Abstract
Inhibition of mitochondrial permeability transition pore (MPTP) opening at reperfusion is critical for cardioprotection by ischemic preconditioning (IP). Some studies have implicated mitochondrial protein phosphorylation in this effect. Here we confirm that mitochondria rapidly isolated from pre-ischemic control and IP-hearts show no significant difference in calcium-mediated MPTP opening, whereas IP inhibits MPTP opening in mitochondria isolated from IP-hearts following 30 min global normothermic ischemia or 3 min reperfusion. Analysis of protein phosphorylation in density-gradient purified mitochondria was performed using both 2D and 1D electrophoresis with detection of phosphoproteins using Pro-Q Diamond or phospho-amino specific antibodies. Several phosphoproteins were detected, including voltage-dependent anion channels isoforms 1 and 2, but none showed significant IP-mediated changes either before ischemia or during ischemia and reperfusion. Nor did either Western blotting or 2-D fluorescence difference gel electrophoresis (DIGE) detect translocation of protein kinase C (α, ε or δ isoforms), glycogen synthase kinase 3β (GSK3β), or Akt to the mitochondria following IP. In freeze-clamped hearts changes in phosphorylation of GSK3β, Akt and AMP-activated protein kinase (AMPK) were detected following ischemia and reperfusion but no IP-mediated changes correlated with MPTP inhibition or cardioprotection. However, measurement of mitochondrial protein carbonylation, a surrogate marker for oxidative stress, suggested that a reduction in mitochondrial oxidative stress at the end of ischemia and during reperfusion might account for IP-mediated inhibition of MPTP. The signalling pathways mediating this effect and maintaining it during reperfusion are discussed.
Keywords: Mitochondrial permeability transition, preconditioning, reperfusion injury, protein phosphorylation, oxidative stress
INTRODUCTION
A critical factor mediating reperfusion injury of the heart is the mitochondrial permeability transition pore (MPTP) whose opening causes mitochondrial swelling with release of pro-apoptotic proteins and uncoupling of mitochondrial oxidative phosphorylation. The resulting ATP deprivation causes disruption of ionic homeostasis and contractile function and ultimately sarcolemma rupture and necrosis1. Inhibition of MPTP opening during reperfusion protects hearts from reperfusion injury1. Effective cardioprotection is also mediated by ischemic preconditioning (IP) before prolonged ischemia is initiated2, and this also involves inhibition of MPTP opening3-6.
Extensive evidence points to protein kinase C (PKC) playing a central role in IP, although controversy remains over which PKC isoform(s) are involved and their translocation to mitochondria7;8. The strongest evidence implicates PKCε since PKCε-knockout mice do not exhibit IP and transgenic mice with cardiac-specific over-expression of PKCε or expression of a PKCε activator are protected from reperfusion injury8. Several studies have reported PKCε translocation to the particulate fraction, including mitochondria9;10 where it might phosphorylate putative components of the MPTP such as the voltage-dependent anion channel (VDAC)9-11. Others have proposed that activation of cyclic-GMP-dependent protein kinase (PKG) by nitric oxide activates a mitochondrial intermembrane pool of PKCε leading to opening of mitochondrial ATP-sensitive potassium channels followed by ROS formation, activation of a distinct mitochondrial PKCε pool and finally inhibition of the MPTP12;13. Activation of pro-survival kinases such as Akt, especially during reperfusion, has been implicated by others14 whilst Sollott15 has proposed that protection by all these kinase may converge to phosphorylate and inhibit glycogen synthase kinase 3β (GSK3β). However, no mitochondrial phosphoprotein has been identified that might mediate protection by these kinases.
In this paper we investigate which protein kinases associate with carefully purified mitochondria from control and IP-hearts and analyse whether consistent changes in mitochondrial protein phosphorylation can be detected. Neither approach provided evidence for IP mediating its effects by mitochondrial protein phosphorylation, but we confirm that mitochondrial protein oxidation is decreased by IP at the end of ischemia and early reperfusion. We propose that this reduction in oxidative stress experienced by mitochondria, rather than protein phosphorylation, may be responsible for IP-mediated inhibition of MPTP at the start of reperfusion.
MATERIALS AND METHODS
Materials
The sources of all materials are described in Supplementary Information.
Heart Perfusion
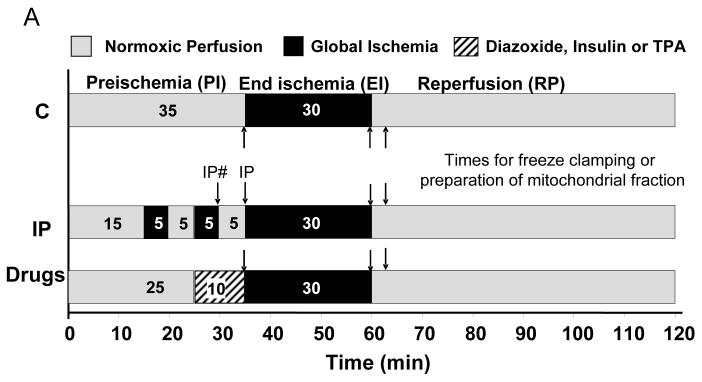
All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Langendorff perfusions of hearts from Male Wistar rats (250-260 g) were performed as described previously3;16 and detailed in Supplementary Methods. All hearts experienced 35 min preischemia, which included the required treatment shown schematically in Figure 1. Perfusate was sampled for determining lactate dehydrogenase (LDH) activity. Supplementary Table 1 presents data on hemodynamic function and LDH release confirming cardioprotection by IP similar to that observed previously.3-5;17;18 At the required time (Fig. 1), hearts were either rapidly homogenised for the preparation of mitochondria or freeze-clamped using liquid nitrogen-cooled tongues, ground under liquid nitrogen and stored at −80°C for later analysis.
Figure 1. Protocols used for heart perfusion.
The times at which control (C) and IP-hearts were freeze clamped or homogenised for mitochondrial preparation are indicated by arrows. For pre-ischemic IP hearts samples were taken at the two points indicated as IP# and IP.
Isolation and analysis of particulate and mitochondrial fractions
All procedures were carried out at 0-4°C in buffers containing protease and phosphatase inhibitors. Two protocols were used to prepare mitochondrial and particulate fractions from homogenised hearts or frozen heart powder as detailed in Supplementary Methods.
PKC translocation and protein phosphorylation
Fractions (10-25 μg protein) were analysed by SDS-PAGE and western blotting with antibodies against both specific phosphoproteins and the corresponding total protein, and then quantification by scanning (see Supplementary Methods). The ratio of the band intensity for phosphoprotein to total protein was used as a measure of phosphorylation state. Purity of mitochondrial fractions was assessed by western blotting with antibodies against the adenine nucleotide translocase (ANT) and monocarboxylate transporter 1 (MCT1- a specific plasma membrane marker19).
2D-gel electrophoresis and 2D-difference gel electrophoresis (DIGE)
These were performed in the University of Bristol Proteomics Facility as described in Supplementary Methods, and gels visualised fluorescently (DIGE) or stained for phosphoproteins and total protein (Pro-Q Diamond and Sypro-Ruby, Invitrogen).
Measurement of MPTP opening in vitro and protein carbonylation assays
MPTP opening was determined at 25°C under de-energized conditions by monitoring A5203 whilst protein carbonyls were analyzed by derivatization with dinitrophenylhydrazine followed by western blotting5;18 as detailed in Supplementary Methods.
Statistical Analysis
Data are presented as means ± S.E. Statistical significance was evaluated using one-way ANOVA followed by two-tailed Students t-test (for simple comparisons between control and preconditioned hearts) or Tukey's multiple comparison post hoc test (for comparisons between multiple groups of hearts) using Graphpad Prism v4.0 software. Differences were considered significant where P<0.05.
RESULTS
MPTP opening was inhibited in mitochondria isolated from IP hearts only after ischemia
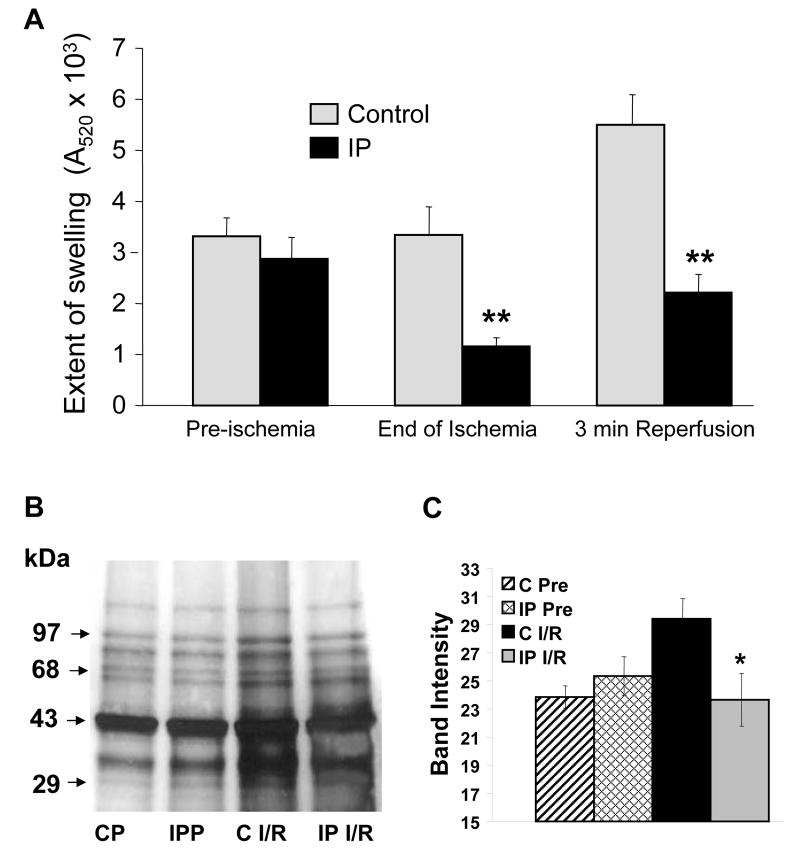
Figure 2A confirms that mitochondria isolated from IP-hearts at end-ischemia or after 3 min reperfusion exhibited less calcium-induced MPTP opening than those isolated from control hearts, whilst no differences were observed in mitochondria isolated immediately after the IP protocol3-5. This lack of effect of IP was independent of the [Ca2+] used (Supplementary Figure 2). Since our protocol uses de-energised mitochondria with buffered [Ca2+] in the presence of calcium-ionophore (A23187), the IP-mediated inhibition of MPTP opening cannot be caused by either differences in mitochondrial calcium loading or membrane potential. Rather it must reflect a change in calcium-sensitivity of the MPTP. Two possible mechanisms might account for this; either phosphorylation of some MPTP component or oxidative modification of critical thiol groups on the MPTP that sensitise it to [Ca2+]1.
Figure 2. MPTP opening and protein carbonylation in mitochondria from control and IP-hearts.
Panel A reports the maximum decrease in A520 after calcium addition to de-energised mitochondria. Panel B shows a representative western blot for protein carbonylation (CP – non-ischemic control hearts). Data in Panels A and C are presented as means of 6 or 12 (preischemic) separate mitochondrial preparations from each group (IP versus control: * p<0.05, ** p<0.01).
Mitochondrial protein oxidation was decreased by preconditioning
The data of Figure 2B,C confirm that mitochondria isolated from IP-hearts during reperfusion exhibit less protein carbonylation, a measure of protein oxidation and a surrogate marker of mitochondrial oxidative stress5, whereas no effect of IP was observed in preischemic hearts. Previous work has shown a similar reduction in protein carbonylation by IP and other preconditioning stimuli at the end of ischemia5 and with temperature precondioning, urocortin and apomorphine at reperfusion18;20;21.
Translocation of protein kinases to purified mitochondria was not detected following IP
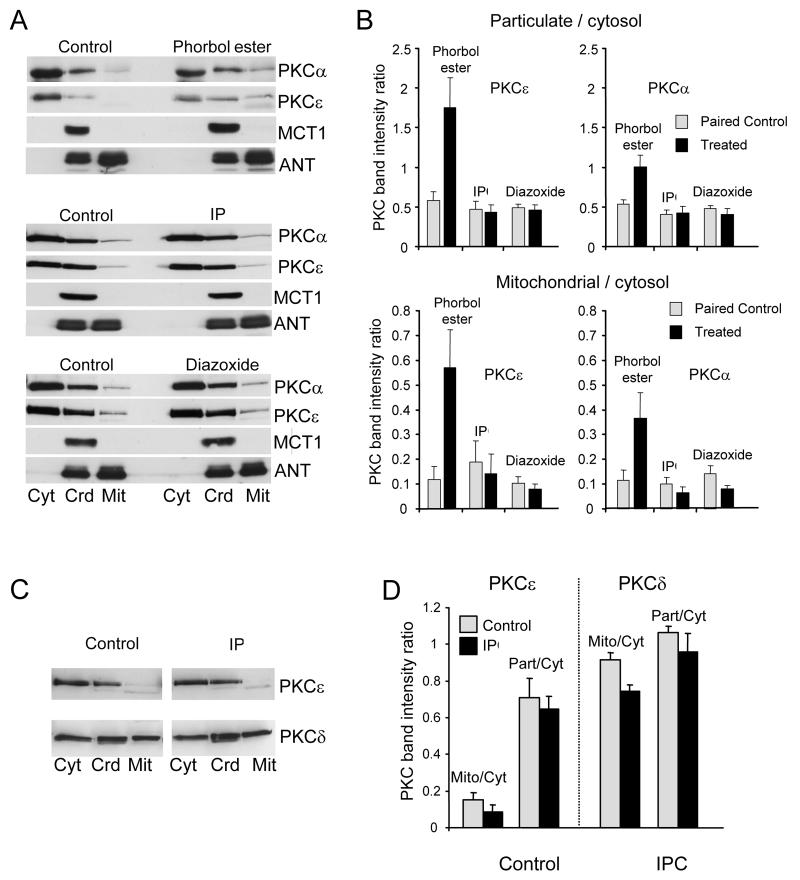
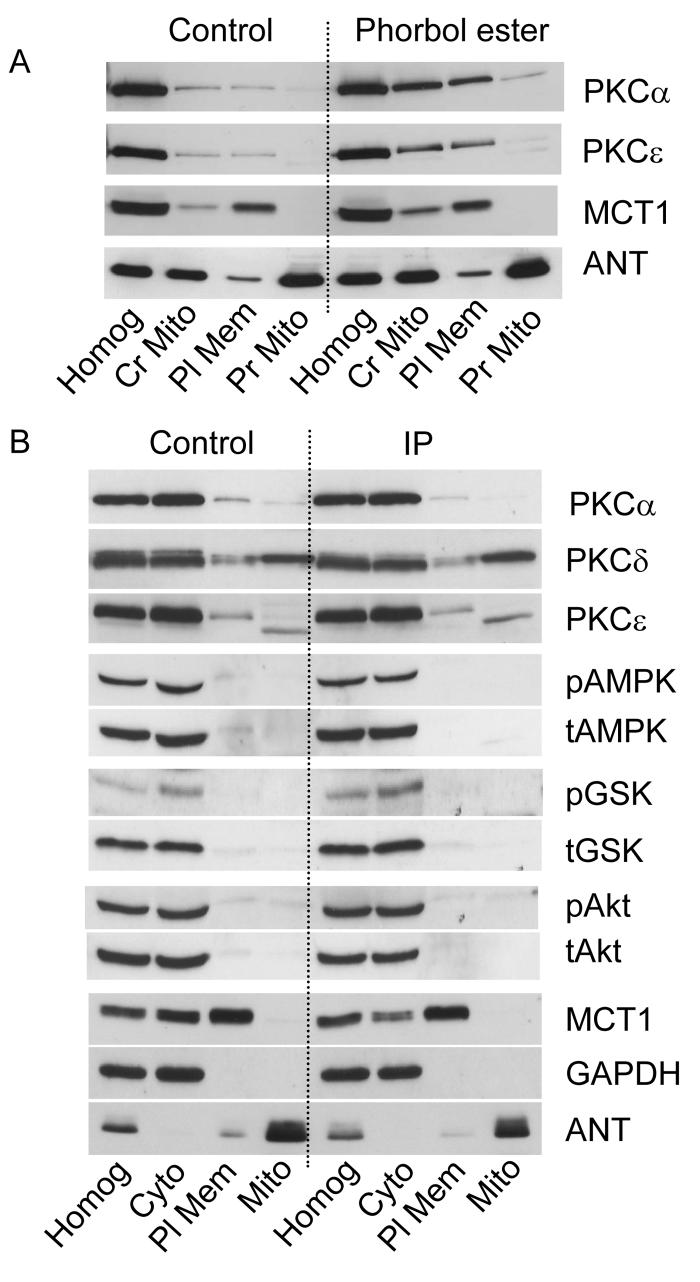
Activation of PKC isoforms causes their translocation to intracellular membranes, including the plasma membrane22. Since conventionally prepared mitochondria are contaminated with such membranes23, their complete removal is essential when studying PKC translocation to mitochondria. Two protocols were used to achieve this as illustrated in Supplementary Figure 1. Either a cytosolic fraction and crude high-speed particulate fraction were prepared, followed by preparation of mitochondria from the latter (protocol 1) or a more conventional mitochondrial preparation was used (protocol 2). In both cases, contaminating plasma membranes were removed by Percoll-gradient centrifugation. Figure 3 shows that protocol 1 produced a crude particulate fraction containing both mitochondria (ANT) and plasma membranes (MCT1) as did the crude mitochondrial preparation of protocol 2 (Figure 4). In both cases Percoll purification removed plasma membranes (MCT1) with the loss of almost all the PKCα and PKCε. Although PKCδ remained, no increase was detected following IP treatment whether mitochondria were isolated before ischemia (Figures 3A and 4A) or during reperfusion (Figure 3C). Nor did treatment with 50 μmol/L diazoxide, an IP-mimic, cause detectable PKCα or PKCε translocation whereas treatment with phorbol ester (200 nmol/L phorbol ester (phorbol-12-myristate-13-acetate) -TPA) produced the anticipated loss of both isoforms from the cytosol and a slight increase in the crude particulate fraction (Figure 3A). A larger PKC increase was detected in the plasma membrane fraction (Figure 4A) ensuring a robust response in the particulate:cytosolic ratio (Figure. 3B). A small increase in PKC in the mitochondrial fraction was also detected following TPA treatment although this might reflect contamination with residual plasma membranes that are highly enriched in PKC following the substantial TPA stimulus (Figures 3A, 4A).
Figure 3. IP does not cause translocation of protein kinase C isoforms to mitochondria.
Sub-cellular fractionation of hearts into cytosolic (Cyt), crude mitochondria (Crd) and pure mitochondria (Mit) was performed according to protocol 1 (Supplementary Fig. 1S). Panels A and B represent fractions isolated immediately after IP or following 10 min treatment with 50 μmol/L diazoxide or 0.2 μmol/L phorbol ester, whilst Panels C and D represent fractions isolated after 30 min ischemia and 3 min reperfusion. Proteins were separated by SDS-PAGE followed by western blotting with the appropriate antibody. Panels B and D present mean data (n = 6) of the ratio of mitochondrial or crude particulate PKC to cytosolic PKC.
Figure 4. IP does not cause mitochondrial recruitment or phosphorylation of other protein kinases.
Sub-cellular fractionation of heart homogenates (Homog) into cytosolic (Cyt), plasma membrane (Pl Mem) and pure mitochondria (Mit) was performed according to protocol 2 (see Supplementary Figure 1S) immediately after the IP protocol (Panel B) or after 10 min with 0.2 μmol/L phorbol ester (Panel A ). Representative western blots with the antibodies indicated are representative of at least 3 experiments. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a cytosolic marker.
The data of Figure 4B illustrate that we were also unable to detect significant amounts of either the non-phosphorylated or phosphorylated forms of AMP-activated protein kinase (AMPK), GSK3β or Akt in the purified mitochondrial fraction isolated from either control or IP-hearts.
Determination of AMPK, GSK3β and Akt phosphorylation in freeze-clamped hearts
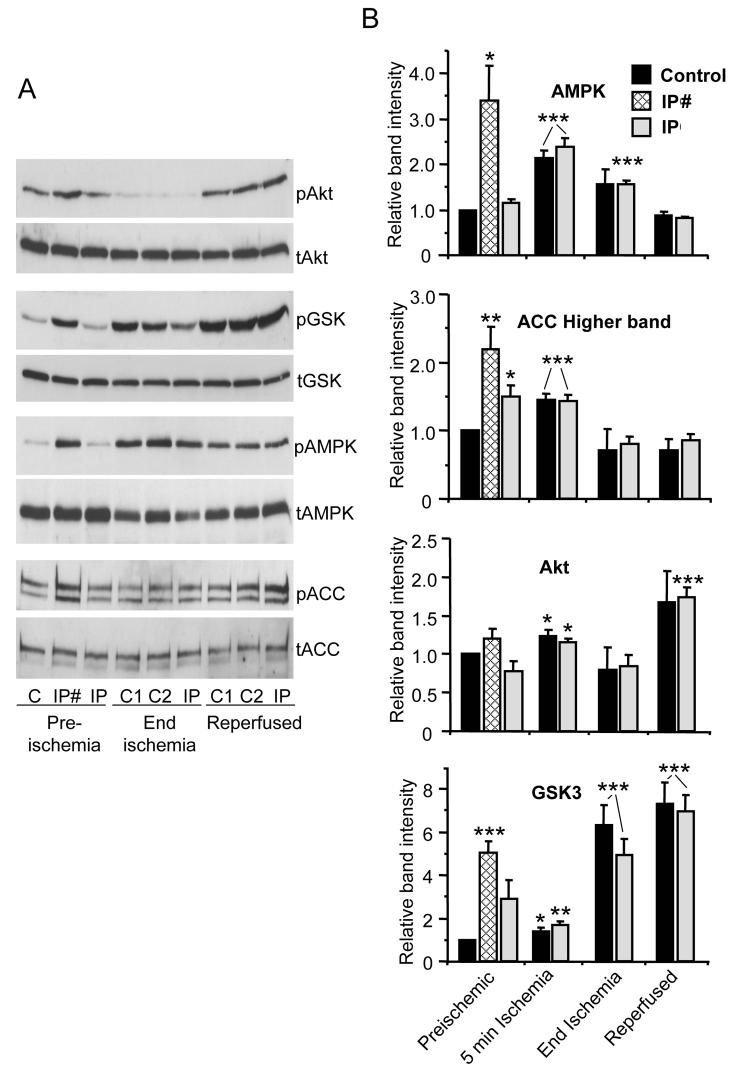
We investigated the phosphorylation state of AMPK, GSK3β and Akt in freeze-clamped control and IP-hearts. Extracts were rapidly prepared in the presence of phosphatase and protease inhibitors and proteins separated by SDS-PAGE before western blotting with antibodies against the phosphorylated and total kinases. Representative blots and mean data for the ratio of phosphorylated to total protein are shown in Figure 5. Before index ischemia, samples from IP-hearts were taken either at the end of the third brief ischemic phase of preconditioning (IP#) or after a further 5 min normoxic recovery (IP). Significant increases in AMPK and GSK3β phosphorylation relative to control hearts were detected in both IP- and IP#-hearts with the latter showing the greater effect. We confirmed the activation of AMPK by measuring an increase in phosphorylation state of acetyl-CoA carboxylase (ACC). However, no significant effects of IP on Akt phosphorylation were detected prior to ischemia. Samples taken after 5 min of index ischemia showed a substantial increases in AMPK phosphorylation relative to preischemic values and small increases in the phosphorylation of ACC, GSK3β and Akt, but in no case could we detect a significant difference between control and IP-hearts. After 30 min ischemia, AMPK phosphorylation remained slightly elevated compared to the preischemia whereas ACC phosphorylation at both time points was less than preischemic values. Akt phosphorylation was slightly reduced after 30 min ischemia and elevated after 3 min reperfusion, whilst GSK3β phosphorylation was greater than preischemic values at both end-ischemia and 3 min reperfusion. Importantly, however, none of the phosphoproteins showed a significant difference between control and IP-hearts whether measured at 5 min ischemia, 30 min ischemia or 3 min reperfusion. Neither did we observe changes in phosphorylation of AMPK, AkT, ACC or GSK3β in response to preconditioning by 50 μM diazoxide (Supplementary Figure 3).
Figure 5. Cytosolic AMPK, Akt, GSK3β and ACC phosphorylation state in freeze-clamped hearts.
Control and IP hearts were freeze-clamped at the times shown in Figure 1 and a cytosolic fraction produced according to the freeze-clamp protocol of Supplementary Figure 1S. Proteins were separated by SDS-PAGE followed by western blotting with the appropriate antibody for the total (t) or phosphorylated (p) kinases indicated or for acetylCoA carboxylase (ACC). Panel A shows representative blots whilst panel B shows mean data (n = 6) of scanned blots where the ratio of phosphorylated to total protein is expressed relative to the ratio for the control preischemic sample run on the same gel. IP# and IP samples represent samples taken from IP hearts at the end of the third brief ischemic phase of preconditioning or immediately before ischemia as indicated in Figure 1. In Panel A, where two adjacent control samples (C) are shown, they represent 2 separate hearts. * p<0.05; ** p<0.02; *** p<0.01 versus preischemic control.
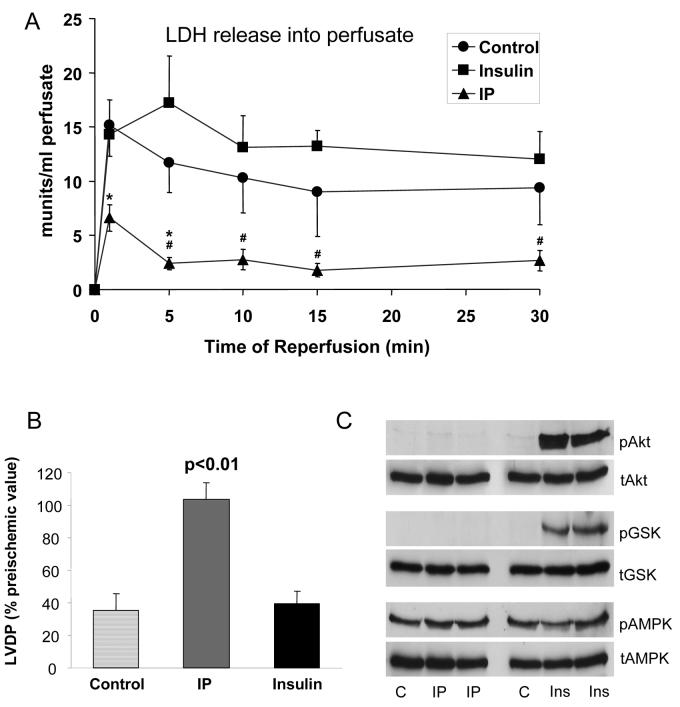
Further evidence that preischemic phosphorylation of Akt and GSK3β may be unimportant for the triggering events of IP is presented in Figure 6. Pre-treatment of hearts for 19 min with 0.7 nM insulin and 1 minute washout prior to ischemia increased the phosphorylation of both Akt and GSK3β several-fold, yet no protection from reperfusion following 30 min ischemia was observed. These data are consistent with previous observations that insulin must be present during reperfusion to be protective15. Additional evidence against a role for AMPK in IP was provided by using compound C, an established inhibitor of AMPK, which exerted no effect on the ability of IP to improve hemodynamic function or lower LDH release (Supplementary Table 1) but does inhibit AMPK-mediated changes in ACC phosphorylation18. By contrast, exposure to 10 μmol/L chelerythrine (CHE) blocked the IP-mediated decrease in LDH release during reperfusion (Supplementary Table 1 and Supplementary Figure 4s).
Figure 6. Insulin treatment prior to ischemia increases Akt and GSK3β phosphorylation but is not cardioprotective.
Hearts were pre-treated with 0.7 nmol/L insulin for 19 min followed by 1 minute of washout prior to 30 min ischemia and then reperfusion for the time shown. Panel A shows the LDH released into the perfusate during reperfusion whist Panel B shows the recovery of LVDP after 30 min reperfusion as a percentage of the pre-ischemic value. Panel C presents data from hearts that were freeze-clamped prior to ischemia and then treated as described for Figure 5.
Preconditioning gave no detectable changes in mitochondrial protein phosphorylation
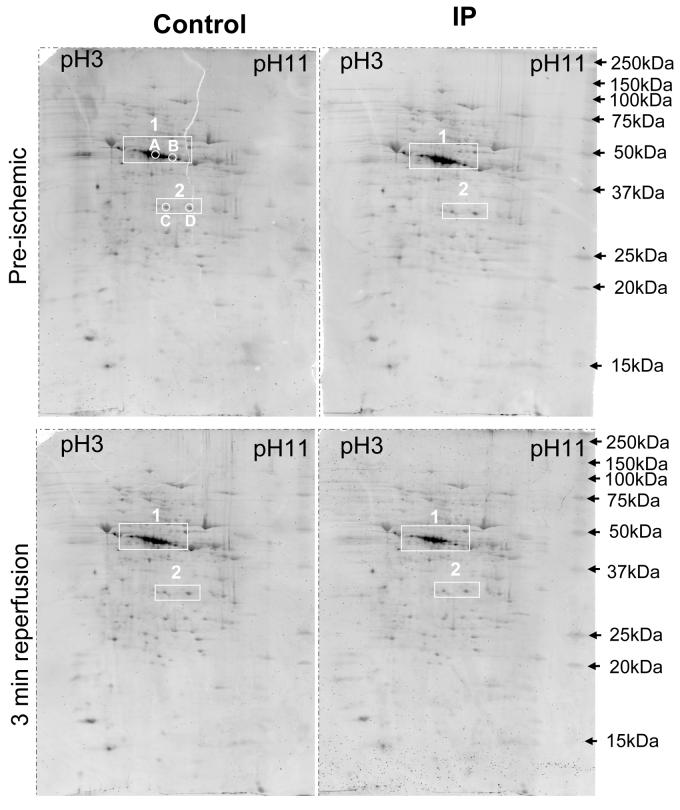
The phosphorylation state of proteins in mitochondria rapidly isolated in the presence of phosphatase and protease inhibitors was determined following their separation by 2D-gel electrophoresis. Pro-Q Diamond and Sypro-Ruby were used to detect phosphoproteins and total proteins respectively24. Figure 7 presents phosphoprotein data for mitochondria from control and IP-hearts isolated both prior to ischemia and after 30 min ischemia and 3 min reperfusion. In Supplementary Figure 5A these data are overlaid (red) on the Sypro-Ruby protein data (green) to allow discrimination between truly phosphorylated proteins and proteins stained non-specifically with the Pro-Q Diamond. Supplementary Figure 5B shows similar data from another set of pre-ischemic, end-ischemic and reperfused hearts. A significant number of proteins were preferentially stained with the Pro-Q Diamond stain, implying that they were phosphorylated. By far the strongest signal was in the 40 kDa region (box 1) in the correct location for multiple phosphorylation states of the E1α subunit of pyruvate dehydrogenase (PDHE1α, Ac. No. P26284; Mw 40.2 kDa) that we have previously shown to be the dominant matrix phosphoprotein whose phosphorylation turns over rapidly25. PDHE1α is known to exhibit multiple phosphorylation states26 and the theoretical pI values of the zero, one, two and three phosphorylated states of 6.82, 6.52, 6.31 and 6.15 are consistent with the spots observed. In Supplementary Figure 9s we provide data to confirm the identity of these spots as PDH1Eα by using mass spectrometry (see Supplementary Table 2), and by western blotting and dephosphorylation studies. Supplementary Figure 9s and Table 2 also provide data on the identity of the two phosphoprotein spots at about 31 kDa in box 2 of Figure 7. These were shown to be singly phosphorylated forms of the voltage dependent anion channel isoforms 1 and 2 (VDAC1 - theoretical pI 7.83 and VDAC2 - theoretical pI 6.68). Evidence has been presented that VDAC1 can be phosphorylated27 and that this might lead, directly11 or indirectly28, to inhibition of MPTP opening. However our data revealed no changes in phosphorylation of either VDAC1 or VDAC 2 in response to IP.
Figure 7. IP-mediated changes in mitochondrial protein phosphorylation were not detected.
Mitochondria were isolated from control or IP hearts just prior to ischemia (Pre) or following 3 min reperfusion (Rep), separated by 2D-gel electrophoresis and then stained with the phosphoprotein stain, Pro-Q Diamond. In supplementary Figure 2A the same data are shown in red overlaid on the total protein stain (Sypro-Ruby) in green. The data shown in Supplementary Fig. 9s and Table 2 establish the identity of the spots in box 1 as different phosphorylation states of PDHE1α and those in box 2 as singly phosphorylated VDAC1 and VDAC2 .
In Supplementary Figure 6 we confirm that we were able to detect IP-mediated changes in cytosolic protein phosphorylation. As additional confirmation that IP exerted no effect on mitochondrial protein phosphorylation we used 2-D fluorescence difference gel electrophoresis (DIGE) in which mitochondrial proteins of control and IP hearts were labelled with red and green fluorescent probes before mixing and separating on the same 2-D gel. Proteins unchanged by IP treatment run in the same place and show as yellow spots whereas any differences are revealed as red or green spots. Data are shown in Supplementary Figure 7s and Table 2 for mitochondria from pre-ischemic, end-ischemic and reperfused hearts but once again no consistent IP-mediated changes were revealed that might account for MPTP inhibition.
We considered that IP might cause changes in the phosphorylation state of proteins such as the ANT that do not readily enter the isolectric focussing gel and so would not be detected using the 2D-gels. Thus we also performed 1-D SDS-PAGE with Pro-Q diamond staining but again found no evidence for changes in any protein phosphorylation following IP (Supplementary Figure 8sA). We have also used antibodies against phosphotyrosine, phosphoserine and phosphothreonine as an alternative strategy to detect IP-mediated changes in protein phosphorylation. Here too, no effects of IP were observed (Supplementary Figure 8s B,C,D).
DISCUSSION
Mitochondrial protein phosphorylation may not be required for IP-mediated inhibition of MPTP opening
Mitochondria isolated immediately after IP showed no decrease in sensitivity to calcium-induced MPTP opening (Figure 2A and refs 3,5). These data argue against phosphorylation of a component of the MPTP during the IP protocol mediating inhibition of pore opening, and our inability to detect a change in mitochondrial protein phosphorylation at this time (Figure 7 and Supplementary Figures 5, 7-9) support this conclusion. However, we were also unable to detect an IP-induced change in the phosphorylation state of any mitochondrial protein in IP-hearts at the end of ischemia or during reperfusion (Figure 7 and Supplementary Figures 5, 7-9) at which time MPTP opening was inhibited by IP (Figure 2A and refs 4;5). Thus it seems unlikely that inhibition of MPTP opening at reperfusion is mediated by protein phosphorylation. Our inability to detect translocation of AMPK, Akt, GSK3β or protein kinase C isoforms to the mitochondria (Figures 3-4) or changes in their phosphorylation state (Figure 4, Supplementary Figures 7,8) is consistent with this conclusion. Although several groups have reported that IP causes translocation of PKC isoforms, particularly PKCε, to the particulate fraction9;11;29-33 not all reported PKCε translocation to mitochondria.18;31;32 This may be because PKCε translocation is transient in the rat heart, being lost after three brief ischemic periods as used in the present study34. Some studies have reported IP-mediated PKCδ translocation to mitochondria.31;32 Although we confirmed the presence of PKCδ in the mitochondria we did not observe IP-mediated translocation (Fig. 3).
We have not attempted to identify all the phosphoproteins detected in the mitochondria, but phosphorylated forms of PDHE1α were the dominant spots (Figure 7 and Supplementary Fig. 9s). PDHE1α is the major phosphoprotein in the mitochondrial matrix known to be rapidly phosphorylated and dephosphorylated together with a small amount of the E1α subunit of branch-chain keto-acid dehydrogenase (BCKDH E1α)25. The additional phosphoproteins detected by us and others24 may turn over more slowly and so are not detected using rapid labelling with 32P. Alternatively, they might represent proteins integral to the outer mitochondrial membrane such as VDAC1 and VDAC2 (Supplementary Figure 9s and ref 27) or bound to it using scaffolding proteins35;36. Their phosphorylation could be regulated by cytosolic kinases associating weakly with mitochondria but not remaining bound during isolation. Indeed, we have shown previously that when mitochondria are isolated from hepatocytes incubated with 32Pi, additional phosphoproteins are observed with two proteins of 30-35 kDa demonstrating increased phosphorylation following glucagon treatment37;38. Whatever their identity, it seems unlikely that the phosphoproteins we detect are involved in IP-mediated inhibition of MPTP opening since their phosphorylation is not changed by IP. This includes VDAC1 whose phosphorylation has been proposed by others to regulate the MPTP11;28.
We cannot totally rule out a role for protein phosphorylation regulating the MPTP since there might be phosphoproteins present at levels below the detection limit of Pro-Q Diamond, DIGE or phospho-amino acid specific antibodies. Additionally, dephosphorylation may have occurred during the mitochondrial preparation despite the presence of phosphatase inhibitors, although this is unlikely since we detected many phosphoproteins in both mitochondrial and cytosolic fractions with IP-mediated changes in the latter (Supplementary Figure 6).
Reduction in oxidative stress may explain the inhibition of MPTP opening by IP
Preconditioning by a variety of means reduces oxidative stress following ischemia and reperfusion39-43 and we have previously shown that this is associated with less oxidative damage to mitochondria as monitored by protein carbonylation.5;18;20;21 Here we confirm that this is the case for IP (Figures 2B,C). Since thiol oxidation greatly sensitises the MPTP to [Ca2+]1, an IP-mediated decrease in oxidative damage provides sufficient explanation for the observed inhibition of MPTP opening at reperfusion, without the need to invoke mitochondrial protein phosphorylation. This would explain why there is no decrease in calcium-sensitivity of MPTP opening immediately following IP when there is no significant oxidative damage (Figure 2, Supplementary Figure 2 and refs 4;5). Such a mechanism is also consistent with the strong cardioprotection afforded by antioxidants specifically targeted to mitochondria44. Thus we propose that decreasing oxidative stress at the end of ischemia and during early reperfusion represents the common mechanism by which preconditioning stimuli inhibit MPTP opening.
The signalling pathways by which ischemic preconditioning reduces oxidative stress
Our data lead us to conclude that AMPK, Akt or GSK3β are unlikely to be involved in reducing oxidative stress at the end of ischemia and early in reperfusion, since we found no appropriate changes in their phosphorylation state in response to IP (Figure 5) or diazoxide (Supplementary Figure 3s). Nor did the AMPK inhibitor, compound C, prevent protection by IP as measured by either hemodynamic function or LDH release (Supplementary Table 1). Indeed, if anything this reagent enhanced the hemodynamic recovery of IP hearts, which might reflect a metabolic effect of the inhibitor such as inhibition of fatty acid oxidation18. By contrast, the inability to precondition PKCε-knockout mice45, the ability of chelerythrine (Supplementary Table 1) and other PKC-inhibitors to prevent IP, and the ability of PKC activation to mimic preconditioning7;29;46;47 all argue for a critical role of PKCε in this process. Furthermore, the decrease in oxidative stress at reperfusion caused by both urocortin21 and temperature preconditioning18 are mediated by PKC. However, the mechanism by which this is achieved and whether decreased ROS production or increased ROS removal is involved remains to be elucidated.
Additional signalling pathways may inhibit MPTP opening as reperfusion continues
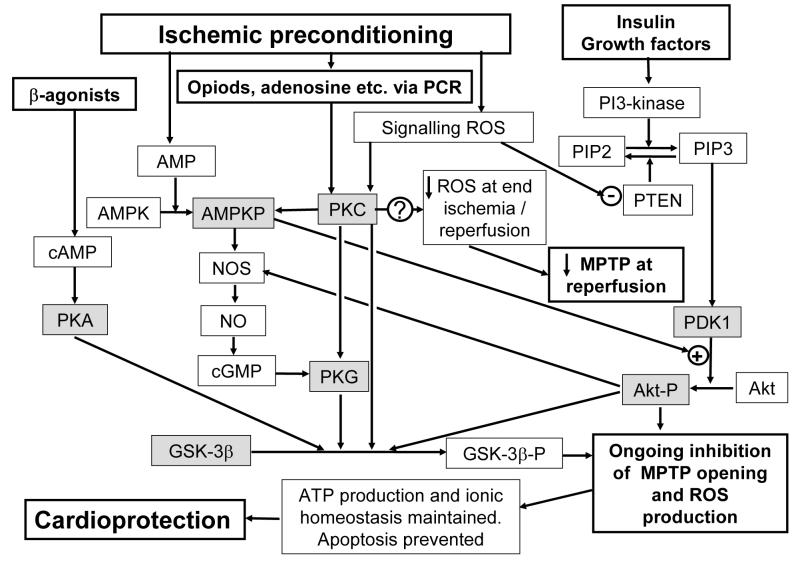
Activation of pro-survival kinases such as members of the MAPK family and the PI3K-Akt cascade during reperfusion have been proposed to play a key role in IP-mediated cardioprotection48. Maintenance of the MPTP inhibition during reperfusion appears to be critical for cardioprotection since treatment with cyclosporin-A and sanglifehrin-A (MPTP inhibitors) within the first 15 min of reperfusion is sufficient to produce a profound reduction in infarct size49;50. This may reflect an ongoing cascade of MPTP opening whereby the initial pore opening at reperfusion stimulates ROS production that goes on to cause further MPTP opening as reperfusion continues51-52. This MPTP-mediated ROS production could involve cytochrome c release, caspase activation and subsequent cleavage of the p75 subunit of complex I53;54. Prevention of MPTP opening at the start of reperfusion, as occurs through IP reduction in ROS levels, will also prevent the subsequent MPTP opening and thus represents a protective mechanism with “memory” as defined by Sollott15. By contrast, those stimuli such as insulin which affect the survival kinase pathway may act on the caspase-mediated pathway and so only work during reperfusion and thus lack “memory”. In Figure 8 we present a scheme that summarises these proposals.
Figure 8. Suggested pathways by which IP may lead to inhibition of MPTP opening during reperfusion.
Active protein kinases are shaded grey. Further details are given in the text.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by a programme grant (RG/03/002) from the British Heart Foundation to APH.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures
One
References
- 1.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion - a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 3.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KHH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. doi: 10.1016/j.cardiores.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Khaliulin I, Schwalb H, Wang P, Houminer E, Grinberg L, Katzeff H, Borman JB, Powell SR. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med. 2004;37:1–9. doi: 10.1016/j.freeradbiomed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol. 2004;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms and in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 10.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKC epsilon and MAPK form signaling modules in the murine heart - Enhanced mitochondrial PKC epsilon-MAPK interactions and differential MAPK activation in PKC epsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 11.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping PP. Protein kinase C epsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 13.Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 15.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3 beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol. 1999;276:H496–H502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- 17.Lim KHH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman M-S, Halestrap AP. Temperature preconditioning of isolated rat hearts - a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–1161. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halestrap AP, Meredith D. The SLC16 gene family - from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 20.Khaliulin I, Schneider A, Houminer E, Borman JB, Schwalb H. Apomorphine prevents myocardial ischemia/reperfusion-induced oxidative stress in the rat heart. Free Radic Biol Med. 2004;37:969–976. doi: 10.1016/j.freeradbiomed.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Townsend PA, Davidson SM, Clarke SJ, Khaliulin I, Carroll CJ, Scarabelli TM, Knight RA, Stephanou A, Latchman DS, Halestrap AP. Urocortin prevents mitochondrial permeability transition in response to reperfusion injury indirectly, by reducing oxidative stress. Am J Physiol. 2007;293:H928–H938. doi: 10.1152/ajpheart.01135.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 23.Halestrap AP. The regulation of the oxidation of fatty acids and other substrates in rat heart mitochondria by changes in matrix volume induced by osmotic strength, valinomycin and Ca2+ Biochem J. 1987;244:159–164. doi: 10.1042/bj2440159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulenberg B, Aggeler R, Beechem JM, Capaldi RA, Patton WF. Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. J Biol Chem. 2003;278:27251–27255. doi: 10.1074/jbc.C300189200. [DOI] [PubMed] [Google Scholar]
- 25.Hughes WA, Halestrap AP. The regulation of branched-chain 2-oxoacid dehydrogenase of liver, kidney and heart by phosphorylation. Biochem J. 1981;196:459–469. doi: 10.1042/bj1960459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau KS, Phillips CE, Randle PJ. Multi-site phosphorylation in ox-kidney branched-chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1983;160:149–152. doi: 10.1016/0014-5793(83)80955-7. [DOI] [PubMed] [Google Scholar]
- 27.Liberatori S, Canas B, Tani C, Bini L, Buonocore G, Godovac-Zimmermann J, Mishra OP, Delivoria-Papadopoulos M, Bracci R, Pallini V. Proteomic approach to the identification of voltage-dependent anion channel protein isoforms in guinea pig brain synaptosomes. Proteomics. 2004;4:1335–1340. doi: 10.1002/pmic.200300734. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase-3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 29.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein Kinase C-ε is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–1948. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995;76:73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Ashraf M. Role of protein kinase C in mitochondrial KATP channel-mediated protection against Ca2+ overload injury in rat myocardium. Circ Res. 1999;84:1156–1165. doi: 10.1161/01.res.84.10.1156. [DOI] [PubMed] [Google Scholar]
- 32.Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003;99:138–147. doi: 10.1097/00000542-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, Shimamoto K. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am J Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 34.Simonis G, Weinbrenner C, Strasser RH. Ischemic preconditioning promotes a transient, but not sustained translocation of protein kinase C and sensitization of adenylyl cyclase. Basic Res Cardiol. 2003;98:104–113. doi: 10.1007/s00395-003-0397-8. [DOI] [PubMed] [Google Scholar]
- 35.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free RadicBiol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci. 2006;31:26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Armston AE, Halestrap AP, Scott RD. The nature of the changes in liver mitochondrial function induced by glucagon treatment of rats. The effects of intramitochondrial volume,aging and benzyl alcohol. Biochim Biophys Acta. 1982;681:429–439. doi: 10.1016/0005-2728(82)90185-2. [DOI] [PubMed] [Google Scholar]
- 38.Vargas A, Halestrap AP, Denton RM. The effects of glucagon, phenylephrine and insulin on thephosphorylation of cytoplasmic, mitochondrial and membrane-bound proteins of intact liver cells from starved rats. Biochem J. 1982;208:221–229. doi: 10.1042/bj2080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 40.Vanden-Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 41.Narayan P, Mentzer RM, Jr, Lasley RD. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol. 2001;33:121–129. doi: 10.1006/jmcc.2000.1282. [DOI] [PubMed] [Google Scholar]
- 42.Kevin LG, Camara AKS, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O-2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 43.Morihira M, Hasebe N, Baljinnyam E, Sumitomo K, Matsusaka T, Izawa K, Fujino T, Fukuzawa J, Kikuchi K. Ischemic preconditioning enhances scavenging activity of reactive oxygen species and diminishes transmural difference of infarct size. Am J Physiol. 2006;290:H577–H583. doi: 10.1152/ajpheart.00817.2004. [DOI] [PubMed] [Google Scholar]
- 44.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 45.Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res. 2002;55:672–680. doi: 10.1016/s0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 46.Chen W, Wetsel W, Steenbergen C, Murphy E. Effect of ischemic preconditioning and PKC activation on acidification during ischemia in rat heart. J Mol Cell Cardiol. 1996;28:871–880. doi: 10.1006/jmcc.1996.0082. [DOI] [PubMed] [Google Scholar]
- 47.Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C. Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production. J Mol Cell Cardiol. 2002;34:361–367. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 48.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 50.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 53.Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricci JE, MunozPinedo C, Fitzgerald P, BaillyMaitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.