Abstract
Recent studies have emphasized the importance of T cells with regulatory/suppressor properties in controlling autoimmune diseases. A number of different types of regulatory T cells have been described with the best characterized being the CD25+ population. In addition, it has been shown that regulatory T cells can be induced by specific Ag administration. In this study, we investigate the relationship between peptide-induced, CD4+ regulatory T cells and naturally occurring CD4+CD25+ cells derived from the Tg4 TCR-transgenic mouse. Peptide-induced cells were FoxP3− and responded to Ag by secreting IL-10, whereas CD25+ cells failed to secrete this cytokine. Both cell types were able to suppress the proliferation of naive lymphocytes in vitro although with distinct activation sensitivities. Depletion of CD25+ cells did not affect the suppressive properties of peptide-induced regulators. Furthermore, peptide-induced regulatory/suppressor T cells could be generated in RAG−/−, TCR-transgenic mice that do not spontaneously generate CD25+ regulatory cells. These results demonstrate that these natural and induced regulatory cells fall into distinct subsets.
Regulatory/suppressor T cells play an important role in both preventing the collateral damage that can arise from the immune response to infection (1) and controlling the immune response to self-Ags that may lead to autoimmune disease (2, 3). A number of different types of regulatory cells have been described (3), and these may, for convenience, be defined as natural or induced. Natural regulatory cells are part of the normal T cell repertoire and are fully functional, whereas induced regulatory cells may be generated from naive T cells by deliberate administration of Ag. Possibly the best-characterized subset of natural regulators is the CD4+CD25+FoxP3+ population. These cells are most probably selected in the thymus (4, 5), and there is evidence that recognition of Ag presented by cortical epithelium is sufficient for their generation (6). Their induction is Ag specific but their suppressive function is Ag nonspecific (7). These cells can be generated from either IL-10 (8) or TGF-β knockout (2) mice but are dependent on cell-cell contact for their function in vitro (8).
Some natural regulatory cells require lymphokines for their function. This has been demonstrated in models of immune pathology where disease is induced by the transfer of naive lymphocytes into lymphopenic animals. Interestingly, IL-10-secreting regulatory cells were required for suppression of inflammatory bowel disease (9), whereas CD25+ regulatory cells were sufficient for prevention of gastritis (10). Belkaid et al. (11) tested whether CD25+ cells, derived from wild-type or IL-10 deficient mice, would mediate suppression in vivo in an infectious disease model. They found that suppression of immunity to Leishmania major, resulting in maintenance of a persistent infection, was dependent on the presence of CD25+ cells and that these cells needed to produce IL-10 to function. These independent models, therefore, demonstrate that IL-10 may play a more important role in the function of regulatory T (TReg)6 cells in vivo. Furthermore, transfer experiments in TCR-transgenic models of experimental autoimmune encephalomyelitis (EAE) have shown that both CD25+ and CD25− fractions of cells can suppress disease (12).
TReg cells can be induced by the administration of Ags in different forms and by various routes. Thus, TGF-β-secreting Th3 cells were induced following mucosal administration of whole proteins (13), whereas administration of peptides by the same route tended to induce IL-10-secreting cells (14). IL-10-producing regulatory cells can be grown in vitro by culture in either IL-10 (15) or a combination of vitamin D3, dexamethasone, and cognate Ag (16). Furthermore, IL-10-secreting TReg cells can be induced in vivo following transplantation under the cover of immunosuppressive agents (17, 18) or by repeated administration of either superantigens (19, 20) or soluble peptide Ags (21).
The diversity of different regulatory/suppressor T cell types raises fundamental questions. In particular, it is important to understand the relationship between the induced regulators and naturally occurring populations of regulatory cells. Previously (22, 23), we have shown that soluble peptide administration suppresses immune responses to Ag. Tolerance could be induced in thymectomized mice (24) and the induced regulatory cells could mediate linked (25) and bystander suppression (26). Peripheral tolerance was induced in the Tg4 TCR-transgenic mouse by repeated intranasal (i.n.) administration of a high-affinity, Au-binding analog of the N-terminal peptide of myelin basic protein (MBP) (21). Repeated i.n. administration of Ac1-9[4Y] rendered Tg4 T cells unresponsive, as measured by proliferation. The peptide-induced regulatory (PI-TReg) T cells, nevertheless, responded to Ag by secreting IL-10, although the production of other cytokines, including IL-2, IL-4, TGF-β, and IFN-γ, was suppressed (21, 27). Furthermore, suppression of proliferation and IL-2 production by PI-TReg cells in vivo was abrogated by neutralization of IL-10 (27).
Tg4 mice treated with i.n. peptide showed raised levels of T cells expressing CD25 and CTLA-4 (28). There are two possible explanations for this enhanced expression. First, the i.n. peptide treatment could expand an existing population of CD25+ cells. Second, the i.n. peptide treatment might act on naive CD25− lymphocytes, stimulating them to transiently up-regulate CD25 and CTLA-4 and to differentiate into IL-10-secreting regulatory cells. Recent studies, using T cell lines in vitro, suggest that human CD25+ cells can be distinct from cytokine-selected type 1 T regulatory cells (29). The latter IL-10-secreting regulatory cells were generated from CD25− cells. Contrasting data from Dieckmann et al. (30) suggest, however, that CD25+ cells serve to promote the generation of IL-10-secreting regulatory cells in vitro. In this study, we investigate the origin and function of peptide-induced, IL-10-secreting regulatory cells in vivo. We compare and contrast these cells with natural, CD25+ regulatory cells derived from the same animal.
Materials and Methods
Mice
All mice were bred under specific pathogen-free conditions and housed in a barrier unit. The Tg4-transgenic mouse expresses TCR α- and β-chains derived from the 1934.4 T cell hybridoma, specific for the N-terminal, acetylated CD4 T cell epitope of MBP (Ac1-9). This mouse strain was established as previously described (31) and backcrossed onto the B10.PL (H-2u) background. RAG−/− Tg4 mice were generated by breeding Tg4 mice with a RAG−/− B10 strain, originally bred by Dr. D. Kioussis (Mill Hill, London, U.K.) and provided by Dr. A. Pullen (University of Bristol, Bristol, U.K.) to the F generation. RAG−/−, H-2u+, and Tg4+ offspring were selected and bred to provide a homozygous line.
Peptides
The acetylated N-terminal peptide of murine MBP (Ac1-9, AcASQKRPSQR) and the high MHC-affinity analog with a tyrosine substituting the wild-type lysine in position four (Ac1-9[4Y]) were synthesized using standard F-moc chemistry on an AMS 422 multiple peptide synthesizer (Abimed).
Tolerance induction
Tolerance was induced by treating the Tg4 mice with 10 i.n. doses of 100 μg Ac1-9[4Y] in PBS at regular intervals over a period of 5 wk.
Cell separation
PI-TReg splenocytes were routinely expanded in the presence of recombinant human IL-2 for 5 days before CD4 cell isolation (32). Spleens were isolated from tolerant mice and cells were cultured in IMDM medium supplemented with 5 × 10−5 M 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Invitrogen Life Technologies), and 5% FCS (Sigma-Aldrich) (complete medium). Ac1-9 was added at a final concentration of 10 μg/ml and rhIL-2 was added at 20 U/ml. Cultures were incubated in upright tissue culture flasks for 5 days before isolation of CD4+ T cells. Purified CD4+ T cells (>95% CD4+ as determined by FACS analysis) were obtained by positive selection using magnetic beads coated with anti-CD4 mAb (Miltenyi Biotec) according to the manufacturer's instructions. Depletion of CD25+ cells was performed by negative selection using anti-CD25-biotin (BD Pharmingen) followed by streptavidin-coated magnetic beads (Miltenyi Biotec). CD4+ T cells were then obtained by positive selection as stated above. Purified CD25+ T cells were obtained by first negatively selecting CD8+ and CD19+ cells using Ab-coated magnetic beads (Miltenyi Biotec). The remaining fraction was then separated into CD4+CD25+ and CD4+CD25− populations by staining with anti-CD4-FITC and anti-CD25-PE (BD Pharmingen) and sorting on the FACSVantage cell sorter (BD Biosciences).
CD4 T cell proliferation assays
For proliferation assays, T cells were cultured in complete medium at 5 × 104 cells/well, unless stated otherwise, at 37°C in the presence of different concentrations of Ac1-9. Irradiated CD4-depleted splenocytes at 1 × 105 cells/well were used as APC. At the indicated times, cells were pulsed with 0.5 μCi [3H]thymidine overnight, and the incorporated radioactivity was measured on a liquid scintillation beta counter (1450 Microbeta; Wallac).
Cytokine protein levels
Cell culture supernatants were tested for cytokine content by specific ELISA (BD Pharmingen) according to instructions from the manufacturer.
FACS analysis (flow cytometry)
Cells were stained by direct immunofluorescence for two-color flow cytometry. Data were analyzed using CellQuest software (BD Pharmingen) or FCS Express (De Novo software). Intracellular staining for FoxP3 was performed using the PE anti-mouse/rat FoxP3 staining set and an isotype control from eBioscience.
CFSE staining and cell transfer
Labeling of naive Tg4 splenocytes with CFSE was performed as described previously (33). A total of 5 × 107 CFSE-labeled splenocytes was transferred i.v. into the tail vein of naive or tolerant RAG−/− Tg4 recipients. One day after the transfer, mice received one i.n. treatment of Ac1-9[4Y] or PBS, and T cell proliferation was monitored 2 days later in the spleen. Chimerism of CFSE+ cells in the spleen of recipient mice varied between 1 and 3% in all instances.
Quantitative real-time PCR
Cells for FoxP3 RNA analysis included MACS-purified CD4+CD25+ cells from a naive Tg4 mouse and MACS-purified CD4 cells from either naive Tg4, PI-tolerant RAG+/+ Tg4, or PI-tolerant RAG−/− Tg4. Total RNA was extracted from the cell pellets with TRIzol (Invitrogen Life Technologies) as described by the manufacturer. Extracted RNA was reverse transcribed with Superscript III RNase H− Reverse Transcriptase (20 U/μl−1) and random primer oligonucleotides (15 ng/μl−1) (Invitrogen Life Technologies). To exclude the contribution of genomic DNA in PCR amplification, Superscript III RTase was omitted from control reactions. The expression level of FoxP3 and the housekeeping gene β2-microglobulin was measured by amplification of cDNA generated. FoxP3 primers were purchased from Qiagen (QT00138369) and used as directed. For β2-microglobulin, the forward primer sequence was 5′-GCTATCCAGAAAACCCCTCAA-3′ and the reverse primer sequence was 5′-CGGGTGGAACTGTGTTACGT-3′, synthesized by Sigma Genosys and used at a concentration of 0.2 μM. PCR were conducted in a final volume of 20 μl containing cDNA, PCR buffer, 3 mM MgCl2, 0.4 mM dNTP mix, forward and reverse primers, 0.05 U/μl−1 platinum Taq polymerase (Invitrogen Life Technologies) and quantitated with SYBR Green I (X30000 dilution of stock; Molecular Probes). The PCR cycling conditions were as follows: an initial denaturation step at 95°C for 2 min, followed by amplification of 35 cycles of 15 s at 94°C, annealing for 30 s at 58°C or 63°C for β2-microglobulin and FoxP3, respectively, and extension for 30 s at 72°C.
Results
Both CD25+ and PI-TReg cells are anergic to antigenic stimulation in vitro
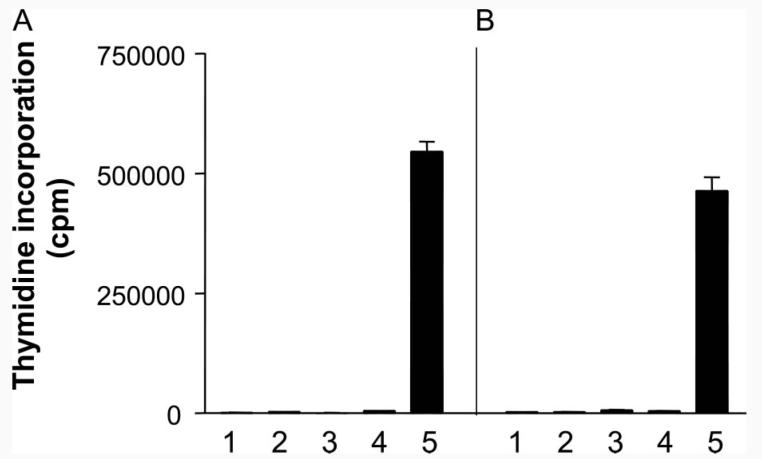
Previous results from our laboratory have shown that PI-TReg cells are predominantly CD25− (27), although these cells do up-regulate cell surface CD25 on exposure to Ag (32). In this study, we have analyzed the role of natural CD25+ cells in both the function and generation of PI-TReg cells. A direct comparison of the anergic state of purified CD25+ and PI-TReg cells was made. CD25+ cells were purified by FACS from the spleens of naive Tg4 mice and the resulting population proved to be >98% pure (data not shown). PI-TReg cells were collected from the spleens of Tg4 mice treated with soluble peptide as previously described (27). Both populations of cells were expanded briefly in vitro by stimulation with Ac1-9 peptide Ag in the presence of IL-2 for 5 days (32). The cells were harvested from culture by magnetic bead purification of CD4 cells. As shown in Fig. 1, both natural CD25+ TReg and PI-TReg cells were profoundly anergic to restimulation with Ac1-9 in vitro, whereas Tg4 cells responded vigorously to the same Ag. Previous experiments (21, 27, 32) from our laboratory demonstrated that PI-TReg cells fail to secrete IL-2, IL-4, TGF-β, or IFN-γ but selectively secrete IL-10. ELISA analysis of the culture supernatants from the experiment shown in Fig. 1 revealed that while naive Tg4 cells secreted IL-2 but no IL-10, PI-TReg cells secreted IL-10 and no IL-2, whereas Ag-stimulated CD25+ TReg cells did not secrete either IL-2 or IL-10 at detectable levels (data not shown). This, therefore, suggests a fundamental difference between natural and induced TReg cells from Tg4 mice. Thus, although both TReg populations are able to suppress naive cell proliferation in vitro, PITReg cells secrete IL-10 at high levels whereas Tg4 CD25+ cells do not.
FIGURE 1.
Both CD25+ and PI-TReg cells maintain their anergic phenotype following expansion in IL-2 in vitro. CD25+ and PI-TReg cells were cultured with IL-2 and Ac1-9 (10 μg/ml) for 5 days and CD4+ cells were purified using magnetic beads. Purified CD25+ (A) or PI-TReg cells (B) were cultured alone (1), with Ac1-9 peptide (2), with irradiated CD4-depleted splenocytes as APC (3), or with irradiated APC and Ac1-9 peptide for 72 h before addition of tritiated thymidine. Proliferation of TReg cells was compared with the response of naive Tg4 cells to Ac1-9 and APC (5). CD4+ T cells were cultured at 5 × 104 cells per well with 1 × 105 cells per well of APC in vitro with 100 μg/ml Ac1-9. Results are depicted as mean thymidine incorporation from triplicate samples ± SEM. The experiment has been repeated three times with similar results.
Both CD25+ and PI-TReg cells suppress proliferation of naive Tg4 cells
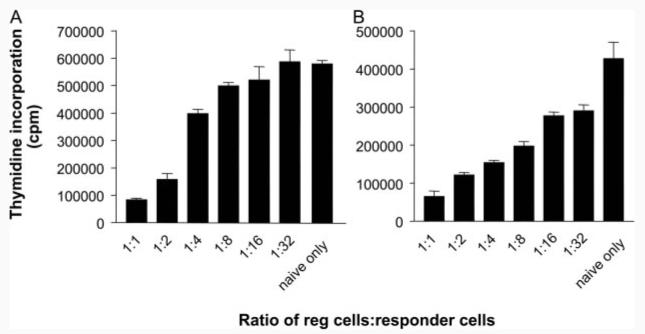
T cells from mice rendered tolerant by i.n. peptide treatment are capable of linked (25) and/or bystander suppression (26). Previous experiments from this laboratory had shown that PI-TReg cells were capable of suppressing proliferation of naive Tg4 cells both in vitro and in vivo (27). We wished to compare the suppressive properties of purified CD25+ and PI-TReg cells. A titration was established whereby naive CD4+ cells were stimulated in vitro with Ac1-9 in the presence of APCs and varying ratios of regulatory T cells. As shown in Fig. 2A, purified CD25+ cells suppressed proliferation of naive CD4+ cells at ratios ranging from 1:1 to 1:16. PI-TReg cells routinely displayed a similar degree of regulation and gave significant suppression at a ratio of 1:32 (p = 0.002) over a series of three experiments (Fig. 2B).
FIGURE 2.
Both CD25+ and PI-TReg cells maintain their suppressive phenotype following expansion in IL-2 in vitro. CD25+ and PI-TReg cells were cultured with IL-2 and Ac1-9 (10 μg/ml) for 5 days and CD4+ cells were purified using magnetic beads. Purified CD25+ (A) or PI-TReg cells (B) were cultured with freshly isolated naive CD4+ Tg4 T cells at the indicated ratios. CD4+ T cells were cultured at 5 × 104 cells per well with 1 × 105 cells per well of irradiated CD4-depleted splenocytes in vitro with 100 μg/ml Ac1-9. Tritiated thymidine was added after 72 h. Results are depicted as mean thymidine incorporation for duplicate cultures ± SEM. The experiment has been repeated three times with similar results.
Depletion of CD25+ cells does not abrogate the suppressive function of PI-TReg cells
PI-TReg cells from peptide-treated Tg4 mice contain ∼10% CD25+ cells (27). It is, therefore, possible that suppression mediated by PI-TReg cells could be accounted for by this residual population of CD25+ cells. A further experiment involved removal of CD25+ cells from the PI-TReg population before expansion in vitro. Levels of CD25+ cells in this depleted population were below the level of detection (data not shown). Nevertheless, we needed to confirm that undetectable natural TReg cells would not grow out during the in vitro expansion of these cells in IL-2-containing medium. This could not be assessed by measuring CD25 expression because PI-TReg cells transiently up-regulate CD25 following Ag stimulation (32). PI-TReg cells do not, however, express FoxP3 when stimulated with Ag (34). FoxP3 expression was therefore chosen as a marker for contaminating natural CD25+ TReg cells. As shown in Fig. 3, CD25-depleted, PI-TReg cells did not up-regulate FoxP3 over the 5-day period of expansion in the presence of IL-2. Fig. 4 reveals the degree of suppression mediated by PI-TReg cells when compared with a population of CD25-depleted PI-TReg cells. There was no significant difference in the suppression generated by these two cell populations in vitro. As such, we can conclude that CD25+ TReg cells are not required for the in vitro suppression mediated by PI-TReg cells.
FIGURE 3.
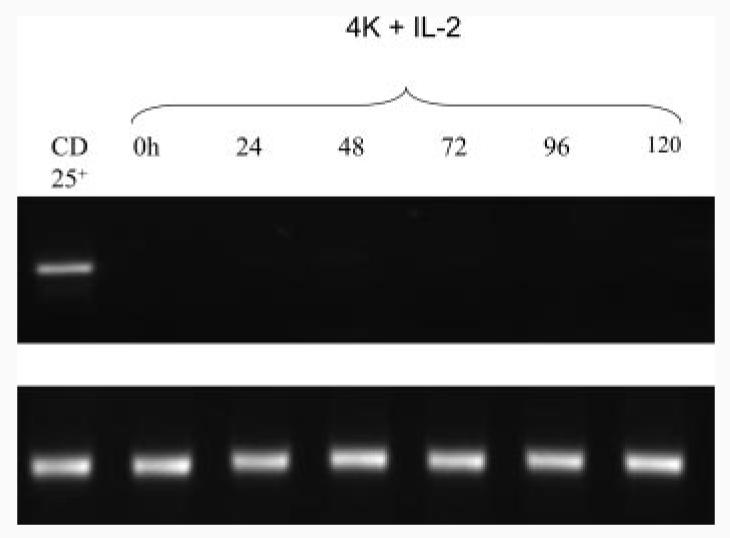
PI-TReg cells do not express FoxP3.PI-TReg cells depleted of CD25+ cells using magnetic beads were cultured with Ac1-9 and IL-2 for 5 days. At 24-h intervals, CD4+ cells were isolated using magnetic beads, RNA was extracted using TRIzol, and RT-PCR performed for expression of FoxP3 mRNA. Purified CD25+ cells served as a positive control and equivalent quantities of RNA were compared, as shown for the β-actin control. The experiment has been repeated three times with similar results.
FIGURE 4.
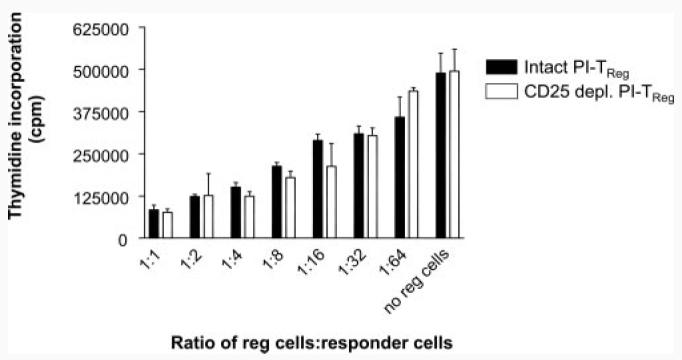
Removal of CD25+ T cells does not alter the suppression mediated by PI-TReg cells. Spleens from peptide-treated mice were either depleted of CD25+ cells or left intact before culture for 5 days in the presence of IL-2 and Ac1-9. CD4+ T cells were purified using magnetic beads and cocultures containing 5 × 104 TReg cells were established using freshly isolated naive CD4+ Tg4 T cells (5 × 104), irradiated APC (1 × 105), and Ac1-9 peptide (100 μg/ml). Tritiated thymidine was added after 72 h. Results are depicted as mean thymidine incorporation for triplicate cultures ± SEM. The experiment has been repeated three times with similar results.
RAG knockout Tg4 mice fail to generate CD25+ TReg cells
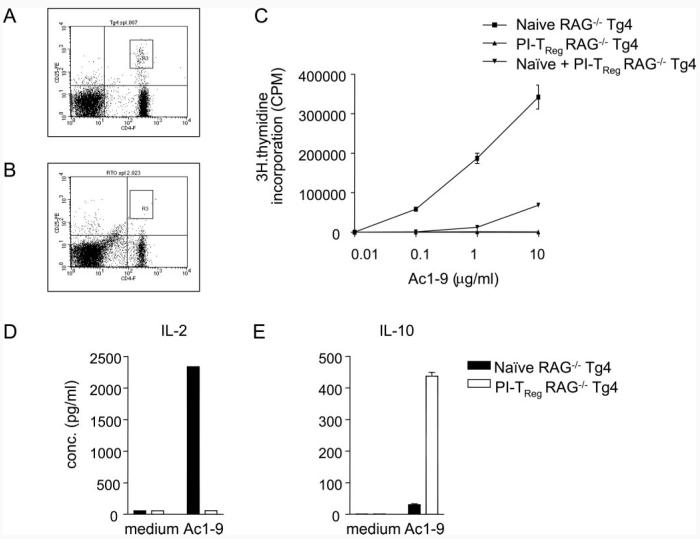
Cell depletion experiments revealed that CD25+ cells are not required for the suppressive phenotype of PI-TReg cells. This does not, however, guarantee that the two cell types represent distinct lineages. It could be, for example, that the CD25− PI-TReg cells are derived from CD25+ TReg cells that simply down-regulate CD25 as a result of repeated Ag encounter. Furthermore, it could be that CD25+ cells are required for the induction of PI-TReg cells in vivo as suggested by recent studies of human CD25+ cells in vitro (30). We therefore studied different cell populations in the RAG−/− Tg4 mouse. RAG+/+ Tg4 mice are highly susceptible to induction of EAE and yet do not suffer from spontaneous disease (31). Tg4 mice bred onto the RAG−/− background, however, develop EAE spontaneously, as previously shown in an independent line of transgenic mice bearing a similar TCR specific for the N-terminal peptide of MBP (35). Recent studies have shown that TCR-transgenic mice bred onto the RAG−/− background tend not to generate CD25high TReg cells (5, 36). We have therefore compared the proportion of CD25high cells in the peripheral lymphoid organs of RAG−/− and RAG+/+ mice by FACS staining. Splenic CD4+ cells from a RAG+/+ Tg4 mouse contained a distinct population of CD25high cells (Fig. 5A). CD4+CD25high cells were, however, barely detectable in the RAG−/− splenocyte population (Fig. 5B). Therefore, RAG−/− Tg4 mice are devoid of natural CD25+ TReg cells.
FIGURE 5.
PI-TReg cells from RAG−/− mice are suppressive in vitro and respond to Ag by IL-10 production. Spleen cells from (A) RAG+/+ and (B) RAG−/− mice were stained with FITC-anti-CD4 and PE-anti-CD25 and analyzed by FACS. The boxed area denotes CD4−CD25high cells constituting 1.1% of RAG+/+ and 0.05% of RAG−/− spleen cells. C–E, RAG−/− mice were rendered tolerant by 10 i.n. doses of Ac1-9[4Y]. Spleen cells were harvested 3 days after the final treatment and CD4+ T cells purified. CD4+ T cells were cultured at 5 × 104 cells per well with 1 × 105 cells per well of irradiated CD4-depleted splenocytes in vitro with 10 μg/ml Ac1-9 unless otherwise indicated. After 3 days, supernatants were harvested for cytokine ELISA or cultures pulsed with tritiated thymidine. The results are representative of groups of at least three mice per treatment group, and the experiment was repeated three times with similar results. Error bars indicate the SEM of triplicate cultures.
Induction of peripheral tolerance in the RAG−/− Tg4 mouse
RAG−/− Tg4 mice were subjected to the same tolerance induction protocol known to induce tolerance and the generation of TReg cells in RAG+/+ mice. This resulted in the generation of a profoundly anergic T cell population as revealed by their incapacity to respond to Ac1-9 (Fig. 5C). The anergic RAG−/− cells were also capable of functioning as TReg cells as shown by the suppression of the normal response of naive Tg4 cells. Previous experiments from this laboratory demonstrated that PI-TReg cells fail to secrete IL-2, IL-4, TGF-β, or IFN-γ but selectively secrete IL-10 (20, 26, 31). Supernatants from the cultures of the naive or PI-TReg RAG−/− cells, stimulated in vitro with Ac1-9, were collected to assess whether cells from these mice shared similar properties. As shown in Fig. 5, D and E, naive RAG−/− Tg4 cells produced high levels of IL-2 but relatively little IL-10. By contrast, cells from tolerant RAG−/− Tg4 mice produced little IL-2 but secreted IL-10 at relatively high levels.
Suppression of naive cell proliferation in tolerant RAG−/− mice
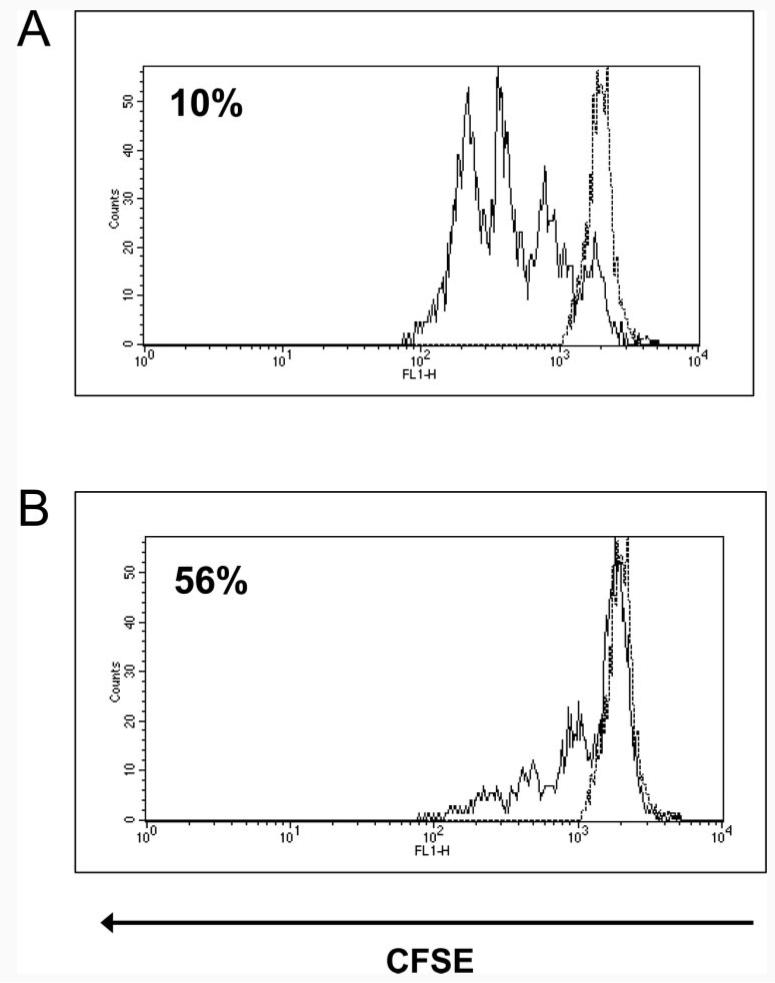
We recently established an in vivo assay for T cell suppression involving transfer of CFSE-labeled cells (27). Splenocytes obtained from naive Tg4 mice were labeled with CFSE and transferred either into naive or tolerant RAG−/− recipients by i.v. injection. One day after cell transfer, the recipient mice were challenged with a single dose of Ac1-9[4Y] by the i.n. route, a procedure previously shown to cause activation of Tg4 cells (27, 28). A single mouse in each group was left untreated to determine baseline CFSE levels. The spleens from these recipient mice were harvested on day 3 after transfer, disaggregated to yield a single-cell suspension, and counterstained with anti-CD4-tricolor Ab. FACS analysis of these splenocyte populations was performed by gating on the CD4-tricolor, CFSE double-positive cells. Fig. 6A shows the response of naive cells transferred into naive RAG−/− recipients; the cells divided readily in response to Ag with only 10% remaining in the undivided fraction. Fig. 6B shows that some cells also divided when transferred into tolerant recipients; however, a greater proportion of these cells (56%) remained in the undivided fraction or underwent lower numbers of divisions. Taken together with the results shown in Fig. 5, the results of this transfer model show that regulatory cells from RAG−/− Tg4 mice are capable of suppressing the proliferation of naive T cells both in vitro and in vivo.
FIGURE 6.
In vivo suppression of proliferation by PI-tolerant RAG−/− cells. Splenocytes obtained from naive Tg4 mice were labeled with CFSE and 5 × 107 cells transferred into either naive or peptide-treated RAG−/− recipients, containing PI-TReg cells, by i.v. injection. Recipient mice received a single dose of Ac1-9[4Y] i.n. after 24 h or were left untreated. Splenocytes were harvested 48 h later and counterstained with anti-CD4-tricolor Ab. FACS analysis was performed applying a live gate around CD4-tricolor, CFSE double-positive cells. A, Naive cells transferred into naive RAG−/− recipient. B, Naive cells transferred into peptide-treated RAG−/− recipient. Percentages indicate the proportion of cells in the undivided fraction. The dotted lines represent cells from recipients that did not receive peptide challenge. The results are representative examples of experimental groups containing three mice. The experiment has been repeated three times with similar results.
PI-TReg cells from both RAG−/− and RAG+/+ mice do not express FoxP3
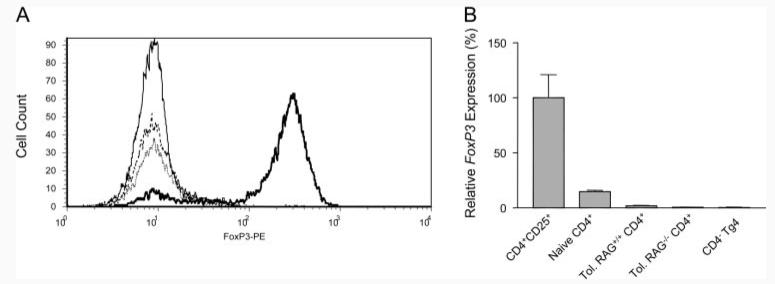
Previous studies in TCR-transgenic mice have shown that continuous antigenic stimulation will induce FoxP3+ regulatory cells among naive CD25− precursors (37). Although preliminary experiments indicated that PI-TReg cells from RAG+/+ Tg4 mice did not express FoxP3 (Ref. 34 and see also Fig. 3), we wished to confirm that this was also the case for tolerant RAG−/− Tg4 mice. Intracellular staining of naive RAG+/+CD4+ cells revealed a distinct population of FoxP3+ cells in the CD25+ gate (Fig. 7A). Tolerant Tg4 cells were stimulated with Ag and cultured in the presence of IL-2 for 5 days. The resulting CD4+ cells were predominantly CD25+ but nevertheless this fraction of cells did not express FoxP3. This was also true for cells grown from tolerant RAG−/− Tg4 mice (Fig. 7A). Furthermore, real-time PCR analysis of FoxP3 gene transcription revealed that neither RAG+/+ nor RAG−/− CD4+ cells from tolerant Tg4 mice expressed FoxP3 (Fig. 7B). This is in contrast to CD4+ cells from naive mice that expressed FoxP3 in proportion to the percentage of CD25+, natural TReg cells. It is notable that the induction of tolerance with soluble peptide led to a reduction in the proportion of FoxP3-expressing cells (compare to naive CD4+ with Tol.RAG+/+CD4+). Presumably, the resident population of natural CD25+ cells was outgrown by PI-TReg cells, as a result of repeated peptide administration in vivo.
FIGURE 7.
Expression of FoxP3 in naive and peptide-treated, tolerant Tg4 cellsTg4 cells from naive and tolerant mice were subjected to surface staining for CD4 and CD25 and intracellular staining for FoxP3. A, Levels of FoxP3 expression among CD4+CD25+ cells freshly isolated from a naive mouse or from tolerant mice following culture with Ag and IL-2 for 5 days. Solid line, isotype control; bold line, gated CD4+CD25+ cells from a naive mouse; dotted line, gated CD4+CD25+ cells from a RAG+/+ peptide-treated, tolerant mouse, and hatched line, gated CD4+CD25+ cells from a RAG−/− peptide-treated, tolerant mouse. B, Relative levels of FoxP3 RNA expression, normalized to β2-microglobulin, in the cell fractions depicted in A. The results represent the mean of two to three separate analyses and the experiments were repeated twice with similar results.
Discussion
The major conclusion from this work is that CD4+ cells in the normal peripheral lymphoid environment can develop into IL-10+FoxP3− regulatory/suppressor cells when their cognate Ag is delivered in vivo in soluble form. The IL-10+ PI-TReg cells described in this article were induced by mucosal administration of soluble peptide. The induction of these cells was not restricted to mucosal surfaces, however, because repeated i.p. administration of peptide also led to their generation (23). Our aim was to compare these Ag-induced cells with natural (FoxP3+) regulatory cells.
Both natural (CD25+) and peptide-induced Tg4 regulatory cells were unresponsive to Ag after expansion with Ag and IL-2 in vitro and were able to suppress the response of naive T cells. The first notable difference between these populations lies in their cytokine production. Previous studies have shown that Tg4 PI-TReg cells failed to secrete IL-2, IFN-γ, TGF-β, and IL-4 but secreted IL-10 at high levels (21, 32). Furthermore, prevention of EAE, following peptide treatment of the Tg4 mouse, was reversed by coinjection of an anti-IL-10 Ab (21). The natural CD25+ regulatory cells did not, however, secrete IL-10 in response to Ag. This result supports previous observations that both mouse (8, 38) and human (39-42) CD25+ regulatory cells did not depend on this or other cytokines for suppression in vitro. It seems likely that the cells characterized in these previous studies were of the natural, thymus-derived phenotype (2, 5, 6). There is, however, evidence for regulatory cells that express CD25 and depend on cytokines such as IL-10 for their function. Here one could include CD25+ cells induced by oral tolerance that secrete both IL-10 and TGF-β in response to Ag (43). Furthermore, CD25+ cells capable of suppressing colitis in a lymphopenic transfer model were dependent on IL-10 for their function (10). Further work is required to assess whether or not CD25+ regulatory cell populations identified in different models share the gene expression profile characteristic of natural FoxP3+ TReg cells (44). Assessing the precise role of IL-10 in regulatory cell function is further complicated by the realization that this depends on the nature of the model studied. Two recent reports have described how regulatory T cells can suppress naive T cell responses in an IL-10-independent fashion in vitro, whereas their in vivo suppressive properties were IL-10 dependent (11, 27).
Peptide-induced regulatory cells need not express CD25 for their function. We routinely found <10% of CD4 cells expressing CD25 in the RAG+/+ Tg4 mouse PI-TReg population (27). The remaining 90% CD25− population was nevertheless highly suppressive in vitro and, furthermore, secreted IL-10 at high levels. This observation implies that PI-TReg and CD25+ cells represent distinct subsets of cells. A counterargument would be that both cell types are derived from the same precursor but that induction of PI-TReg cells leads to down-regulation of CD25 and coincident up-regulation of IL-10 secretion. It is now widely accepted, however, that CD25 is not a reliable marker for regulatory cells, whereas FoxP3 has been shown to correlate with regulatory activity in both CD25+ and CD25− populations of CD4+ T cells (45). Previous experiments (34) showed that PI-TReg cells do not express FoxP3 at high levels. We, therefore, chose to use FoxP3 expression as a means of distinguishing between natural and PI-TReg cells. Depletion of CD25+ cells from PI-TReg cells produced a population of cells that remained stably FoxP3− in vitro. These cells were equally as suppressive as unfractionated PI-TReg cells, confirming that CD25+ cells are not required for the suppressive function of PI-TReg cells in vitro. Furthermore, this demonstrates that although FoxP3 is an excellent marker for natural TReg cells, its expression is not a prerequisite for cells with regulatory activity. Thus, IL-10+ TReg cells generated either in the presence of vitamin D3 and dexamethasone in vitro (34), or by repetitive Ag administration in vivo, as described in this article, are FoxP3 negative. Although CD25+ cells are clearly not required for the function of PI-TReg cells in vitro, the question remains as to the role of CD25+ cells in the generation of PI-TReg in vivo.
A recent study by Dieckmann et al. (30) demonstrated that CD25+ regulatory cells were able to induce IL-10-secreting Tr1-like cells in vitro. This observation implies that CD25+ cells may contribute to the generation of PI-TReg cells in vivo. Previous studies had shown that RAG−/− mice, transgenic for a specific TCR, do not have CD25+ cells in their peripheral lymphoid organs (5, 7). Mice expressing a TCR specific for MBP, such as the Tg4 mouse described in this study, generally do not suffer spontaneous disease. The anti-MBP TCR-transgenic mouse generated by Lafaille et al. (35) behaved similarly and yet suffered a high incidence of spontaneous disease when bred onto the RAG−/− background. The RAG−/− Tg4 mice used in this study developed spontaneous disease, with all mice showing signs of encephalomyelitis by 12 wk of age. MBP-specific CD25+ T cells have been shown to prevent spontaneous EAE in RAG+/+ anti-MBP TCR-transgenic mice (36) and this, in part, explains why their RAG−/− counterparts suffer from spontaneous disease. Most importantly, however, we were still able to induce tolerance in RAG−/− Tg4 mice by i.n. administration of peptide and thereby to prevent spontaneous disease in these mice. This provides proof that cells of the CD25+ lineage are not required for generation of induced regulatory cells in vivo and demonstrates that PI-TReg cells are derived from naive CD25− cells. Furthermore, PI-TReg cells behaved in the same way when derived either from RAG+/+ or RAG−/− mice, both in terms of cytokine production and their ability to suppress naive cells in vitro. The implication of this finding is that PI-TReg cells in both types of mouse are identical and that such cells are normally derived from CD25− precursors in the periphery. We cannot exclude that CD25+ cells may enhance the generation of PI-TReg cells in RAG+/+ mice, but this seems unlikely given that there was no difference between the level and function of PI-TReg cells in RAG+/+ and RAG−/− mice. These observations in vivo therefore support studies by Vieira et al. (34) using mouse cells and Levings et al. (29), with isolated human blood cells, both of which implied that CD25+ cells were not required for the generation of IL-10-secreting regulatory cells in vitro.
Both regulatory CD25+ cells and naive CD25− cells are generated in the thymus. CD25− naive cells may then differentiate into a variety of effector cells including Th1, Th2, Th3, and the more recently described Tr1 cells. We believe that PI-TReg cells differentiate into IL-10+ TReg cells because they encounter Ag presented by semimature APC. The fact that these cells can be generated in RAG-deficient mice demonstrates that B cells are not required for their differentiation. A number of recent articles have shown, however, that semimature dendritic cells induce naive cells to differentiate into IL-10-secreting regulatory cells when presenting Ag to them (46-48). In this article, we provide evidence that PI-TReg cells need neither to express CD25 for their suppressive activity nor to require the presence of CD25+ TReg cells for their differentiation and expansion in vivo. This clearly places these two important types of regulatory cells in distinct subsets distinguishable on the basis of cytokine secretion and FoxP3 expression.
CD25+ TReg cells may be selected in peripheral lymphoid tissues following encounter with Ag. There is evidence that human CD25+ TReg cells may be generated from CD25− precursors (49). Furthermore, continuous peptide release from osmotic pumps led to expansion of CD25+ cells from CD25− precursors in a thymectomized TCR-transgenic mouse model (37). These cells were similar in cell surface phenotype to natural CD25+ TReg, expressed high levels of FoxP3, and suppressed immune responses in vitro. T cell encounters with continuous Ag in the absence of an inflammatory stimulus might thus mimic conditions under which natural, CD25+ TReg cells are generated in the thymus (5) and possibly other lymphoid organs (41). These conditions appear to be different, however, from pulsed, peptide administration that drives the generation of CD25−, FoxP3−, IL-10+ TReg cells in the Tg4 mouse (27, 34). There are two possible explanations for this difference. It may be that continuous Ag encounter drives cells that escape deletion toward the CD25+FoxP3+ phenotype, whereas pulsed Ag administration may encourage cells to differentiate into IL-10-secreting, FoxP3− TReg cells. Alternatively, it may be that the particular TCR transgenics studied by Apostolou et al. (37) is of an appropriate affinity to trigger differentiation of CD25+ cells from CD25− precursors. Additional study is required to distinguish these possibilities.
Pulsed administration of soluble peptides enhances Ag-specific IL-10-secreting regulatory cells in humans (50-52). These IL-10-secreting cells may be the human equivalent of the PI-TReg cells described in this article. Further characterization of this important population of TReg cells is clearly required whether these cells are to be safely manipulated for treatment of human autoimmune and allergic conditions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was supported by a Wellcome Trust Programme Grant. A.S. was supported by a fellowship from The Swedish Foundation for International Cooperation in Research and Higher Education. E.J.O. was supported by a Wellcome Trust Veterinary Studentship. K.N. was supported by the RJ Daniels Family Trust.
6 Abbreviations used in this paper: TReg, regulatory T; EAE, experimental autoimmune encephalomyelitis; MBP, myelin basic protein; i.n., intranasal.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Trinchieri G. Regulatory role of T cells producing both interferon γ and interleukin-10 in persistent infection. J. Exp. Med. 2001;194:F53–F57. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 3.Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr. Opin. Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells: expression of IL-2R α chain, resistance to clonal deletion., and IL-2 dependency. Int. Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 5.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 6.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 8.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 12.Furtado GC, Olivares-Villagomez D, Curotto de Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol. Rev. 2001;182:122–134. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 14.Maron R, Melican NS, Weiner HL. Regulatory Th2-type T cell lines against insulin and GAD peptides derived from orally- and nasally-treated NOD mice suppress diabetes. J. Autoimmun. 1999;12:251–258. doi: 10.1006/jaut.1999.0278. [DOI] [PubMed] [Google Scholar]
- 15.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J, Roncarolo MG. A CD4+ T cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 16.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 18.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 19.Sundstedt A, Hoiden I, Rosendahl A, Kalland T, Van Rooijen N, Dohlsten M. Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J. Immunol. 1997;158:180–186. [PubMed] [Google Scholar]
- 20.Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J. Exp. Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for interleukin-10. Int. Immunol. 1999;11:1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 22.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int. Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 23.Liu GY, Wraith DC. Affinity for class II MHC determines the extent to which soluble peptides tolerize autoreactive T cells in naive and primed adult mice—implications for autoimmunity. Int. Immunol. 1995;7:1255–1263. doi: 10.1093/intimm/7.8.1255. [DOI] [PubMed] [Google Scholar]
- 24.Metzler B, Wraith DC. Inhibition of T cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T cell-dependent functions. Immunology. 1999;97:257–263. doi: 10.1046/j.1365-2567.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J. Exp. Med. 1993;178:1783–1788. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur. J. Immunol. 1998;28:1251–1261. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Sundstedt A, O'Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 2003;170:1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 28.Metzler B, Burkhart C, Wraith DC. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int. Immunol. 1999;11:667–675. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- 29.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 32.Anderson PO, Sundstedt A, Yazici Z, Minaee S, Woolf R, Nicolson K, Whitley N, Li L, Li S, Wraith DC, Wang P. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells. J. Immunol. 2005;174:310–319. doi: 10.4049/jimmunol.174.1.310. [DOI] [PubMed] [Google Scholar]
- 33.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 34.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 35.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 36.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apostolou I, Von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 39.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–199. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J. Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 46.Roncarolo M-G, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 2001;193:5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhodapker MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonuliet H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, Ebeling SB, Lombardi G, Rustin MH, Bijlsma JW, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J. Clin. Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Feld1on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 52.Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, de Kleer I, Bonnin D, et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2004;101:4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]