Abstract
Objective:
Family history (FH) of alcohol-use disorders (AUDs) has been associated with frontal lobe deficits, more positive expectations for alcohol effects, and increased risk of developing AUDs. We tested the hypothesis that anterior brain regions mediate the relationship between FH of AUDs and alcohol expectancies in adolescents.
Method:
Nondrinking adolescents (N = 50) ages 12-14 completed measures of FH of AUDs, alcohol expectancies, and substance use and performed spatial working memory and vigilance tasks during functional magnetic resonance imaging.
Results:
Activation of the anterior cingulate significantly predicted alcohol expectancies (R2Δ = 9%, β = .32, Fchange = 6.09, 1/43 df, p < .05). However, FH of AUDs was not associated with brain response or alcohol expectancies.
Conclusions:
Although a mediational model was not supported, activation in the anterior cingulate was linked to alcohol expectancies, such that adolescents with less neural differentiation to task demands had more positive expectancies for alcohol's effects. These results provide a greater understanding of the interrelations among risk factors for AUD and point to individuals who might be targeted for early, cognitively based interventions.
Afamily history (fh) of alcohol-use disorders (AUDs) has repeatedly been demonstrated as a risk factor for personal AUD (e.g., Schuckit et al., 1972), and parental AUD has been linked to several cognitive anomalies in nondrinking offspring (Weinberg, 1997). Spatial deficits have been observed in preschool children (Schandler et al., 1995), nondrinking adolescents (Ozkaragoz and Noble, 1995), and nondependent adult children of alcoholic individuals (Garland et al., 1993). The frontal lobes, including anterior cingulate cortex (ACC; Streeter et al., 1998), appear to be key to the aberrancies observed in youths who are FH positive (FHP) for AUDs.
Functional magnetic resonance imaging (fMRI) studies have suggested that frontal areas such as the ACC (Kinder-mann et al., 2004) and middle frontal gyrus (Leung et al., 2002; McCarthy et al., 1996) are associated with spatial working memory (SWM) deficits. Deckel and colleagues (1995) found that frontal electroencephalogram (EEG) functioning and frontally mediated cognitive test scores were linked to AUD risk through alcohol expectancies. This suggested that anterior brain regions may be involved in the formation of alcohol expectancies and that dysfunctions in this area may indicate a risk for excessive alcohol use.
Alcohol expectancies refer to the anticipated effects of alcohol use and are associated with the likelihood of alcohol consumption and related problems (e.g., Anderson et al., 2005). Some studies have shown elevated positive expectancies in youths and adults FHP for AUDs (Brown et al., 1987; Mann et al., 1987), whereas others have not (Wiers et al., 1998). More positive alcohol expectancies are often predicted by a positive FH (Conway et al., 2003) as well as dysfunction in the frontal lobes (Deckel et al., 1995). However, it has not been determined if the relationship between FH and alcohol expectancies might be explained by frontal brain functioning in adolescents. Based on our prior work showing that (1) SWM is a robust probe of ACC response in youths (Nagel et al., 2005) and (2) SWM response in the cingulate is different in FHP than in FH-negative youths (Spadoni et al., 2008), we hypothesized that increased frontal SWM activation would mediate the relationship between FH and greater alcohol expectancies.
Method
Participants
Fifty adolescents (54% female), ages 12-14 (mean [SD] = 13.54 [0.89]) participated in the study (94% white; family income: mean = $116,000 [$54,000]). Participants were recruited through fliers mailed to middle schools in San Diego County and were compensated for participation (Anderson et al., 2005; Schweinsburg et al., 2004). Exclusionary criteria were premature birth; reports of maternal drinking or drug use during pregnancy; parental history of psychosis, bipolar I, or antisocial personality disorder; history of neurological problems, head trauma, learning disabilities, Axis I psychiatric disorder other than conduct disorder or oppositional defiant disorder, or psychiatric medication use; lifetime use of 10 or more drinks; MRI contraindications (e.g., braces); noncorrectable sensory impairments; nonfluency in English; and left-handedness.
Measures
The Customary Drinking and Drug Use Record (Brown et al., 1998) was used to assess frequency and quantity for use of alcohol, nicotine, illicit drugs, and prescription drug misuse (lifetime), and the Timeline Followback was used to query use patterns in the 7 days before scanning (Sobell and Sobell, 1992) to ensure participants had very limited substance-use histories. The Family History Assessment Module (Rice et al., 1995) and Computerized Diagnostic Interview Schedule (Robins et al., 1996) were used to assess parents' drinking behaviors and related consequences (parent and adolescent reports). To maximize sensitivity, a continuous composite FH density score was computed using diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994); each parent with an AUD added 0.50 and each grandparent with an AUD added 0.25 to the score (range: 0-2; Stoltenberg et al., 1998). The FH density score ranged from 0 to 1.5 in this sample (mean = 0.31 [0.45]). The Alcohol Expectancy Questionnaire for Adolescents (AEQ-A; Brown et al., 1987) was used to measure enhancement/impedance of social behavior, sexual enhancement, cognitive and motor impairment, improved cognitive and motor abilities, relaxation and tension reduction, arousal, and global positive (range: 15-65; mean = 38.85 [10.21]) expectancies for alcohol, with the latter score used in analyses.
Procedures
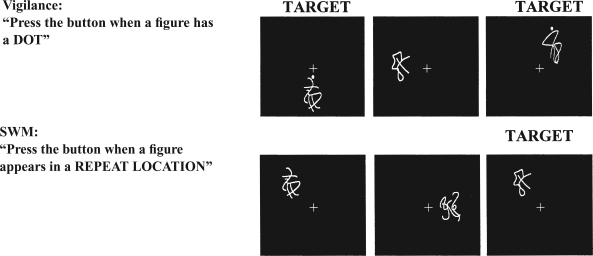
On arrival at the imaging session, interviews were administered and participants were trained on the SWM task (Kindermann et al., 2004; Tapert et al., 2001) (Figure 1). Images were acquired on a 1.5 Tesla GE scanner (Milwaukee, WI). A high-resolution structural image was collected using a spiral fast spin-echo sequence (repetition time = 2,000 ms; echo time =16 ms; field of view = 240 mm; resolution = 0.94 mm × 0.94 mm × 1.33 mm) (Wong et al., 2000). Functional imaging was collected using T2-weighted spiral gradient recall echo imaging (repetition time = 3,000 ms; echo time = 40 ms; flip angle = 90˚; field of view = 240 mm; 20 continuous slices; slice thickness = 7 mm; in-plane resolution = 1.88 mm × 1.88 mm; 156 repetitions). The fMRI task (Kindermann et al., 2004; Tapert et al., 2001) consisted of 18 20-second blocks alternating between SWM and vigilance conditions, with blocks of rest interspersed. In the SWM condition, abstract drawings appeared in one of eight locations, and participants were asked to press a button when a design appeared at a location that had already been occupied in that block (30% of trials; repeats were 2-back). In the vigilance condition, the same stimuli were presented in the same locations, but a dot appeared above figures on 30% of trials, and participants were requested to press a button when a dot appeared. In both conditions, stimuli were presented for 1,000 ms with a 1,000 ms interstimulus interval (total time: 7 minutes, 48 seconds). Data were processed and analyzed with Analysis of Functional NeuroImages (Cox, 1996; Spadoni et al., 2008). Hierarchical linear regression (SPSS, Version 11.0; SPSS Inc., Chicago, IL) was used to test mediation of FH and alcohol expectancies through SWM using criteria outlined by Baron and Kenny (1986).
Figure 1.
Examples of baseline (vigilance) and spatial working memory (SWM) task conditions. Identical stimuli were used in both conditions (Kindermann et al., 2004; Tapert et al., 2001). The purpose of the vigilance condition was to control for simple motor and attention processes involved in the SWM conditions.
Results
In the simple vigilance condition, participants performed with 96% accuracy and 655 (±58) ms mean response time. In the SWM condition, participants performed with 90% accuracy and 590 (±78) ms mean response time. Analyses focused on the ACC and middle frontal gyrus because they are commonly implicated in SWM tasks (e.g., Kindermann et al., 2004), and alcohol expectancies were linked to frontal functioning (Deckel et al., 1995). A cluster centered on the ACC was sufficiently large so as to include parts of the medial frontal gyrus and the superior frontal gyrus and was significantly activated during vigilance relative to SWM. The middle frontal cluster was significantly more activated by the SWM task relative to the vigilance task.
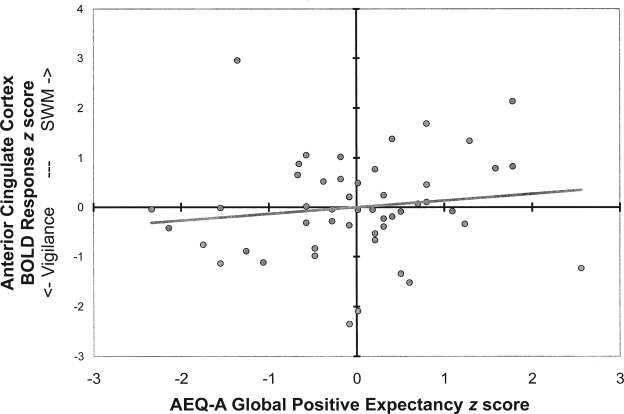
Hierarchical linear regressions tested the preliminary relations necessary for mediation (FH → SWM; FH → expectancies; SWM → expectancies) while controlling for the influence of demographic variables (i.e., gender, age, ethnicity, and socioeconomic status). Demographic variables accounted for significant variance in SWM performance (11%) and alcohol expectancies (20%). FH of AUDs did not predict SWM activation or alcohol expectancies above and beyond these characteristics, precluding the possibility of mediation. However, ACC activation, above and beyond demographics, predicted global positive alcohol expectancies (R2Δ = 9%; Fchange = 6.13, 1/44 df, p < .05; see Figure 2). Middle frontal gyrus activation did not significantly add to the estimation of AEQ-A scores. A hierarchical model including demographics (Block 1), FH (Block 2; included to demonstrate the magnitude of its influence), and brain activation (Block 3) predicted 36% of the variance in AEQ-A global positive scores (Table 1).
Figure 2.
Scatter plot of anterior cingulate activation by Alcohol Expectancy Questionnaire for Adolescents (AEQ-A) global positive scores
Table 1.
Hierarchical regressions of family history of alcohol-use disorders and frontal brain activation predicting Alcohol Expectancy Questionnaire for Adolescents (AEQ-A) global positive expectancy scores (N = 50), controlling for sociodemographic variables
| AEQ-A global positive expectancy scores |
|||||
| Predictor variable | R2 | R2Δ | β | t | F model, df |
| Block 1 | .27 | .27† | 4.1 1,† 4/45 df | ||
| Gendera | -.32 | -2.41* | |||
| Age | .44 | 3.42† | |||
| Ethnicityb | -.16 | -1.17 | |||
| Socioeconomic status | -.06 | -0.44 | |||
| Block 2 | .27 | .00 | 3.24,* 5/44 df | ||
| Gendera | -.32 | -2.37* | |||
| Age | .44 | 3.31† | |||
| Ethnicityb | -.16 | -1.19 | |||
| Socioeconomic status | -.04 | -0.24 | |||
| Family history | -.05 | -0.34 | |||
| Block 3 | .36 | .09* | 4.03,† 6/43 df | ||
| Gendera | -.41 | -3.07† | |||
| Age | .50 | 3.93† | |||
| Ethnicityb | -.19 | -1.45 | |||
| Socioeconomic status | .00 | 0.01 | |||
| Family history | -.06 | -0.45 | |||
| Anterior cingulate activation | .32 | 2.47* | |||
Positive β's are in the direction of being female;
ethnicity coded as white versus nonwhite; positive β's are in the direction of being nonwhite.
p<.05;
p<.01.
Discussion
This study focused on whether frontal brain activation mediated the relationship between FH of AUDs and alcohol expectancies in early adolescence. Activation to the vigilance condition relative to the SWM condition in the ACC (including medial and superior frontal gyri) significantly predicted global positive alcohol expectancies. These results refine EEG findings relating anterior brain functioning to alcohol expectancies (Deckel et al., 1995) by implicating the ACC, in particular, as activation in the middle frontal gyrus did not predict alcohol expectancy scores. As part of the interoceptive system (Craig, 2003), the ACC is key to conflict monitoring (Carter et al., 1998) and activates when cocaine-dependent adults expect to receive cocaine (Kufahl et al., 2008). ACC response modulation covaries with level of reward expectancy in nonhuman primates (Shidara and Richmond, 2002). Based on these findings, adolescents who have similar levels of ACC activation to SWM as to vigilance tasks (i.e., low task-specific allocation of neural resources) have more positive expectancies for the effects of alcohol, and thus are at greater risk for drinking problems. These adolescents may have less neural specificity for task-related demands and default mode neural responding. This tendency could relate to attention and monitoring functions conducive to developing expectancies based on immediate stimulus-response observations, rather than linking a behavior (i.e., drinking) with delayed effects (e.g., hangover, embarrassment, injury). Imaging paradigms probing learning, delayed discounting, cue reactivity, and interoception will help refine the role of the ACC in alcohol expectancy formation.
Although brain response was associated with global positive alcohol expectancies, it was not a mediator, because FH of AUDs did not predict frontal activation to SWM or vigilance task conditions. Similarly, FH was not associated with alcohol expectancies in this sample of youths, which is consistent with some studies (e.g., Wiers et al., 1998), but inconsistent with other reports, possibly owing to sample characteristics such as relatively limited exposure to alcohol-dependent family members (i.e., some parents quit drinking before birth of the index child). With increased sample size, some undetected results may be revealed and results may become more robust. For linear regression with N =50 at α = .05 (two tailed), power for detecting β's of .32 is .72, and bringing power to .80 requires a sample of 62. However, the negative FH results are not likely to change, given the negligible effect observed under current conditions.
Although these findings are preliminary, owing to the relatively small sample size and high sociodemographics of this sample, the literature on the relationship between alcohol expectancies and brain activation is just emerging. Previous findings from our laboratory (Anderson et al., 2005) suggested that neural activation on inhibition tasks was associated with alcohol expectancies in this age group. More studies in diverse samples with other measures of cognitive functioning are needed to clarify the relationship between blood oxygen level-dependent response and alcohol expectancies. The goal of this line of research is to better understand the interplay of neurological and cognitive factors associated with the formation of alcohol expectancies and developmental transitions into alcohol use, hazardous use, and AUDs.
Acknowledgment
Appreciation is expressed to Gregory Brown, Ph.D., Sandra Kinder-mann, Ph.D., Valerie Barlett, Lisa Caldwell, Lauren Killeen, and Alecia Schweinsburg for their help with this project.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant R01 AA13419 awarded to Susan F. Tapert and a minority supplement grant R01 AA013419-02S1 awarded to Carmen Pulido. Portions of this work were presented at the annual meeting of the Research Society on Alcoholism, Baltimore, MD, June 23-29, 2006.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: 1994. [Google Scholar]
- Anderson KG, Schweinsburg A, Paulus MP, Brown SA, Tapert S. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. J. Stud. Alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Social Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: An instrument for the assessment of adolescent and adult alcohol expectancies. J. Stud. Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen JD, Merikangas KR. Alcohol expectancies, alcohol consumption, and problem drinking: The moderating role of family history. Addict. Behav. 2003;28:823–836. doi: 10.1016/s0306-4603(02)00265-4. [DOI] [PubMed] [Google Scholar]
- COX RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Hesselbrock V, Bauer L. Relationship between alcohol-related expectancies and anterior brain functioning in young men at risk for developing alcoholism. Alcsm Clin. Exp. Res. 1995;19:476–481. doi: 10.1111/j.1530-0277.1995.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Garland MA, Parsons OA, Nixon SJ. Visual-spatial learning in nonalcoholic young adults with and those without a family history of alcoholism. J. Stud. Alcohol. 1993;54:219–224. doi: 10.15288/jsa.1993.54.219. [DOI] [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophr. Res. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kufahl P, LI Z, Risinger R, Rainey C, Piacentine L, Wu G, Bloom A, Yang Z, Li SJ. Expectation modulates human brain responses to acute cocaine: A functional magnetic resonance imaging study. Biol. Psychiat. 2008;63:222–230. doi: 10.1016/j.biopsych.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Leung H-C, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J. Cog. Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable T, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb. Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Barlett VC, Schweinsburg AD, Tapert SF. Neuro-psychological predictors of BOLD response during a spatial working memory task in adolescents: What can performance tell us about fMRI response patterns? J. Clin. Exp. Neuropsychol. 2005;27:823–839. doi: 10.1080/13803390490919038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaragoz TZ, Noble EP. Neuropsychological differences between sons of active alcoholic and non-alcoholic fathers. Alcohol Alcsm. 1995;30:115–123. [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcsm Clin. Exp. Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Marcus L, Reich W, Cunningham R, Gallagher T. NIMH Diagnostic Interview Schedule—Version IV (DIS-IV) St. Louis, MO: Department of Psychiatry, School of Medicine, Washington University; 1996. [Google Scholar]
- Schandler SL, Thomas CS, Cohen MJ. Spatial learning deficits in preschool children of alcoholics. Alcsm Clin. Exp. Res. 1995;19:1067–1072. doi: 10.1111/j.1530-0277.1995.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Amer. J. Psychiat. 1972;128:1132–1136. doi: 10.1176/ajp.128.9.1132. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann.. N.Y. Acad. Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcsm Clin. Exp. Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: Density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Ciraulo DA, Harris GJ, Kaufman MJ, Lewis RF, Knapp CM, Ciraulo AM, Maas LC, Ungeheuer M, Szulewski S, Renshaw PF. Functional magnetic resonance imaging of alpra-zolam-induced changes in humans with familial alcoholism. Psychiat. Res. 1998;82:69–82. doi: 10.1016/s0925-4927(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcsm Clin. Exp. Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Weinberg NZ. Cognitive and behavioral deficits associated with parental alcohol use. J. Amer. Acad. Child Adolesc. Psychiat. 1997;36:1177–1186. doi: 10.1097/00004583-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gunning WB, Sergeant JA. Do young children of alcoholics hold more positive or negative alcohol-related expectancies than controls? Alcsm Clin. Exp. Res. 1998;22:1855–1863. [PubMed] [Google Scholar]
- Wong EC, Luh W-M, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc. Int. Soc. Magn. Reson. Med. 2000;8:683. [Google Scholar]