Abstract
Innovative methods are needed to systematically track the HIV epidemic and appropriately target prevention and care programs in vulnerable populations of women. We conducted sentinel surveillance among women entering the jail system of San Francisco from 1999 to 2001 to track trends in HIV incidence, HIV prevalence, and related risk behavior. Using geographic information software (GIS), we triangulated findings to examine the spatial distribution of risk and disease. A total of 1,577 female arrestees voluntarily screened for sexually transmitted diseases at intake were included. HIV incidence, estimated using the serologic testing algorithm for recent HIV seroconversion (STARHS), was 0.4% per year (95% confidence interval [95%CI] = 0.1–2.1). HIV prevalence was 1.8% (95%CI = 1.1–2.4). HIV infection was independently associated with age 30 to 39 years compared to all other ages, African-American race/ethnicity vs. non-African-American, and recent injection drug use. Maps showed that the communities in which arrested women reside are also those with the highest concentrations of newly detected female HIV cases, AIDS cases, and clients of substance use programs. The combined strategy of using sentinel surveillance in the jail setting and GIS to map the spatial distribution of disease provides a useful tool to identify patterns of risk in hard-to-reach, vulnerable populations of women.
Keywords: Arrestees, HIV, Sentinel surveillance, GIS, Women, Sexually transmitted diseases
Introduction
The proportion of AIDS cases among women in the US grew from 8% in 1986 to 26% in 2006.1 In San Francisco, over 1,000 women had been diagnosed with AIDS and over 400 with HIV-non-AIDS through 2006.2 Compared to men, women diagnosed with HIV/AIDS are more likely to be African American, younger age, and dependent on public insurance. Also compared to men, there is a relative scarcity of epidemiological studies on HIV prevalence and incidence among female populations at risk in San Francisco. While HIV and AIDS case reporting for men and women is very complete in San Francisco, these data only include those who have been diagnosed with HIV. There are currently few studies that include sufficient numbers of women who may not be accessing HIV testing or care programs and, therefore, are unaware of their infection status.
In the late 1980s through the 1990s, HIV prevalence was tracked among women outside of HIV testing and care programs through a series of seroprevalence studies known as the “family of surveys” or “sentinel surveillance.”3 The basic approach entailed conducting HIV antibody testing on consecutive samples of blood collected for routine purposes from persons visiting selected facilities. The approach used an unlinked and anonymous HIV testing protocol where the specimens and abstracted data were stripped of personal identifying information prior to HIV testing. Because HIV testing was done on blood collected for other purposes, the measure of prevalence was not biased by including only those who would consent and volunteer for HIV testing. The rationale behind sentinel surveillance was to identify HIV infection and characterize trends in prevalence at sites where populations at highest risk are concentrated. In the US and in San Francisco, HIV prevalence was tracked in this manner among child-bearing women and among women and men at sexually transmitted disease (STD) clinics, drug treatment centers, and jails outside of the US. Sentinel surveillance among women at antenatal clinics continues to form the backbone of HIV surveillance systems in areas with generalized epidemics, such as the region of sub-Saharan Africa.4 Furthermore, sentinel surveillance is conducted among women and men at STD clinics in areas of the world where there are concentrated epidemics, such as Asia and Central America.5–9 Due to the wide availability of HIV testing and treatment, the CDC discontinued unlinked anonymous sentinel surveillance surveys in the US in the early 2000s leaving a gap in the ability to monitor trends in HIV prevalence among vulnerable groups on an ongoing basis.
Two major limitations of sentinel surveillance may be addressed by recent technological developments. First, HIV prevalence trends have become difficult to interpret in the era of HIV treatment; therefore, HIV incidence trends provide more useful information for making inferences on emerging subepidemics.10 Laboratory assays, including CDC’s serologic testing algorithm for recent HIV seroconversion (STARHS), including the “detuned” assay and the BED IgG capture enzyme immunoassay, can distinguish recent from longer-standing infection in HIV-positive specimens and thus estimate population HIV-1 incidence in cross-sectional settings.11,12 A second limitation was the uncertainty in which populations or communities were represented in the facilities included in sentinel surveillance, in particular with respect to the geographic distribution of the target population. Through the use of geographic information software (GIS), geographic data can provide a means to examine the spatial distribution of disease in an urban setting, factors that affect the spread of disease, link public health resources to individuals in locations demonstrating greatest need for targeted prevention and treatment programs, and evaluate whether current programs are adequately reaching these locations. Used in combination with sentinel surveillance data, GIS data is a powerful tool to assist with the epidemiologic interpretation of surveillance data.13,14
In this paper, we present data from the last nationally coordinated sentinel surveillance survey in San Francisco conducted at the central intake county jail from 1999 to 2001 with a particular focus on women. The correctional setting represents a unique opportunity to access a large number of persons at risk for HIV who might otherwise not be seen or tested for HIV at health care facilities. In the United States in 2002, an estimated 2.2% of state prison inmates and 1.7% of local jail inmates were infected with HIV. Because most inmates are booked and released within 24 hours (in the San Francisco County Jail system, half of men and over two-thirds of women are released within 24 hours), HIV prevalence estimates observed in local jails at any cross-sectional point in time may reflect the epidemic in the communities from which they originate. Therefore, the greatest opportunity for surveillance and prevention interventions among arrestees and the communities from which they originate exists at their initial intake rather than among the resident jail population. Our study combines several innovative approaches to sentinel surveillance, including the priority sampling of women upon intake into jail, the application of STARHS to identify recent infection, and the triangulation of AIDS and HIV surveillance, demographic, and service data using GIS mapping.
Methods
Study Site
The San Francisco County Jail system is comprised of six county jails, including one central intake jail (CJ9) where all arrestees are initially processed. The average daily census of the entire system is 2,200 inmates, of which 86% are male and 14% are female. It is estimated that an average of 90,000 to 100,000 persons pass through the jail system each year. At CJ9, inmates are provided with medical, psychiatric, and substance abuse care. Since 1996, voluntary sexually transmitted disease (STD) screening and treatment services have been offered to male inmates ages 18 to 30 years and female inmates ages 18 to 45 years within hours of arrest. Options for voluntary HIV counseling, testing and treatment referral are presented to inmates within the jail health system, as well as referrals for anonymous testing outside the jail upon release. We conducted our sentinel surveillance survey among arrestees screened for STD at CJ9 using leftover sera collected for syphilis screening and information routinely recorded for the STD screening program.
Study Subjects
This sentinel surveillance study is focused on women. All women voluntarily screened for syphilis at CJ9 from July 1999 to June 2001 were eligible. This included female arrestees aged 18 to 45 years who did not have a previous syphilis serology in the jail health service records in the past 6 months. Furthermore, all eligible women were required to be physically able to provide consent for syphilis screening. Therefore, any inmate who was acutely intoxicated, acutely withdrawing, or posing a safety threat was not eligible for syphilis screening. From 1999 to 2001, staff coverage ranged from 16 to 24 hours per day, 7 days per week, for 12 months each year. Some inmates were not offered STD screening due to incomplete shift coverage and high jail volume. Given previously documented higher prevalence of STD among women in San Francisco jails and quicker rates of release of women from jail,15 priority for STD screening was given to women over men. For example, women engaging in sex work were more likely to be arrested and processed in CJ9 during late night hours; therefore, midnight shifts were prioritized to target the largest number of women. Also, in times of high inmate volume, women were offered STD screening first, after which male inmates were offered screening on a consecutive cell-by-cell basis.
Study Procedures
Procedures for the sentinel surveillance study followed standardized protocols for unlinked anonymous testing from the CDC.3 The steps were integrated into the usual procedures to provide STD screening and treatment to arrestees upon intake. Within a few hours of arrest, eligible inmates were individually approached in their holding cells by the program staff, offered health services, and, if interested, escorted to a private screening room where they were informed of the voluntary STD screening services available to them. Participants were asked to provide a 5-ml tube of blood for syphilis testing and a 30-ml cup of first void urine for gonorrhea and chlamydia testing. An interviewer completed a basic intake form that recorded information on demographics, sexual and drug use behavior, reproductive and sexual health, and reasons for arrest. After data collection, risk reduction counseling and educational information on STDs and HIV infection were provided. Specimens were sent to the San Francisco Department of Public Health Laboratory for STD testing following standard protocols. Results and treatment for STD were given to inmates within 1 week following standard clinical protocols, either in jail if they were still incarcerated or at their contact address provided.
HIV testing was done on the leftover sera after syphilis testing was completed. Several steps were taken to merge sociodemographic data and risk information with HIV test results in a manner that precluded the possibility of linking results back to individuals. After syphilis testing, residual sera were transferred into vials labeled only with unique, nonidentifying study identification numbers. The vials were then transferred to the HIV testing lab where they were categorized, logged, and stored at −35°C for future testing. An STD screening database was created by the jail health program that included a quarterly roster of names and the information collected during screening and later linked to the laboratory results for syphilis, gonorrhea, and chlamydia. A temporary linkage database was created that included a quarterly roster of names, date of birth, and the study numbers of all participants. Once data were checked for completeness, all personal identifiers were deleted from the study database and HIV testing was performed on the stored sera. The results of HIV testing were linked to the database containing the study numbers and intake information, but no personally identifying information.
Laboratory Methods
Blood samples were screened for syphilis antibodies by a rapid plasma reagin (RPR) assay. If reactive, the sample was screened by a T. pallidium particle agglutination (TPPA) assay. A syphilis case was defined by a reactive RPR and confirmed by TPPA. Urine samples were screened for gonorrhea and chlamydia infection (BD Probtech, Sparksville, MD, USA). Serum samples were initially screened for HIV antibodies by a standard enzyme immunoassay (EIA) (Vironostika HIV-1 Microelisa, Organon Teknika, Durham, NC, USA) and confirmed as positive by immunofluorescent antibody (Flourognost HIV-1 IFA, Waldheim Pharmazeutika, Vienna, Austria). To identify recent seroconversion, STARHS was applied following standard CDC protocols.11,16 Confirmed positive specimens were tested with the Vironostika Less-Sensitive HIV-1 EIA, modified from the standard Vironostika HIV-1 EIA, to be less sensitive by an increased specimen dilution and a decreased specimen incubation period. Standard optical densities (SOD) from the less-sensitive assay were calculated. Based on the slow rise in HIV antibody titer over the early period of infection, subjects whose specimen tested nonreactive on the less-sensitive EIA (median SOD < 1.0) were categorized as having recently seroconverted (i.e., within the last 170 days).
Measures
Only information routinely collected for jail health services purposes was used for sentinel surveillance; no questions were asked specifically for the purpose of this study. Persons screened for STDs provided information on demographic characteristics and HIV/STD risk-related behavior in a structured face-to-face, clinical interview. Additional data on booking charges and previous STD screening through the jail health system were abstracted from a computerized database and entered onto the form. Key measures included basic demographic characteristics, risk behavior in the past 6 months, category of arrest offense, and STD test results. Demographic data included date of birth, gender, race, and inmate residence (later converted to US Census block group). Residence information was missing for 59% of inmates because the inmate reported no known address. The high percentage of missing residence data can be attributed to a high percentage of missing residence data that should have been reported to the jail STD program by the San Francisco Sheriff’s Department and is not the result of a systematic bias such as housing status.
Jail-related information included date of incarceration, booking charges, and previous incarceration history. Risk behavior recorded for the past 6 months included number of male partners; number of female partners; sexual orientation; substance use, including noninjection and injection drug use and sharing syringes; sexual behavior, including sex with men who have sex with men, sex with injection drug users, sex with a known HIV-positive partner, receipt of money or drugs for sex, and providing money or drugs for sex. Additional sexual and reproductive health data included past history of STDs, date of last syphilis serology in the San Francisco County Jail, current STD symptoms, and antibiotic use in the past 30 days.
Statistical Methods
Data were analyzed using SAS version 6.0 (SAS, Cary, NC, USA) for univariate, bivariate, and multivariate analysis, including point prevalence, 95% confidence intervals (95%CI), and crude and adjusted odds ratios. Analysis of temporal trends was conducted for 6-month time intervals from July 1999 to June 2001 using the Cochrane Mantel Haenzel chi-square test for trend. Geographic data were analyzed using the ArcGIS 3.0 software (ESRI, Redlands, CA, USA). Multiple logistic regression models for HIV infection were performed including potential predictors that were associated with HIV at p < 0.2 in bivariate analysis. Variables that remained associated with HIV infection at p < 0.05 or those that were important confounders were retained in the final multivariate model.
HIV incidence was calculated from STARHS results following a standard formula provided by the CDC.11 Crude incidence was obtained by dividing the number of persons with recent infection by the total number of persons with recent infection plus uninfected persons (i.e., the “susceptible population”). The estimate was annualized as “percent per year” using the formula: 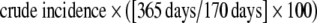 . Ninety-five percent confidence intervals for estimated HIV incidence were constructed using a Bonferroni procedure that assumes a Poisson distribution, an alpha of 0.025, and adjusts for variability around the 170-day less-sensitive EIA window period (132–212 days).
. Ninety-five percent confidence intervals for estimated HIV incidence were constructed using a Bonferroni procedure that assumes a Poisson distribution, an alpha of 0.025, and adjusts for variability around the 170-day less-sensitive EIA window period (132–212 days).
Geographic Analysis
We triangulated several sources of data for geographic analysis, including individual inmate addresses converted to the US Census block group level, AIDS case reporting data from the San Francisco Department of Health HIV Surveillance Unit, client data from the San Francisco Department of Health Community Substance Abuse Services, selected demographic data using projections to 1999 from a commercial source (Geolytics, East Brunswick, NJ, USA), and the 1990 US Census. Geographic data in the maps were displayed to the US Census block group level. According to the 1990 Census, San Francisco had 651 block groups. Block groups usually represent several city blocks, bounded by streets or bodies of water. In order to protect confidentiality of individuals, block groups containing greater than one but fewer than five cases were collapsed into one level representing one to five cases.
Ethical Considerations
The Institutional Review Boards for the CDC and the University of California San Francisco reviewed and approved the protocol. Both bodies included a prisoner’s advocate.
Results
From June 1999 to June 2001, staff approached 3,637 arrested women to offer STD screening services. Of these, 2,771 (76.2%) were eligible, and of those, 2,064 (74.5%) agreed to participate. Overall, 1,577 women, or 57% of those eligible, provided a serological specimen for syphilis testing.
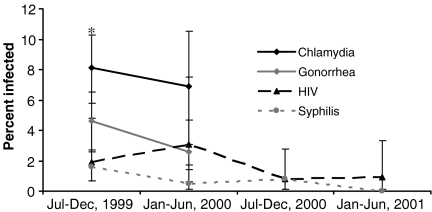
HIV-1 antibody was detected in 1.8% (95%CI = 1.1–2.4) of the 1,577 women who provided a serological specimen, with a borderline significant decline from 1.9% in 1999 to 0.9% in 2001 (p = 0.09) (Figure 1). A similar borderline significant decline was noted for syphilis infection, from 1.7% in 1999 to 0% in 2001 (p = 0.09). Overall syphilis prevalence was 1.0% (95%CI = 0.5–1.5). Data for gonorrhea and chlamydia were available from June 1999 to June 2000 only. Gonorrhea infection was detected in 4.6% (95%CI = 2.9–6.2) of women and chlamydia in 7.9% (95%CI = 5.9, 9.9) with no statistically significant change in the prevalence for either infection. Eighty-six percent of HIV infections and 87% of syphilis infections were observed among participants who were previously incarcerated (Table 1).
FIGURE 1.
Trends in HIV prevalence and sexually transmitted infections, women upon intake in San Francisco County Jail, 1999–2001. The asterisk represents the 95% CI.
Table 1.
Demographic characteristics, reason for arrest, risk behavior, and history of sexually transmitted diseases in women voluntarily screened for syphilis in San Francisco County Jail, 1999–2001
| Variable | N (%) |
|---|---|
| All female arrestees screened for syphilis | 1,577 (100) |
| Age group | |
| 29 years or younger | 1,107 (70.2) |
| 30–39 years | 388 (24.6) |
| 40–45 years | 82 (5.2) |
| Race/ethnicity | |
| White | 292 (18.6) |
| African American | 845 (53.9) |
| Latino | 236 (15.1) |
| Other | 194 (12.4) |
| Sexual orientation | |
| Heterosexual | 1,401 (90.2) |
| Bisexual/lesbian | 152 (9.8) |
| Female sex partner, last 6 months | 150 (9.6) |
| Reason for arrest | |
| Drug offense | 500 (31.7) |
| Burglary | 423 (26.8) |
| Commercial sex work | 132 (8.4) |
| Homicide/assault | 27 (1.7) |
| Sex offense | 13 (0.8) |
| Previous incarceration | 952 (60.4) |
| Used non injection drugs, last 6 months | 925 (58.7) |
| Injected drugs, last 6 months | 108 (6.9) |
| Commercial sex work, last 6 months | 230 (14.6) |
| Number of male partners, last 6 months | |
| 0 | 5 (0.5) |
| 1 | 828 (61.7) |
| 2 or more | 509 (37.9) |
| Sex with a known HIV-infected man, last 6 months | 18 (1.1) |
| Sex with a man who has sex with men, last 6 months | 16 (1.0) |
| Sex with an injection drug user, last 6 months | 62 (3.9) |
| History of chlamydia, ever | 288 (18.3) |
| History of gonorrhea, ever | 233 (14.8) |
| History of syphilis, ever | 57 (3.6) |
Categories do not always add to total due to missing data
In bivariate analysis (Table 2), HIV infection was significantly associated with older age, bisexual or lesbian sexual orientation, being arrested for homicide or assault, previous incarceration, reported history of injection drug use, sex with a known HIV-positive man, sex with a man who has sex with men, history of gonorrhea, and history of syphilis. In addition, women whose current syphilis test was positive were more likely to also be HIV-positive (7.0%) than women whose test was negative (1.6%) (p < 0.01). After adjusting for age, only African-American race/ethnicity and recent injection drug use were independent predictors of HIV infection (Table 3).
Table 2.
HIV positivity and bivariate associations in women voluntarily screened for syphilis in San Francisco County Jail, 1999–2001 (N = 1,577)
| Variable | HIV-infected, N (%) | Odds ratio (95% CI) | p value |
|---|---|---|---|
| Total | 28 (1.8) | – | – |
| Age group | |||
| 29 years or younger | 9 (0.8) | 1.0 | |
| 30–39 years | 16 (4.1) | 5.2 (2.3–12.0) | <0.01 |
| 40–45 years | 3 (3.7) | 4.6 (1.2–17.5) | 0.02 |
| Race/ethnicity | |||
| White | 6 (2.1) | 4.0 (0.4–33.9) | 0.2 |
| African American | 21 (2.5) | 4.4 (0.6–33.4) | 0.1 |
| Latino | 2. (0.9) | 1.7 (0.1–18.3) | 0.7 |
| Other | 1 (0.5) | 1.0 | na |
| Sexual orientation | |||
| Heterosexual | 21 (1.5) | 1.0 | na |
| Bisexual/lesbian | 7 (4.6) | 3.2 (1.3–7.6) | <0.01 |
| Female sex partner | 4 (2.7) | 1.5 (0.4–4.5) | 0.4 |
| Reason for arrest | |||
| Drug offense | 10 (2.0) | 1.2 (0.6–2.6) | 0.6 |
| Burglary offense | 11 (2.6) | 1.5 (0.7–3.3) | 0.2 |
| Commercial sex work | 2 (1.5) | 0.8 (0.2–3.6) | 0.8 |
| Homicide/aggravated assault | 2 (7.4) | 4.7 (1.1–20.8) | 0.03 |
| Sex offense | 0 | na | na |
| Previous incarceration | 24 (2.5) | 4.0 (1.4–11.6) | <0.01 |
| Used noninjection drugs | 20 (2.2) | 1.4 (0.7–3.1) | 0.4 |
| Injected drugs | 5 (4.6) | 3.1 (1.1–8.2) | 0.02 |
| Commercial sex work | 5 (2.2) | 1.3 (0.5–3.4) | 0.6 |
| Number of male partners | |||
| 0 | 0 | na | na |
| 1 | 13 (1.6) | 1.0 | na |
| 2 or more | 9 (1.8) | 1.1 (0.5–2.7) | 0.8 |
| Sex with a known HIV-infected man | 4 (22.2) | 18.3 (5.6–59.6) | <0.01 |
| Sex with a man who has sex with men | 3 (18.8) | 14.2 (3.8–52.9) | <0.01 |
| Sex with an injection drug user | 2 (3.2) | 1.7 (0.2–7.2) | 0.4 |
| History of chlamydia, ever | 8 (2.8) | 1.8 (0.8–4.1) | 0.2 |
| History of gonorrhea, ever | 8 (3.4) | 2.4 (1.0–5.4) | 0.04 |
| History of syphilis, ever | 4 (7.0) | 4.7 (1.6–14.0) | <0.01 |
The referent does not have risk factor unless a category is stated. Risk behavior is in the last 6 months unless otherwise stated.
Table 3.
Independent predictors of HIV infection in women voluntarily screened for syphilis in San Francisco County Jail, 1999–2001 (N = 1,577)
| Variable | Adjusted odds ratioa | 95% CI | p value |
|---|---|---|---|
| Age group | |||
| 29 years or younger | 1.0 | na | na |
| 30–39 years | 5.0 | 2.2–11.4 | <0.01 |
| 40 years or older | 4.2 | 1.1–15.9 | 0.06 |
| Race/ethnicity | |||
| White | 2.4 | 0.6–10.2 | 0.2 |
| African American | 3.7 | 1.1–12.6 | 0.03 |
| Other | 1.0 | na | na |
| Injection drug use in last 6 months | 2.8 | 0.9–8.5 | 0.06 |
aAdjusted for other variables listed in the table
Several trends in risk behavior over time were notable. Reported history of recent injection drug use in the past 6 months increased significantly from 4.5% in June 1999 to 7.9% in June 2001 (p < 0.01). However, among participants who reported ever injecting drugs, syringe sharing in the past 6 months decreased from 45.2% to 17.7% from 1999 to 2001 (p = 0.02). Reported history of sex work in the past 6 months decreased significantly from 19.9% in June 1999 to 5.1% in June 2001 (p < 0.01). No significant changes over time were detected in the proportion of persons reporting multiple partners, sex with a known HIV-positive man, and sex with men who have sex with men in the past 6 months.
Using STARHS, HIV incidence was estimated to be 0.4% per year (95%CI = 0.1, 2.1) based on three detected recent infections overall. The small number of recent infections precluded further analysis of trends and risk factors for HIV incidence. Nonetheless, incidence tended to be high among women under age 30 years (1.9% per year), those reporting injecting drugs (2.7% per year), and those arrested for burglary (1.5% per year).
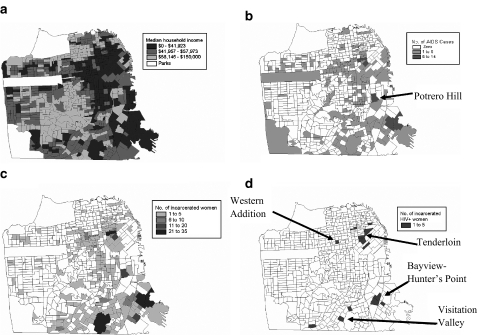
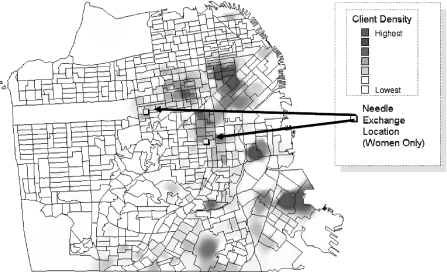
Figure 2 illustrates the distribution of household income, women living with AIDS, women arrested, and women detected with HIV infection in the jail sentinel surveillance study. Of note, women living with AIDS and women arrested are highly concentrated in a few poor neighborhoods located in the inner-city eastern and southern parts of San Francisco. The same neighborhoods were largely the areas of residence for women detected with HIV in the jail sentinel surveillance with the additional detection of a cluster in the Western Addition, a poor, predominantly African-American neighborhood in the center of the city. We further mapped the concentration of female clients who had ever used or requested services related to their injection drug use (e.g., detoxification for injecting heroin, methamphetamine, or cocaine, or methadone maintenance), and findings intersected similar neighborhoods (Figure 3). Of note, at the time of the study, the two women-only syringe exchange program sites were not located in any of these neighborhoods. It is important to note that several other syringe exchange programs were operating at the time in these neighborhoods of “high risk” though it is unclear whether or not women in our study used their services.
FIGURE 2.
Spatial distribution of female arrestees identified at San Francisco County Jail, 1999–2001. a Median household income, 2000; b reported AIDS cases in women, 2002; c women arrested, 1999–2001; d HIV-positive women arrested, 1999–2001.
FIGURE 3.
Concentration of female clients for injection drug use services (e.g., detoxification, methadone maintenance) and women-only syringe exchange sites. The kernel density estimation method in ArcGIS software was applied to display the data on this map.
A substantial proportion (59%) of participants did not report residence data and, therefore, could not be mapped. Compared to participants with residence information, participants without residence information were significantly less likely to be HIV-positive (1.2% vs. 2.6%, p = 0.03), more likely to be White (21.2% vs. 14.5%, p < 0.01), and more likely to be young at age 18–19 years (24.4% vs. 16.9%, p < 0.01). No differences were observed in injection drug use in the past 6 months between the two groups (7.1% vs. 6.7%, p = 0.8).
Discussion
Our analysis illustrates how unlinked anonymous sentinel surveillance in jails, at intake, is a feasible, practical approach to tracking the HIV epidemic among vulnerable populations of women. We identified a large sample of women at risk and observed high participation rates for voluntary STD screening among women who traditionally underutilize health care services elsewhere.17–19 We applied the STARHS methodology to estimate HIV incidence in a cross-sectional design and geographic analysis to enhance our understanding of the communities being sampled. Geographic analysis also offered practical suggestions for targeting new and evaluating existing HIV prevention programs for women by neighborhood. Furthermore, our data provided a unique opportunity to identify the correlates and drivers of HIV transmission to women using preexisting data collected on HIV, STD, demographics, and risk behavior. In this jail population, similar to San Francisco as a whole, recent injection drug use was strongly associated with HIV infection.
HIV prevalence estimates in this sample were over tenfold higher than the HIV estimates in the general female population in San Francisco (1.8% vs. 0.2%),2 highlighting the utility of jail-based surveillance to effectively reach the most vulnerable segments of society. Our estimates were comparable to those observed among female inmates entering the California Department of Corrections facilities (1.7%)20 and to national estimates of HIV infection among local jail inmates in 1999 (1.7%).21 Of note, our blinded estimates, which were not subject to the selection biases observed in a voluntary HIV testing setting, were considerably lower than the voluntary HIV testing program located in the San Francisco jail premises where prevalence was 5.8% among females in 2001. Given the stigma of HIV infection in jails, the higher prevalence suggests female inmates seeking testing may represent a biased sample of women who are aware of their elevated risk of infection. Thus, relying on program testing data would overestimate HIV prevalence in the jail population, lending further support to the blinded methodology used in this study for surveillance purposes. For example, communicable diseases such as HIV infection are marked on the jail records of inmates which precludes them working in the food services and limits their job opportunities.
Other findings of our data are noteworthy. Detection of chlamydia, gonorrhea, and syphilis cases was high. In fact, because the jail-based STD screening program enabled the detection of a high proportion of all diagnosed STD cases in San Francisco,22 many of the STD cases identified in the jail may have otherwise gone undetected. The young age of our study population (over two-thirds were under 30 years) also presents a unique HIV prevention opportunity for young people who represent the leading edge of the epidemic and may become newly infected without access to comprehensive HIV and STD prevention programs. The relatively low prevalence of HIV among sex workers in our data suggests that sex workers in San Francisco with a recent history of arrest and incarceration are at low risk for HIV infection. Community-based efforts in San Fancisco such as the St. James Infirmary, a comprehensive health and occupational clinic, may be critical to maintaining this low risk.23 Our data are also encouraging in that HIV prevalence among women in San Francisco remained relatively low and possibly declined during a period of resurgence of HIV incidence among men who have sex with men.24 Other indicators of sexual risk correlated with high HIV prevalence in our study also appear to be stable or declining, including a recent history of sex work, sex with men who have sex with men, syphilis prevalence, sex with known HIV-infected persons, and number of recent male partners. Injection-related risk such as syringe sharing decreased during the study period even though the reported history of injection drug use increased among female arrestees. It is possible that with the legalization of syringe exchange programs in San Francisco in 2000, women had increased access to sterile syringes yet were also subject to increased police surveillance and subsequent arrest for the possession of syringes and other injection paraphernalia.25
We note that the locations of the two women-only syringe exchange programs in San Francisco during the study period were not located in the neighborhoods with the highest concentration of arrests related to injection drug use and female injection drug users living with HIV infection. Although these programs are well-received, their impact may be greater if they are replicated in additional neighborhoods. The location of syringe exchange programs, and specifically those that target their services specifically to women, may be critical to their success;26 a study in New York found that program use significantly dropped with increases in travel distance.27
We recognize several limitations to the study approach and interpretation of data. First, apparent changes in risk behaviors and subsequently HIV prevalence may reflect changes in interdiction strategies and police activities rather than changes in the community as a whole. Further research aimed at understanding police practices and locations of drug-related arrests might further inform changes in risk behavior as well as document potential structural barriers to optimizing HIV prevention strategies such as syringe exchange programs. Second, while acceptability overall was high for voluntary STD screening services, data were not collected for 36% of eligible persons. Therefore, there exists the potential that persons declining STD screening may differ from those who accepted. Of note, a comparison of aggregate demographic characteristics between inmates included in the study and the jail population as a whole found no differences in age or race/ethnicity. Third, not all inmates were offered STD screening due to limitations in shift coverage. However, we find no reason to believe this would result in substantial selection bias because the reasons for lack of shift coverage were logistical and not related to arrestee behavior. Furthermore, while priorities in screening were given to women, the quick turnover rates in arrest for sex workers made it difficult to screen a large numbers of sex workers. Fourth, we were not able to infer much regarding HIV incidence due to the low number of recent seroconverters (n = 3) in our sample. However, the data presented in this study corroborate other data in San Francisco that suggest that new HIV infections remain rare and sporadic among women.2 Finally, a substantial proportion of residence data was uncollected; therefore, the GIS mapping results cannot be generalizable to all female jail inmates in San Francisco. Though without complete residence data, the GIS maps were still successful in identifying potential hotspot locations for HIV infection that corresponded directly to the locations of highest densities of reported AIDS cases in San Francisco at the time of the survey.
Despite those limitations, the linkage of surveillance data enabled us to identify emerging trends in the HIV epidemic in hard-to-reach populations as well as identify new areas for enhanced prevention. It is critical that HIV surveillance data be routinely used to inform HIV prevention and care efforts. The high arrest and incarceration rates in the United States enable the jail system to act as a sentinel site for tracking vulnerable populations that may cycle in and out of the communities where HIV infection is concentrated. The dual epidemics of incarceration and HIV/AIDS in the United States predominately affect poor communities of color28, and additional efforts to understand the context of risk in these communities will contribute to the gaps in understanding the health needs of this vulnerable population.
Footnotes
Funding
This work was supported by a cooperative agreement between the San Francisco Department of Public Health and the US Centers for Disease Control and Prevention (U62/CCU906255).
References
- 1.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2005. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007.
- 2.San Francisco Department of Public Health. HIV/AIDS Epidemiology Annual Report, 2005. San Francisco: HIV/AIDS Statistics and Epidemiology Section; 2006.
- 3.Pappaioanou M, Dondero T, Petersen L, Onorato I, Sanchez C, Curran J. The family of HIV seroprevalence surveys: objectives, methods, and uses of sentinel surveillance for HIV in the United States. Public Health Rep. 1990;105(2):113–119. [PMC free article] [PubMed]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) (2003) Guidelines for conducting HIV sentinel surveys among pregnant women and the other groups / UNAIDS / WHO Working Group on Global HIV/AIDS and STZ Surveillance. Geneva, UNAIDS.
- 5.Pal BB, Acharya AS, Satyanarayana K. Seroprevalence of HIV infection among jail inmates in Orissa. Indian Journal of Med Research. 1999;109:199–201. [PubMed]
- 6.Lye NS, Archibald C, Ghazali AA, et al. Patterns of risk behaviour for patients with sexually transmitted diseases and surveillance for human immunodeficiency virus in Kuala Lumpur, Malaysia. Int J STD AIDS. 1994;5(2):124–129. [DOI] [PubMed]
- 7.Soto R, Ghee A, Nunez GMR, et al. Sentinel surveillance of sexually transmitted infections/HIV and risk behaviors in vulnerable populations in 5 Central American countries. J Acquir Immune Defic Syndr. 2007;46(1):101–111. [PubMed]
- 8.Dandona L, Lakshmi V, Sudha T, Kumar G, Dandona R. A population-based study of human immunodeficiency virus in south India reveals major differences from sentinel surveillance-based estimates. BMC Med. 2006;4:31 doi:10.1186/1741-7015-4-31. [DOI] [PMC free article] [PubMed]
- 9.Hien NT, Long HT, Chi PK, et al. HIV monitoring in Vietnam: system, methodology, and results of sentinel surveillance. J Acquir Immune Defic Syndr. 1999;21(4):338–346. [DOI] [PubMed]
- 10.Rutherford G, Schwarcz S, McFarland W. Surveillance for incident HIV infection: new technology and new opportunities. J Acquir Immune Defic Syndr. 2000;25(Suppl 2):S115–S119 doi:10.1097/00126334-200012152-00005. [DOI] [PubMed]
- 11.Janssen R, Satten G, Stramer S, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280(1):42–48 doi:10.1001/jama.280.1.42. [DOI] [PubMed]
- 12.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18(4):295–307. [DOI] [PubMed]
- 13.Kaukinen C, Fulcher C. Mapping the social demography and location of HIV services across Toronto neighbourhoods. Health Soc Care Community. 2006;14(1):37–48 doi:10.1111/j.1365-2524.2005.00595.x. [DOI] [PubMed]
- 14.Pierce SJ, Miller RL, Morales MM, Forney J. Identifying HIV prevention service needs of African American men who have sex with men: an application of spatial analysis techniques to service planning. J Public Health Manag Pract. 2007;(Supplement):72–79. [DOI] [PubMed]
- 15.Barry P, Kent C, Scott K, Snell A, Goldenson J, Klausner J. Optimising sexually transmitted infection screening in correctional facilities: San Francisco, 2003–2005. Sex Transm Infect. 2007;83(5):416–418. [DOI] [PMC free article] [PubMed]
- 16.Kothe D, Byers R, Caudill S, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33(5):625–634 doi:10.1097/00126334-200308150-00012. [DOI] [PubMed]
- 17.Mertz K, Schwebke J, Gaydos C, Beidinger H, Tulloch SWCL. Screening women in jails for chlamydial and gonococcal infection using urine tests: feasibility, acceptability, prevalence, and treatment rates. Sex Transm Dis. 1997;29(5):271–276 doi:10.1097/00007435-200205000-00004. [DOI] [PubMed]
- 18.Mertz K, Voigt RHK, Levine WJSPMG. Findings from STD screening of adolescents and adults entering corrections facilities: implications for STD control strategies. Sex Transm Dis. 2002;29(12):834–839 doi:10.1097/00007435-200212000-00016. [DOI] [PubMed]
- 19.Fickenscher A, Lapidus J, Silk-Walker P, Becker T. Women behind bars: health needs of inmates in a county jail. Public Health Rep. 2001;116(3):191–196. [DOI] [PMC free article] [PubMed]
- 20.Ruiz J, Molitor F, Plagenhoef J. Trends in hepatitis C and HIV infection among inmates entering prisons in California, 1994 versus 1999. AIDS. 2002;16(16):2236–2237 doi:10.1097/00002030-200211080-00023. [DOI] [PubMed]
- 21.Maruschak L. HIV in prisons and jails 1997. Washington D.C., US Department of Justice, Bureau of Justice Statistics Bulletin, 1999, Bureau of Justice Statistics Bulletin publication NCJ 178284.
- 22.STD Control Section. San Francisco Sexually Transmitted Disease Annual Summary, 2006. San Francisco, CA: San Francisco Department of Public Health; 2007 July.
- 23.Cohan D, Lutnick A, Davidson P, et al. Sex worker health: San Francisco style. Sex Transm Dis. 2006;82(5):418–422. [DOI] [PMC free article] [PubMed]
- 24.Katz MSSK, Kellogg TA, Klausner JD, Dilley J, Gibson S, McFarland W. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92(9):1387–1388. [DOI] [PMC free article] [PubMed]
- 25.Martinez A, Bluthenthal R, Lorvick J, Anderson RNF, Kral A. The impact of legalizing syringe exchange programs on arrests among injection drug users in California. J Urban Health. 2007;84(3):423–435 doi:10.1007/s11524-006-9139-1. [DOI] [PMC free article] [PubMed]
- 26.Welton A, Adelberger K, Patterson K, Gilbert D. Optimal placement of syringe-exchange programs. J Urban Health. 2004;81(2):268–277 doi:10.1093/jurban/jth113. [DOI] [PMC free article] [PubMed]
- 27.Rockwell R, Des Jarlais D, Friedman SPTE, Paone D. Geographic proximity, policy and utilization of syringe exchange programmes. AIDS Care. 1999;11(4):437–442 doi:10.1080/09540129947811. [DOI] [PubMed]
- 28.Pettit B, Western B. Mass imprisonment and the life course: race and class inequality in US incarceration. Am Sociol Rev. 2004;69(2):151–169.