of 3 weeks so is more strongly predicted by previous than current sunlight exposure. We adjusted the total hours of monthly sunshine for seasonal variation in UV-B radiation (Wh/m2) using the SoDa-IS web service for professionals in solar energy and radiation (http://www.soda-is.com/eng/index.html). At the same time as the serum sample was taken in late pregnancy, women completed a food frequency questionnaire that assessed frequency of consumption of 100 foods or food groups in the previous 3 months.(Robinson et al. 1996) Average daily vitamin D intake was calculated by multiplying the vitamin D content of a standard portion of each food by reported frequency of use. Women also provided information on the vitamin supplements they were taking.
After delivery, research nurses recorded weight, length, head circumference and mid-upper arm circumference as described previously.(Godfrey et al. 1996b) Figure 1 shows the number of participants in the initial study and the derivation of the study sample used to study associations between maternal 25(OH)-vitamin D concentrations and outcomes in the child.
Figure 1.
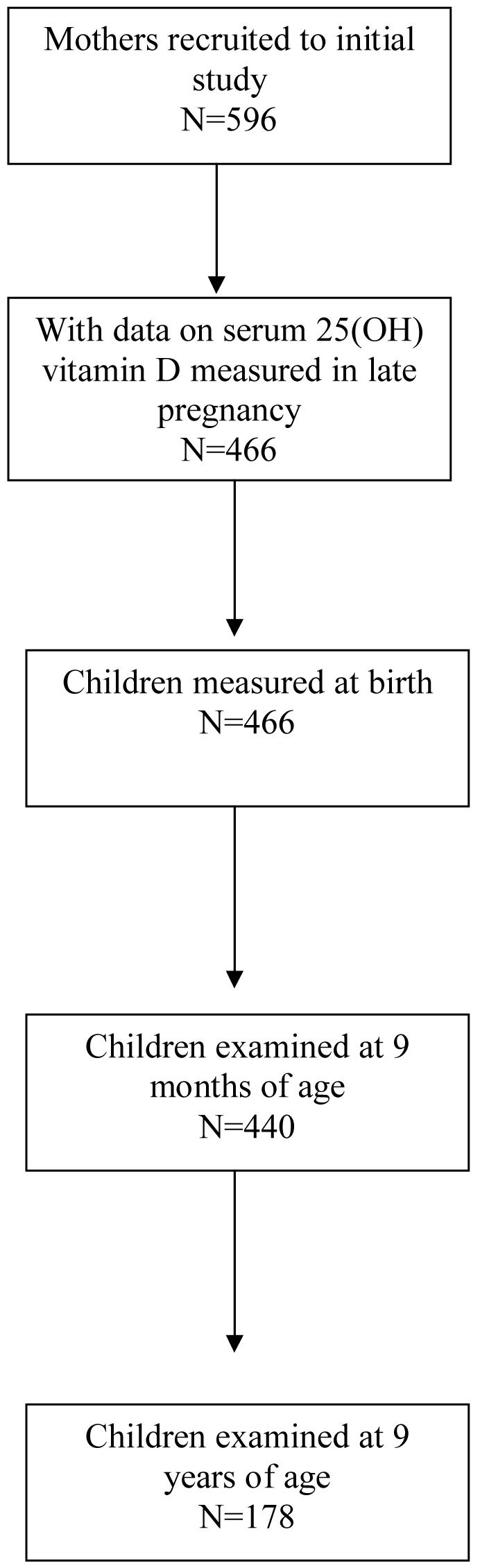
Derivation of the study sample used to study associations between maternal 25(OH)-vitamin D concentrations in late pregnancy and child outcomes
Follow-up at 9 months of age
Of the 466 children who had information on maternal 25(OH)-vitamin D concentrations in late pregnancy, 440 (94%) were followed up at the age of 9 months. We chose to study the children at this age in order to assess the postnatal growth trajectory and ascertain infantile atopic eczema. The research nurses recorded weight, length, head circumference and mid-upper arm circumference. They examined the child's skin, looking for evidence of visible atopic eczema. Mothers were also asked whether the child had dry skin or an itchy skin condition since birth and a diagnosis of atopic eczema was defined using a modified version of the UK Working Party's diagnostic criteria for atopic dermatitis.(Williams et al. 1994) We omitted a history of asthma or hay fever as a criterion because the children were too young to have developed these disorders. Mothers were asked whether a doctor had ever diagnosed pneumonia or bronchiolitis, a chest infection or bronchitis, or an ear infection in the child, and whether the child had had any bouts of diarrhoea (frequent unformed stools) lasting 2 days or longer.
Follow-up at 9 years of age
Assessment of anthropometry, blood pressure and cognitive and psychological function
When the children approached age 9 years, we asked the Community Paediatric Service in Southampton to write to their parents with an invitation to take part in a further follow-up study to investigate the effect of early growth on the structure and function of the heart and blood vessels, on cognitive and psychological function and on bone mass. This age was chosen because we wanted to study the children before they reached puberty. All the children in the cohort had previously been flagged on the child health computer at the Central Health Clinic in Southampton. Letters were sent to all families still living in the Southampton area. Of 440 children seen at 9 months who had data on maternal 25(OH)-vitamin D concentrations, 178 were still living in the city and agreed to participate.
The child's height and weight were measured during a home visit. Mothers were asked whether their child had any long-standing illness and for details of any current prescribed medication. After a five-minute rest, systolic and diastolic blood pressures were measured three times on the left arm placed at the level of the heart whilst the child was seated. Measurements were made using a Dinamap 1846 (Critikon, UK), with manufacturer's recommended cuff sizes based on the child's mid upper arm circumference. The mean of the three measurements was used in the analysis. Cognitive function was measured using the Wechsler Abbreviated Scale of Intelligence (WASI)(Wechsler 1999) and psychological health was assessed with the Strengths and Difficulties Questionnaire (SDQ), which was completed by the mother.(Goodman 1997) The SDQ is made up of 5 subscales assessing prosocial behaviour, hyperactivity, emotional symptoms, conduct problems and peer problems.
Assessment of cardiac structure, arterial compliance and carotid intima-media thickness
Children attended a clinic at the Princess Anne Hospital. At the clinic they sat quietly in a temperature-controlled room (20° C ± 2° C) for at least 10 minutes. When pulse rate and blood pressure measurements indicated hemodynamic stability, transthoracic echocardiography (Acuson 128 XP and a 3.5 MHz phased array transducer) was performed by a single ultrasonographer with the child in the left lateral recumbent position. Two-dimensional, M-mode, and Doppler echocardiograms were recorded over five consecutive cardiac cycles and measurements were made off-line. Aortic root diameter, left ventricular diameter, left atrial diameter, left ventricular outflow tract diameter, left ventricular mass (according to American Echocardiography Society convention) and total coronary artery diameter were measured as reported previously.(Jiang et al. 2006) While the child was recumbent the ultrasonographer used a 7 MHz linear-array transducer to measure intima-media thickness in the right distal portion of the common carotid artery. Three longitudinal views, including lateral, antero-posteral and antero-oblique sections, were recorded at the end of the diastolic phase. Intima-media thickness of the far wall was measured 10 mm proximal to the beginning of bifurcation, using CVI software (National Instruments; Austin, Texas). The mean of the three measurements was used in the analysis.
Arterial compliance was measured by a non-invasive optical method that determines the transit time of the wave of dilatation propagating in the arterial wall, as a result of the pressure wave generated by contraction of the left ventricle. Measurement of the time taken for the wave to travel a known distance allows the velocity of the pulse wave to be calculated. The optical method has been validated against intra-arterial determinations of pressure wave velocity.(Bonner et al. 1995) Pulse wave velocities were measured in two arterial segments, aorta to femoral, extending from the common carotid artery near the arch of the aorta into the femoral artery just below the inguinal ligament, and aorta to foot, extending to the posterior tibial artery. Pulse wave velocity is inversely related to the square root of the compliance of the vessel wall. High pulse wave velocity therefore indicates a stiffer arterial wall.
Assessment of body composition
The child underwent measurements of fat mass and lean mass by DXA (Lunar DPX-L instrument using specific paediatric software [v 4.7c, GE Corporation, Madison, Wisconsin, USA]). The instrument was calibrated each day and all scans were performed with the children wearing light clothing. The short-term and long-term coefficients of variation (CV) of the instrument were 0.8% and 1.4% respectively.
Statistical methods
Anthropometric measurements made at the 9-month and 9-year examinations were adjusted for age at the time of examination. We used the British 1990 growth reference data on weight, height and head circumference,(Cole et al. 1998) obtained from the Child Growth Foundation, in combination with our measurements and data on gestation and sex, to estimate for each child what his or her weight, length and head circumference would have been at exactly 9 months and 9 years of age. In the absence of agreed normal ranges for 25(OH)-vitamin D during pregnancy, we divided mothers into quarters of the distribution of 25(OH)-vitamin D. We used the thresholds >50 nmol/l, 27.5-50 nmol/l and <27.5 nmol/l to classify mothers according to whether they were vitamin D replete, insufficient or deficient respectively.(Malabanan et al. 1998; Department of Health 1998b) ANOVA and χ2 test were used to examine maternal characteristics in relation to quarters of the distribution of 25(OH)-vitamin D. ANOVA, spearman correlation, linear and logistic regression were used to examine the relations between outcomes in the child and maternal vitamin D status. Where necessary, variables were transformed using logarithms to satisfy statistical assumptions of normality. Based on the existing literature, our a priori hypothesis was that high maternal vitamin D status would not have adverse effects on cardiovascular and neuro-cognitive outcomes, but might be associated with an increased risk of atopic disorders.(Raby et al. 2004a; Poon et al. 2004a; Hypponen et al. 2004b)
The Local Research Ethics Committee approved the study protocols and written informed consent was obtained from the children (at the 9-year follow-up) and their mothers. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Results
Among the 466 women who took part in the original study, the median serum 25(OH)-vitamin D concentration in late pregnancy was 50 nmol/l (inter-quartile range 30 to 75.3). 235 women (50.4%) had 25(OH)-vitamin D >50 nmol/l, 132 women (28.3%) had concentrations that between 27.5-50 nmol/l and 99 (21.2%) had concentrations <27.5 nmol/l. Table 1 shows the characteristics of these women according to quarters of the distribution of 25(OH)-vitamin D. Compared to women with low concentrations of 25(OH)-vitamin D, women with high concentrations had a considerably higher estimated UV-B exposure in late pregnancy. The correlation coefficient between estimated ultraviolet B exposure and maternal 25(OH)-vitamin D concentration was 0.60, P<0.0001. Women with high vitamin D concentrations also had a higher mean daily intake of vitamin D from food and supplements combined. 6.5% of women were taking supplements containing vitamin D in late pregnancy. Supplement use was more frequent in women with higher concentrations of 25(OH)-vitamin D but this trend was of borderline significance. Only 3 women had an intake of vitamin D that met the UK recommendation of 10 micrograms a day. There were no statistically significant relations between 25(OH)-vitamin D concentrations and age (p=0.08), height (p=0.57), pre-pregnant weight (p=0.24), social class (p=0.31), educational qualifications (p=0.78) or smoking status (p=0.16).
Table 1.
Anthropometric and lifestyle characteristics of 466 mothers according to quarters of the distribution of serum 25(OH)-vitamin D
| 25(OH)-vitamin D, nmol/l | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | <30 | −50 | −75 | >75 | P value1 |
| Age, yr | 26.2 (4.8) | 26.3 (5.2) | 26.3 (4.6) | 27.4 (5.2) | 0.074 |
| Height, cm | 163.3 (5.7) | 162.8 (6.6) | 164.0 (6.2) | 163.4 (7.2) | 0.574 |
| Pre-pregnant weight, kg | 61.4 (1.24) | 58.8 (1.20) | 62.7 (1.19) | 59.0 (1.17) | 0.238 |
| Smoker in late pregnancy, n (%) |
22 (22.8) | 33 (28.2) | 24 (20.3) | 19 (16.2) | 0.106 |
| Vitamin D intake from food and supplements in late pregnancy, μg |
2.66 (1.59) | 2.90 (1.55) | 2.89 (1.56) | 3.04 (1.55) | 0.034 |
| Vitamin D supplements in late pregnancy, n (%) |
5 (4.4) | 8 (6.8) | 7 (5.9) | 13 (11.1) | 0.071 |
| UV-B exposure in late pregnancy, Wh/m2 |
2974 (6997) |
7333 (12195) |
22397 (20976) |
32017 (20808) |
<0.001 |
| Social class, non-manual, n (%) |
77 (67.5) | 77 (65.8) | 83 (70.3) | 87 (70.4) | 0.190 |
| Qualifications, A level or Degree, n (%) |
50 (44.2) | 44 (37.6) | 37 (31.4) | 44 (27.6) | 0.199 |
P values calculated from linear regression for continuous variables or χ2 for categorical variables. Values are means (SD) or number (%). Values for weight and vitamin D intake from food are geometric means (SD)
Women whose children were followed up to age of 9 years (n=178) had a slightly higher geometric mean concentration of 25(OH)-vitamin D than the remainder of the cohort (52 nmol/l compared to 45.8 nmol/l, p=0.03); they were also slightly older (27.1 yr compared to 26.1 yr, p=0.014), more likely to be educated to at least A level standard (42% compared to 34%, p=0.06), and less likely to have smoked in late pregnancy (18% compared to 25%, p=0.034) but there was no difference between them in social class (p=0.14), height (p=0.36) or pre-pregnant weight (p=0.16). There was no difference in weight at birth (p=0.10) between the children who were followed up to age 9 years and the rest of the cohort but they had a slightly lower median gestational age at birth (280 vs 282 days, p=0.02).
Anthropometry and body composition
We found no statistically significant associations between maternal 25(OH)-vitamin D concentrations and any measure of the child's size at birth or at age 9 months (Table 2). At age 9 years, weight, fat mass and lean mass tended to be lower in children whose mothers were in the lowest quarter of the distribution of 25(OH)-vitamin D, but there were no statistically significant linear trends between weight, fat mass and lean mass and maternal 25(OH)-vitamin D concentrations. Children whose mothers had higher 25(OH)-vitamin D concentrations had a significantly larger head circumference at age 9 years than those whose mothers had lower concentrations.
Table 2.
Mean (SD) anthropometric measurements at birth, 9 months and 9 years and fat and lean mass at 9 years according to quarters of the distribution of maternal serum 25(OH)-vitamin D
| 25(OH)-vitamin D, nmol/l | |||||
|---|---|---|---|---|---|
|
| |||||
| Child anthropometry and body composition |
<30 | −50 | −75 | >75 | P value1 |
| At birth (n=466) | |||||
| Birthweight, kg | 3.38 (0.46) | 3.40 (0.56) | 3.49 (0.57) | 3.43 (0.51) | 0.247 |
| Length, cm | 50.0 (1.83) | 50.0 (2.29) | 50.5 (2.25) | 50.1 (2.09) | 0.150 |
| Head circumference, cm | 35.1 (1.29) | 35.1 (1.44) | 35.3 (1.45) | 35.1 (1.28) | 0.557 |
| Mid upper arm circ, cm | 11.5 (0.98) | 11.6 (1.12) | 11.8 (1.08) | 11.7 (0.94) | 0.080 |
| At 9 months (n=440) | |||||
| Weight, kg | 9.10 (1.07) | 9.03 (1.14) | 9.24 (1.34) | 9.07 (1.06) | 0.873 |
| Length, cm | 71.2 (2.85) | 71.4 (2.60) | 71.7 (2.89) | 71.1 (2.67) | 0.861 |
| Head circumference, cm | 45.7 (1.33) | 45.7 (1.28) | 45.7 (1.59) | 45.6 (1.39) | 0.532 |
| Mid upper arm circ, cm | 15.9 (1.14) | 15.8 (1.26) | 16.1 (1.341) | 15.9 (1.09) | 0.581 |
| At 9 years (n=178) | |||||
| Weight, kg | 27.4 (1.19) | 29.4 (1.21) | 30.0 (1.20) | 29.3 (1.19) | 0.100 |
| Height cm | 129.6 (5.88) | 131.5 (6.66) | 131.8 (5.09) | 130.6 (6.45) | 0.188 |
| Head circumference, cm | 52.6 (1.59) | 53.2 (1.68) | 53.5 (1.59) | 53.6 (1.43) | 0.012 |
| BMI, kg/m2 | 16.4 (1.14) | 17.0 (1.15) | 17.2 (1.14) | 17.3 (1.12) | 0.165 |
| Fat mass, kg | 5.01 (1.54) | 6.03 (1.76) | 6.67 (1.70) | 6.14 (1.56) | 0.090 |
| Lean mass, kg | 20.4 (1.14) | 21.8 (1.15) | 21.5 (1.13) | 21.5 (1.14) | 0.090 |
P values calculated from linear regression. Values for weight, BMI, fat and lean mass at 9 yrs are geometric means (SD)
Eczema and asthma
Of the 440 children followed up at 9 months of age, 27 had visible atopic eczema on skin examination (6.1%) and 48 had atopic eczema defined using the modified UK Working Party's diagnostic criteria (10.9%). Of the 178 children followed up at age 9 years, the mother reported long-standing eczema in 16 (9%) and asthma in 19 (10.6%). Risk of eczema on examination at age 9 months was higher in children whose mothers had been in the top quarter of the distribution of serum 25(OH)-vitamin D compared to those whose mothers had been in the bottom quarter (Table 3), though the odds ratios for eczema in children whose mothers were in the middle two quarters of the 25(OH)-vitamin D distribution provided no indication of a dose-response effect. Risk of atopic eczema using the modified UK Working Party's diagnostic criteria at age 9 months tended to be higher in children whose mothers had been in the top two quarters of the distribution compared to those in the bottom quarter, but this difference was not significant. At age 9 years, risk of having a reported history of eczema was also higher in children whose mothers were in the top quarter of the distribution, though this trend was not statistically significant. Exposure to high maternal concentrations of 25(OH)-vitamin D was associated with an increased risk of reported asthma at age 9 years compared to children whose maternal concentrations had been in the bottom quarter of the distribution (OR 5.40, 95% CI 1.09-26.65). There was a similar, though slightly weaker, increase in risk of currently taking medication prescribed for asthma (OR 4.66, 95% CI 0.93-23.30) (data not shown).
Table 3.
Odds ratios (95% CI) for eczema or atopic eczema on examination at age 9 months and for a reported history of eczema or asthma at age 9 years according to quarters of the distribution of maternal serum 25(OH)-vitamin D
| 25(OH)-vitamin D, nmol/l | ||||
|---|---|---|---|---|
|
| ||||
| <30 | −50 | −75 | >75 | |
| At 9 months (n=440) | ||||
| Visible eczema on examination |
1.0 | 0.59 | 0.79 | 3.26 |
| (0.14-2.50) | (0.21-3.00) | (1.15-9.29) | ||
| No of cases | 5 | 3 | 4 | 15 |
| Atopic eczema (modified UK Working Party criteria) |
1.0 | 1.11 | 1.75 | 1.62 |
| (0.43-2.84) | (0.73-4.17) | (0.67-3.89) | ||
| No of cases | 9 | 10 | 15 | 14 |
| At 9 years (n=178) | ||||
| Reported eczema |
1.0 | 0.71 | 0.47 | 1.89 |
| (0.15-3.39) | (0.08-2.68) | (0.51-6.99) | ||
| No of cases | 4 | 3 | 2 | 7 |
| Reported asthma | 1.0 | 2.05 | 2.05 | 5.40 |
| (0.36-11.80) | (0.36-11.80) | (1.09-26.65) | ||
| No of cases | 2 | 4 | 4 | 9 |
Eczema on examination at age 9 months was more common in babies born to mothers educated to at least A-level standard (9.5% vs 4.1%, p=0.021) and in those born during the summer (June to August) (12% vs 4.6%, p=0.007). Adjustment for maternal education had little effect on the odds ratio for visible eczema in children whose mothers were in the top quarter of the distribution of 25(OH)-vitamin D (OR 3.55, 95% CI 1.22 to 10.25), but adjustment for season of birth weakened the association (OR 2.50, 95% CI 0.80 to 7.77). Atopic eczema at age 9 months diagnosed using the modified UK Working Party's diagnostic criteria and reported asthma at age 9 years were not associated with season of birth or with maternal characteristics.
Infections in infancy
At age 9 months, 24 infants were reported by their mothers to have been diagnosed by a doctor as having pneumonia or bronchiolitis, 113 had had a diagnosis of chest infection or bronchitis, 106 had had a diagnosis of ear infection and 132 of diarrhoea. Children whose mothers were in the top quarter of 25(OH)-vitamin D in late pregnancy were significantly more likely to have had a reported diagnosis of pneumonia or bronchiolitis than those whose mothers were in the bottom quarter (OR 4.80, 95% CI1.01-22.73) However, there were no significant associations between maternal 25(OH)-vitamin D concentrations and risk of reported chest infections or bronchitis or respiratory infections overall. Children whose mothers' 25(OH)-vitamin D concentrations had been in the top quarter of the distribution were more likely to be reported as having had one or more bouts of diarrhoea, compared to those whose mothers were in the bottom quarter (OR 1.87, 95% CI 1.01-3.46). We found no relation between maternal 25(OH)-vitamin D concentrations and risk of having a diagnosis of ear infection.
Arterial structure and function at age 9 years
We found no statistically significant associations between maternal 25 (OH)-vitamin D status and the child's blood pressure, pulse wave velocity, carotid intima-media thickness or any measure of cardiac structure (Table 4).
Table 4.
Mean (SD) blood pressure, pulse wave velocity, carotid intima-media thickness and cardiac structure variables in 178 children aged 9 years according to quarters of the distribution of maternal serum 25(OH)-vitamin D
| Measures | 25(OH)-vitamin D, nmol/l | ||||
|---|---|---|---|---|---|
|
| |||||
| <30 | −50 | −75 | >75 | P value1 | |
| Blood pressure | |||||
| Systolic blood pressure, mm Hg |
103.4 (7.94) | 102.2 (7.26) | 101.9 (8.18) | 102.9 (8.10) | 0.468 |
| Diastolic blood pressure, mm Hg |
59.8 (5.25) | 60.1 (5.49) | 60.2 (5.7) | 59.9 (6.2) | 0.746 |
| Arterial compliance | |||||
| Aortic/femoral pulse wave velocity, m/sec |
2.70 (0.25) | 2.72 (0.24) | 2.82 (0.28) | 2.73 (0.26) | 0.397 |
| Aortic/foot pulse wave velocity, m/sec |
4.67 (0.32) | 4.75 (0.36) | 4.80 (0.38) | 4.73 (0.34) | 0.510 |
| Carotid artery | |||||
| Intima-media thickness, mm |
0.33 (0.05) | 0.36 (0.07) | 0.34 (0.06) | 0.34 (0.07) | 0.660 |
| Cardiac structure | |||||
| Total coronary artery diameter, mm |
4.20 (0.26) | 4.29 (0.28) | 4.25 (0.27) | 4.28 (0.25) | 0.524 |
| Left atrial diameter, cm | 2.36 (0.21) | 2.43 (0.27) | 2.40 (0.23) | 2.44 (0.21) | 0.141 |
| Aortic root diameter, cm | 2.15 (0.17) | 2.22 (0.17) | 2.22 (0.16) | 2.17 (0.17) | 0.274 |
| Left ventricular outflow tract diameter, cm |
1.56 (1.07) | 1.58 (1.06) | 1.57 (1.06) | 1.55 (1.06) | 0.472 |
| Left ventricular diameter, cm |
3.93 (1.06) | 3.99 (1.07) | 4.01 (1.06) | 3.94 (1.07) | 0.219 |
| Left ventricular mass, g | 76.2 (1.23) | 82.4 (1.237) | 83.2 (1.18) | 79.9 (1.21) | 0.304 |
P values calculated from linear regression. Values for aortic root diameter, LV mass, LV ventricular diameter and LV outflow tract diameter are geometric mean (SD)
Cognitive function at age 9 years
There were no statistically significant associations between maternal 25 (OH)-vitamin D concentrations and full-scale, verbal or performance IQ, assessed by the Wechsler Abbreviated Scale of Intelligence (Table 5).
Table 5.
Mean (SD) full-scale, verbal and performance IQ in 178 children aged 9 yrs according to quarters of the distribution of maternal serum 25 (OH)-vitamin D
| 25(OH)-vitamin D, nmol/l | |||||
|---|---|---|---|---|---|
|
| |||||
| Wechsler Abbreviated Scale of Intelligence |
<30 | −50 | −75 | >75 | P value1 |
| Full scale IQ | 107.0 (12.1) | 107.9 (15.5) | 105.1 (16.2) | 104.9 (14.8) | 0.937 |
| Verbal IQ | 105.6 (11.2) | 109.2 (15.3) | 105.0 (17.7) | 105.7 (14.9) | 0.986 |
| Performance IQ | 106.9 (14.0) | 104.9 (16.1) | 103.8 (14.5) | 103.1 (16.9) | 0.951 |
P values calculated from linear regression
Psychological health at the age of 9 years
177 mothers completed the Strengths and Difficulties Questionnaire about their child's behaviour. Of these, using the recommended cut-points for a high score, 24 (13.6%) had a high score for total difficulties, 33 (18.6%) were classified as having hyperactivity, 26 (14.7%) conduct problems, 26 (14.7%) emotional symptoms and 24 (13.6%) peer problems. Only 3 children were classified as having problems with prosocial behaviour. In logistic regression, there were no statistically significant associations between maternal 25(OH)-vitamin D status in late pregnancy and risk of high scores for total difficulties, conduct problems, emotional symptoms or peer problems (Table 6). Risk of hyperactivity was higher in children whose mothers had been in the top three quarters of the distribution of serum 25(OH)-vitamin D compared to those in the bottom quarter, but this relation too was not statistically significant. As a further check, we examined the relations between serum 25(OH)-vitamin D concentration as a continuous variable and score on each subscale, plus total difficulties score. Children whose mothers had higher concentrations of 25(OH)-vitamin D tended to gain lower scores on the peer problems scale (rs = −0.153, p=0.038), but the correlations between maternal 25(OH)-vitamin D and all other scores, including hyperactivity, were very weak and non-significant (p>0.5).
Table 6.
Odds ratios (95% CI) for high scores on Strengths and Difficulties scales in 177 children aged 9 years according to quarters of the distribution of maternal serum 25(OH)-vitamin D
| 25(OH)-vitamin D, nmol/l | ||||
|---|---|---|---|---|
|
| ||||
| <30 | −50 | −75 | >75 | |
| Total difficulties | 1.0 | 2.11 (0.59-7.62) |
2.44 (0.69-8.64) |
0.75 (0.16-3.58) |
| No of cases | 4 | 8 | 9 | 3 |
| Hyperactivity | 1.0 | 2.94 (0.84-10.2) |
2.57 (0.73-9.1) |
3.12 (0.90-10.9) |
| No of cases | 4 | 10 | 9 | 10 |
| Conduct problems |
1.0 | 0.66 (0.19-2.26) |
1.36 (0.46-4.04) |
0.70 (0.20-2.38) |
| No of cases | 7 | 5 | 9 | 5 |
| Emotional problems |
1.0 | 0.67 (0.21-2.13) |
1.12 (0.39-3.25) |
0.32 (0.08-1.33) |
| No of cases | 8 | 6 | 9 | 3 |
| Peer problems | 1.0 | 2.79 (0.80-9.69) |
1.53 (0.40-5.89) |
1.03 (0.24-4.40) |
| No of cases | 4 | 10 | 6 | 4 |
Discussion
Our findings in this cohort of children followed up to the age of 9 years suggest that for the most part exposure to higher maternal concentrations of 25(OH)-vitamin D during late pregnancy poses little risk to the child. We found no indications to suggest that maternal 25(OH)-vitamin D status during late pregnancy had any effect on the child's growth, cognitive function, psychological health or cardiovascular system. There was some evidence that children exposed to higher maternal 25(OH)-vitamin D concentrations in pregnancy were more likely to be reported by their mothers as having had pneumonia or bouts of diarrhoea in the first 9 months of life, though risk of other respiratory or ear infections was not increased. There was a more consistent pattern as regards atopic disorders. Children whose mothers had high 25(OH)-vitamin D concentrations (>75 nmol/l) in late pregnancy were more likely to have visible eczema on examination at 9 months of age and tended to have a higher risk of atopic eczema diagnosed using the modified UK Working Party's diagnostic criteria. By 9 years of age, their risk of having a history of asthma was 5-times that of children who had been exposed to low maternal concentrations of vitamin D, though the number of cases was small.
One limitation of our study was our inability to follow-up to age 9 years all children in the original cohort. This was partly because some had moved away from the area in which the original study took place and it was not possible to trace them and partly because some declined to participate. Mean maternal concentrations of 25(OH)-vitamin D in late pregnancy were slightly higher in the children who took part in the 9-year follow-up compared to the remainder of the cohort. Their mothers were slightly older, tended to be better educated and were less likely to have smoked in pregnancy but there were no significant differences between the two groups in size at birth, gestation or maternal social class. Non-response or our inability to follow-up all children in the original cohort would only have introduced bias if the relations between maternal 25(OH)-vitamin D and outcomes differed in children whose families had moved away or did not respond to our invitation. We think this is unlikely.
Although skin exposure to UV-B is thought to be the major determinant of vitamin D status (Holick 2004), assessment of UV-B exposure from Meteorological Office sunlight data alone is imprecise because of substantial seasonal variations in UV-B intensity. By combining information on the hours of sunshine per month of pregnancy from a local Meteorological Office weather station with an adjustment for seasonal energy variation in UV-B radiation, we derived a measure of ultraviolet B exposure that was strongly related to vitamin D status in pregnancy. The current UK recommendation is that pregnant women take supplements containing 10 micrograms of vitamin D each day.(Department of Health 1998a) In this cohort, only 3 women had dietary intakes, from food or supplements, in excess of this figure so we were not able to examine whether eating the recommended amount of vitamin D had effects on their child's health and development.
Existing information on the effect of higher maternal 25(OH)-vitamin D concentrations during pregnancy in relation to the health of the child is sparse. The few randomised controlled trials of vitamin D supplements in pregnant women suggest that supplementation may lead to an increase in birthweight,(Brooke et al. 1980; Marya et al. 1981) and in size at age one year,(Brooke et al. 1981) though this is not a consistent finding.(Mallet et al. 1986) In this cohort, we found no evidence that higher maternal 25(OH)-vitamin D influenced the child's body size at birth, in infancy or at age 9 years , though it was associated with greater bone mass at that age.(Javaid et al. 2006) There has been only one previous report into the long-term effects of higher maternal 25(OH)-vitamin D concentrations on the child.(Goodenday & Gordon 1971b) Children born to hypoparathyroid mothers who had received a daily dose of 2.5 mg vitamin D throughout pregnancy were monitored for signs of hypercalcaemia or cardiovascular abnormalities over a 4-year period. No evidence of ill effects was found but only 15 children were studied.(Goodenday & Gordon 1971a) There has been some evidence from animal studies that exposure to high doses of vitamin D has an adverse effect on the developing vasculature, resulting in coronary lesions, supravalvular aortic stenosis and reduced aortic elastogenesis.(Friedman & Roberts 1966; Toda et al. 1985; Norman et al. 2002) Among the 178 children we examined at age 9 years, there were no indications that exposure to higher maternal concentrations of 25(OH)-vitamin D in pregnancy had any influence on their cardiac structure, arterial compliance, blood pressure or carotid intima-media thickness. We also found no evidence that such exposure had any effect on their cognitive function or their psychological health.
We did find, however, that children born to mothers who were in the top quarter of the distribution of 25(OH)-vitamin D in pregnancy were more likely to have visible atopic eczema on skin examination at age 9 months and to have a reported history of asthma at age 9 years. Whilst misclassification of these conditions is of course possible the diagnosis of visible atopic eczema was made by experienced research nurses who had been trained to diagnose the disorder, and we found that high maternal vitamin D status had similar associations with both self-reported doctor-diagnosed asthma and with current use of medications prescribed for asthma. Several recent studies have indicated that vitamin D might play a role in the development of atopic disorders. Mice given supplements of vitamin D in the first weeks of life show a sustained proliferation of the allergy-inducing Th2 cells.(Matheu et al. 2003) Children who received supplements of vitamin D in infancy have been found to have an increased risk of atopic disorders as adults.(Hypponen et al. 2004a) There is also some evidence to link risk of atopy and asthma with polymorphisms in the vitamin D receptor.(Raby et al. 2004b; Poon et al. 2004b) It was suggested some years ago that exposure to higher doses of vitamin D during fetal development might be a risk factor for the development of atopy and atopic disorders.(Wjst & Dold 1999) Our finding of an association between maternal 25(OH)-vitamin D concentrations in late pregnancy and risk of eczema at age 9 months and asthma at 9 years provides some support for this hypothesis. Numbers of cases were small, however, and it is possible that other factors may play a part. It is also worth noting that the 20.5% prevalence of asthma in the group exposed to the highest maternal vitamin D concentrations in our study is similar to the prevalence rates found in other studies of children of this age.(Sproston & Primatesta 2003; Butland et al. 2006) In the Health Survey for England 2002, for example, prevalence of asthma in children aged 7-9 years was 20% in girls and 27% in boys. The prevalence of asthma among the children in our study whose mothers had 25(OH)-vitamin D concentrations of less than 30 nmol/l was, at 4.5%, lower than would be expected from these figures. These results need to be confirmed in larger studies.
Many women are deficient in vitamin D both before and during pregnancy.(Alfaham et al. 1995b; Datta et al. 2002a; Nesby-O'Dell et al. 2002b; Dawodu et al. 2003; Schroth et al. 2005a; Meddeb et al. 2005a; Sachan et al. 2005b) The detrimental effect of such deficiency on their child's skeletal development and its likely long-term impact on their risk of osteoporotic fracture(Javaid et al. 2006) raises questions about the public health benefits of vitamin D supplementation during pregnancy. Our data suggest that exposure to higher maternal concentrations of 25(OH)-vitamin D during pregnancy (>75 nnol/l) does not influence the child's growth, cognitive function, psychological health or cardiovascular system. Our finding that risk of atopic disorders was increased in children exposed to higher maternal vitamin D concentrations needs to be confirmed in other studies, but it suggests that vitamin D supplementation during pregnancy may need to be targeted at women who are known to be deficient rather than offered to all regardless of vitamin D status.
Acknowledgments
We thank the children and their families for their help with this study and the research nurses who collected data.
Sponsorship: The study was supported by the Medical Research Council and WellChild (previously known as Children Nationwide)
References
- Alfaham M, Woodhead S, Pask G, Davies D. Vitamin D deficiency: a concern in pregnant Asian women. Br J Nutr. 1995b;73:881–887. doi: 10.1079/bjn19950093. [DOI] [PubMed] [Google Scholar]
- Alfaham M, Woodhead S, Pask G, Davies D. Vitamin D deficiency: a concern in pregnant Asian women. Br J Nutr. 1995a;73:881–887. doi: 10.1079/bjn19950093. [DOI] [PubMed] [Google Scholar]
- Bonner SE, Dawson R, Martyn CN, Greenwald SE. Validation and use of an optical technique for the measurement of pulse wave velocity in conduit arteries. Fortschritt Berichte. 1995;107:43–52. [Google Scholar]
- Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J (Clin Res.Ed) 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland BK, Strachan DP, Crawley-Boevey EE, Anderson HR. Childhood asthma in South London: trends in prevalence and use of medical services 1991-2002. Thorax. 2006 doi: 10.1136/thx.2005.043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;28:407–429. [PubMed] [Google Scholar]
- Datta S, Alfaham M, Davies DP, Dunstan F, Woodhead S, Evans J, Richards B. Vitamin D deficiency in pregnant women from a non-European ethnic minority population--an interventional study. BJOG. 2002b;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- Datta S, Alfaham M, Davies DP, Dunstan F, Woodhead S, Evans J, Richards B. Vitamin D deficiency in pregnant women from a non-European ethnic minority population--an interventional study. BJOG. 2002a;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- Department of Health . Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. HMSO; London: 1998a. [PubMed] [Google Scholar]
- Department of Health . Nutrition and Bone Health. Report on Health and Social Subjects 49. Stationery Office; London: 1998b. [Google Scholar]
- Friedman WF, Roberts WC. Vitamin D and the supravalvar aortic stenosis syndrome. The transplacental effects of vitamin D on the aorta of the rabbit. Circulation. 1966;34:77–86. doi: 10.1161/01.cir.34.1.77. [DOI] [PubMed] [Google Scholar]
- Godfrey K, Robinson S, Barker DJP, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. British Medical Journal. 1996a;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Hales CN, Osmond C, Barker DJ, Taylor KP. Relation of cord plasma concentrations of proinsulin, 32-33 split proinsulin, insulin and C-peptide to placental weight and the baby's size and proportions at birth. Early Hum.Dev. 1996b;46:129–140. doi: 10.1016/0378-3782(96)01752-5. [DOI] [PubMed] [Google Scholar]
- Goodenday LS, Gordon GS. No risk from vitamin D in pregnancy. Ann.Intern.Med. 1971b;75:807–808. doi: 10.7326/0003-4819-75-5-807_2. [DOI] [PubMed] [Google Scholar]
- Goodenday LS, Gordon GS. No risk from vitamin D in pregnancy. Ann.Intern.Med. 1971a;75:807–808. doi: 10.7326/0003-4819-75-5-807_2. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Stengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatr. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004a;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004b;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Pittard WB., III Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol.Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann.N.Y.Acad Sci. 2004b;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann.N.Y.Acad Sci. 2004a;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Arden NK, Godfrey KM, Cooper C, the Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Jiang B, Godfrey KM, Martyn CN, Gale CR. Birth weight and cardiac structure in children. Pediatrics. 2006;117:e257–e261. doi: 10.1542/peds.2005-1325. [DOI] [PubMed] [Google Scholar]
- Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy. The Cochrane Database of Systematic Reviews. 1999;(Issue 1) doi: 10.1002/14651858.CD000228. Art. No. CD000228. DOI:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet.Gynecol. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol.Obstet.Invest. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- Meddeb N, Sahli H, Chahed M, Abdelmoula J, Feki M, Salah H, Frini S, Kaabachi N, Belkahia C, Mbazaa R, Zouari B, Sellami S. Vitamin D deficiency in Tunisia. Osteoporos.Int. 2005a;16:180–183. doi: 10.1007/s00198-004-1658-6. [DOI] [PubMed] [Google Scholar]
- Meddeb N, Sahli H, Chahed M, Abdelmoula J, Feki M, Salah H, Frini S, Kaabachi N, Belkahia C, Mbazaa R, Zouari B, Sellami S. Vitamin D deficiency in Tunisia. Osteoporos.Int. 2005b;16:180–183. doi: 10.1007/s00198-004-1658-6. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002a;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002b;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Norman P, Moss I, Sian M, Gosling M, Powell J. Maternal and postnatal vitamin D ingestion influences rat aortic structure, function and elastin content. Cardiovascular Research. 2002;559:369–374. doi: 10.1016/s0008-6363(02)00444-3. [DOI] [PubMed] [Google Scholar]
- Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir.Crit Care Med. 2004a;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir.Crit Care Med. 2004b;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir.Crit Care Med. 2004a;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir.Crit Care Med. 2004b;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- Robinson S, Godfrey KM, Osmond C, Cox V, Barker D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50:302–308. [PubMed] [Google Scholar]
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005b;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005a;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- Schroth RJ, Lavelle CL, Moffatt ME. Review of vitamin D deficiency during pregnancy: who is affected? Int J Circumpolar.Health. 2005b;64:112–120. doi: 10.3402/ijch.v64i2.17964. [DOI] [PubMed] [Google Scholar]
- Schroth RJ, Lavelle CL, Moffatt ME. Review of vitamin D deficiency during pregnancy: who is affected? Int J Circumpolar.Health. 2005a;64:112–120. doi: 10.3402/ijch.v64i2.17964. [DOI] [PubMed] [Google Scholar]
- Sproston K, Primatesta P. Health Survey for England 2002: a survey carried out on behalf of the Department of Health. The Stationery Office; London: 2003. [Google Scholar]
- Toda T, Toda Y, Kummerow FA. Coronary arterial lesions in piglets from sows fed moderate excesses of vitamin D. Tohoku J Exp.Med. 1985;145:303–310. doi: 10.1620/tjem.145.303. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- Weiler H, Fitzpatrick-Wong S, Veitch R, Kovasc H, Schellenberg J, McCloy U, Kin Yuen C. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The UK Working Party's diagnostic criteria for atopic dermatitis. Br J Dermatol. 1994;131:416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54:757–759. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabedian M. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr. 1997;65:771–778. doi: 10.1093/ajcn/65.3.771. [DOI] [PubMed] [Google Scholar]


