Abstract
Hepatitis B vaccination and targeted testing for hepatitis C virus (HCV) are recommended for jails with medical services available. This study estimates hepatitis B virus (HBV) and HCV infection prevalence among jail inmates, since most previous studies have been conducted among prison inmates. Prison and jail populations differ: jails hold a wide spectrum of persons for an average of 10–20 days, including persons awaiting arraignment, trial, conviction, or sentencing, while prisons typically hold convicted criminals for at least 1 year. A stratified random sample of sera obtained during routine syphilis testing of inmates entering jails in Chicago (March–April 2000), Detroit (March–August 1999), and San Francisco (June 1999–December 2000) was tested for serologic markers of HBV and HCV infection. All sera had been previously tested for antibody to HIV (anti-HIV). A total of 1,292 serum samples (12% of new inmates) was tested. Antibody to HCV (anti-HCV) prevalence was 13%. Antibody to hepatitis B core antigen (anti-HBc) prevalence was 19%, and hepatitis B surface antigen (HBsAg) prevalence was 0.9%; 12% had serologic evidence of hepatitis B vaccination. Hispanics had high rates of chronic HBV infection (3.6% HBsAg positive) along with Asians (4.7% HBsAg positive). Among HIV-infected persons, 38% were anti-HCV positive and 8.2% were HBsAg positive. Anti-HBc positivity was associated with anti-HCV positivity (aOR = 4.58), anti-HIV positivity (aOR = 2.94), syphilis infection (aOR = 2.10), and previous incarceration (aOR = 1.78). Anti-HCV-positivity was associated with anti-HBc positivity (aOR = 4.44), anti-HIV-positivity (aOR = 2.51), and previous incarceration (aOR = 2.90). Jail entrants had high levels of HCV and HBV infection and HIV co-infection; HBV prevalence was comparable to previous prison studies, and HCV prevalence was lower than prison studies. Hispanics had an unexpectedly high rate of chronic hepatitis B infection and had the lowest rate of hepatitis B vaccination. The finding that hepatitis B vaccination coverage among jail entrants is lower than the general population, despite this population’s increased risk for infection, highlights the need to support vaccination in jail settings.
Keywords: Viral hepatitis, Co-infections, Correctional facilities
Background
Prisons and jails in the U.S. have had about two million persons within their custody annually since 2000.1 Most previous studies of prevalence of HBV and HCV infection among incarcerated persons have been conducted in prison settings rather than jail settings, mainly for logistical reasons.2 The prevalence of hepatitis C virus (HCV) infection among prison inmates is at least seven times greater than that of the general population, and the prevalence of hepatitis B virus (HBV) infection is at least two times greater.2,3 A key contributor to the increased prevalence is injection drug use, the main risk factor for HCV infection and an important risk factor for HBV infection. Although the proportion of inmates who have injected drugs is not regularly measured nationally, approximately 65% of inmates reported being regular drug users in a 2004 survey of federal and state prisons.4 Along with increased infection prevalence, national surveillance data provide evidence for an opportunity to prevent further HBV infection, since 40% of persons with reported acute hepatitis B had been incarcerated at some time before their infection.5
Although prevalence estimates from prison settings provide insight to what one might expect in jails, prison and jail populations are inherently different: jails hold a wide spectrum of persons, including persons awaiting arraignment, trial, conviction, sentencing, or serving short sentences in jail, while prisons typically hold convicted criminals. Most jails estimate an average length of stay of 10–20 days, while prisons typically hold inmates for at least 1 year.6 An estimated 12.6 million admissions and 12.6 million releases from local jails in 2002 reflects a high turnover rate (1,300% in jails compared to 40% in prisons).2 This high turnover rate illustrates the dynamic flow between jails and communities, suggesting that jails could be important venues for preventing and controlling viral hepatitis both in and outside of facilities.
The purpose of this study was to estimate the prevalence of HBV and HCV infection, co-infection with HIV, and serologic evidence of hepatitis B vaccination among incoming jail inmates in Chicago, Detroit, and San Francisco. Estimating the prevalence of infection among jail inmates provides insight into burden of disease in jails and opportunities for preventing infection and disease.
Materials and Methods
A parent HIV seroprevalence study conducted anti-HIV testing on stored sera collected for syphilis testing of inmates entering four jails. Three of the four jails were able to provide serum samples required for the present study; these jails were located in Chicago, Detroit, and San Francisco. Blood collection originally took place at intake usually within 24–48 h of arrival to the facility. A total of 12,576 persons entered into the three jail systems and during March–April 2000 in Chicago (n = 2,495), March–August 1999 in Detroit (n = 4,949), and June 1999–December 2000 in San Francisco (n = 5,132). Of the jail entrants, 11,170 (89%) had blood collected and HIV results and basic demographic information available.
The present study conducted HBV and HCV testing on a subset of stored blood on this population. Information on demographics, previous incarcerations, reasons for incarceration, and syphilis test results were previously abstracted from medical or intake records. This study involved no contact with inmates. The parent study was approved by human subject review committees at all participant sites and at CDC. This study was approved as a nonhuman-subjects addendum to the larger study because identifying information was removed prior to testing, and no information can be linked with identifiable human subjects.
Study population and sampling methods A randomized stratified sampling scheme was used to select approximately 500 sera per site to test for HCV and HBV serologic markers. The sampling strata included jail site, sex, age, race/ethnicity, and HIV infection status. Random sampling within strata was conducted, and a final selection criterion was the availability of at least 1.5 mL of sera. No sera from anti-HIV-positive persons from Detroit (n = 85) were available for testing. Due to small numbers in the jail population, anti-HIV-positive persons in San Francisco and Chicago, persons in lower and upper age categories (aged 15–19 years and >40 years), and Asians in San Francisco were oversampled to ensure a large enough sample size. Due to large numbers in the jail population, Blacks were undersampled.
Serologic testing In the parent study, a positive anti-HIV result was defined as having two reactive enzyme immunoassays and a positive Western blot. For the present study, serologic specimens were shipped from local storage sites and tested for HBV and HCV serologic markers at the CDC Hepatitis Reference Laboratory. All sera were tested for total antibodies to hepatitis B core antigen (anti-HBc). San Francisco sera were tested using the CORZYME anti-HBc assay (Abbott Laboratories, Abbott Park, IL, USA). All Chicago sera were tested using both the CORZYME anti-HBc assay and the VITROS anti-HBc EIA (Ortho-Clinical Diagnostic, Raritan, NJ, USA). The results from both assays were >99% concordant; the VITROS anti-HBc EIA results are presented in this report. Detroit specimens were tested using the VITROS anti-HBc EIA assay. Anti-HBc-negative specimens were tested for antibodies to hepatitis B surface antigen (anti-HBs) using the AUSAB EIA assay (Abbott Laboratories, Abbott Park, IL, USA); anti-HBc-positive specimens were tested for hepatitis B surface antigen (HBsAg) using the AUZYME Monoclonal assay (Abbott Laboratories, Abbott Park, IL, USA).Results of HBV serologic testing were used to define three serostatus categories: (1) past infection (anti-HBc-positive; this definition includes persons who were infected in the past and have either resolved infection or have chronic infection), (2) chronic infection (anti-HBc-positive and HBsAg-positive; this definition could also represent recent resolving infection, but since recent, non-chronic infection is transient, this serologic finding would most likely represent chronic infection), and (3) evidence of hepatitis B vaccination (anti-HBc-negative, HBsAg-negative, and anti-HBs-positive).All sera were tested for anti-HCV using the VITROS HCV 3.0 EIA assay (Ortho-Clinical Diagnostic, Raritan, NJ, USA). Specimens with a signal-to-cut-off ratio ≥ 3.8 were considered to be positive;7 confirmation testing using the HCV 3.0 RIBA assay (Chiron Corporation, Emeryville, CA, USA) was conducted for EIA-positive specimens with a signal-to-cutoff ratio of <3.8. HCV RNA testing to identify chronic HCV infection was not performed; among the general U.S. population, approximately 75–85% of anti-HCV-positive persons are HCV RNA-positive.8
Statistical Analysis Analyses were weighted by assigning a multiplier to each observation that equaled the number of observations in strata of the study population divided by the number that were sampled in that strata. Chi-square analyses were used to determine statistical differences in prevalence of viral hepatitis markers; p values < 0.05 were considered significant. The Cochran–Armitage test was used to measure trends in prevalence across ordered categories. Fisher’s exact test was used to calculate upper confidence bounds around zero frequencies. Adjusted odds ratios (aORs) and 95% confidence intervals were obtained from multiple logistic regression models that included fixed effects (variables found to be significant in univariate analysis) and significant interaction terms (combinations of age, sex, race, and jail site). Interaction terms were considered significant if they yielded significant likelihood ratio tests (p < 0.05) when comparing the fixed effect model with the fixed effect model plus the interaction term; interaction terms were sequentially added if they continued to yield significant likelihood ratio tests compared to the previous model. Confounding and effect modification were further explored using stratified analysis. All data management and analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Of the 11,170 jail entrants with HIV and basic demographic information available, 1,292 (12%) were tested for HCV and HBV serologic markers. The distributions of demographic characteristics of the selected sample are shown in Table 1; however, these are unweighted and not meant to be generalized. HIV prevalence in the sample ranged from 0% (HIV-positive specimens from Detroit were not available for testing) to 21% reflecting oversampling of HIV-positive persons; HIV-prevalence in the parent study ranged from 1.7% to 2.6% (unpublished data).
Table 1.
Distribution of characteristics among sampled group by site (unweighted proportions)
| Characteristic | Chicago % (n = 447) | Detroit % (n = 340) | San Francisco % (n = 505) | Total % (n = 1,292) |
|---|---|---|---|---|
| Female sex | 51 | 39 | 43 | 43 |
| Age | ||||
| 15–19 | 15 | 15 | 22 | 18 |
| 20–29 | 26 | 29 | 40 | 33 |
| 30–39 | 33 | 28 | 52 | 31 |
| 40 + | 25 | 27 | 6 | 18 |
| Race | ||||
| White | 24 | 33 | 23 | 26 |
| Black | 53 | 55 | 34 | 46 |
| Hispanic | 21 | 9 | 21 | 18 |
| Asian | 2 | 3 | 23 | 11 |
| Previous Incarcerationa | 63 | 81 | 65 | 69 |
| Drug offenceb | 47 | 23 | 40 | 37 |
| HIV-positivec | 13 | NA | 21 | 13 |
| Syphilis-positived | 5.2 | 5.6 | 1.0 | 3.6 |
a1 missing value
b14 missing values
cHIV prevalence among all inmates (n = 11,170) was 2.6% in Chicago, 1.7% in Detroit and 2.2% in SF.
dSyphilis prevalence among all inmates (n = 11,170) was 5.7% in Chicago, 4.5% in Detroit, and 0.6% in SF.
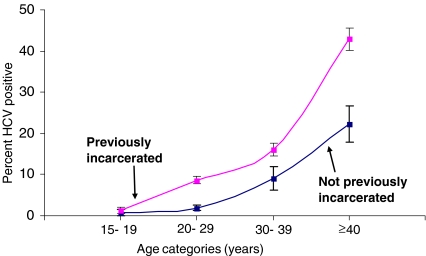
HCV Infection The overall weighted anti-HCV prevalence was 13% (Table 2). In univariate analysis, anti-HCV prevalence was higher among inmates in Chicago and Detroit than San Francisco. Other factors significantly associated with HCV infection in univariate analysis included female sex, increasing age, non-Hispanic white race/ethnicity, HIV infection, past HBV infection, and previous incarceration (Table 2). Persons who were previously incarcerated had consistently higher HCV prevalence than those not previously incarcerated across all age groups (Figure 1). In multivariate analysis, an increased risk of HCV infection was found among persons with past HBV infection (aOR = 4.44), anti-HIV-positive persons (aOR = 2.51), and persons who had been previously incarcerated (aOR = 2.90; Table 3). This model also included significant interaction terms between sex, age, race, and jail site (data not shown).
Table 2.
Univariate analysis: prevalence of viral hepatitis markers among inmates by jail site and other characteristics
| Characteristic | Weighted percent (95% confidence intervals)a | |||
|---|---|---|---|---|
| HCV infection (n = 11,168) | Past HBV infection (n = 11,166) | Chronic HBV infection (n = 11,165) | Serologic evidence of vaccinationb (n = 11,166) | |
| Overall | 13 (12–14) | 19 (18–19) | 0.9 (0.8–1.1) | 12 (12–13) |
| Chicago | 14 (13–16) | 19 (17–20) | 0.6 (0.3–0.9) | 9 (8–10) |
| Detroit | 15 (14–16) | 21 (20–22) | 0.2 (0.1–0.3) | 10 (9–11) |
| San Francisco | 10 (9–11) | 16 (15–17) | 2.0 (1.6–2.4) | 17 (16–19) |
| Female | 16 (15–18) | 26 (24–27) | 0.9 (0.5–1.2) | 13 (11–14) |
| Male | 12 (11–13) | 16 (15–17) | 1.0 (0.8–1.2) | 12 (12–13) |
| Age (years) | ||||
| 15–19 | 1 (1–2)c | 5 (4–6)c | 0.2 (0–0.4) | 35 (33–38)c |
| 20–29 | 7 (6–8) | 13 (12–14) | 1.1 (0.8–1.3) | 9 (8–10) |
| 30–39 | 15 (14–16) | 27 (25–29) | 1.3 (0.9–1.7) | 10 (9–11) |
| 40 + | 39 (36–41) | 33 (31–35) | 0.6 (0.2–0.9) | 9 (7–10) |
| Race | ||||
| White | 24 (22–25) | 14 (12–15) | 1.0 (0.6–1.4) | 12 (10–13) |
| Black | 11 (10–12) | 21 (20–22) | 0.2 (0.1–0.3) | 13 (12–13) |
| Hispanic | 8 (7–10) | 11 (10–13) | 3.6 (2.7–4.6) | 10 (9–12) |
| Asian | 3 (1–4) | 31 (26–35) | 4.7 (2.5–6.8) | 25 (21–30) |
| Previously incarcerated | 15 (14–16) | 21 (20–22) | 0.7 (0.6–0.9) | 11 (11–12) |
| Not previously incarcerated | 5 (5–6) | 10 (9–11) | 1.6 (1.1–2.0) | 16 (15–17) |
| Drug related offence | 14 (12–15) | 20 (18–21) | 1.2 (0.9–1.5) | 12 (11–13) |
| Not drug related offence | 13 (12–13) | 18 (17–19) | 0.8 (0.6–1.0 | 13 (12–13) |
| HIV-positive | 38 (31–42) | 50 (42–57) | 8.2 (4.0–12.4) | 13 (8–18) |
| HIV-negative | 13 (12–13) | 18 (17–19) | 0.8 (0.7–1.0) | 12 (12–13) |
| Syphilis-positive | 27 (22–32) | 48 (43–54) | 2.0 (0.4–3.7) | 3 (1–5) |
| Syphilis-negative | 13 (12–13) | 16 (17–18) | 0.9 (0.7–1.1) | 13 (12–14) |
| HBV core antibody-positive | 33 (31–35) | NA | NA | NA |
| HBV core antibody-negative | 8 (8–9) | |||
| HCV-positive | NA | 48 (46–51) | 1.3 (0.6–1.9) | 11 (9–13) |
| HCV –negative | 14 (14–15) | 0.9 (0.7–1.1) | 13 (12–13) | |
aResults in bold font highlight nonoverlapping 95% CIs. Chicago, the youngest age group and white race were used as references for comparing polytomous variables.
bPresence of HBV surface antibodies and absence of HBV core antibodies
cCochran–Armitage trend test, p < .05
Figure 1.
Age-specific HCV prevalence and 95% confidence limits comparing previously and not previously incarcerated persons from the present jail study population.
Table 3.
Adjusted odds ratios and 95% confidence intervals for HBV and HCV history, chronic HBV, and vaccination statusa
| Variableb | Adjusted odds ratios (95% confidence intervals) | |||
|---|---|---|---|---|
| HCV infectionc | Past HBV infectiond | Chronic HBV infectione | Serologic evidence of vaccinationf | |
| Site (Chicago) | ||||
| Detroit | 0.95 (0.79–1.14) | 0.93 (0.64–1.36) | 0.84 (0.34–2.02) | 1.05 (0.87–1.26) |
| San Francisco | 1.36 (1.11–1.66) | 0.75 (0.40–1.39) | 2.63 (1.34–5.16) | 2.00 (1.70–2.38) |
| Sex—female | 3.99 (2.33–6.83) | 4.20(2.60–6.79) | 0.47 (0.02–8.84) | 1.02 (0.88–1.18) |
| Age (15–19 years) | ||||
| 20–29 | 3.06 (1.69- 5.55) | 1.72 (1.16–2.54) | 5.42 (1.45–20.53) | 0.18 (0.15–0.21) |
| 30–39 | 2.85 (1.42- 5.72) | 2.70 (1.51–4.85) | 10.35 (2.62–41.07) | 0.25 (0.21–0.29) |
| ≥40 | 8.69 (3.69–20.45) | 2.32 (1.10–4.88) | 7.20 (1.26–41.27) | 0.20 (0.16–0.26) |
| Race/ethnicity (White) | ||||
| Black | 0.07 (0.05–0.10) | 2.63 (2.22–3.11) | 0.26 (0.12–0.59) | 1.06 (0.90–1.24) |
| Hispanic | 0.04 (0.02–0.09) | 1.72 (1.33–2.23) | 4.73 (2.30–9.70) | 0.56 (0.45–0.70) |
| Asian | 0.00 (0.00–0.01) | 8.67 (6.27–11.99) | 7.04 (3.01–16.48) | 1.49 (1.11–2.00) |
| Previous incarceration | 2.90 (2.36–3.56) | 1.76 (1.51–2.05) | 0.62 (0.40–0.96) | 0.94 (0.81–1.08) |
| Anti-HIV-positive | 2.51 (1.71–3.68) | 2.88 (2.02–4.10) | 6.20 (3.18–12.06) | 1.23 (0.77–1.97) |
| Syphilis-positive | 1.27 (0.93–1.73) | 2.13 (1.66–2.74) | 6.31 (2.33–17.11) | 0.24 (0.12–0.47) |
| Anti-HCV-positive | NA | 4.51 (3.90–5.21) | 0.97 (0.53–1.78) | 0.91 (0.73–1.15) |
| Anti-HBc-positive | 4.44 (3.85–5.14) | NA | NA | NA |
aVariables included in the multiple logistic regression are listed in the table unless specified by NA (not applicable).
bReference group is the category not listed for dichotomous variables and is the category in parentheses for polytomous variables.
cJail and age, sex and age, and race and age interaction terms were included in this model.
dRace and sex, jail and age, and jail and sex interaction terms were included in this model.
eSex and age, and sex and race interaction terms were included in this model.
fAbsence of anti-HBc and presence of anti-HBs.
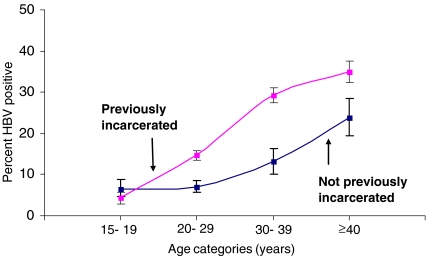
Past HBV Infection Overall, 19% of inmates had serologic evidence of past HBV infection (i.e., anti-HBc-positive) (Table 2). In univariate analysis, anti-HBc prevalence was higher in Chicago (19%) and Detroit (21%) than San Francisco (16%). Factors associated with increased anti-HBc prevalence in univariate analysis included female sex, older age, HIV infection, syphilis infection, HCV infection, and previous incarceration. Persons who were previously incarcerated had consistently higher HBV prevalence then those not previously incarcerated across all age groups (Figure 2). Multivariate analysis showed an increased anti-HBc prevalence among persons who were anti-HCV-positive (aOR = 4.58), anti-HIV-positive (aOR = 2.94), syphilis-positive (aOR = 2.10), and previously incarcerated (aOR = 1.78). This model also included significant interaction terms between sex, age, race, and jail site (data not shown).
Figure 2.
Age-specific HBV prevalence and 95% confidence limits comparing previously and not previously incarcerated persons from the present jail study population.
Chronic HBV Infection The overall HBsAg prevalence was 0.9%; prevalence was higher in San Francisco (2.0%) than in Chicago (0.6%) and Detroit (0.2%). In univariate analysis, HBsAg prevalence was higher among persons aged 20–29 years (1.1%) and 30–39 years (1.3%) than those aged 15–19 years (0.2%). In addition, HBsAg prevalence was higher among Asians (4.7%) and Hispanics (3.6%) than among whites (1.0%), and higher among anti-HIV-positive persons (8.2%) than anti-HIV-negative persons (0.8%).Multivariate analysis indicated increased likelihood of chronic HBV infection among inmates: in San Francisco (compared with Chicago), aged >20 years (compared to 15–19 years old), with HIV infection, with reactive VDRL test results, and who are Hispanic or Asian (Table 3). This model also included significant interaction terms between sex, age, and race (data not shown).
HIV Co-infection Anti-HIV prevalence in the Detroit sampled population was 0% because no sera were available from persons with HIV infection in Detroit. In Chicago and San Francisco, 0.6% of inmates were positive for both HIV and HCV; 0.7% were positive for both HIV and past HBV infection. Of persons with HIV in Chicago and San Francisco, 8.2% had chronic HBV infection and 38% were anti-HCV positive.
Syphilis Co-infection Overall, 0.8% of inmates were both syphilis-positive and anti-HCV positive; 1.4% were syphilis-positive and had past HBV infection; 0.1% were syphilis-positive and HIV-positive.
Hepatitis B Vaccination Overall, 12% of inmates had serologic evidence of hepatitis B vaccination (Table 2). Inmates in San Francisco had a significantly higher prevalence of vaccination (17%) than inmates in Chicago (9%) and Detroit (10%). Overall, vaccination prevalence was higher among younger inmates (35% among those aged 15–19 years compared with ≤10% in the older age groups), Asians (25%, compared with <15% among other races/ethnicities), those not previously incarcerated (16%, compared with 11% among those previously incarcerated), and syphilis-negative persons (13%, versus 3% among syphilis positive; Table 2). Young age (i.e., 15–19 years) was the only consistent predictor of vaccination across all jail sites (data not shown). Multivariate analysis detected no significant interactions, and all significant univariate findings were also significant risk factors after controlling for confounding (Table 3).
Discussion
High prevalences of HCV infection (13%), past HBV infection (19%), and chronic HBV infection (0.9%) were estimated in the three jail populations and makes a case for the need for viral hepatitis prevention activities in jails. In general, viral hepatitis prevention objectives and activities include: (1) prevent infection through vaccination and risk reduction education; (2) prevent transmission through identifying cases of acute viral hepatitis and prophylaxing (HBV) or counseling (HCV) cases and contacts; and (3) reduce liver disease through screening, counseling, medical evaluation, and treatment, if indicated. Other measures for preventing viral hepatitis transmission, such as needle exchange or condom distribution programs, have been implemented in correctional settings internationally but are not widely implemented in the U.S.; no U.S. correctional facilities have needle exchange programs, and two prison and five local jail systems make condoms available to inmates.9
This study provides compelling data on the opportunity to prevent HCV and HBV infection among younger inmates. A dramatic increase in the prevalence of HCV infection (from 1% among 15- to 19-year olds to 39% among ≥40-year olds) and HBV infection (from 5% among 15–19 year olds to 33% among ≥40 year olds) with age was observed. While data from the 1999–2002 NHANES study of the non-institutionalized U.S. population also showed an increase, HCV prevalence at all ages was considerably smaller (from 0.01% among 15- to 19-year olds to 2.4% among ≥40 year olds, CDC, unpublished data).10 Furthermore, >50% of inmates aged 15–29 years in this study had previous incarcerations, indicating an opportunity for jails to provide prevention interventions including education, vaccination, testing, and counseling before younger inmates become infected with HBV and HCV.
The most important factors associated with chronic HBV infection in this study were race/ethnicity and HIV infection. Higher rates of chronic HBV infection were expected among Asians (4.7%), among whom a high prevalence has been well documented.11,12 However, high rates among Hispanics (3.6%) was an unexpected finding since this has not been reported previously. This finding coupled with Hispanics having the lowest rate of hepatitis B immunization puts an even greater importance on ensuring awareness of the benefits of vaccination and access to vaccine.
The highest prevalence of chronic HBV infection was observed among persons with HIV (8.2%). Identifying chronic or acute HBV infection is important for preventing HBV transmission, since HBV is highly contagious, and multiple opportunities for transmission exist, such as sharing of inmate quarters, sexual activity, injection-drug use, and tattooing. Transmission opportunities are further increased because the majority of persons with chronic infection have no symptoms and can unknowingly transmit the virus.13 Several studies have documented transmission among inmates in correctional facilities.14–16 An investigation of HBV transmission in a state correctional facility found 22 inmates with acute or chronic hepatitis B, only one of whom was aware of his infection status, thus delaying treatment and activities aimed at preventing transmission.16 The potential for efficient undetected HBV transmission is the basis for CDC recommendations that correctional facilities vaccinate all inmates against hepatitis B and consider routine testing of long-term inmates for chronic HBV infection.2,17
To put this into context with other estimates, HCV infection prevalence from this jail population was six times higher among jail inmates than the general civil population (1.8%); was lower compared to two other jail studies in Texas and Maryland (27% and 31%, respectively);18,19 and was lower compared to prison-based studies (16–41%).2,14,19–21 HCV prevalence reflects the proportion of inmates who have injected drugs, data unavailable in this study. Knowing this would most likely explain the difference in HCV prevalence across studies. This study shows that jail entrants have a high prevalence of HCV infection compared to the general population and, together with the two previous jail studies, indicates that prevalence is comparable to that among prison populations.
The prevalence of past HBV infection in this study was almost four times the prevalence of the general civil population (5%);22 was similar to the jail study conducted in Maryland (17%); and was within the range of prison-based estimates (14–30%).14,19,23 The prevalence of chronic HBV infection in this study was twice that of the general population (0.4–0.5%);24 was substantially lower than the study of jail inmates in Maryland (11.4%); and was within the range of prison estimates (0.9%–2.2%).19,23,25
Prevalent co-infections (38% HCV-positive and 50% HBV-positive among persons with HIV) suggest that correctional medical providers should be prepared for complex case management. Treating HBV and HCV infections among persons with HIV has become an increasing priority in HIV care. As the use of highly active anti-retroviral therapy makes many opportunistic infections less common and results in longer survival, liver disease progression resulting from HBV or HCV co-infection in HIV-infected patients becomes a more salient medical concern.26
Given the high admission and release rates, prevalence of infections in jails is likely to reflect high incidence rates within segments of the community from which these individuals came. A study of reincarcerated women from 1996 through 1997 showed incidence rates of 18.2 and 12.2 per 100 person years for HCV and HBV, respectively, compared to the 1996 national rates of 1.4 and 4.0 per 100,000 population estimated using the National Notifiable Disease Surveillance System.27 The majority of the participants’ study time was spent in the community, leading the authors to conclude that new infections occurred in the community.
Only 12% of the study inmates had serologic evidence of hepatitis B immunization, a low rate compared with 25% self-reported hepatitis B vaccination coverage among the general U.S. population in 2000–2002.28 Asians had the highest immunization coverage in all sites when compared to other race/ethnic groups, which probably reflects awareness of HBV infection risk among this population. Hispanics, who had equal risk to Asians for chronic hepatitis B in this study, had the lowest vaccination coverage, perhaps indicating a gap in awareness or access to vaccination.
The oldest cohorts of children vaccinated under universal childhood vaccination recommendations are turning 17 years of age in 2008. Therefore, current jail populations are likely to be similar to those included in this 1999–2000 study in terms of vaccine-induced immunity resulting from early childhood vaccinations. However, in 1995, CDC and ACIP recommended vaccinating adolescents against hepatitis B,29 which has led to greater vaccine-induced immunity among persons in certain older age cohorts. This is consistent with the finding that persons aged 15–19 years had the highest vaccination coverage in all sites.
Serologic evidence of hepatitis B vaccination varied across facilities (17% in San Francisco versus ≤10% in other sites) and likely reflects facilities’ different hepatitis B vaccination polices, and those of the juvenile justice systems inmates may have encountered prior to admission. In 2005, all three of the study jails participated in a National Institutes of Justice/CDC survey where they reported practices and policies regarding hepatitis B vaccination. Of the three sites, San Francisco was the only one that reported having a hepatitis B vaccination policy in 2005 (CDC, unpublished data); San Francisco jail has provided ~1,500 doses of hepatitis B vaccine during June 2004–July 2007, but their ability to vaccinate inmates has been declining and is anticipated to decline further due to staffing shortages. (Goldenson J., personal communication, April 29, 2008). Recent communication with the Detroit jail indicates that they do not have funds to support vaccination and only provide hepatitis B vaccine to persons who have started and wish to complete the series (Griffin V., personal communication, May 2, 2008). The Chicago jail reported that their health care provider gave 2,460 doses to 1,348 persons during June 2003–September 2007 (Wagaw F., personal communication, May 15, 2008). Lack of funding is a common theme for these jails and a major barrier to implementing hepatitis B vaccination programs in correctional facilities in general.30
Including jails as venues for implementing hepatitis prevention activities should be a priority component to a comprehensive strategy to prevent and control viral hepatitis. National recommendations exist for prevention and control of HBV and HCV infection in correctional settings.2 However, these recommendations are more likely to be implemented in longer-term facilities, since jails face multiple obstacles including limited health care staff, medical evaluation facilities and, most importantly, short duration of stays which often impedes initiation of the hepatitis B vaccination series and treatment.2 Recommendations to administer hepatitis B vaccination regardless of an inmate’s projected length of stay have been published to encourage vaccination even if it is not clear if the series can be completed.2,17 Studies have shown that achieving high vaccination coverage is both feasible and cost-effective and treating appropriately screened individuals has been considered a reasonable expenditure for correctional facilities.15,31–33 A recent Federal initiative to provide adult hepatitis B vaccine to a variety of health care venues will monitor implementation in correctional facilities and other venues to evidence the need and interest in vaccinating at-risk adults at these points of contact with medical care.
This study has a few limitations. First, 11% of the study population was not included due to missing stratification variables; we do not believe this bias was systematic, and there is no reason this should affect prevalence estimates. Second, we could have underestimated HBV and HCV infection prevalence, since we were not able to include HIV-infected inmates from Detroit, and because our requirement for 1.5 ml of sera might have systematically excluded some persons who inject drugs and may have less successful blood draws. Finally, study periods differed slightly by site, and these estimates date back to 1999–2000. However, few prevalence studies have been conducted in jail settings, and these data contribute substantially to the knowledge of hepatitis prevalence in jails.
This study found high levels of HCV and HBV infection and HIV co-infection in the three jails. Active and untreated HCV or HBV infection among jail inmates can lead to transmission in both civilian and incarcerated populations. Over 80% of the jail inmates had been previously incarcerated illustrating the close tie between civilian and incarcerated populations. Jails not only represent important venues for addressing current infection and disease but for also preventing viral hepatitis through vaccination. The finding that hepatitis B immunization coverage among jail entrants is lower than the general population despite this population’s increased risk for infection, and the continuing inability of jail programs to procure sufficient funds and staff to implement successful vaccination programs, highlights the need to support vaccination in jail settings.
Acknowledgements
The authors would like to thank Stephanie Neitzel for her thoughtful review of this manuscript and Annemarie Wasley for calculating the age-specific HCV prevalence using NHANES data of Division of Viral Hepatitis, CDC, and Joe Goldenson, Director/Medical Director Jail Health Services San Francisco Department of Public Health, and Fikirte Wagaw, Director of Community Based Services, Chicago Department of Public Health for information on the current vaccination policy from the jail study sites.
Financial disclosures None. CDC funds were used to conduct this study.
Conflict of Interest None of the authors has any commercial interest or other association that might pose a possible conflict of interest.
References
- 1.Bureau of Justice Statistics. Prison and jail inmates at midyear 2005. US Department of Justice. 2006.
- 2.Weinbaum C, Lyerla R, Margolis HS. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–36. [PubMed]
- 3.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releases from US correctional facilities, 1997. Am J Public Health. 2002;92:1789–1794. [DOI] [PMC free article] [PubMed]
- 4.Bureau of Justice Statistics. Drug Use and Dependence, State and Federal Prisoners, 2004. US Department of Justice. 2006.
- 5.Williams I, Boaz K, Openo K, et al. Missed Opportunities for Hepatitis B Vaccination in Correctional Settings, Sexually Transmitted Disease (STD) Clinics, and Drug Treatment Programs. Paper presented at: Annual Meeting of the Infectious Disease Society of America, San Francisco, CA 2005.
- 6.National Intitute of Corrections. Jail crowding: understanding jail population dynamics. US Department of Justice. 2002.
- 7.CDC. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Morb Mortal Wkly Rep. 2003;52:1–16. [PubMed]
- 8.CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed]
- 9.Bick J. HIV and Viral Hepatitis in Corrections. In: Greifinger R, ed. Public Health Behind Bars From Prisons to Communities. Dobbs Ferry: Springer; 2007:103–126.
- 10.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. [DOI] [PubMed]
- 11.CDC. Screening for chronic hepatitis B among Asian/Pacific Islander populations—New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:505–509. [PubMed]
- 12.Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158–S168. [DOI] [PubMed]
- 13.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. [DOI] [PubMed]
- 14.Macalino GE, Vlahov D, Sanford-Colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. [DOI] [PMC free article] [PubMed]
- 15.CDC. Hepatitis B vaccination of inmates in correctional facilities–Texas, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53:681–683. [PubMed]
- 16.Khan AJ, Simard EP, Bower WA, et al. Ongoing transmission of hepatitis B virus infection among inmates at a state correctional facility. Am J Public Health. 2005;95:1793–1799. [DOI] [PMC free article] [PubMed]
- 17.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. [PubMed]
- 18.Baillargeon J, Wu H, Kelley MJ, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. [DOI] [PubMed]
- 19.Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence Of HIV, Syphilis, Hepatitis B, and Hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25–37. [DOI] [PMC free article] [PubMed]
- 20.Ruiz JD, Molitor F, Plagenhoef JA. Trends in hepatitis C and HIV infection among inmates entering prisons in California, 1994 versus 1999. AIDS. 2002;16:2236–2238. [DOI] [PubMed]
- 21.Fox RK, Currie SL, Evans J, et al. Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005;41:177–186. [DOI] [PubMed]
- 22.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. [DOI] [PMC free article] [PubMed]
- 23.Tucker RM, Gaffey MJ, Fisch MJ, Kaiser DL, Guerrant RL, Normansell DE. Seroepidemiology of hepatitis D (delta agent) and hepatitis B among Virginia State prisoners. Clin Ther. 1987;9:622–628. [PubMed]
- 24.McQuillan GM, Townsend TR, Fields HA, Carroll M, Leahy M, Polk BF. Seroepidemiology of hepatitis B virus infection in the United States. 1976 to 1980. Am J Med. 1989;87:5S–10S. [DOI] [PubMed]
- 25.Ruiz JD, Molitor F, Sun RK, et al. Prevalence and correlates of hepatitis C virus infection among inmates entering the California correctional system. West J Med. 1999;170:156–160. [PMC free article] [PubMed]
- 26.Soriano V, Barreiro P, Nunez M. Management of chronic hepatitis B and C in HIV-coinfected patients. J Antimicrob Chemother. 2006;57:815–818. [DOI] [PubMed]
- 27.Macalino GE, Vlahov D, Dickinson BP, Schwartzapfel B, Rich JD. Community incidence of hepatitis B and C among reincarcerated women. Clin Infect Dis. 2005;41:998–1002. [DOI] [PubMed]
- 28.Billah K, Weinbaum C, Culver D, Fiore A, Finelli L, Stokley S. Trends in self-reported hepatitis B vaccine coverage rates among adults in the United States, 2000–2002. Paper presented at: National Immunization Conference, Atlanta, Georgia 2005.
- 29.CDC. Update: recommendations to prevent hepatitis B virus transmission—United States. MMWR. 1999;48:33–34. [PubMed]
- 30.Charuvastra A, Stein J, Schwartzapfel B, et al. Hepatitis B vaccination practices in state and federal prisons. Public Health Rep. 2001;116:203–209. [DOI] [PMC free article] [PubMed]
- 31.Spaulding A, Greene C, Davidson K, Schneidermann M, Rich J. Hepatitis C in state correctional facilities. Prev Med. 1999;28:92–100. [DOI] [PubMed]
- 32.Pisu M, Meltzer M, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. In: Margolis HS, Alter MJ, Liang TJ, Dienstag JL, eds. Viral Hepatitis and Liver Disease. London: International Medical Press; 2002:236–238.
- 33.Herlihy EJ, Klein SJ, Newcomb ML, Blog DS, Birkhead GS. Expansion of adult hepatitis A and B vaccination in STD clinics and other settings in New York State. Public Health Rep. 2007;122(Suppl 2):36–41. [DOI] [PMC free article] [PubMed]