Abstract
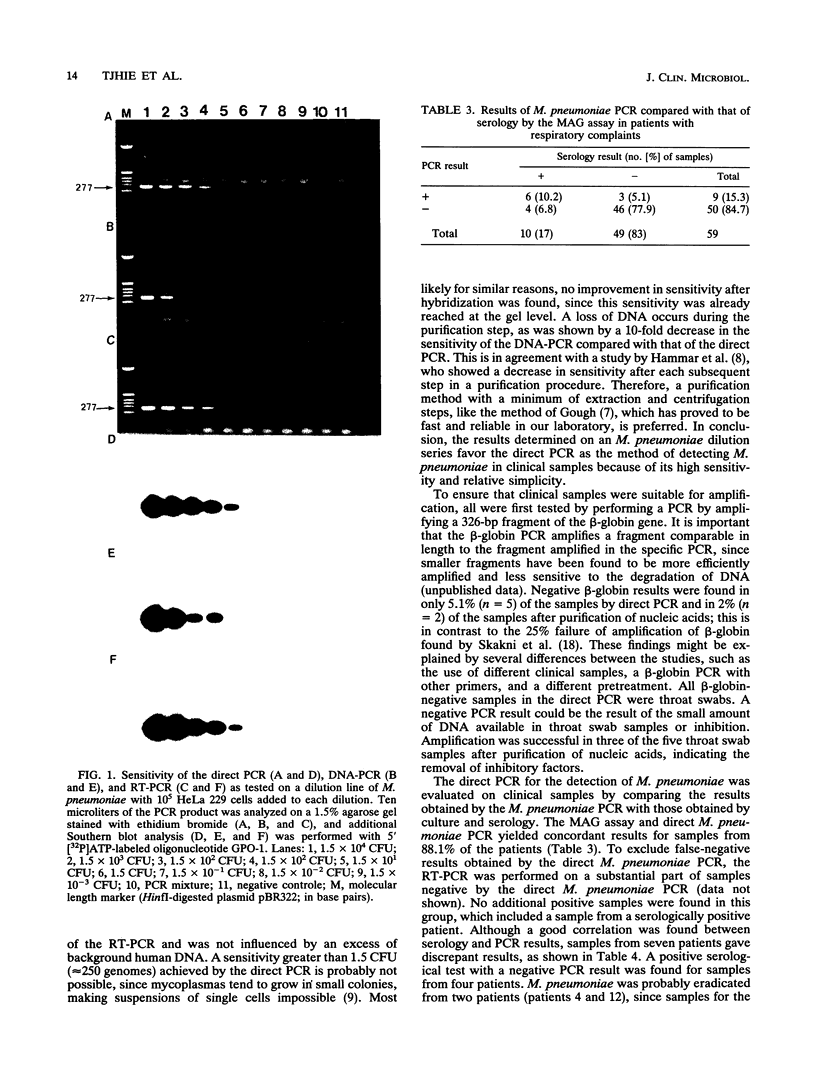
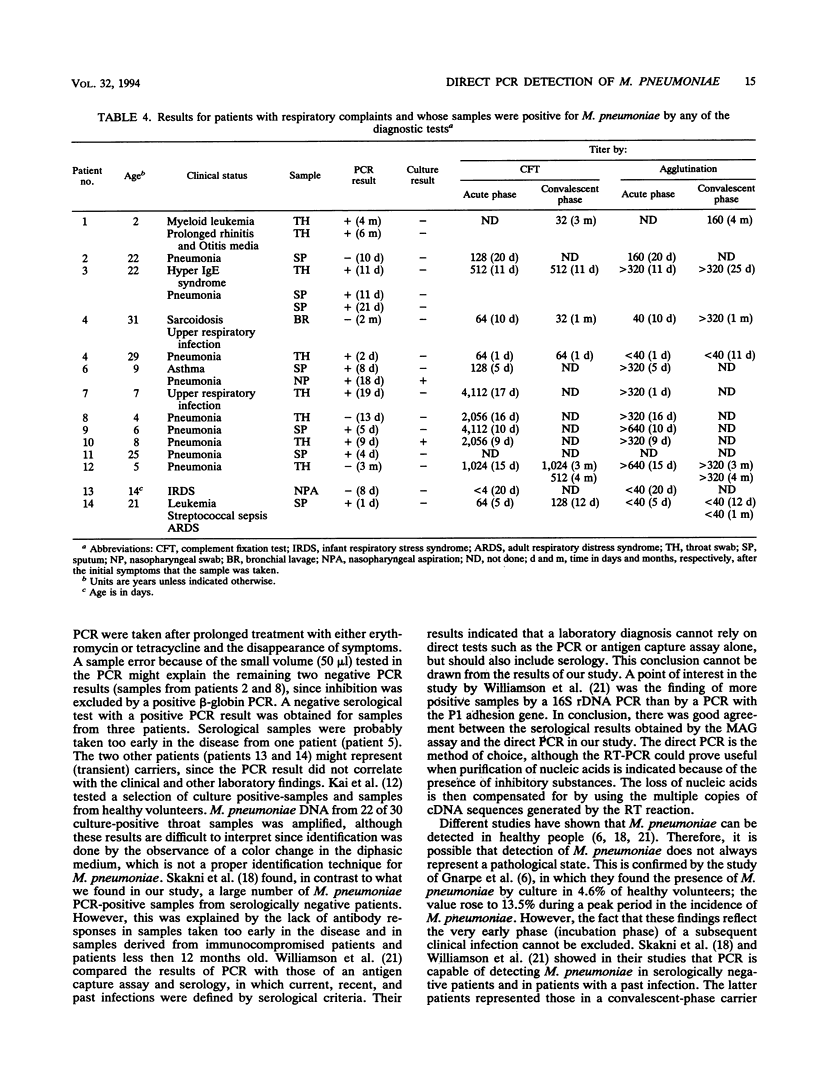
The sensitivities of three methods of detection of Mycoplasma pneumoniae by a 16S rDNA PCR were compared by using a serial dilution of M. pneumoniae. These methods consisted of a PCR performed directly on cells after a proteinase K pretreatment (direct PCR), a PCR after purification of nucleic acids (DNA-PCR), and a PCR with rRNA sequences as the target after reverse transcription. The direct PCR and the reverse transcription PCR had a sensitivity of 1.5 CFU (approximately 250 genomes). By purification, a 10-fold loss of target DNA occurred, as shown by a 10-fold decrease in sensitivity (15 CFU) of the DNA-PCR. The presence of an excess of human background DNA did not influence the sensitivity of either PCR. The direct PCR was evaluated on samples from patients with respiratory complaints. Direct PCR amplification was possible in 94.9% of the samples, which were tested by amplification of a 326-bp fragment of the beta-globin gene, which was performed to test for the suitability of amplification. Nucleic acid purification was performed on the beta-globin-negative samples, after which only 2% remained negative. A positive correlation between the direct M. pneumoniae PCR and serology, as tested by the microparticle agglutination assay (MAG assay), was found in 88.1% of the cases. A positive MAG assay result was found for samples from 10 (17%) of the patients; samples from 6 (10.2%) of these patients were also positive by PCR. Samples from three patients were found to be positive by the M. pneumoniae PCR and negative by the MAG assay. Persistence of M. pneumoniae, as detected by PCR was observed in three patients. These results indicate that the direct PCR with 16S rDNA could prove to be useful in the detection of M. pneumoniae in respiratory tract samples, although more studies are needed to evaluate the correlation between clinical symptoms and positive test results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría J. M., León P., Balfagón P., López J. A., Fernández M. V. Diagnosis of Mycoplasma pneumoniae infection by microparticle agglutination and antibody-capture enzyme-immunoassay. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):217–220. doi: 10.1007/BF01963842. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. New molecular techniques for microbial epidemiology and the diagnosis of infectious diseases. J Infect Dis. 1990 Apr;161(4):595–602. doi: 10.1093/infdis/161.4.595. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Gnarpe J., Lundbäck A., Sundelöf B., Gnarpe H. Prevalence of Mycoplasma pneumoniae in subjectively healthy individuals. Scand J Infect Dis. 1992;24(2):161–164. doi: 10.3109/00365549209052607. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Hammar M., Tyszkiewicz T., Wadström T., O'Toole P. W. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):54–58. doi: 10.1128/jcm.30.1.54-58.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R., Marmion B. P., Varkanis G., Kok T., Lunn B., Martin J. Laboratory diagnosis of Mycoplasma pneumoniae infection. 2. Comparison of methods for the direct detection of specific antigen or nucleic acid sequences in respiratory exudates. Epidemiol Infect. 1988 Dec;101(3):685–694. doi: 10.1017/s0950268800029563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. A., Hoyle J. A., Lewis F. A., Secker A. D., Cross D., Mapstone N. P., Dixon M. F., Wyatt J. I., Tompkins D. S., Taylor G. R. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol. 1991 Nov;29(11):2543–2549. doi: 10.1128/jcm.29.11.2543-2549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Søndergård-Andersen J., Uldum S. A., Lind K. Detection of Mycoplasma pneumoniae in simulated clinical samples by polymerase chain reaction. Brief report. APMIS. 1989 Nov;97(11):1046–1048. doi: 10.1111/j.1699-0463.1989.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Kai M., Kamiya S., Yabe H., Takakura I., Shiozawa K., Ozawa A. Rapid detection of Mycoplasma pneumoniae in clinical samples by the polymerase chain reaction. J Med Microbiol. 1993 Mar;38(3):166–170. doi: 10.1099/00222615-38-3-166. [DOI] [PubMed] [Google Scholar]
- Narita M., Matsuzono Y., Togashi T., Kajii N. DNA diagnosis of central nervous system infection by Mycoplasma pneumoniae. Pediatrics. 1992 Aug;90(2 Pt 1):250–253. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. 1990 Dec;33(4):253–258. doi: 10.1099/00222615-33-4-253. [DOI] [PubMed] [Google Scholar]
- Skakni L., Sardet A., Just J., Landman-Parker J., Costil J., Moniot-Ville N., Bricout F., Garbarg-Chenon A. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2638–2643. doi: 10.1128/jcm.30.10.2638-2643.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J., Marmion B. P., Worswick D. A., Kok T. W., Tannock G., Herd R., Harris R. J. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol Infect. 1992 Dec;109(3):519–537. doi: 10.1017/s0950268800050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppeveld F. J., van der Logt J. T., Angulo A. F., van Zoest M. J., Quint W. G., Niesters H. G., Galama J. M., Melchers W. J. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992 Aug;58(8):2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule A. J., Meijer C. J., Bakels V., Kenemans P., Walboomers J. M. Rapid detection of human papillomavirus in cervical scrapes by combined general primer-mediated and type-specific polymerase chain reaction. J Clin Microbiol. 1990 Dec;28(12):2739–2743. doi: 10.1128/jcm.28.12.2739-2743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]