Abstract
High hepatitis C virus (HCV) prevalence has been documented among many injecting drug user (IDU) populations worldwide; however, there is limited published data on trends in incidence of infection in these epidemics over time. To address this, we used a novel method of analyzing data collected via repeat, cross-sectional sero-surveys by injection initiation cohorts to investigate trends in HCV seropositivity among a population of needle and syringe program (NSP) attendees in Australia between 1995 and 2004, and thereby infer annual incidence trends. Injection initiation cohorts were defined by their time of entry into the IDU population. We also investigated the associations between HCV antibody seroprevalence and risk factor data, and trends in risk factor data over the decade. Approximately 20,000 NSP attendees participated in the study over the 10-year period. Within each injection initiation cohort, we found an increase in HCV prevalence over time, with prevalence appearing to reach saturation around 90%. There was little indication that the slopes of increase had changed with more recent initiation cohorts. While duration of injecting was most strongly associated with HCV seropositivity in this study, we also found that self-reported history of needle and syringe sharing and imprisonment were independently associated with higher HCV prevalence regardless of duration of injecting, with the exception of IDUs who have 15 or more years injecting experience. In this group, recent risk behavior had no relationship to prevalence. In summary, our findings suggest a persistent HCV epidemic despite significant harm reduction efforts in Australia since the mid-1980s, with HIV incidence effectively constant in successive initiation cohorts.
Keywords: Hepatitis C virus, Epidemiology, Injecting drug use, Injecting cohorts
Introduction
Two decades have passed since needle and syringe programs (NSPs) were introduced in Australia. During this period, widespread methadone maintenance treatment (MMT) services have also formed an integral part of Australia’s harm reduction strategy, and, more recently, a medically supervised injecting facility was established at a single site in Sydney. NSPs in particular have been shown to be effective in reducing blood borne viral infections, as well as being low cost and highly cost effective.1–3 This has led to Australia’s success in maintaining low human immunodeficiency virus (HIV) prevalence among injecting drug users (IDUs) since the 1980s.4,5 In contrast, hepatitis C virus (HCV) prevalence and incidence in various clinic- and community-based IDU populations throughout Australia, mostly in major cities and regional urban centers, have remained high.6–10
Routine surveillance data have the potential to increase our understanding of HCV transmission trends and consequently inform an improved public health response in the context of a well-established epidemic. To date, there has been limited published data internationally on trends in HCV infection and related risk factors in IDU populations over significant time periods, with studies examining trends in prevalent HCV infection being limited to IDUs recruited via drug treatment facilities11 or comparison of different data sources over time.12 While studies published in HIV13–15 and cancer epidemiology16 have used various statistical methods to estimate trends in incident infection from prevalence data when cohort studies were not feasible, we are not aware of similar methods being applied to routinely collected HCV prevalence data.
In Australia, a national annual sero- and risk factor survey of HIV and HCV infections among IDUs has been conducted via NSPs since 1995, involving approximately 20,000 IDUs throughout the first decade. In this study, our primary objective was to apply a novel method of analyzing this data by injection initiation cohorts, defined by the time of entry into the study population, as a surrogate for incidence estimation and a basis for disentangling the relationship between duration of injecting and time period effects on prevalence. We also describe trends in HCV infection and related risk factors in this unique, large sample of community-based IDUs.
Methods
Subject Recruitment
The Australian Needle and Syringe Program (NSP) Survey is a cross-sectional study that has been conducted annually over a 1-week period since 1995. The number of participating NSP sites increased gradually from 21 in 1995 to more than 40 sites in 2002–2004, with representation from all Australian states and territories. Sites were initially selected based on needle and syringe distribution, attendance levels, geographic coverage, and willingness to participate. Twenty four sites participated in eight or more of the ten survey years, with the vast majority being located in major cities or regional urban centers.
The survey methods have been described elsewhere in detail.5,7 In brief, all IDUs who attended participating NSP sites during the designated survey week were invited to participate by NSP workers. Participation was anonymous and voluntary. There was no reimbursement for participation. Annual participation rates ranged from 41% to 60% between 1995 and 2004.4,17 Participants were asked to complete a brief, self-administered questionnaire on demographic characteristics and injecting and sexual risk behaviors (www.web.med.unsw.edu.au/nchecr/Publications, National Data Report) and to provide a capillary blood sample for antibody HIV and HCV testing. Sharing of needles and syringes was defined as reuse of a needle and syringe after someone else had used it.
HCV Antibody Testing
Capillary blood was obtained by finger prick using single-use, disposable lancets and cotton-fiber blotting paper. Specimens were kept at room temperature at the survey sites, then couriered to a central collection point before they were forwarded to the laboratory. HIV antibody was detected using Genetic Systems HIV-1 ELISA tests. Repeatedly reactive specimens were subjected to Western blot confirmatory testing. A modified, third generation enzyme immunoassay (Abbott hepatitis C 3.0, Chicago, IL, USA) was used to test for HCV antibody. A modified cutoff value for optical density was calculated to capture greater than 95% of the sero-negative population. Specimens were considered positive for HCV antibody if the optical density to cutoff ratio was greater than or equal to one on initial and subsequent testing.
Statistical Analysis
We restricted all analyses reported in this paper to data collected at a sub-set of 24 NSP sites that participated in at least eight of the ten survey years to minimize sampling inconsistency over time. For the logistic regression analysis only, we used a de-duplicated version of the dataset so that individuals who participated in multiple survey years would not be overrepresented when data from all survey years were combined. De-duplication was performed using the SAS version 9.1 computer package (SAS Institute Inc., Cary, NC, USA) and involved identification and selection of the first occasion of participation attributed to each individual during the 10-year period, using a combination of the following variables as a unique identifier: birth month and year; first name and surname codes (first two letters of each); and sex and Indigenous status.
Data were analyzed using the STATA version 8.2 computer package (StataCorp LP, College Station, TX, USA). p values less than 0.05 were considered statistically significant. All tests for trend or homogeneity were performed using logistic regression analysis, with missing data excluded. Pearson chi-square tests were used to assess any difference in proportions between groups. Participants with missing data for variables presented graphically were excluded from the relevant analyses.
Associations between HCV antibody prevalence and covariates were explored using univariate and multivariate logistic regression analysis among participants with anti-HCV serology in the de-duplicated dataset. Missing data for covariates were included in the analysis. Covariates were entered into the multivariate model if they had a p value less than 0.10 in the univariate analysis. The forward stepwise regression method was used using the likelihood ratio statistic to assess contribution to the model.
For all other analyses, duplicates were included in the dataset. To investigate temporal trends in HCV antibody prevalence by the time of entry into the study population, an analysis of injection initiation cohorts was performed. We also investigated temporal trends in HCV prevalence across injection duration groups. In this study, injection initiation cohorts are based on the same concept as birth cohorts in age–period–cohort analyses, commonly used in cancer epidemiology,16,18 with the year of first injection effectively replacing year of birth. Similarly, injection duration groups in our analyses are similar to age groups in age–period–cohort analyses. First injection year and duration of injection experience were calculated from the continuous variables age at first injection and age at survey participation. Participants were grouped into 16 2-year injection initiation cohorts on the basis of their first year of injecting, ranging from 1973–1974 to 2003–2004. Participants with a calculated year of first injection prior to 1973 were excluded because of the small numbers in these cohorts. The term “older cohorts” will be used to refer to cohorts that commenced injecting prior to other cohorts.
Temporal trends in recent sharing of needles and syringes and recent imprisonment in different duration of injecting groups were also explored and presented graphically. Duration of injection was categorized using a log2 scale. Those with 31 or more years injecting duration were combined with the 15 to 30 years injecting duration group to avoid comparison of small numbers of participants.
Ethics
Verbal consent was obtained from all participants. Ethic approval for the study was received by all relevant institutional review boards.
Results
Between 1995 and 2004, 21,839 surveys were collected at participating NSP sites, including multiple occasions of survey participation attributed to the same individual across different survey years. Of these, 15,232 were recruited at NSPs that participated in more than eight of the ten survey years.
Following de-duplication of the dataset, a total of 12,754 participants who had available antibody HCV results were included in the logistic regression analysis. Of these, 65% were male and approximately half reported ≥8 years duration of injecting, daily or more frequent injection in the month prior to the survey, opiates as the last drug injected, and prior hepatitis B virus (HBV) vaccination. Fifteen percent reported imprisonment in the last year and sharing a needle and syringe at least once in the month prior to the survey. Seven percent of participants identified as Indigenous Australians, and 8% reported sex work in the month prior to survey participation.
There was a significant difference in the distribution of all covariates by HCV antibody status, with the exception of HBV vaccination. After adjustment for covariates, HCV antibody seropositivity remained associated with a longer duration of injecting, older age, participation in the state of New South Wales, opiates as the last drug injected, imprisonment in the last year, female sex, daily or more frequent injection, sharing needles and syringes in the last month, sex work, and survey participation in 2000–2004 (Table 1). Indigenous status did not remain significant in the final multivariate model. The same covariates were significantly associated with HCV seropositivity when the multivariate analysis was performed on the unrestricted primary dataset (data not shown). Of all the covariates included in the model, duration of injecting, followed by age, were most strongly associated with antibody HCV prevalence.
Table 1.
Sample characteristics and associations between HCV antibody seropositivity and covariates, The Australian Needle and Syringe Program Survey, 1995–2004
| Covariates | Total | Hepatitis C+ | OR | p overalla | AOR | p value | p overalla |
|---|---|---|---|---|---|---|---|
| N | N (%) | (95% CI) | (95% CI) | ||||
| Age group | N = 12,754 | N = 6,726 | |||||
| <25 years | 3,882 | 1,143 (29) | 1.00 | 0.000 | 1.00 | 0.001 | |
| 25–29 years | 2,822 | 1,266 (45) | 1.9 (1.8, 2.2) | 1.1 (1.0, 1.3) | 0.046 | ||
| 30–34 years | 2,368 | 1,463 (62) | 3.9 (3.5, 4.3) | 1.9 (1.7, 2.2) | 0.000 | ||
| 35+ years | 3,667 | 2,850 (78) | 8.4 (7.5, 9.3) | 4.0 (3.5, 4.6) | 0.000 | ||
| Missing | 15 | 4 (27) | 0.9 (0.3, 2.7) | 0.7 (0.2, 2.3) | 0.516 | ||
| Sex | |||||||
| Males | 8,358 | 4,274 (51) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Females | 4,307 | 2,397 (56) | 1.2 (1.1, 1.3) | 1.4 (1.3, 1.6) | 0.000 | ||
| Transgender | 50 | 31 (62) | 1.6 (0.9, 2.8) | 1.3 (0.7, 2.5) | 0.456 | ||
| Missing | 39 | 24 (62) | 1.5 (0.8, 2.9) | 1.7 (0.8, 3.8) | 0.175 | ||
| Indigenous status | |||||||
| No | 11,551 | 6,010 (52) | 1.00 | 0.000 | 1.00 | 0.970 | |
| Yes | 881 | 528 (60) | 1.4 (1.2, 1.6) | 1.0 (0.9, 1.2) | 0.050 | ||
| Missing | 322 | 188 (58) | 1.3 (1.0, 1.6) | 1.1 (0.9, 1.5) | 0.347 | ||
| State/territory | |||||||
| New South Wales | 4,282 | 2,959 (69) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Queensland | 4,124 | 1,438 (35) | 0.2 (0.2, 0.3) | 0.4 (0.3, 0.4) | 0.000 | ||
| Other | 4,348 | 2,329 (54) | 0.5 (0.5, 0.6) | 0.6 (0.5, 0.6) | 0.000 | ||
| Survey year | |||||||
| 1995–1999 | 6,718 | 3,373 (50) | 1.00 | 0.000 | 1.00 | 0.009 | |
| 2000–2004 | 6,036 | 3,353 (56) | 1.2 (1.2, 1.3) | 1.1 (1.0, 1.2) | 0.009 | ||
| Imprisonment last year | |||||||
| No | 10,672 | 5,269 (49) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Yes | 1,863 | 1,333 (72) | 2.6 (2.3, 2.9) | 2.3 (2.0, 2.6) | 0.000 | ||
| Missing | 219 | 124 (57) | 1.3 (1.0, 1.8) | 1.3 (1.0, 1.8) | 0.092 | ||
| Duration of injecting | |||||||
| <3 years | 1,868 | 345 (18) | 1.00 | 0.000 | 1.00 | 0.000 | |
| 3–8 years | 3,658 | 1,297 (35) | 2.4 (2.1, 2.8) | 2.1 (1.8, 2.4) | 0.000 | ||
| >8 years | 6,889 | 4,921 (71) | 11.0 (9.7,12.5) | 5.1 (4.4, 5.9) | 0.000 | ||
| Missing | 339 | 163 (48) | 4.1 (3.2, 5.2) | 3.0 (2.3, 4.0) | 0.000 | ||
| Last drug injected | |||||||
| Opiatesb | 6,567 | 3,903 (59) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Psychostimulantsc | 3,826 | 1,380 (36) | 0.4 (0.4, 0.4) | 0.5 (0.4, 0.5) | 0.000 | ||
| Other | 2,228 | 1,402 (63) | 1.2 (1.0, 1.3) | 0.9 (0.8, 1.0) | 0.105 | ||
| Missing | 133 | 41 (31) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.000 | ||
| Frequency of injection last month | |||||||
| Less than daily | 5,375 | 2,581 (48) | 1.00 | 0.000 | 1.00 | 0.006 | |
| Daily of more | 6,359 | 3,695 (58) | 1.5 (1.4, 1.6) | 1.1 (1.0, 1.2) | 0.007 | ||
| Missing | 1,020 | 450 (44) | 0.9 (0.7, 1.0) | 0.8 (0.7, 1.1) | 0.144 | ||
| Shared needle/syringe in last month | |||||||
| No | 9,454 | 4,907 (52) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Yes | 2,102 | 1,232 (59) | 1.3 (1.2, 1.4) | 1.4 (1.2, 1.5) | 0.000 | ||
| Missing | 1,198 | 587 (49) | 0.9 (0.8, 1.0) | 1.0 (0.9, 1.3) | 0.726 | ||
| Sex work in last month | |||||||
| No | 11,355 | 5,864 (52) | 1.00 | 0.000 | 1.00 | 0.000 | |
| Yes | 1,048 | 664 (63) | 1.6 (1.4, 1.8) | 1.3 (1.1, 1.6) | 0.000 | ||
| Missing | 351 | 198 (56) | 1.2 (1.0, 1.5) | 1.1 (0.8, 1.4) | 0.601 | ||
| HBV vaccination | |||||||
| No/not sure | 6,422 | 3,344 (52) | 1.00 | 0.140 | 1.00 | 0.075 | |
| Yes | 6,014 | 3,211 (53) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.2) | 0.036 | ||
| Missing | 318 | 171 (54) | 1.1 (0.9, 1.3) | 0.9 (0.7, 1.2) | 0.589 |
OR odds ratio, AOR adjusted odds ratio, CI confidence interval
ap values reported for test of homogeneity in nominal covariates and test for trend for ordinal covariates. Patients with missing data were not included
bIncludes heroin and morphine
cIncludes amphetamine/methamphetamine and cocaine, injected alone or with other drugs
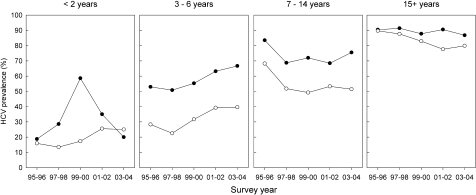
To further investigate the effect of duration of injecting on HCV status, participants were stratified into 2-year injection initiation cohorts. Figure 1a shows temporal trends in HCV antibody prevalence within each cohort between 1995 and 2004. There was a significant increase in HCV prevalence across survey years among the younger cohorts (1989–1990 to 1999–2000) (p < 0.01). Older cohorts (1973–1974 to 1987–1988) did not show a significant trend in HCV prevalence across survey years (p > 0.05), though successively older cohorts had successively higher prevalence of HCV.
FIGURE 1.
Temporal trends in hepatitis C virus prevalence among a injection initiation cohorts and b injection duration groups: The Australian NSP Survey 1995–2004. a: dashed lines indicate injection initiation cohorts with non-significant trends over time; solid lines indicate injection initiation cohorts with significant trends over time. b: dashed lines indicate injection duration groups with non-significant trends over time; solid lines indicate injection duration groups with significant trends over time.
From 1995 to 2004, HCV prevalence increased significantly among IDUs with less than 2 years, 2–4 years, 4–6 years, and 6–8 years injecting experience (all p < 0.01) (Figure 1b). These four injection duration groups represent 43% of all surveys collected at consistently participating NSP sites. There were significant decreasing trends in HCV prevalence among injection duration groups with more than 12 but less than 24 years duration of injecting.
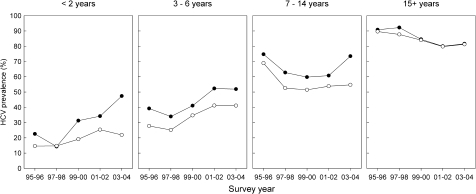
Overall, there was significant variation in self-reported sharing of needles and syringes in the month prior to survey participation, ranging from 14% to 30% (p < 0.01), with no observable differences between injection duration groups (Figure 2). There was a decreasing trend in the proportion of respondents reporting sharing of needles and syringes among all injection duration groups (p < 0.02) except those with less than 1 year injecting experience. There was higher HCV prevalence in respondents reporting recent sharing of needles and syringes among duration of injecting groups with less than 15 years injecting experience, while there was no observable difference after 15 years of injecting when the population prevalence almost reached saturation (Figure 3). A similar pattern was observed with recent imprisonment (Figure 4).
FIGURE 2.
Temporal trends in the proportion of NSP attendees reporting recent sharing of needles and syringes by injection duration groups: The Australian NSP Survey 1995–2004.
FIGURE 3.
Temporal trends in hepatitis C prevalence by recent sharing of needles and syringes among injection duration groups: The Australian NSP Survey 1995–2004. Closed black circles = recent sharing of needles and syringes, open black circles = no recent sharing of needles and syringes.
FIGURE 4.
Temporal trends in hepatitis C prevalence by recent imprisonment status among injection duration groups: The Australian NSP Survey 1995–2004. Closed black circles = recent imprisonment; open black circles = no recent imprisonment.
Discussion
Despite widespread availability of NSPs throughout Australia, we found little indication that transmission of HCV infection among more recent initiates to injecting changed between 1995 and 2004. We found an increase in HCV prevalence within injection initiation cohorts over time, with prevalence appearing to reach saturation around 90% in the older cohorts. While duration of injecting was most strongly associated with HCV seropositivity in this study, we also found that self-reported recent needle and syringe sharing and imprisonment were independently associated with higher HCV prevalence regardless of duration of injecting. However, for those who had been injecting for 15 years or more, recent risk behavior had no relationship to prevalence. Our novel injection initiation cohort analysis suggests that there are opportunities to overcome the challenges in estimating trends in HCV incidence in large IDU populations using routinely collected HCV prevalence data, and to realize the potential of existing surveillance data that have been invested in over long periods of time.
Our injection initiation cohort analysis clearly illustrates the rapid accumulation of HCV during the early years following injection initiation within a series of cohorts defined by year of injection initiation. This observation is consistent with studies of HCV incidence among young IDUs over shorter time periods, both locally and internationally.9,19–22 While there was no detectable increase in prevalence among the older cohorts during the decade of study, we observed that successively older cohorts had a successively higher prevalence of HCV. This pattern may represent decreasing incidence rates within cohorts over time, with a progressive increase in HCV prevalence towards a common plateau of approximately 90%. Alternatively, HCV prevalence may have reached a plateau at a lower level in the younger cohorts in this sample. The latter has been observed among IDUs accessing drug treatment centers in New York between 1990 and 2001 following large-scale expansion of harm reduction services in the city over that time period.11 Overall, this study suggests that routinely collected, cross-sectional survey data may provide a useful indication of trends in HCV incidence in the context of limited resources and the challenges associated with conducting long-term incidence studies of HCV infection among IDU populations.
We found little indication that transmission of HCV infection among more recent initiates to injecting changed between 1995 and 2004, with significantly increasing trends in HCV prevalence among IDUs with less than 8 years injecting experience over the study period. Given the majority of prevalent cases among IDUs with less than 2 years injecting experience reflects recent transmission, our findings suggest that there may have even been an increase in HCV incidence among new initiates to injecting over the decade. However, we are limited by the cross-sectional nature of the data when it comes to inferring trends in transmission of HCV among those with more than 2 years injecting experience.
Our data indicate that self-reported recent sharing of needles and syringes was independently associated with higher HCV prevalence regardless of duration of injecting, with the exception of those injecting for 15 years or more. Among those with longer injecting duration, the population prevalence of HCV was close to saturation and recent risk behavior had no relationship to prevalence. This is consistent with what one would expect to observe in a group in which the period of susceptibility is long past for the majority of individuals. It is most likely that survey respondents moved in and out of the high risk behavior group throughout their injecting drug use career, providing continual opportunity for naïve infection, and therefore an incrementally higher prevalence in successively more experienced drug injection groups. This would for the most part explain the incremental increases in HCV prevalence across duration of injection groups both among survey respondents who reported, and did not report, recent sharing of needles and syringes in our study. It is also possible that some cases of infection occurred via use of other contaminated drug preparation equipment9,23 rather than sharing of needles and syringes at some point in time since injection initiation; however, we were unable to account for this in our analysis. While it is likely that the prevalence of sharing of needles and syringes was underreported in our study due to the highly stigmatized nature of the behavior and the perceived need to provide socially desirable responses in the survey context,24 it is unlikely that the direction of the trends observed would have been affected by underreporting over time.
Importantly, our study indicates that recent imprisonment is also strongly associated with higher HCV prevalence, except among IDUs with more than 15 years injecting experience. Imprisonment may be an indicator of more marginalized IDUs in community settings who may find themselves in high risk environments more often because of factors such as unstable accommodation and who may also participate in more high risk injecting behavior. However, some survey respondents are clearly moving in and out of the prison environment, which poses a high level of risk of HCV exposure, with approximately half of incarcerated IDUs injecting while in prison.25 This could potentially explain the incremental increases in HCV prevalence across duration of injection groups among those who reported, and did not report, recent imprisonment. While we cannot draw firm conclusions as to why or how IDUs reporting recent imprisonment have higher HCV prevalence from this study, our results confirm the vulnerability of this marginalized population.
To our knowledge, this is the first study of prevalent HCV infection in an IDU population that has adopted a injection initiation cohort analysis approach. Although we do not have verification of incidence in this population, the estimated trends in incidence from our injection initiation cohort analysis seem plausible because they increase over time within each cohort, defined by year of first injection. Ideally, we would follow the same IDUs over time to determine trends in incident HCV infection; however, this is not feasible. Therefore, our injection initiation cohort method offers an alternative method that may be useful for inferring trends in incident HCV infection using routinely collected data.
This study has several other strengths, including multi-site sampling from a range of geographical areas and different models of NSP service delivery across all states and territories of Australia, and recruitment of a large, heterogeneous sample of community-based IDUs who access NSPs. Further, we restricted our analysis to data from NSP sites that consistently participated in at least eight of the ten survey years, and used the same risk factor study questions over time, both of which aim to reduce any bias that may have been incurred from inconsistent survey methods over the study period.
Our study also has some limitations. The sample was not selected randomly given the inherent difficulties involved with identifying a sample frame for what is effectively a “partially hidden” population.26 Further, survey participation rates were approximately 50%, which may have incurred some selection bias. While both of the above factors may have affected the generalizability of our results to the broader IDU population in Australia, a recent investigation into the representativeness of the Australian NSP Survey study population found that survey responders were representative of the broader group of IDUs attending NSPs, including those who chose not to participate in the survey.27 Based on these data, IDUs who were male, aged less than 25 years or reported last injecting cocaine at the time of the survey may have been less likely to participate in the survey compared to IDUs who were female, aged over 35 years, or reported last injecting other drugs. Given the association between cocaine use and injection frequency,28 it is possible that there was a selection bias against IDUs with this particular high risk behavior. In this circumstance, our estimates are likely to be conservative.
In summary, our injection initiation cohort approach to analyzing routinely collected HCV prevalence data suggests that there are opportunities to overcome the challenges in estimating trends in HCV incidence in large IDU populations in the context of a well-established epidemic. Despite widespread availability and uptake of NSPs in Australia, we found little indication of change in transmission of HCV among recent initiates to injecting between 1995 and 2004, suggesting that community-based NSPs alone are not sufficient to prevent HCV transmission among IDUs. Further, we showed that recent sharing of needles and syringes and imprisonment are associated with higher HCV prevalence across duration of injecting groups and time, until the stage when population prevalence approximates saturation. While NSPs have played a significant role in maintaining low HIV prevalence among IDUs in Australia to date,5,29 current harm reduction efforts need to be enhanced, taking into account high risk environments such as prison, in order to reduce HCV transmission and ensure that HIV prevalence remains low among this vulnerable population.
Acknowledgements
We acknowledge the late Dr. Margaret MacDonald who co-founded the Australian NSP Survey, the study participants, the NSP staff involved in data collection, the laboratory staff at the Centre for Immunology at St Vincent’s Hospital, Sydney, the Australian NSP Survey advisory group and Jiong Li for assistance with database management, and the NCHECR postgraduate academic writing group for their feedback on the draft manuscript.
Author Contributions KF conducted the literature review, designed and conducted the statistical analysis, and drafted the manuscript. JK was involved in the initial design and implementation of the study, supervision of the statistical analysis plan for the current manuscript, and provided comments on drafts of the manuscript. LM directed the study’s implementation since 2004 including supervision of data collection, and was involved in the design and implementation of the study and commented on all drafts of the manuscript. All contributions of authors were on behalf of the Collaboration of Australian Needle and Syringe Programs.
Statement of Interests None declared.
Footnotes
FUNDING SOURCE: Australian Government Department of Health and Ageing
References
- 1.Hurley SF, Jolley DJ, Kaldor JM. Effectiveness of needle-exchange programmes for prevention of HIV infection. Lancet. 1997;349(9068):1797–1800 doi:10.1016/S0140-6736(96)11380-5. [DOI] [PubMed]
- 2.Health Incomes International PTY LTD., National Centre in HIV Epidemiology and Clinical Research, Drummond M. Return on Investment in Needle and Syringe Programs in Australia: Final Report: Commonwealth Department of Health and Ageing; 2002.
- 3.MacDonald M, Law M, Kaldor J, Hales J, Dore GJ. Effectiveness of needle and syringe programmes for preventing HIV transmission. Int J Drug Policy. 2003;14:353–357 doi:10.1016/S0955-3959(03)00133-6. [DOI]
- 4.National Centre in HIV Epidemiology and Clinical Research. Australian NSP Survey National Data Report 1995–2002. Sydney: National Centre in HIV Epidemiology and Clinical Research, The University of NSW; 2003.
- 5.MacDonald M, Wodak AD, Ali R, et al. HIV prevalence and risk behaviour in needle exchange attenders: a national study. The Collaboration of Australian Needle Exchanges. Med J Aust. 1997;166(5):237–240. [PubMed]
- 6.Crofts N, Hopper JL, Bowden DS, Breschkin AM, Milner R, Locarnini SA. Hepatitis C virus infection among a cohort of Victorian injecting drug users. Med J Aust. 1993;159(4):237–241. [DOI] [PubMed]
- 7.MacDonald MA, Wodak AD, Dolan KA, van Beek I, Cunningham PH, Kaldor JM. Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995–1997. Med J Aust. 2000;172:57–61. [DOI] [PubMed]
- 8.Maher L, Chant K, Jalaludin B, Sargent P. Risk behaviors and antibody hepatitis B and C prevalence among injecting drug users in south-western Sydney, Australia. J Gastroenterol Hepatol. 2004;19(10):1114–1120 doi:10.1111/j.1440-1746.2004.03438.x. [DOI] [PubMed]
- 9.Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101(10):1499–1508 doi:10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed]
- 10.van Beek I, Dwyer R, Dore GJ, Luo K, Kaldor JM. Infection with HIV and hepatitis C virus among injecting drug users in a prevention setting: retrospective cohort study. BMJ. 1998;317(7156):433–437. [DOI] [PMC free article] [PubMed]
- 11.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19(suppl 3):S20–S25 doi:10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed]
- 12.Burt RD, Hagan H, Garfein RS, Sabin K, Weinbaum C, Thiede H. Trends in hepatitis B virus, hepatitis C virus, and human immunodeficiency virus prevalence, risk behaviors, and preventive measures among Seattle injection drug users aged 18–30 years, 1994–2004. J Urban Health. 2007;84(3):436–454 doi:10.1007/s11524-007-9178-2. [DOI] [PMC free article] [PubMed]
- 13.Baryarama F, Bunnell R, McFarland W, et al. Estimating HIV incidence in voluntary counseling and testing clients in Uganda (1992–2003). J Acquir Immune Defic Syndr. 2007;44(1):99–105 doi:10.1097/01.qai.0000245879.36015.54. [DOI] [PubMed]
- 14.Hallet TB, Zaba B, Todd J, et al. Estimating incidence from prevalence in generalised HIV epidemics: methods and validation. PLoS Medicine. 2008;5(4):0611–0622. [DOI] [PMC free article] [PubMed]
- 15.Stoneburner RL, Low-Beer D, Tembo GS, Mertens TE, Asiimwe-Okiror G. Human immunodeficiency virus infection dynamics in east Africa deduced from surveillance data. Am J Epidemiol. 1996;144(7):682–695. [DOI] [PubMed]
- 16.Osmond C, Gardner MJ. Age, period and cohort models applied to cancer mortality rates. Stat Med. 1982;1(3):245–259 doi:10.1002/sim.4780010306. [DOI] [PubMed]
- 17.National Centre in HIV Epidemiology and Clinical Research. Australian NSP Survey National Data Report 2000–2004. Sydney: National Centre in HIV Epidemiology and Clinical Research, The University of NSW; 2005.
- 18.Chiu YL, Yu IT, Wong TW. Time trends of female lung cancer in Hong Kong: age, period and birth cohort analysis. Int J Cancer. 2004;111(3):424–430 doi:10.1002/ijc.20265. [DOI] [PubMed]
- 19.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15(5):543–549 doi:10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed]
- 20.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186(11):1558–1564 doi:10.1086/345554. [DOI] [PubMed]
- 21.Judd A, Hickman M, Jones S, et al. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ. 2005;330(7481):24–25 doi:10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed]
- 22.Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in injecting drug users: a policy failure. Aust N Z J Public Health. 2007;31(1):30–35. [DOI] [PubMed]
- 23.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91(1):42–46. [DOI] [PMC free article] [PubMed]
- 24.Hunter GM, Stimson GV, Judd A, Jones S, Hickman M. Measuring injecting risk behaviour in the second decade of harm reduction: a survey of injecting drug users in England. Addiction. 2000;95(9):1351–1361 doi:10.1046/j.1360-0443.2000.95913516.x. [DOI] [PubMed]
- 25.Butler T, Levy M, Dolan K, Kaldor J. Drug use and its correlates in an Australian prisoner population. Addict Res Theory. 2003;11(2):89–101 doi:10.1080/1606635021000021403. [DOI]
- 26.Des Jarlais DC, Dehne K, Casabona J. HIV surveillance among injecting drug users. AIDS. 2001;15(Suppl 3):S13–S22 doi:10.1097/00002030-200104003-00003. [DOI] [PubMed]
- 27.Topp L, Iversen J, Wand H, Day C, Kaldor J, Maher L. Representativeness of injecting drug users who participate in HIV surveillance: results from Australia’s Needle and Syringe Program Survey. J Acquir Immune Defic Syndr. 2008;47(5):632–638 doi:10.1097/QAI.0b013e31816a1d68. [DOI] [PubMed]
- 28.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17(6):887–893 doi:10.1097/00002030-200304110-00014. [DOI] [PubMed]
- 29.National Centre in HIV Epidemiology and Clinical Research. Australian NSP Survey National Data Report 2001–2005. Sydney: National Centre in HIV Epidemiology and Clinical Research, The University of NSW; 2006.