Abstract
Amnestic mild cognitive impairment (aMCI) is characterized by decline in anterograde memory as measured by the ability to learn and remember new information. We investigated whether retrograde memory for autobiographical information was affected by aMCI. Eighteen control (age 66–84 years) and 17 aMCI (age 66–84 years) participants described a personal event from each of five periods across the lifespan. These events were transcribed and scored according to procedures that separate episodic (specific happenings) from semantic (general knowledge) elements of autobiographical memory. Although both groups generated protocols of similar length, the composition of autobiographical recall differentiated the groups. The aMCI group protocols were characterized by reduced episodic and increased semantic information relative to the control group. Both groups showed a similar pattern of recall across time periods, with no evidence that the aMCI group had more difficulty recalling recent, rather than remote, life events. These results indicate that episodic and semantic autobiographical memories are differentially affected by the early brain changes associated with aMCI. Reduced autobiographical episodic memories in aMCI may be the result of medial-temporal-lobe dysfunction, consistent with multiple trace theory, or alternatively, could be related to dysfunction of a wider related network of neocortical structures. In contrast, the preservation of autobiographical semantic memories in aMCI suggests neural systems, such as lateral temporal cortex, that support these memories, may remain relatively intact.
Keywords: remote memory, autobiographical memory, mild cognitive impairment, Alzheimer’s disease, multiple trace theory
Introduction
Amnestic mild cognitive impairment (aMCI) is a high-risk factor for Alzheimer’s disease (AD) (Petersen et al., 2001; Gauthier, et al., 2006) and is characterized by decline in anterograde memory as measured by the ability to learn and remember new information. In contrast to AD, in aMCI there is no evidence of decline, relative to same aged peers, in cognitive domains outside of memory and no functional decline involving daily activities (reviewed in Petersen, 2004). Whether there also is a decline in remote, episodic memory, however, is not known. The purpose of this study was to investigate whether there is any loss of remote memory in aMCI, particularly autobiographical memory, since in many patients with focal lesions involving the medial temporal lobes, anterograde amnesia often is accompanied by loss of remote memories.
Most behavioural studies indicate that the degree of impairment exhibited by individuals with aMCI on various types of tests of memory for recently acquired information, is intermediate to normal aging and AD (e.g., Perri et al., 2005, Troyer & Murphy, 2007). The same can be said of the neuroanatomical changes associated with aMCI, which show region-specific volume loss in the hippocampus and entorhinal cortex of the medial temporal lobes that are again intermediate to normal aging and AD (reviewed in Masdeu et al., 2005). Not surprisingly, the volumes of the hippocampus and its related structures are correlated with anterograde memory performance in normal aging (Rosen et al., 2003; Van Petten, 2004) and in MCI (Chételat et al., 2003), and atrophy in these regions is a sensitive predictor of progression from aMCI to AD (deToledo-Morrell, Goncharova, Dickerson, Wilson, & Bennett, 2000; Jack et al., 1999; Killiany et al., 2000). There is also evidence that semantic memory, such as retrieval of names of famous faces or names of animals, for example, is reduced in MCI (e.g., Estévez-González et al., 2004; Dudas et al., 2005; Murphy et al., 2006). Reduced semantic memory in MCI is consistent with neuroanatomical evidence of neuropathology extending beyond the hippocampal region into a network of related, neocortical structures that also show degeneration, albeit more marked, in Alzheimer’s disease (Chételat et al., 2002; Chételat & Baron, 2003; Karas et al., 2004). Together, these studies indicate that episodic memory for recently acquired information and semantic memory for remote impersonal information (e.g., news events, famous faces) are affected in MCI and may be related to early degenerative changes in a network that extends from the medial temporal lobe to related neocortical regions.
In this study, we were interested in whether there is any remote memory loss for personal past events because the changes in MTL and related areas noted in MCI correspond closely to the hipppocampal-neocortical network implicated in lesion and functional neuroimaging studies of autobiographical memory (reviewed in Moscovitch et al., 2005; Maguire, 2001; Svoboda et al., 2006). We were further interested in whether any memory loss for personal past events, if evident, affects episodic and semantic autobiographical memory equally. Similar to laboratory tasks of episodic and semantic memory, naturalistic autobiographical discourse can be used to examine memory for episodic and semantic information. For example, in recounting your childhood, remembering an autobiographical event when you were sent to the corner for talking out of turn is episodic; remembering the name of your first grade teacher is semantic. Substantial research suggests that episodic and semantic autobiographical memory can be dissociated (Tulving, 2002; Wheeler, Stuss & Tulving, 1997). Moreover, neuropsychological studies of patients (Moscovitch et al., 2005; Gilboa et al., 2005; Kapur, 1999), and functional neuroimaging studies of healthy adults (Maguire, 2001; Svoboda et al., 2006), suggest that the two forms of autobiographical memory differ in nature of the recruitment of brain regions, with medial temporal lobes especially involved in episodic, and lateral temporal cortex (particularly on the left) especially involved in semantic, autobiographical memory.
Past research investigating autobiographical memory in normal aging has shown that age-related decline is particularly marked in episodic, as compared to semantic, autobiographical memory (Levine et al., 2002; Piolino et al., 2002). It is well established that autobiographical memory is impaired in AD (e.g., Piolino et al., 2003; Meeter et al., 2006; Starkstein et al., 2005; Eustache et al., 2004), with both personal semantic and episodic autobiographical recall affected (Hou et al., 2005; Ivanoiu et al., 2006). Further, there is evidence suggesting these different forms of autobiographical memory are differentially correlated with region-specific brain volume loss in AD: semantic autobiographical memory is correlated with volume loss in bilateral anterior and posterior lateral temporal cortex (particularly on the left) and right frontal cortex, and episodic autobiographical memory is correlated with bilateral medial temporal regions and anterior lateral temporal cortex, particularly on the right (Gilboa et al., 2005). Consistent with this observation, Fujii et al. (2000), in reviewing the literature on patients with focal lesions, noted that remote episodic, autobiographical memory is impaired even with lesions confined to the medial temporal lobes whereas remote personal semantic memories do not become affected until the lesion extends into lateral temporal cortex.
In the existing literature, most investigations of autobiographical memory use separate tests to assess memory for episodic events and memory for personal semantics (e.g., names of friends, locations lived, schools attended, etc.), with the most widely used measure being the Autobiographical Memory Interview (AMI; Kopelman et al., 1989). These separate measures appear to artificially divide these two forms of autobiographical memory, which co-occur and interact in naturalistic autobiographical discourse, assessing them with tasks unmatched in sensitivity, content, and psychometric characteristics. Furthermore, episodic autobiographical memory is characterized by ordinal scale ratings that encompass both generic (e.g., repeated or not temporally specific) and specific autobiographical events. While performance on such measures is considered to reflect episodic autobiographical memory, contamination by semantic autobiographical memory cannot be ruled out.
The Autobiographical Interview (Levine et al., 2002), circumvents the above limitations by deriving both episodic and semantic information using the same test. This is accomplished with a reliable system for classifying episodic and semantic details from within a single transcribed autobiographical narrative. Consistent with research using the AMI developed by Kopelman et al., (1989) or closely similar variations (i.e., Piolino et al., 2002; Gilboa et al., 2005; Meeter et al., 2006; Bayley et al., 2006), the Autobiographical Interview measure has proven to be sensitive to normal aging (Levine et al., 2002), changes due to dementia (McKinnon et al., 2006; McKinnon et al., 2008), emotion manipulation (St.-Jacques & Levine, 2007), and especially to medial-temporal-lobe damage (Steinvorth et al., 2005; Rosenbaum et al., 2004; Rosenbaum et al., 2008; Addis et al., 2007; Kirwan et al., 2008).
The goal of the present study was to assess the effect of aMCI on episodic and semantic autobiographical memory for events across the lifespan. Two groups of older adults, one with age normal memory (controls) and one with amnestic mild cognitive impairment (aMCI), were administered an Autobiographical Interview, requiring them to recollect a personal past event for each of five different life periods ranging from early childhood to the past year.
We hypothesized that the aMCI group would produce less autobiographical information overall, as compared to controls, and that this would be due to impoverished output for episodic details in the aMCI group. Reduced episodic output would be in keeping with the fact that early changes in aMCI predominate in the entorhinal cortex and hippocampus (Masdeu et al., 2005), structures believed to be critical to the ability to recall personal episodic events (Moscovitch et al, 2005), though different theories make different predictions about its temporal extent (for review of an alternative account see Squire & Bayley, 2007). Although the neuropathology of MCI has been shown to extend beyond the hippocampus (e.g., Chételat & Baron, 2003), these extended changes are not as profound and consequently more difficult to quantify. One might expect that personal semantic memory, theorized to be less dependent on the hippocampus, would be less affected by the neuropathology associated with MCI. According to two influential theories of memory, multiple trace theory-MTT; Nadel & Moscovitch, 1997) and consolidation theory (Squire, 1992; Squire & Alvarez, 1995), the hippocampus initially contributes to retention and retrieval of semantic memory, but is not needed for it, so that semantic memory should be spared relative to episodic memory. Because personal semantic memory is spared in people with damage restricted to the medial temporal lobes with deficits emerging only when lateral temporal cortex is involved (Fujii et al., 2000), we expected that people with aMCI would have relatively preserved personal semantic memories.
Methods
Participants
Older adults with age normal memory (controls) and with mild memory decline greater than expected for their age (aMCI) were recruited for this study. A general estimate of cognitive status was obtained with the Mini-Mental Status Examination (MMSE; Folstein, et al., 1975), and an estimate of verbal intellectual ability was obtained for all participants with a vocabulary test (Wechsler, 1997; 3 controls received the Shipley, 1946).
Control group
Eighteen healthy older adults (age 66–84 years) were recruited from community talks, newspaper advertisements, and databases of research volunteers. On interview we verified that there was no history of neurological, medical, or psychiatric disorder, substance abuse, or medications affecting cognition. Performance was within normal limits for age and education on measures of: (a) general cognitive status (MMSE; Folstein et al., 1975); (b) immediate and delayed memory (Hopkins Verbal Learning Test-Revised, HVLT-R; Brandt & Benedict, 2001); and (c) self-reported mood (i.e., Geriatric Depression Scale, GDS; Yesavage et al., 1983; Hospital Anxiety and Depression Scale, HADS; Zigmond & Snaith, 1983; or clinical interview).
aMCI group
Seventeen individuals (age 66–84 years), 14 recruited from physician referrals and 3 from databases of research volunteers and newspaper advertisement, were classified as aMCI by consensus (K.J.M. & A.K.T.) according to criteria suggested by Petersen (2004). Specifically, each participant: (a) reported a memory complaint during interview; (b) exhibited objective memory impairment for age on cognitive testing (i.e., HVLT-R; Brief Visuospatial Memory Test-Revised, BVMT-R; Benedict, 1997; Logical Memory or Verbal Paired-Associates, Wechsler, 1987), defined as memory scores on two or more memory measures lower than expected based on age, education, and estimated IQ (Petersen, 2004). No particular cut-off score was used, although as Table 1 shows, recall scores on the HVLT-R were, on average, 1.5 standard deviations lower than demonstrated verbal ability in the aMCI group; (c) demonstrated normal general cognitive status for age and education, that is, performance was within 1 standard deviation of the mean based on age normative data, on the MMSE and on measures of attention (Digit Span; Wechsler, 1997), confrontation naming (Boston Naming Test; Kaplan, et al., 1983), visuospatial construction (Rey-Osterrieth Complex Figure copy; Spreen & Strauss, 1998), and Trail Making Test (Spreen & Strauss, 1998; or Delis, Kaplan, & Kramer, 2001); (d) no substantial interference with normal daily activities as determined by detailed clinical interview (e.g., personal banking, grocery shopping); and (f) no dementia, determined by taking into consideration all previous criteria with specific emphasis on the absence of functional impairment. An additional required criterion included the absence of medical or psychiatric conditions that could account for the memory decline, other than possible incipient AD, determined by review of medical history and current self-reported mood status on the GDS or HADS.
Table 1.
Demographic and Descriptive Data for the Participant Groups
| Control (n = 18) |
aMCI (n = 17) |
Cohen’s d | |
|---|---|---|---|
| Age (years) | 74.2 (6.4) | 76.2 (5.7) | 0.33 |
| Female:Male ratio | 8:10 | 10:7 | |
| Education (years) | 13.6 (3.5) | 14.5 (2.8) | 0.27 |
| MMSE+ | 28.2 (1.7) | 27.3 (2.0) | 0.45 |
| Vocabulary SS | 13.6 (2.9) | 13.8 (2.7) | 0.07 |
| HVLT-R Immediate SS | 10.1 (2.1) | 6.9 (2.2) | 1.21** |
| HVLT-R Delay SS | 10.7 (1.8) | 4.29 (2.6) | 1.63** |
Note. Mean scores with standard deviations in parentheses.
group differences p < .001. aMCI = amnestic mild cognitive impairment; MMSE = Mini-Mental Status Exam; HVLT = Hopkins Verbal Learning Test-Revised; SS = age-corrected scaled score.
MMSE scores were not available for 3 control participants.
As Table 1 shows there was no significant difference in the ratio of females to males between the two participant groups χ = .72; p < .39 nor were there group differences on demographic variables relating to age, education, estimated verbal ability, and a general index of cognitive status (all p’s > .05).
Procedure
Participants were given the Autobiographical Interview test (see Levine et al., 2002) as part of a larger battery of tests. They were asked to tell the examiner about a personal past memory event that happened at a specific time and place for each of five different life periods in the following order: early childhood (up to age 11), teenage years (age 11 to 18), early adulthood (age 18 to 30), middle adulthood (age 30 to 55), and in the past year.
Participants were told to choose any events they wished subject to the following conditions: they must be (a) events in which they were personally involved, (b) events they could recollect as opposed to events they heard about from others, and (c) events specific to a time and place, such as an incident that occurred one day on vacation, as opposed to events that extended over a long period, such as an entire three-week vacation. Participants were also told to provide as many details as possible because the examiner was interested in how the event was described as much as what the event was about.
For each life period, a card was placed in front of the participant with specific instructions, for example, “Tell me about an event that happened at a specific time and place during your early childhood (up to age 11).” The participant was then allowed to speak without interruption until he or she was finished or five minutes had elapsed, whichever came first. General probes were given to encourage recall of detailed information, particularly if the participant had trouble coming up with a specific detailed memory or provided a very brief recollection. The examiner adhered to a strict protocol for administering general probes, which, while not limited in number, were limited in nature to non-specific statements and clarifications of instructions (e.g., “Is there anything else you can tell me? Do you remember any other details?”). Because of time constraints, the specific probing condition, described in Levine et al., (2002), that involves a structured interview designed to assess retrieval support effects, was not administered.
Responses were audio-recorded and later transcribed for scoring. The standardized scoring protocol developed by Levine et al., (2002) is available upon request (from B.L.). Scoring involved segmenting each event recalled into details. Details were defined as unique occurrences, observations, or thoughts, typically bound within a grammatical unit. These details were then categorized as internal or external. The identification of internal and external details and their subtypes is described subsequently, and detail scoring is additionally illustrated in two examples of autobiographical recall, one generated by a participant with aMCI and one generated by a control participant (see Appendix).
Internal details were defined as details about the main memory event described that were specific to time and place; thus, internal details reflect episodic re-experiencing of the incident. These internal/episodic details were subcategorized into: (a) event (happenings, people involved, actions and reactions of self and others, nature of the environment i.e., weather conditions), (b) place (information about where the event occurred), (c) time (date, season, or time of day references), (d) perceptual (sensory information relating to sights, sounds, smells, etc.) and (e) emotion/thought (feelings and thoughts relating to the event).
External details pertained to extraneous information that was not uniquely specific to the main memory event being described and not anchored to the time and place of the incident of interest. These external details were subcategorized into: (a) semantic (general facts/knowledge related to the context of the event), (b) a detail repetition, (c) details concerning unrelated events and (d) other (editorializing, metacognitive statements).
The detail subtypes within each category were summed to form Internal and External detail composite scores, which were the primary measures of interest. The ratio of internal-to-total details generated was also calculated (i.e., internal composite / (internal composite + external composite)) to provide information about the proportion of details per memory out of the total number of details generated that reflected episodic re-experiencing.
Inter-rater reliability for two independent raters on a subset of one memory from each of 10 participants (3 controls and 7 aMCI) indicated high agreement with respective coefficients (r) of 0.96 and 0.99 for the episodic and semantic detail composite scores.
Autobiographical narratives reflect personal subjective experiences that are by nature difficult to verify. Thus, recall accuracy is typically not analyzed in procedures investigating personal remote memories (e.g., Levine et al., 2002; Kopelman et al., 1989; Piolino et al., 2002; Meeter et al., 2006; and for further discussion of this issue see Moscovitch et al., 2006). Confabulation in aMCI was considered an unlikely influence as this is not an associated feature. Thus, our emphasis was not on accuracy, but rather on how details were distributed across internal and external categories among the participant groups.
For our main analyses, we used mixed-design ANOVAs with planned comparisons conducted at p < .05. These analyses were conducted with data pooled across the between-subject factor of sex because no significant main effects or interpretable interactions were obtained for analyses involving this factor. To explore possible relations between autobiographical variables and traditional neuropsychological measures, we conducted specific correlational analyses. We also calculated sensitivity and specificity of autobiographical scores in classifying participants as normal controls versus individuals with aMCI in order to assess the degree to which performance on the Autobiographical Interview can differentiate these two participant groups.
Results
As we shall show in the subsequently described statistical analyses, the aMCI group produced autobiographical narratives that were characterized by reduced internal (episodic) and increased external details, which were predominantly semantic, relative to the control group.
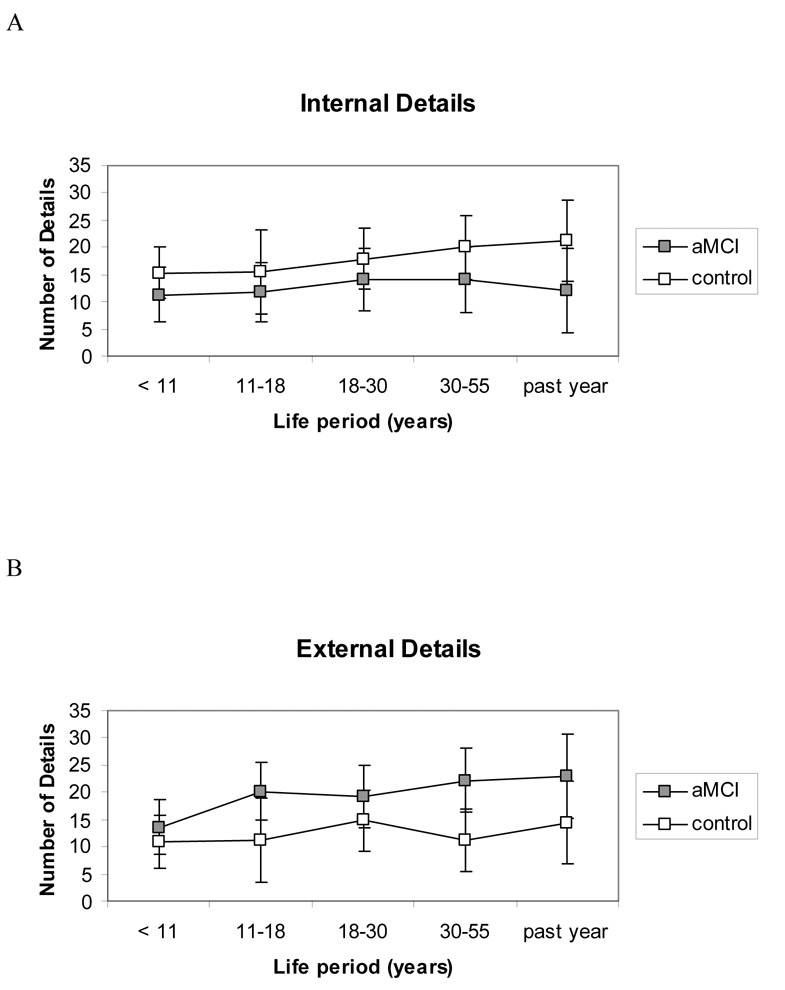
The total number of details generated, summed over all life periods, was comparable between the groups (aMCI M = 161.29; SD = 61.76 and control M = 152.22; SD = 59.07; F(1,33) = 0.20, p = 0.66, ηp2 = 0.01) ), indicating that both groups produced protocols of similar lengths. There was a main effect of life period, F(4,132) = 4.12, p < .01, ηp2 = 0.11, whereby fewer details were generated in the earlier life periods as compared to the later life periods (<11 years M = 25.54; SD = 12.18; 11–18 years M = 29.17; SD = 13.09; 18–30 years M = 33.02; SD = 15.32; 30–55 years M = 33.60; SD = 16.17; past year M = 35.29; SD = 20.20). A group-by-detail composite score interaction, F(1,33) = 16.12, p < .001, ηp2 = 0.33, revealed that the control group recalled more internal details (M = 89.83; SD = 39.21) than the aMCI group (M = 63.18; SD = 22.12) whereas the aMCI group recalled more external details (M = 98.12; SD = 54.92) than the control group (M = 62.39; SD = 27.42) (see Figure 1a and b). There was no significant interaction involving life period, indicating that the group effects were consistent across the lifespan.
Figure 1.
The number of details generated during autobiographical recall, for each life period according to (A) internal and (B) external detail categories. There is no group difference in the total number of details generated, but there is a significant group-by-detail-category interaction. Controls generate more internal details and aMCI participants more external details when recollecting personal events. Error bars represent 95% confidence intervals. aMCI = amnestic mild cognitive impairment.
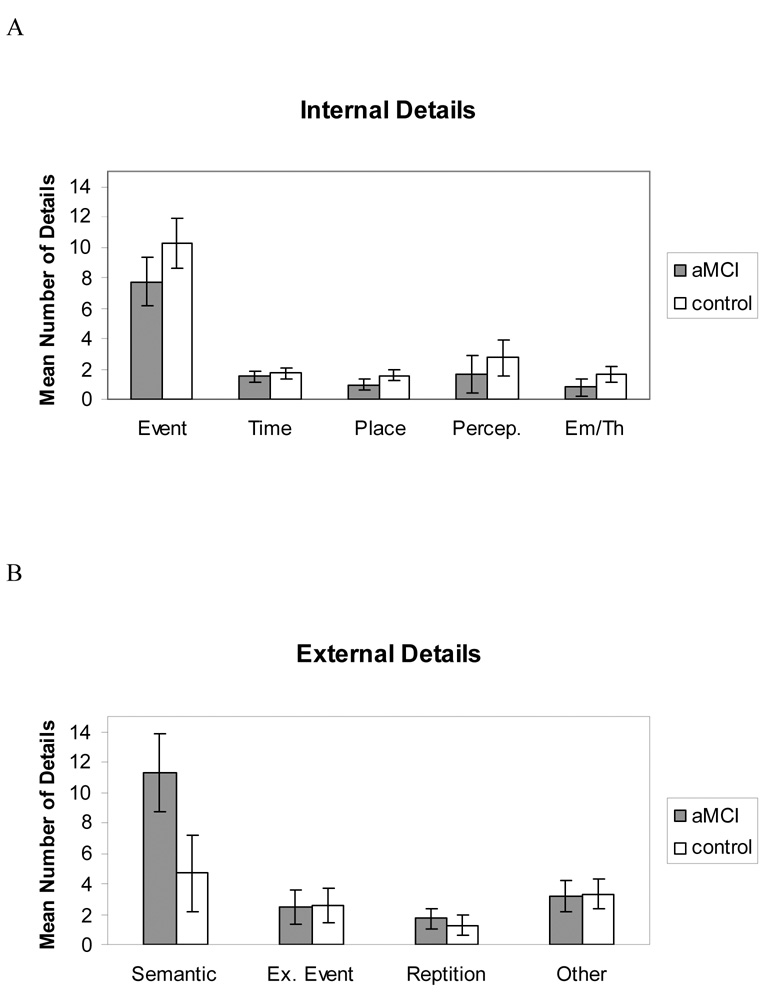
We next examined the effects of specific detail categories within each composite score and found the pattern of predominant detail subtypes (seen in Figures 2a and 2b) was consistent with data reported by Levine et al. (2002) in normal older and younger adults. For internal details, there was a main effect of detail category (F(4,30) = 64.91, p < .001, ηp2 = 0.89), with event details predominating over other detail types (all p’s < .001). The detail-category-by-group interaction was not significant (F(4,30) = 1.78, p = .16, ηp2 = 0.19). There was a main effect of external detail category (F(3,30) = 40.36, p < .001, ηp2 = 0.79), but this was qualified by a significant interaction with group (F(3,30) = 5.59, p < .01, ηp2 = 0.35). For each group, and consistent with past research (e.g., Levine et al., 2002), semantic details predominated over all other detail types (all p’s < .05). The interaction was due to a significant elevation of semantic details for the aMCI group over the control group (p < .001).
Figure 2.
Categorization of details comprising autobiographical memories, with the number of details averaged across the five life periods, for each participant group. (A) Internal details representing episodic information. The event subcategory represents the majority of the output. (B) External details are comprised primarily of semantic information, with the semantic subcategory representing the majority of the output. There is also a significant group-by-external-detail-type interaction whereby semantic details are significantly elevated in the aMCI group as compared to the control group. Error bars represent 95% confidence intervals. Percep. = Perceptions, Em/Th = Emotion / Thought, Ex. Event = External Event, and aMCI = amnestic mild cognitive impairment.
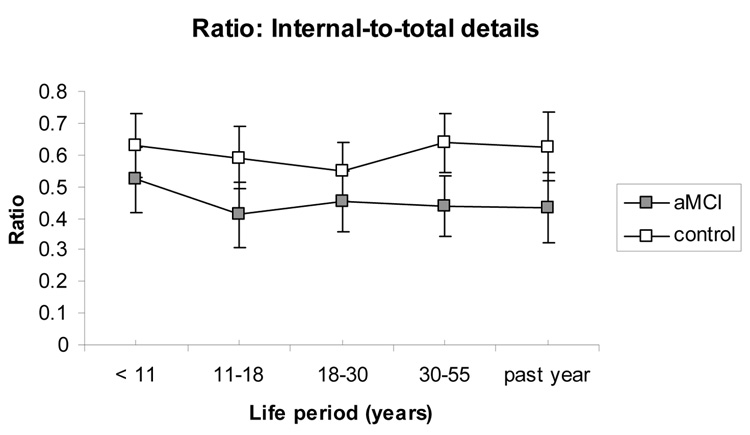
As seen in Figure 3, and consistent with the previously described interaction, the internalto-total-detail ratio was significantly different between groups, F(1,33) = 10.95, p < .01, ηp2 = 0.25, indicating control group autobiographical memories contained more episodic re-experiencing as compared to the aMCI group. There was no effect of life period, F(4,132) = 1.41, p = 0.34, ηp2 = 0.03, and no interaction, F(4,132) = 0.76, p = 0.55, ηp2 = 0.02, for the ratio data.
Figure 3.
The ratio of internal-to-total number of details generated, representing an index of episodic re-experiencing in autobiographical recall, shows no clear temporal gradient and is greater in the control as compared to the aMCI group. Error bars represent 95% confidence intervals. aMCI = amnestic mild cognitive impairment.
Correlations were calculated, within the aMCI group, between the internal-to-total ratio score and selected neuropsychological measures, including delayed recall on HVLT-R and BVMT-R, semantic and phonemic fluency, Boston Naming test, and Trails switching. All correlations were small to medium in size (r’s = .22 to .44), and none were statistically significant (p’s = .10 to .39), which is not surprising given the small sample size of 17.
Sensitivity and specificity in classifying aMCI and control participants were also calculated using the internal-to-total ratio score averaged across the five time periods. We selected a cutoff score of 0.48, because this score showed the highest overall accuracy (i.e., 86%; 95% confidence interval = 74% – 97%) in classifying the two participant groups. This score showed a sensitivity of 76% (i.e., 13 of 17 participants with aMCI scored below the cutoff) and a specificity of 94% (i.e., 17 of 18 control participants scored at or above the cutoff).
Discussion
The performance of our aMCI participants indicates that memory for autobiographical episodes is impaired whereas personal semantic memory remains relatively preserved. Compared to controls, the aMCI group produced fewer episodic, event-specific details in their recollections, as indicated by their internal detail composite score. By contrast, their external detail score was elevated relative to controls, which was due primarily to increases in the number of semantic details the aMCI group produced. Importantly, the total number of details across all categories was equivalent in the two groups, indicating that the deficit that is observed cannot be ascribed to general loss of fluency or lack of motivation on the part of people with aMCI.
Reduced episodic but elevated semantic autobiographical memory in aMCI resembles the pattern observed for normal aging on this task, whereby older adults achieved output comparable to that of younger adults, but produced memories that contained fewer episodic and more semantic details (Levine et al., 2002). This pattern simply is magnified further in aMCI. By contrast, in AD, both personal semantics and episodic event memory are impaired. Thus, our results are consistent with the common observation that the impairment in aMCI is intermediate to that of normal aging and AD, although it remains to be seen whether semantic details as assessed by the Autobiographical Interview are reduced or elevated in patients with AD.
The findings are consistent with our predictions based on research showing that region-specific atrophy predominates in the hippocampus and related structures (see Masdeu et al., 2005) and on predictions from Nadel and Moscovitch’s (1997) multiple trace theory (MTT) that episodic memory is disproportionately affected, as compared to personal semantic memory, by hippocampal damage. MTT posits that the hippocampus is necessary for the retrieval of both anterograde and retrograde episodic information, but not for established (retrograde) semantic knowledge. According to MTT and other theories (McClelland, McNaughton, and O’Reilley, 1995), the neocortex extracts regularities across episodes to form the basis of semantic memory, whereas details that are unique to a particular episode continue to be dependent on the hippocampus. The AI captures this distinction in its scoring procedure. Though separable conceptually and neurologically, episodic and semantic memory, nonetheless, interact with one another under most conditions in normal people, such that one can detect the influence of one on the other (see Westmacott & Moscovitch, 2003, Westmacott et al., 2003, and discussion in Moscovitch et al, 2005).
There was no evidence of a temporal gradient across life periods with reduced recall of recent as compared to remote events and no interactions with life period. Our results indicate that memories are affected across the entire lifespan with the most remote events perhaps being the most forgotten, because both aMCI and normal controls generated more details for recent than remote life periods; this pattern is similar to previous reports investigating autobiographical memory and normal aging (e.g., Levine et al, 2002; Piolino et al., 2002). Participants only recalled one event per time period. This event was presumably the most accessible and therefore the most likely to yield detailed recollection, whereas additional events may have been less accessible to recall. We have shown that recall of additional events does not alter the age-related pattern of reduced episodic and increased semantic details (St-Jacques & Levine, 2007). Overall, the findings are consistent with MTT which proposes that the hippocampus is necessary for episodic re-experiencing regardless of the age of the memory, but not for retention and retrieval of remote semantic knowledge (reviewed in Moscovitch et al., 2005).
Alternatively, it is possible that extensive retrograde amnesia for detailed autobiographical episodes is related not only to early degeneration of the medial temporal lobes, but also to degeneration of a related network of neocortical structures implicated in retrieval of autobiographical memories (Addis et al., 2004, 2007; Bright et al., 2006; Maguire et al., 2001; Gilboa et al., 2004; Moscovitch et al., 2005; Svoboda et al., 2006). These structures, which include the medial and anterior temporal lobes, inferior parietal lobule, regions around retrosplenial cortex and the ventromedial prefrontal cortex are part of the default system (Fox & Raichle, 2002; Schacter et al., 2007) that typically also is affected in Alzheimer’s disease (Buckner et al, 2005). Thus, the loss of detailed autobiographical memories we observed in people with aMCI may additionally reflect some very early dysfunction of these regions.
The semantic autobiographical details provided by our aMCI participants pertained to information not tied to a spatial or temporal context and, thus, not dependent on the hippocampus. This would suggest that whereas aMCI might be associated with impaired remote semantic memory related to impersonal information, such as famous faces (reviewed previously), it does not appear to affect autobiographical memory for personal semantic information, especially information connected to a personally-relevant event. This finding is consistent with observation from patients with focal lesions involving the medial temporal lobes (MTL; Steinvorth et al., 2005; Rosenbaum et al., 2008). Although impaired memory for public events and personalities is observed when lesions extend beyond the hippocampus into the MTL, loss of personal semantic information is not evident until the lateral temporal cortex is implicated (Fujii et al, 2000). The significant elevation of semantic autobiographical details in aMCI may reflect either compensation for lack of episodic details or a form of disinhibition, perhaps because they are over-rehearsed and thus more readily available.
In our clinical experience, the memory complaints of clients with aMCI are largely focused on anterograde episodic memory errors relating to failures to remember to carry out intentions, misplacing objects, and difficulty recalling the details of recently acquired information, as well as retrograde semantic memory failures relating to names of people and occasionally to word-finding problems in general conversation. These complaints are consistent with objective weaknesses reported in the literature. Interestingly, individuals with aMCI often contrast their subjective memory failures with their perceived strengths in having a robust memory for remote information relating to their personal past. The current findings suggest that the absence of subjective complaints regarding autobiographical memory is related to their intact access to semantic autobiographical information, as well as access to episodic details (although reduced relative to controls). Previous research into the neural substrates supporting memory indicates that there are shared and unique regions of involvement in mediating episodic and semantic autobiographical and non-autobiographical memory (for review see Svoboda et al., 2006).
The calculations of sensitivity and specificity using our measure of autobiographical recall, specifically the episodic-to-total ratio score, indicated that this measure was equivalent to other recall scores in categorizing aMCI and control participants. Our previous research (Troyer et al., 2008 (a)) showed accuracies of 83% and 92 when using two different associative recall tasks. Both of these accuracies overlap with the 86% accuracy (confidence interval = 74% – 97%) obtained from our measure of autobiographical recall in the present study. We plan to follow these patients to see if the extent of impairment and compensation is predictive of the onset of AD.
Autobiographical memories over the lifespan are comprised of episodic and semantic components that are often tied to an emotional context. These memories are believed to be the means by which we formulate our sense of self and of continuity, and also guide our future behaviour (Conway, 1997; Tulving, 2002). Impaired autobiographical memory in AD is likely a contributing factor to functional decline in this population. Individuals with aMCI have a high probability (i.e., more than 80 percent over six years) of developing a future dementia due to AD (Petersen et al., 2001). We have attempted to prolong the level of independence in aMCI individuals through memory interventions aimed at addressing failures of recent memory (Troyer et al., 2008 (b)) which have obvious functional ramifications (e.g., failure to carry out an intended activity, such as taking medication). The current results indicate intervention directed toward enhancing and preserving episodic re-experiencing of personal past memories in aMCI is also worthy of consideration. Research into providing direction in how to preserve memory for personal past events may also affect the course of functional decline in those with incipient AD by promoting maintenance of awareness of self in temporal context.
In conclusion, the findings reported here show that episodic and semantic autobiographical memories are differentially affected by the early brain changes associated with aMCI, with a significant reduction in episodic as compared to semantic content. These results afford us important insights into how the earliest brain changes associated with the neuropathology of AD evolve and affect memory systems in the brain. Interpretation of these findings within the context of multiple trace theory and autobiographical memory implicates dysfunction of a neural network centred on the medial temporal lobe as predominantly affecting episodic autobiographical memories with possibly preserved neural integrity in systems, such as lateral temporal cortex, that support semantic autobiographical memory.
Acknowledgements
We gratefully acknowledge the participants for generously volunteering their memories, time, and effort. We thank Alex Proulx, Namita Kumar, Triti Namiranian, Brent Hayman-Abello, Linda Moradzadeh, Julia Cheng, and Angelina Polsinelli for assistance with data collection, scoring, and entry. Dr. Eva Svoboda kindly provided her insights into the neural substrates supporting autobiographical memory. Supported by a grant from the Alzheimer’s Society of Canada (Grant # 04–61 to A.K.T., K.J.M. & M.M) and through funding from the Morris Goldenberg Medical Research Endowment, Desjardins Financial, and Richter Usher and Vineberg, and by grants the Canadian Institutes of Health Research (Grant #’s MT-14744, MOP-37535; MOP-108540 to B.L.) and the NIH-NICHD (Grant # HD42385-01 to B.L).
Appendix
Selected representative sections from actual protocols generated by a control participant and a participant with amnestic mild cognitive impairment (aMCI) for an event that occurred within the last year. These examples, demonstrate the application of the scoring method and the pattern whereby aMCI group protocols show reduced internal and increased external information relative to the control group. Please see methods section for description of these detail types and categories.
Control Participant – describing an event on vacation
Time Event Event Place Event
Last year, my wife and I took our three grandkids to Disney World and went on a lot of
Event Event
rides. They were all rollercoaster rides, but there was this one, space mountain, it was the
Emotion Event
most terrifying thing I’ve ever been on in my life. So the five of us get on there and it was
Perceptual Perceptual
like we were sitting in a one man car all the time, all one behind the other and you race
Event Perceptual Event
through this mountain all in darkness. ….. I was almost throwing up a couple of times. …..
Event Event Event
Kids loved it and went right back in again. Not me, I had enough of that one ride. ….. I
Emotion External Emotion Repetition
really enjoyed it. I thought I would hate it, taking three kids to Disney World, drive me nuts;
Event
but, they were great.
aMCI Participant – describing an event during a visit with family
Semantic Semantic
Well our son and daughter live in Michigan and they have a country place and I remember
Other External time External Event
during one of those times, because first of all, six months prior to that, they lost their dog, which
Semantic Event
was a great treasure to them …… We happened to be there shortly after Haddy’s death,
Semantic Semantic Semantic
Which was the name of the dog, and so they had learned of a dog whose owner lived in a two-
Semantic Event
room apartment and wanted to find a decent home for his dog….. So anyway, my daughter-in-
Event Event External Event Other
law and son were ecstatic when they arrived back with this dog. They had to drive, I don’t know
External Perceptual External Event
how far, a hundred miles anyway. They had been told that it might be a dog that they were
Semantic Time
interested in because they have contacted a few breeders and so that did happen last year and
Event
we were there when they brought the dog home.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. The Journal of Neuroscience. 2006;26(51):12211–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test-Revised. Lutz, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Bright P, Buckman J, Fradera A, Yoshimasu H, Colchester ACF, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learning and Memory. 2006;13:545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron J-C. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, Lalevee C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Baron J-C. Early diagnosis of Alzheimer’s disease: contribution of structural neuroimaging. Neuroimage. 2003;18:535–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Conway MA. Inventory experience memory identity. In: Pennebacker JW, Paez D, Rimae B, editors. Collective memory of political events: Social psychological perspectives. Hillsdale, NJ: Lawrence Erlbaum Associates Inc.; 1997. pp. 21–45. [Google Scholar]
- Cooper JM, Shanks MF, Venneri A. Provoked confabulations in Alzheimer’s disease. Neuropsychologia. 2006;44:1697–1707. doi: 10.1016/j.neuropsychologia.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- deToledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From health aging to early Alzheimer’s disease: In vivo detection of entorhinal cortex atrophy. Annals of the New York Academy of Sciences. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Dudas RB, Clague F, Thompson SA, Graham KS, Hodges JR. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia. 2005;43:1266–1276. doi: 10.1016/j.neuropsychologia.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Estévez-González A, García-Sánchez C, Boltes A, Otermín P, Pascual-Sedano B, Gironell A, Kulisevsky J. Semantic knowledge of famous people in mild cognitive impairment and progression to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2004;17:188–195. doi: 10.1159/000076355. [DOI] [PubMed] [Google Scholar]
- Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron J-C, Desgranges B. ‘In the course of time’: A PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain. 2004;127:1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2002;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fujii T, Moscovitch M, Nadel L. Consolidation, retrograde amnesia, and the temporal lobe. In: Boller F, Grafman J J, Cermak LS, editors. The Handbook of Neuropsychology. 2nd Edition. Volume 4. Amersterdam, The Netherlands: Elsevier; 2000. pp. 223–250. [Google Scholar]
- Gauthier S, Reisberb B, Zaudig M, Petersen RC, Ritchie K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Ramirez J, Köhler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer’s disease: Relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15:535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Hou CE, Miller BL, Kramer JH. Patterns of autobiographical memory loss in dementia. International Journal of Geriatric Psychiatry. 2005;20:809–815. doi: 10.1002/gps.1361. [DOI] [PubMed] [Google Scholar]
- Ivanoiu A, Cooper JM, Shanks MF, Venneri A. Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia. 2006;44:1936–1955. doi: 10.1016/j.neuropsychologia.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kapur N, Thompson P, Kartsounis LD, Abbott P. Retrograde amnesia: clinical and methodological caveats. Neuropsychologia. 1999;37(1):27–30. doi: 10.1016/s0028-3932(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, Barkhof Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of Neurology. 2000;47:430–439. [PubMed] [Google Scholar]
- Kirwan CB, Bayley PJ, Galván VV, Squire LR. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proceedings of the National Academy of Sciences. 2008;105:2676–2680. doi: 10.1073/pnas.0712155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesiac patients. Journal of Clinical and Experimental Neuropsychology. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroantamony of episodic and semantic autobiographical remembering: A prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Henson RNA, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12(3):441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Zubieta JL, Arbizu J. Neuroimaging as a marker of the onset and progression of Alzheimer’s disease. Journal of the Neurological Sciences. 2005;236:55–64. doi: 10.1016/j.jns.2005.05.001. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mc Kinnon MC, Black SE, Miller B, Moscovitch M, Levine B. Autobiographical memory in semantic dementia: Implications for theories of limbic-neocortical interaction in remote memory. Neuropsychologia. 2006;44:2421–2429. doi: 10.1016/j.neuropsychologia.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Nica EI, Sengdy P, Kovacevic N, Moscovitch M, Freedman M, Miller BL, Black SE, Levine B. Autobiographical memory and patterns of brain atrophy in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2008.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter M, Eijsackers EV, Mulder JL. Retrograde amnesia for autobiographical memories and public events in mild and moderate Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychiatry. 2006;28:914–927. doi: 10.1080/13803390591001043. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum S. The cognitive neurosicent of remote episodic, semantic, and spatial memory. Current Opinion in Neurobiology. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrew MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Rich JB, Troyer AK. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer’s type dementia. Journal of the International Neuropsycholgoical Society. 2006;12:570–574. doi: 10.1017/s1355617706060590. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Perri R, Carlesimo GA, Serra L, Caltagirone C. Characterization of memory profile in subjects with amnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2005;27:1033–1055. doi: 10.1080/13803390490919317. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevée C, De La Sayette V, Eustache F. Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F. Episodic and semantic remote autobiographical memory in ageing. Memory. 2002;10:239–257. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JDE, Stoub T, O’Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behavioural Neuroscience. 2003;117(6):1150–1160. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, McKinnon MC, Levine B, Moscovitch M. Visual imagery deficits, impaired strategic retrieval, or memory loss: disentangling the nature of an amnesic person's autobiographical memory deficit. Neuropsychologia. 2004;42(12):1619–1635. doi: 10.1016/j.neuropsychologia.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Moscovitch M, Foster JK, Verfaellie M, Gao FQ, Black SE, et al. Patterns of autobiographical memory loss in medial temporal lobe amnesic patients. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2008.20105. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RJ. Remembering the past to imagine the future. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Current Opinion in Neurobiology. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, momkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Boller F, Garau L. A two-year follow-up study of remote memory in Alzheimer’s disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:336–341. doi: 10.1176/jnp.17.3.336. [DOI] [PubMed] [Google Scholar]
- St.-Jacques P, Levine B. Aging and emotional autobiographical memory. Memory. 2007;15:129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: Evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Svoboda E, Mc Kinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time- and event- based prospective memory. Journal of the International Neuropsycholgoical Society. 2007;13:365–369. doi: 10.1017/S1355617707070452. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, Hayman-Abello BA, Craik FIM, Moscovitch M. Item and associative memory in amnestic mild cognitive impairment: Performance on standardized memory tests. Neuropsychology. 2008a;22:10–16. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, Moscovitch M, Craik FIM. Changing everyday memory behaviour in amnestic mild cognitive impairment: A randomized-control clinical trial. Neuropsychological Rehabilitation. 2008b;18:65–88. doi: 10.1080/09602010701409684. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic Memory: From Mind to Brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2003;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Adult Intelligence Scale - III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale –Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Westmacott R, Black SE, Freedman M, Moscovitch M. The contribution of autobiographical significance to semantic memory: evidence from Alzheimer’s disease, semantic dementia, and amnesia. Neuropsychologia. 2003;42:25–48. doi: 10.1016/s0028-3932(03)00147-7. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Memory and Cognition. 2003;31:761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum W, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]