Abstract
Hydraphiles are synthetic ionophores that were designed to mimic some properties of protein channels that conduct such cations as sodium. They use macrocyclic (crown) polyethers as amphiphilic headgroups and as entry and exit portals. Their overall length is controlled by covalent links between the two headgroups (distal macrocycles) and the “central relay” unit, typically also an azacrown. The hydraphiles insert in the bilayer membranes of synthetic phospholipid vesicles or vital cells and mediate the transport of cations. The hydraphiles were intended to be models but they are functional channels. Because they are symmetric, they are non-rectifying but they show open-close behavior characteristic of natural channels. Because they are non-rectifying, when they insert into a microbial membrane, they lead to a rapid change in osmotic balance that proves fatal to bacteria.
Keywords: Azacrown, Biological activity, Ion channel, Ionophore, Membrane, Metal cation transport
1. Introduction
Most biological structures are incredibly complex. Indeed, they perform intricate tasks with remarkable efficiency and exquisite selectivity. Such structures must have evolved from far simpler compounds that performed the requisite function less effectively and with poorer selectivity (often called “specificity”). When a molecule emerged that performed some function, it then further evolved over time into a structure that accomplished the task with increasingly efficacy. In some cases, single molecules aggregated to accomplish certain tasks and the monomers evolved cooperatively [1,2].
The evolutionary key must have been to achieve some level of function that could then be enhanced or elaborated. The same must be true of synthetic organic models systems [3]. The challenge for the chemist/designer is to devise a compound that achieves a function, even if it is at a modest level. This is so for natural evolution or for improving a synthetic design. The non-functional compound provides no starting point for further elaboration. Structural variations in a functional structure will either enhance or diminish the desired function. In either case, information about the structure-function relationship is obtained.
The design of a system to model biological function involves several facets. First, one must identify the function to be mimicked. This does not necessarily require understanding the mechanism by which the function is achieved. Indeed, the model system may help to reveal mechanistic information. Second, one must identify or estimate the environment in which the function is achieved. This may involve distinguishing between cytosol and membrane or identifying and understanding an organelle. A compound that targets an organism requires some appreciation of the differences in organisms. For the organic chemist, the third and critical issue is bringing the desired structure to hand in pure form. This may be a daunting challenge for a complex structure. There will usually be difficulties and surprises in even the best-planned synthetic strategy. Organic chemists will always want to use the best and most exciting synthetic methods in the preparation but the goal at first must be to achieve function rather than a beautiful synthesis. Fourth, the resulting structure must be fully characterized in the chemical sense so that a lack of purity does not mislead the investigation.
1.1. The need for transport
Livings cells are not only complex, they are asymmetric. The major phospholipids [4] found in animal cells are phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin [5-8]. Phosphatidylserine [9] is found predominantly on the cytosolic side of the membrane (i.e., on the inside surface of the cell). Glycolipids are also found in many organelle membranes. They are generally found in the plasma membrane outer leaflet. Taken together, the numerous membrane components [10] pose a formidable barrier through which only gases and small, uncharged molecules can pass [11]. Phospholipid bilayers are impermeable to large molecules, very polar molecules, and ions.
Mammalian cells typically have concentrations of K+ in the 140 mM range within and 5-10 mM outside. The concentrations of Na+ are usually 5-10 mM inside the cell and 140-150 mM in plasma. Chloride ion concentrations are more variable but 5-10 mM inside the cell and 100 mM outside is not uncommon. None of these three ions can readily pass through the bilayer, i.e., all show low membrane permeability [12]. Transporters of some type are essential so that species such as ions that have low membrane permeability can traverse the bilayer. In addition, selectivity (“specificity”) is essential so that the balance of ionic concentrations can be maintained within the cell.
1.2. The challenge presented by group 1 (alkali metal) cations
The most common biological cations, Na+ and K+, are alkali metal cations. They fall in group 1A in the periodic table and each cation’s electronic configuration is s2p6. Their outermost orbital is an empty, spherical s orbital. Thus, the ions are featureless spheres that differ primarily in size and charge density. There are at least two important challenges in dealing with these cations. First, how can one recognize and transport them? Second, how can one select one over the other? It might seem simple, for example, to construct a tunnel that fits Na+ but not K+. That might give selectivity for Na+—unless it was blocked by larger K+. The opposite approach would not be as practical. A tunnel fabricated to fit K+ should also fit smaller Na+.
A further complication is that it is probably not the cation that is transported through the bilayer, but a cation that is solvated to some extent. The sodium cation is smaller than K+ as judged from crystal structures. Solid state structures show that the Na+, K+, and Cl- ions have diameters of 2.04, 3.02, and 3.62 Å, respectively [13]. In contrast, the calculated [14] hydrated diameters of these ions are: Na+ = 5.98 Å, K+ = 5.50 Å, and Cl- = 6.48 Å. Clearly, the size order for Na+ and K+ is reversed depending on whether or not the ion is solvated. A fundamental problem is that the extent of solvation for each ion within a transporter, channel, or the bilayer is uncertain.
1.3. Crown ether cation carriers
The ability of crown ethers to complex alkali metal cations [15] led many to ask whether the complexes would be transported across membranes [16]. The transport of ions such as Na+ and K+ across phospholipid bilayers is a process critical to life and the subject of extensive study [17]. Cations are transported, as noted above, by a variety of processes. Although cation transport by channel proteins is the most common process, most studies of macrocycles have focused on the carrier mechanism of transport. In part, this was a matter of experimental expediency: transport could be assessed in many laboratories using simple equipment. Transport experiments also gave interesting information about the combination of binding strength and selection along with information about the hydrophobic/hydrophilic balance in the host molecule and in the complex.
The carrier transport process is sometimes described by using a “ferryboat” analogy. The ferryboat (receptor or host molecule) diffuses across the river (i.e., membrane) and picks up the passenger (guest molecule). The passenger and ferry (complex) again diffuse across the river, depositing the passenger on the opposite shore (Fig. 1).
Fig. 1.
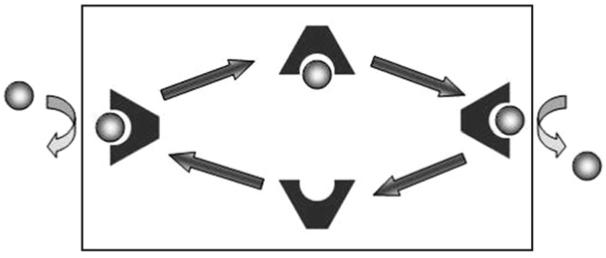
Schematic illustration of carrier transport. The membrane barrier is illustrated by the black box. The source phase is at the left and the receiving phase is at the right.
Many early cation transport studies were done by using a U-shaped glass tube. Chloroform or other dense, hydrophobic solvent was placed in the tube to a height past the bend. Water (or other hydrophilic solvent) was placed in each arm. A solute dissolved in the solvent present in one arm (left in Fig. 2) can reach the opposite hydrophilic phase (right arm in Fig. 2) only by passing through the hydrophobic solvent membrane. A carrier molecule present in the hydrophobic membrane can serve this purpose.
Fig. 2.
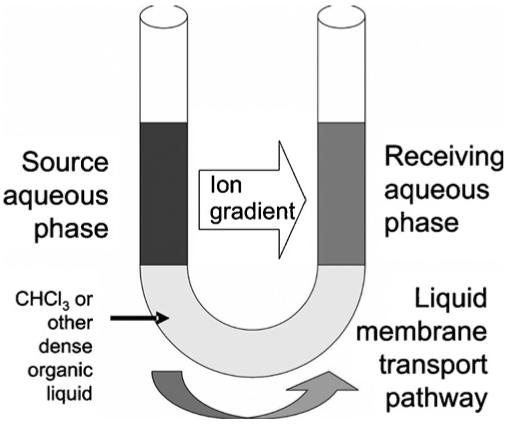
Schematic of the U-tube transport apparatus.
Cation transport was typically monitored by colorimetry, assuming that each cation was paired with a colored anion such as picrate that was deliberately added to serve this purpose. At the start of the experiment, the “source” arm of the U-tube would be bright yellow and the “receiving” arm would be clear and colorless. As transport proceeds, the two arms become similarly colored, assuming a 1:1 concentration of cation and anion. Once a 50:50 (1:1) distribution of the salt is achieved, no further change in concentration is detected, although transport presumably continues. Our own efforts in this area used an alternative, concentric tube device [18]. We attempted to correlate extraction constants, homogeneous binding constants, and transport phenomena for a family of lariat ether compounds [19]. In subsequent work conducted in collaboration with Echegoyen, transport by these compounds was assayed in phospholipid bilayer membranes [20].
A good deal of information was accumulated about the complexation and transport behavior of macrocycles [21]. Although such studies answered a variety of chemical questions, their relevance to the biology was somewhat limited. The comparison of a chloroform or dichloromethane membrane with a phospholipid bilayer is tenuous. Further, only limited examples of ion transport are known in nature to use carrier molecules rather than channels [22].
1.4. The elements of channel design
When chemists considered the design of model ion channels, they were faced with numerous questions, some of which remain unresolved today. Some of the more obvious issues are overall dimension, shape, and polarity [23]. The latter is a particular challenge because one must consider polarity at various positions within the channel structure. The phospholipid bilayer itself exhibits a range of polarity from the polar headgroup to the midpolar glyceryl regime to the hydrocarbon insulator regime [24].
In order for a molecule to function as a channel, it must insert in the bilayer from an aqueous phase. In order to do that, it must be an amphiphile. The issue of bilayer membrane insertion begs the question of rigidity. How rigid must a molecule be to insert in a bilayer and maintain a conduction path? Protein ion channels form very well-defined conductance pores [25]. Evolution has developed these over millions of years so they obviously represent an excellent solution to the chemical transport problem. The earliest channels must have been less than ideal, however. Indeed, the earliest ion transport through the boundary layer of a cell (or what evolved into a cell) likely occurred by leakage (diffusion). As the cell’s boundary layer evolved into a plasma membrane, cellular integrity increased as did the need for a transport mechanism.
Some early attempts to design channels involved the preparation of cylindrical structures or compounds that looked cylindrical [26]. In some of these early studies, only the conceptualization and synthesis were presented. There was no evidence that the compound inserted into bilayers, much less that it transported ions. The field has become more sophisticated over time. Not only must one design and prepare a potential channel, one must demonstrate efficacy. Ideally, one can also demonstrate such properties as open-close behavior that characterize natural channel-forming compounds [27].
Finally, there is a problem that may not be apparent at all. It seems clear that a synthetic channel must be able to span the phospholipid bilayer. Presumably, however, not all bilayers are equally thick. The monomer compositions in bilayer membranes are highly variable and, in nature, incorporate substantial amounts of protein. One can crystallize membrane monomers and obtain their solid state structures. This result of a solid state structure determination does not necessarily reflect the thickness of a dynamic membrane system. The design of a membrane-spanning channel will therefore require a guess concerning its appropriate length.
1.5. Function in artificial model systems
Model systems may be considered in two obvious senses. A compound may be a physical model of another structure. It may possess a size similar to another compound. It may “fit” sterically into a guest site within another structure. We may consider such compounds as structural models. How well they correspond to the structure they are intended to model can be determined by computer modeling involving structural overlap or by building physical models and comparing them.
Compounds that are designed to accomplish a particular task must be assayed by using the appropriate methodology. For example, the partition coefficient (log P) can be calculated for a molecule that is designed to partition into a membrane from water [28]. A value for log P that shows the membrane is favored is important evidence but it is not proof of function. The compound must be exposed to a membrane in contact with water to demonstrate efficacy. One may design a compound that looks physically like a channel. A functional model does not necessarily have to look like the structure it mimics, but it must do at least some of the same things. A functional model need not be as efficient, selective, etc. as the compound being mimicked, but it must exhibit some form or level of efficacy.
Reasonable people of good will may differ on what is an acceptable level of efficacy and how best to demonstrate it. A good example is the pioneering effort of Tabushi and coworkers who reported the preparation of an artificial cation channel in 1982 [29]. Their channel was based on a modified cyclodextrin and its efficacy was demonstrated by measuring Co2+ transport. Divalent cobalt transport through a bilayer is of marginal biological interest, but the measurements were chemically accessible and proved the concept. Thus, Tabushi et al. demonstrated that theirs was a functional model for a compound that supported ion transport through a membrane.
Ideally, the effectiveness of a compound designed for any particular purpose will be assessed by the means typically used to evaluate the natural substance. This may require special equipment, special skill and training, or both. Alternative methods are certainly acceptable, so long as the limitations of the methods are recognized. What is unacceptable, however, is not to demonstrate efficacy for a compound claimed to possess it.
1.6. Mechanisms of cation transport
There are at least four mechanisms by which cations (or other species) can be transported across a membrane. The four transport mechanisms are diffusion, passive transport, active transport, and carrier transport. Among these, the latter has been most extensively studied, especially by using synthetic receptor molecules [21]. We note that in this article “membrane” normally refers to phospholipid bilayers that form the outer boundaries of most cells.
1.6.1. Measurement of carrier transport
The transport of metallic cations by receptor molecules such as crown ethers has received enormous attention. We noted the use of a U-tube device (Fig. 2) in Section 1.3. The approach generally involves the use of a salt that has a colored counterion. Use of the bright yellow picrate anion is described above. The carrier (receptor) molecule is injected into the organic solvent, where it diffuses between the two aqueous phases. At one interface, the host molecule captures a cation and diffuses to the other aqueous phase where the cation is deposited. The cation is not transported alone; it is accompanied by the colored anion, which can be followed quantitatively. As cations transport from one arm to the other, color diminishes in the source phase and intensifies in the receiving phase. Transport of the salt can be followed colorimetrically until equilibrium is reached. Transport continues thereafter, but there is no further color change. Of course, transport can be driven further by varying the pH of the two phases or by various other means.
A concentric tube apparatus performs the same function as the U-tube, but it is more amenable to magnetic stirring of the organic phase. Variations other than the configuration of the apparatus have proved to be more problematic. Many solvents and volumes can be used for the organic phase. Although trends in transport may prove to be similar, rates determined in different solvents such as chloroform, dichloromethane, and tetrachloroethylene are not directly comparable. Another potential problem is the source of the salt that is transported. If sodium picrate is prepared pure and added to water, its concentration may be the same as if picric acid is added to aqueous NaOH. To ensure complete reaction of the alkali metal cation, however, an excess of base is often used, affecting the ionic strength of the aqueous solution. Individually, each method may be valid, but there exists no standard that would facilitate comparisons among transporter molecules.
1.6.2. Measurement of sodium transport by NMR methods
A clever, but intricate, method to measure alkali metal transport was devised by Riddell and coworkers [30,31]. It may be illustrated for sodium cation as follows. Liposomes (vesicles) are prepared from phospholipid monomers in a sodium salt-containing solution. Analysis of this suspension by 23Na NMR shows a single Na+ peak because ions inside and outside the liposomes are magnetically equivalent. A shift reagent such as dysprosium phosphate is added to the bulk aqueous phase, causing the line position for external Na+ cations to shift position. The signal strength for the external Na+ is stronger than for the Na+ that is included within the vesicles because there is so much more of the former present. When a transporter is added to the suspension, it may partition into the membrane. If so, transport can be monitored by changes in 23Na linewidth. From these changes in linewidth, an exchange rate constant can be determined. This is an excellent method, but it is labor intensive and experience suggests that the results tend to be somewhat operator dependent.
1.6.3. Ion selective electrode detection of Na+ release
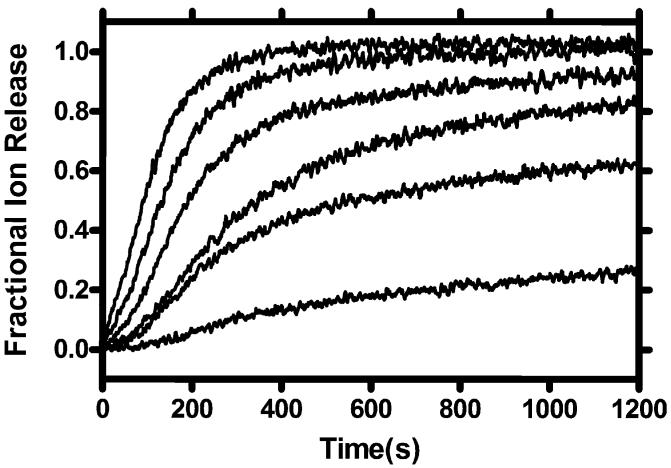
In this method, liposomes are created in the presence of Na+, but the external medium is then exchanged for Na+-free buffer. An ion selective electrode (ISE) sensitive to Na+ is inserted into the aqueous suspension [32]. The ionophore is added to the suspension and a measurement of Na+ concentration is recorded every second. In experiments conducted in our laboratory, we observe release for an arbitrarily set time span, depending on the experiment. The times are typically 1500 or 1800 s. After that time, a detergent such as Triton X-100 is added to lyse the vesicles. The data are normalized to the maximum value recorded by the ISE at this time. Typically, release data follow an exponential curve as shown in Fig. 1. In this figure, the concentration of ionophore increases from the lowest to the highest line. Note that the data set terminates at 1200 s in this case (Fig. 3).
Fig. 3.
Example plot showing fractional sodium cation release mediated by a synthetic transporter molecule at concentrations varied over an approximately 10-fold range.
When similar experiments are run using different compounds at identical concentrations, the percentage release can be compared at a particular time point to determine which compound is most effective in mediating ion release. This method measures transport, but does not confirm that transport occurs exclusively by a channel mechanism. A useful aspect of this method is that it is often applicable across a 10-fold concentration range. As such, the data may be used with the Hill equation [33] to gain insight into the pore’s aggregation state.
1.6.4. Fluorescent methods of analysis
Fluorescence is a powerful tool for visualization of structures, for determining positional relationships, and for probing mechanism. Many fluorescent dyes are highly sensitive and give clear fluorimetric responses at micromolar concentrations. Fluorescence methods have proved to be particularly useful in understanding the behavior and function of synthetic ion channels. The release of a fluorescent dye from liposomes can give an indication of anion channel function [34]. Likewise, Cl- transport can be assayed by monitoring the quenching of the fluorescent dye lucigenin [35,36].
The transport of cations does not typically use fluorescence methods except for the detection of proton flux. A fluorescent dye suitable to this purpose was introduced by Kano and Fendler [37]. Proton flux through a phospholipid bilayer was demonstrated by Menger and coworkers using the oligoethylene glycol ester, CH3(CH2)10COO(CH2CH2O)5CH2Ph, as a synthetic transporter [38]. Fyles and coworkers adapted the method of Gary-Bobo and coworkers [39], by competing proton and cation flux, to quantitate alkali metal cation transport rates and selectivity [40].
1.6.5. Voltage clamp experiments
The Nobel Prize winning method of Sakmann and Neher [41] is now considered the definitive demonstration of ion channel function. In one manifestation of this method, two chambers (“cuvettes”) containing different concentrations of ions are separated by a Teflon plate. Membrane lipids are “painted” into a pinhole in the plate. The membrane serves as an insulator between the two solutions until an ion transporter inserts in the bilayer. A fixed voltage is applied (the voltage is “clamped”) and transport of ions is detected as current by use of a sensitive amplifier and recorder.
Ion channels typically show “open-close” behavior. When the channel is open, ions flow through at a rate dependent in part on the pore diameter. When the opening of two channels is detected, the conductance is twice that observed when a single channel is open. This leads to the square wave type of readout that is characteristic of channel function (Fig. 4).
Fig. 4.
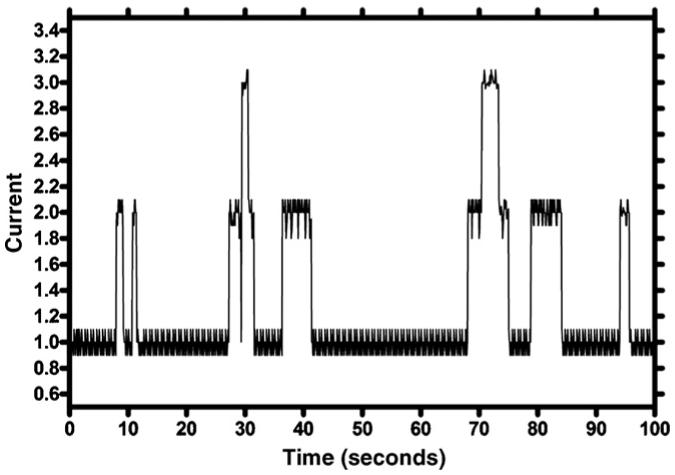
Plot of current vs. time showing typical one and two channel opening behavior.
The tracing shown is simply an example and does not correspond to any of the synthetic channels discussed in this article. It is important only for the reader to recognize that such clear open-close behavior is characteristic of conducting pores and channels and fundamentally different from the profile exhibited by a carrier molecule.
2. Design criteria for hydraphiles
Channel design must begin with a consideration of dimensions and polarity. An initial challenge is to ask what length must a channel be? When we began our studies, no solid state structure of a channel protein was available so no cue could be taken from it. The only synthetic channel model that was available was that of Tabushi et al. [29], which had about the same length dimension as a phospholipid monomer. Of course, even the length of a phospholipid monomer is highly variable because the fatty acid chains can range from about 12 carbons to 24 or more. Neither the extent nor depth to which fatty acid chains interpenetrate (“interdigitate”) where the two bilayer leaflets meet (bilayer midplane) is not known with any precision. Typical bilayer thickness can be guessed from capacitance measurements [42] that reflect the effectiveness of the insulator component of the bilayer. Another vagary is that the biological dimensions are often reckoned in nm or microns (μM) rather than in Å. This may make the uncertainty interval less prominent to some observers [43].
If we use a value of 30-35 Å (3-3.5 nm) as the thickness of the insulator regime (hydrocarbon slab), we must consider how far beyond that dimension a synthetic channel should extend. Chemists often think of a bilayer as having simply a head and tail structure (see Fig. 5). This is certainly true but membrane structure is more subtle than that. Even liposomes formed from a single phospholipid, such as dioleoylphosphatidylcholine (DOPC), possess three distinct polarity regimes. The fatty acid tails are nonpolar and, with those present in the opposite leaflet of the bilayer, combine to form the insulator regime of the membrane. The neutral, but charged, headgroup comprises both an ammonium cation and a phosphate anion. This charged and very polar regime is in contact with the aqueous cytosol and periplasm.
Fig. 5.
Schematic and oversimplified representation of a bilayer membrane in which the headgroups are represented as circles and the fatty acid chains as wavy lines.
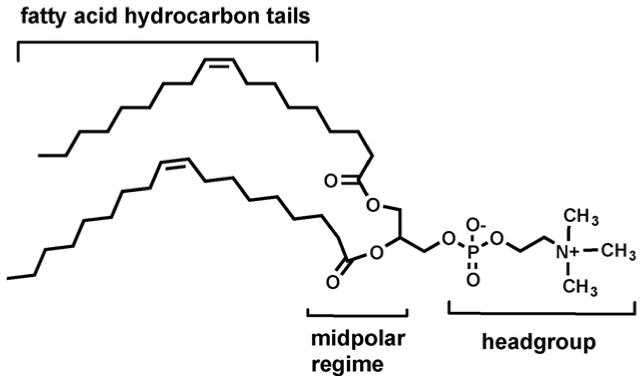
Interactions that position a protein or other structure within the bilayer may involve hydrophobic interactions with the membrane’s hydrocarbon chains. It will be hard for polar interactions such as hydrogen bonds to compete with the bulk aqueous phase. The midpolar regime is reported to have a polarity corresponding to a dielectric constant (ε) of about 25 [44]. Hydrogen bonding and other weak force interactions can certainly play a significant role in a medium of this polarity. An examination of the KcsA voltage gated potassium channel from Streptomyces lividans suggests that the indole residues of all the tryptophans in the structure occur at the insulator-midpolar boundary [45]. Tryptophan is the rarest of the 20 common amino acids and appears to play an important role as a stabilizing element [46-48] (Fig. 6).
Fig. 6.
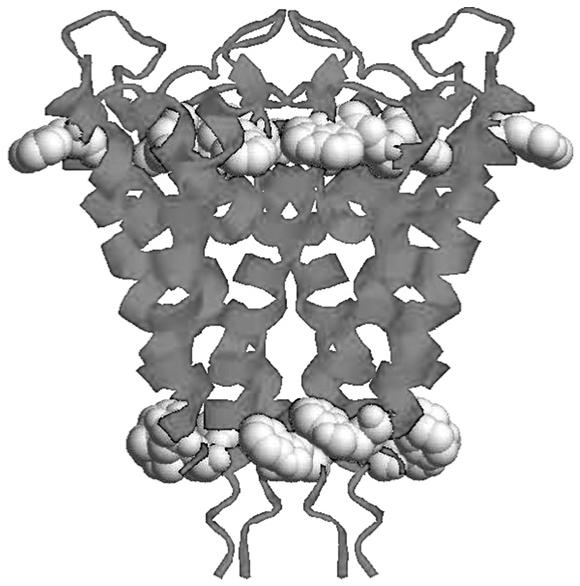
Ribbon structure of KcsA potassium channel drawn from solid state structure coordinates (PDB: 1BL8).
Our initial decision was to devise a structure that fully spanned the insulator regime but did not dramatically exceed it. It was thought that this would be achieved by simple hydrocarbon links. If the structure inserted into the bilayer, such hydrocarbons should align with the long axis of the fatty acyl chains and be stabilized thereby. The alkyl chains also were favored over rigid chains based on our own type of “uncertainty principle.” A critical factor in the design of a biomimetic compound is to achieve function. If there is no function, then there is no basis for comparison when structural changes are made. When structural elements work well, they can be rigidified to make them even more effective—a kind of chemical evolution. In the design phase, our choice was to keep the spacer chains flexible in the hope that a poor estimate about distances would be compensated by adjustment within the structure.
The channel-former must clearly be an amphiphile. The decision was made to use macrocyclic (crown) polyethers as headgroups. Azacrown-based lariat ethers [49,19] proved versatile and flexible. In separate studies, it was found that crown ethers function well as amphiphilic headgroups [50,51]. Crown ethers are, of course, cation selective and it was thought that some selectivity would be imposed on the system if cations used the crown as an entry portal. N,N’-Di-n-dodecyl-4,13-diaza-18-crown-6 binds Na+ and K+ in CH3OH solution with constants Ks (log Ks) of 980 (2.99) and 6300 (3.80) [52]. This is a selectivity of about 6:1 for K+ >Na+.
With the headgroups and spacer residues decided, there remained two structural elements to consider. One of these was the connection of the macrocycles with one or more than one link. Such a connection would produce a tunnel-like structure. This had the advantage of “looking” like a channel but it had the disadvantage of increased rigidity. The more rigid the initial structure, the more likely it seemed that a small error in the estimates made would result in function failure. We therefore decided to attach alkyl chains to the second nitrogens in the two macrocyclic headgroups. These would not be connected to each other so that adjustment of the overall size within the bilayer would not be impeded.
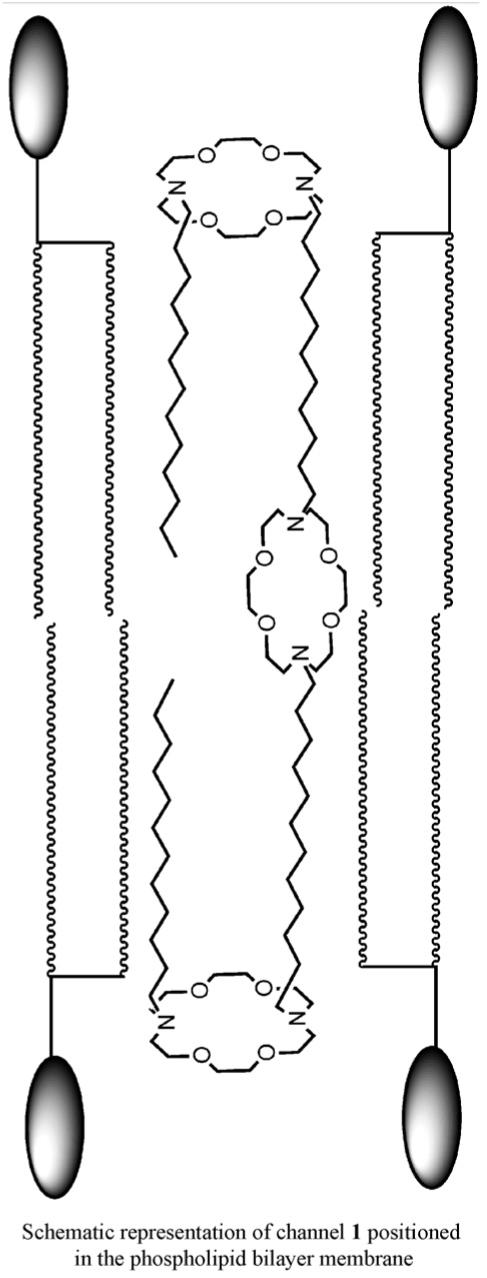
The process of moving a charged ion across a nonpolar pathway ≥30 Å is energetically unfavorable [53]. It was apparent to us that a synthetic channel would have to incorporate a polar element at or near the midplane of the bilayer to reduce the overall energy of transport. Such an element was unknown in protein channels but chemical intuition suggested that some energy reduction mechanism must be present. We decided to incorporate a third macrocycle whose position would be defined by the lengths of the covalent spacer units. We refer to this element as the “central relay.” The elements of the initial channel design are illustrated schematically in Fig. 7.
Fig. 7.
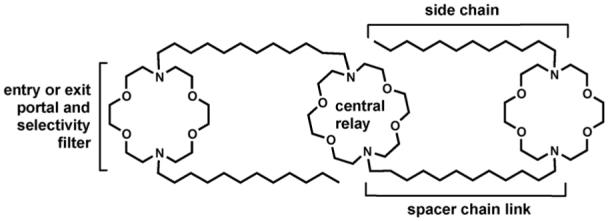
The modular structural elements of compound 1.
The working assumption was that the structure shown would insert in the bilayer and form a conduction path. Ions and water would fill it as cars fill a tunnel and pass through. Ions could enter and exit in either direction because the structure is symmetrical. Thus, transport through the bilayer would occur but the property of rectification (transport in one direction but not the other) is absent. The role of the central relay is to shorten the ion’s “jump” distance and thus lower the energy of transport.
The distal macrocycles serve not only as entry and exit portals, but as selectivity filters. We noted above that in methanol solution, complexation of K+ is favored over Na+ by a factor of ∼6:1. The exact meaning of this selectivity factor in the present context is unclear for two reasons. First, the binding constant is determined in homogeneous solution; the solvent in this case is anhydrous methanol. Second, it is transport rather than binding that will dictate ion selectivity in a channel. Thus, the lower Na+ binding constant exhibited by didodecyldiaza-18-crown-6 suggests that it will be passed more readily than will the more strongly bound K+.
2.1. Synthesis and characterization
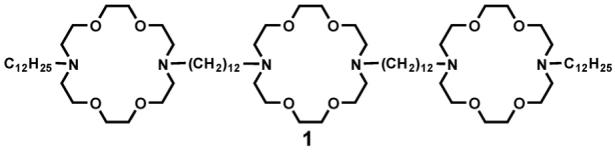
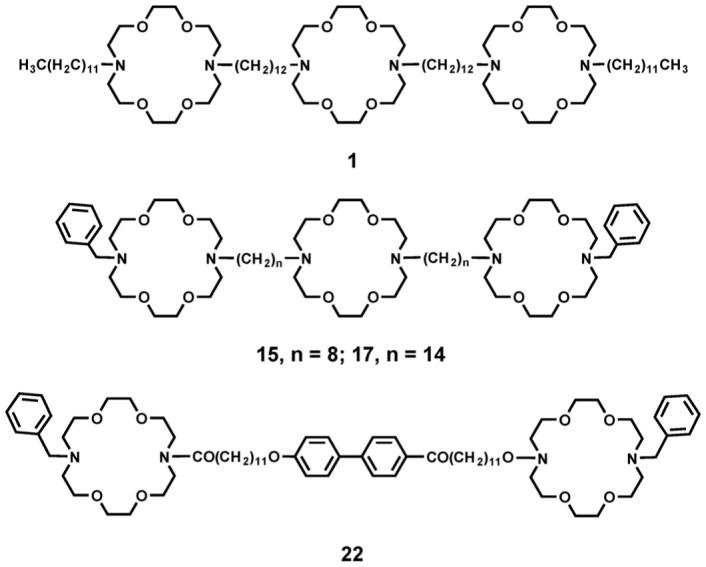
The initial synthetic target, 1, incorporates three diaza-18-crown-6 macrocycles and four 12-carbon alkyl chains. The two distal macrocycles are both attached to dodecyl chains, but the two chains slightly are different. The central (medial) macrocycle is also attached to two dodecyl chains, but these are identical. Thus, the synthetic challenge in creating 1 was to start with a symmetrical crown and to prepare a system that was over all symmetrical but that contained two unsymmetrically substituted macrocycles.
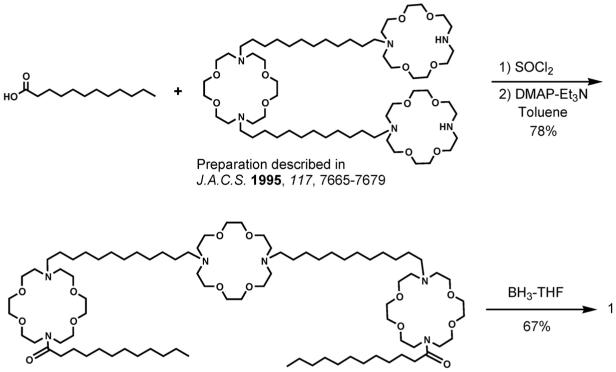
A number of different approaches have been tried over the years since the initial preparation of 1 [54]. The synthetic sequence in current favor in our lab is shown in the scheme below. From the synthetic perspective, the analog of 1 that has benzyl rather than dodecyl sidearms is a flexible starting material. Benzyl compound 4 can be converted into the hydraphile required as starting material in Scheme 1 by hydrogenolysis of the N-benzyl groups [55]. The compounds having terminal NH groups can usually be acylated in good yield to give a diamide. This can typically be reduced to the amine without complications.
Scheme 1.
Preparation of 1.
2.2. Assessing functionality
The value of a model system is in its function. The goal is not to make a previously known structure (target-directed synthesis), but to make a compound that has a particular function or property (property-directed synthesis [3]). As described above, there are several methods to determine whether or not a channel exhibits the desired (or any) function. Confirmation of Na+ transport by compound 1 was initially obtained by use of the Riddell NMR method described in Section 1.6.2 (above).
Numerous structural variants of 1 were prepared and their ability to transport Na+ was assayed in each case by using the NMR method. By this means, we were able to discover how various structural changes altered Na+ transport efficacy. The results of these early studies are shown in Table 1 [55]. In all cases, the sodium transport rate was compared with that obtained at the same time in the same experimental system using gramicidin D as a standard. Rates were normalized to a value of 100 arbitrarily set for gramicidin. Note that the abbreviation <N00N> represents a diazacrown ether of ring size 00. The abbreviation <00N> or <N00> represents an azacrown rather than a diazacrown.
Table 1.
Sodium ion transport rates determined by 23Na NMRa
| No. | Compound | Rateb |
|---|---|---|
| Gramicidin D | 100 | |
| 1 | C12<N18N>C12<N18N>C12<N18N>C12 | 28 |
| 2 | C12<N18N>C12<N15N>C12<N18N>C12 | 25 |
| 3 | C12<N18N>C12(OCH2CH2)3OC12<N18N>C12 | 14 |
| 4 | PhCH2<N18N>C12<N18N>C12<N18N>CH2Ph | 39 |
| 5 | H<N18N>C12<N18N>C12<N18N>H | 28 |
| 6 | <18N>C12<N18N>C12<N18> | <2 |
| 7 | C12[<N18N>(OCH2CH2)3O]2<N18N>C12 | 3 |
| 8 | C12<N18N>C12 | <2 |
| 9 | PhCH2<N18N>CH2Ph | <2 |
| 10 | C12<N18N>C12<N18N>C12 | <2 |
Data obtained at 25 °C by using egg lecithin vesicles.
Transport rate observed in each experiment arbitrarily set to 100 and all other values are normalized to it.
The structural variations shown in Table 1 reveal a number of interesting facts. First, compounds 2 and 3 differ from 1 only in the identity of the central relay. In 1, it is diaza-18-crown-6 and in 2 it is diaza-15-crown-5. The Na+ transport rates for the two compounds are nearly identical suggesting that the transient cation may not be passing through the central relay. The central relay in 4 is not cyclic but 4 is a functional transporter even though there is no cycle through which the ion can pass. This suggested that the initially envisioned conformation was not correct, as discussed in the next section.
A particularly interesting comparison is between 1 and 7. We imagined that by incorporating ethyleneoxy units as spacers instead of dodecylene units, we would reduce the cation’s “jump distance” even further and enhance transport. In fact, the opposite proved to be the case. It now seems clear that although a central relay is required for channel function, the best transport rates are achieved when the two passages leading to it are hydrophobic. It is interesting to note that when the first solid state structure of a potassium channel appeared, MacKinnon and coworkers noted as follows. “From inside the cell (bottom) the pore begins as a tunnel 18 Å in length (the internal pore) and then opens into a wide cavity (∼10 Å across) near the middle of the membrane. A K+ ion could move throughout the internal pore and cavity and still remain mostly hydrated [45].”
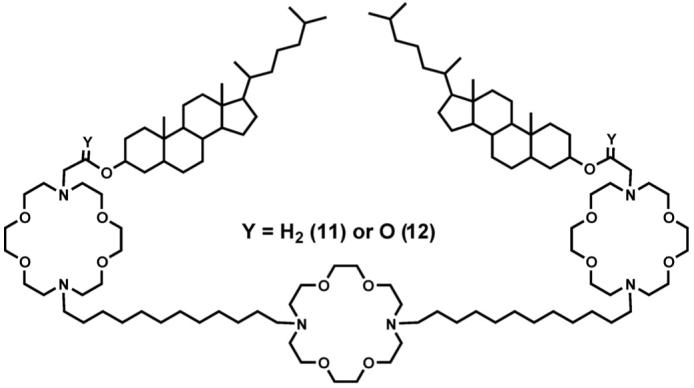
Another interesting observation concerns steroid sidechains (data not shown). Cholestanol was attached as the sidechain to each distal macrocycle in an analog of 1. The connectors between the macrocycle’s nitrogen and the steroid’s 3-hydroxyl group were ethylene (CH2CH2, 11) or acetyl (CH2CO, 12). Cholesterol is an important component of most mammalian membranes and it was thought that including it as part of the sidechain would better organize the conduction pathway. Instead, we found that the ethylene spacer compound was inactive (<2 compared with Table 1 compounds) and the ester’s transport rate was 5 compared to gramicidin (1 0 0) [56]. Crystallographic, NMR, and computational studies led to the conclusion that cholestanol interacted strongly with the spacer chain opposite to it and this closed the conduction pathway. The ester, being less flexible than the ether link, permitted some ion passage because the steroid could not contact the dodecylene unit as effectively. It is interesting to note that a channel designed with steroid walls around a central macrocycle showed extremely slow ion transport [57].
In more recent studies, we used sodium ion-selective micro-electrodes to detect Na+ release from liposomes [32]. When this method is used, Na+ is trapped within phospholipid vesicles. The ionophore is added (typically in 2-propanol) solution. The external buffer is initially Na+-free so release of the ion is readily detected. After an arbitrary time period (typically 1500-1800 s), a detergent such as Triton X-100 is added to lyse the vesicles. The amount of Na+ ions detected after vesicular lysis is set to 1.00 and the fractional release of Na+ is expressed accordingly. This method gives a rapid, convenient, and reproducible means to assess transport efficacy.
2.3. Defining the conformation in the bilayer
Compounds 1 and 2 transported Na+ at the same rate within experimental error. Compound 3, which lacks a cyclic central relay, was still functional although its transport rate was only about half of that observed for 1 and 2. The smaller macroring of 2 compared to 1 was expected to slow transport if the cation passed through it. The functionality of 3 showed that cations did not need to “pass through” anything. It thus seemed likely that the central relay macrocycle was parallel to the fatty acid chain axis rather than parallel to the membrane surfaces. Additional studies confirmed this assumption and suggested that the central relay interacts with the solvated cation rather than the cation itself [58].
The compound we refer to as the “dansyl channel” is one of the most important tools available to us. Its structure is shown as 13. This channel is a fully functional Na+ ion transporter. The two dimethylaminonaphthylsulfonyl (dansyl) residues are fluorescent. The fluorescence maximum for 13 is red shifted as the solvent in which it is dissolved becomes more polar. The fluorescence maximum for 13 was measured when the channel was in the membrane. Its shift suggested that it experienced an environment similar to but slightly more polar than ethanol [59]. If the tris(macrocycle) portion of the molecule spans the insulator regime and the dansyl groups are in the midpolar regime, the observed polarity is as expected.
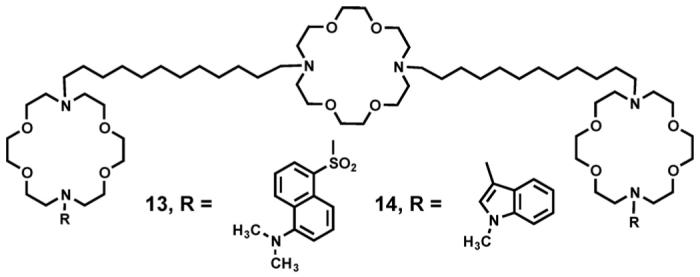
Synthetic phospholipids may be prepared that have nitroxide residues placed at varying distances along one of the fatty acid chains. Nitroxides quench fluorescence in a distance dependent fashion. Thus, we may determine how readily the dansyl group fluorescence is quenched in the presence of nitroxides placed at different distances from the midplane of the bilayer. By so doing, one may triangulate the distance of the dansyl residue from the bilayer midplane [60]. Such studies conducted with 13 indicated that the dansyl residues are located 14 Å from the bilayer midplane [61]. This value places them at a separation of ∼28 Å. Of course, the dansyl group itself has molecular dimensions of about 6 Å × 6 Å so the separation is actually 28-34 Å. This places the dansyl residues at the outside of the hydrocarbon slab and in the midpolar regime, as expected.
A third type of experiment involves the transfer of energy from one molecule to another [62]. If we irradiate 14 at λ = 283 nm, the indole residue absorbs energy and fluoresces at λ = 340 nm. The dansyl residue of 13 absorbs light at this wavelength and fluoresces at λ = 516 nm. This fluorescence resonance energy transfer (FRET) experiment indicates that at least for these two compounds, the aggregation number is 1.1 [59]. This supports the initial unimolecular design criteria.
2.3.1. The central relay
Our initial design concept placed the medial or central macro-cycle of 1 near the midplane of the bilayer and parallel to the distal macrocycles. The successful function of channels having central relay units [58] too small (e.g. 3) to pass Na+, strongly suggested that in 1, the third macrocycle was positioned perpendicular to the other two and parallel to the axes of the fatty acid chains. It is clear from the symmetry of the structure shown in Fig. 7 that cations can enter and exit either side of the channel and pass through the membrane. If a single direction was preferred, the channel would be rectifying.
2.4. Length dependence of channel function
The overall length of the tris(macrocyclic) channel compounds can be varied if the covalent links (spacer chains) are changed. The fact that our initial channel (1) was effective at transporting Na+ did not mean that it was the optimal design. We noted that in transport experiments of the type summarized in Table 1 that benzyl sidechains gave higher transport than did dodecyl chains. We therefore prepared a family of benzyl channels of the type PhCH2<N18N>-(CH2)n-<N18N>-(CH2)n-<N18N>CH2Ph in which the value of n varied from 8 to 16 [63]. Transport was assayed by using the 23Na NMR method described in Section 1.6.2 above. An important difference, however, is that the standard for these experiments was dansyl channel 13 rather than gramicidin. In this case, sodium transport mediated by 13 was set to an arbitrary value of 100.
According to the data obtained in these studies and presented in Table 2, either dodecyl or tetradecyl spacers would have been appropriate covalent links to give a functioning channel. Our expectation was that 15, which has an octylene [(CH2)8] spacer chain, is too short to span the bilayer. In fact, no evidence of transport was observed. As the spacer chains grow longer, we anticipated that transport would diminish because the conductance path would be inappropriately long and therefore less stable.
Table 2.
Length dependence of sodium cation transporta through phospholipid bilayers
| Compound | Structure | Rateb |
|---|---|---|
| 15 | PhCH2<N18N>C8<N18N>C8<N18N>CH2Ph | <2 |
| 16 | PhCH2<N18N>C10<N18N>C10<N18N>CH2Ph | 96 |
| 4 | PhCH2<N18N>C12<N18N>C12<N18N>CH2Ph | 211 |
| 17 | PhCH2<N18N>C14<N18N>C14<N18N>CH2Ph | 201 |
| 18 | PhCH2<N18N>C16<N18N>C16<N18N>CH2Ph | 109 |
| 13 | Dn<N18N>C12<N18N>C12<N18N>Dn | 100 |
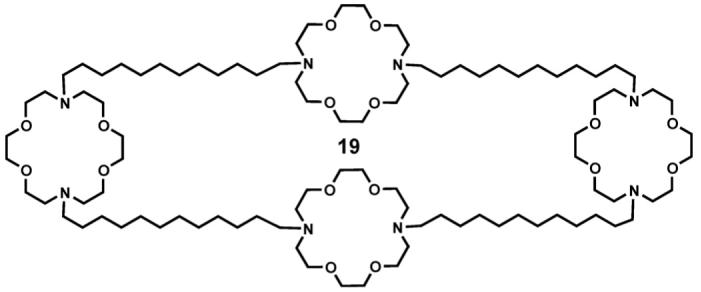
2.5. Formation of a tetramacrocyclic channel compound
If the extended conformation of 1 is present in the bilayer as indicated by the bulk of the experimental evidence, it should be possible to simply insert a fourth macrocycle between each terminus of the side chain residues. Of course, this is not a viable synthetic approach but such a tetramacrocyclic compound (19) was prepared [64]. When Na+ transport was assessed by use of the NMR method, 19 showed a transport rate of 340 compared to 100 for dansyl channel 13.
2.6. The hydraphile name
The success of the tetramacrocyclic system raised a new problem. We had referred for some time to this family of compounds as tris(macrocycle) channels. Addition of a fourth ring (19), or even successful transport of Na+ by 3 (see Table 1) made this designation inappropriate. The definition of hydra in the American Heritage Dictionary caught our attention.
Any of several small freshwater polyps of the genus Hydra and related genera, having a naked cylindrical body and an oral opening surrounded by tentacles.
The tris(macrocycles) have a cylindrical body surrounded by tentacles. The name hydra also suggests hydration or the presence in water. A further definition was “[a] persistent or multifaceted problem that cannot be eradicated by a single effort.” Thus, we dubbed the family “hydraphiles.”
2.7. Confirming transport efficacy by voltage clamp studies
The voltage clamp experiment described in Section 1.6.2 (above) was a critical demonstration of channel function for the early workers in this area. The complexity of channel proteins was well known and it seemed unreasonable to many biologists that a completely synthetic channel of barely 1500 Da could possibly transport ions through a bilayer. The voltage clamp method is a recognized standard in the channel community. Thus, it was incumbent upon us to use this sophisticated methodology to demonstrate ion transport function and to gain credibility.
The trace shown below was obtained with benzyl channel 4 as the ionophore. It was added at a concentration of 8 picomolar. The ion solutions were 500 mM aqueous NaCl, and the applied voltage was +100 mV. In this experiment, more than 30,000 transients were observed over about 4 h. The open-close behavior typical of protein channels is apparent in the traces shown in Fig. 8. Single channel openings dominate but there are some two-channel events as well.
Fig. 8.
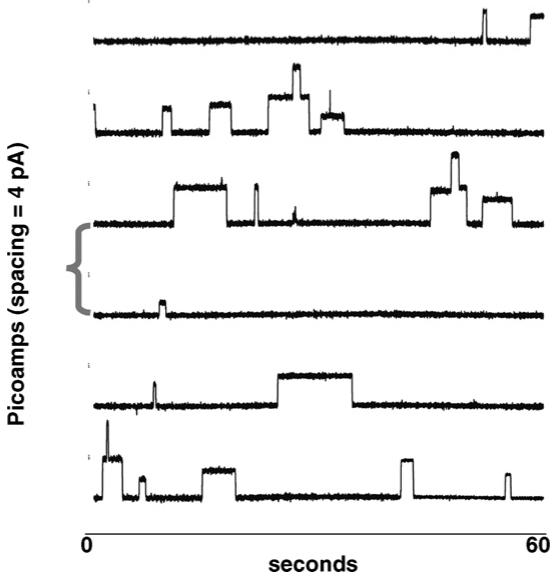
Planar bilayer voltage clamp recordings for sodium cation transport by benzyl channel 4.
There are two particularly interesting features of these traces. First, in some cases, the channels remain open for a period of 10 s or more. This seems surprising considering the flexible nature of the hydraphile compounds. Second, a few sub-conductance events are apparent. These are conductance states that do not correspond to the principal channel openings. The origin of such events is unclear.
It is not known what mechanism leads to the open-close behavior of the hydraphile channels. We postulate that water and ions fill the channel. The channel helps to organize this column of hydrated ions and the ions serve to organize the conductance pathway. Any twist in the channel would compress the conductance pathway and prevent the passage of ions. Likewise, an opposite twist would reopen the channel, again permitting ions to pass.
It is worth noting that experiments that to obtain results such as those shown in Fig. 8 requires specialized and rather expensive equipment. Learning to do such experiments has required both patience and perseverance, at least in our laboratory. It is not uncommon for synthetic chemists to find a collaborator to undertake these measurements.
2.7.1. Rectification
Natural channels are not only entry and exit portals for the cell, they are also regulators. As noted above, sodium concentrations are typically 10-fold or more higher outside than inside the cell. The converse is true for potassium cation. In order to maintain such ionic balances, protein channels typically are rectifiers: they pass ions preferentially in only one direction. The symmetrical hydraphiles are not rectifiers but their channel transport is still well controlled. A plot of current versus voltage (an “I-V”) plot is curved when ions pass preferentially in one direction and linear when there is no such preference. An I-V plot obtained for benzyl channel 4 is shown in Fig. 9.
Fig. 9.
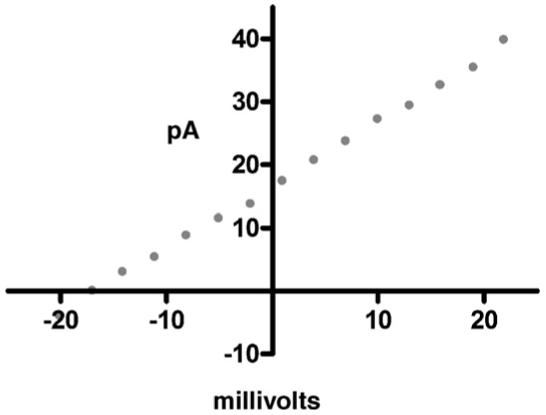
Current-voltage (I-V) graph for 4 transporting sodium cation. The linearity shows that the channel is non-rectifying.
2.8. Confirmation of channel position within the bilayer
In order to function as channels, the hydraphiles inserted into the liposomal bilayer. We expected the hydraphiles to insert into living cells as well. In order to visualize this process, we used the fluorescent dansyl channel, 13. We had previously shown that it was fully functional although not as effective at transporting Na+ as some other hydraphiles. If the channel inserted into the outer membrane of, for example, Escherichia coli, we would expect to detect fluorescence primarily at the cell’s periphery. Fig. 10 shows a photomicrograph of an E. coli cell after exposure to 13 [65].
Fig. 10.
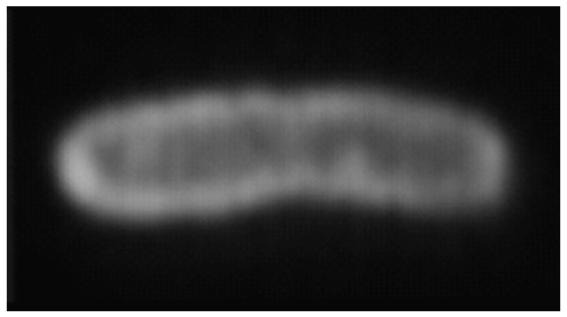
Fluorescent photomicrograph of Escherichia coli bacterium in the presence of hydraphile channel 1.
2.9. Channel function in living cells
The hydraphiles were designed to be channel models but their activity shows that they are functional channels. They insert in phospholipid bilayers and they effectively transport Na+ cations. They have been studied most extensively in phospholipid vesicles. It was of obvious interest to see if hydraphiles functioned in the bilayers of living mammalian cells. To this end, a whole cell patch voltage clamp experiment was conducted using human embryonic kidney (HEK 293) cells.
The experiment was conducted by patching a human embryonic kidney cell and monitoring the membrane electrical activity under voltage clamp conditions. No electrical activity was observed until 1 or 17 (8 μM) was added to the cell-containing medium. An almost immediate increase in conductance was observed upon hydraphile addition. The increased conductance was sustained until the cell was bathed in saline solution to remove the channel. Conductance was gradually lost but the cell remained vital throughout the experiment [66].
The active compounds, 1 and 17, are shown below, along with the close analog, 8. In length dependence studies, 8 was found not to transport Na+ across the phospholipid bilayer of liposomes. In the whole cell experiment, it did not change membrane conductance when administered as were 1 and 17. Compound 22 is long enough to transcend the bilayer but it lacks the central relay necessary for channel function in a bilayer [63]. In the whole cell studies, no change in membrane conductance was observed when it was added as described for 1.
3. Antimicrobial activity of hydraphiles
If a non-rectifying channel inserts in an organism’s exterior or plasma membrane, unregulated ion transport should occur. In such a case, the regulation of osmotic balance will be lost and the consequences may be dire. The hydraphile compounds are abiotic and it was hoped that if they proved to be toxic to microbes, it might also be difficult for bacteria to build a resistance to them.
Our initial studies to determine biological activity were done by placing a cellulose disc that was soaked in a solution of 4 on a media plate. A lawn of E. coli grew overnight except where the hydraphile was present. This simple study was quantitated by determining the minimum inhibitory concentration (MIC) for 4. One of the controls used in this case was the C8 analog, 15, which is identical to 4 except that it is shorter. In studies of length dependence, 15 did not transport Na+ and in these studies, it was 15-fold less toxic to bacteria than was 4 [67].
Additional studies of toxic effects to microbes were performed with the Gram positive bacterium Bacillus subtilis and with the yeast Saccharomyces cerevisiae. With the E. coli studies, this group of organisms gave a span of Gram negative and Gram positive bacteria and an assessment of the primitive eukaryote S. cerevisiae. Some selectivity was in evidence but the compounds in hand do not yet show a broad enough therapeutic index to be considered drug leads [68].
3.1. Length dependence of toxicity
Table 2 in Section 2.4 shows that when the hydraphiles are of different lengths, they exhibit different transport efficacies. When the channel is too short to span the bilayer, it cannot create a conduction pathway. Ions may still be transported, presumably by a carrier mechanism. Of course, the latter process is far slower than channel function. When the thickness of the membrane and the length of the channel are similar, we expect function to be most favorable. When the channel is longer than the membrane is thick, we expect difficulty in forming a stable conduction path. When the path forms, ions should be transported but the path will not necessarily form readily or persist.
If the mechanism of toxicity is a disruption of osmotic balance, then toxicity to the microbe should parallel transport efficacy. We prepared two additional compounds of the general form PhCH2<N18N>Cn<N18N>Cn<N18N>CH2Ph, having spacer chains of (n=) 18 and 20 carbon atoms (20, 21). This provided a family of structures that were identical in all respects except that the spacer chains ranged from 8 to 20 carbon atoms. The overall length of the channels therefore ranged from about 30 to 60 Å.
Minimum inhibitory concentrations were determined for each compound for both E. coli and B. subtilis. For channels having spacer chain lengths between 12 and 16 (i.e. 4, 17, 18), MIC values were <10 μM, in some cases these compounds have proved to be toxic in the high nanomolar range. This compares with effective plasma concentrations for penicillin of about 8 μM. Thus, these compounds are effective. Of course, to be useful as drugs, they must be both efficacious and selective.
Fig. 11 shows a graph in which three data sets are overlaid. Two of the traces show that both B. subtilis and E. coli are most susceptible to hydraphile compounds that have spacer chains in the 12-16 carbon range. The third trace shows the length dependence of Na+ transport. The biological data and the length dependence are approximately mirror images of each other. This is expected if the mechanism of toxicity is disruption of osmotic balance because the better the channel function, the more readily the ionic balance will be lost [69].
Fig. 11.
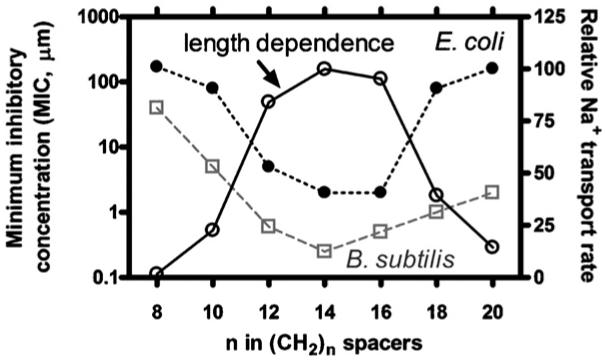
Overlaid traces showing (open circles) the length dependence of Na+ transport and the toxicities of the corresponding hydraphiles to E. coli (filled circles) and Bacillus subtilis (open squares).
3.2. Probing the mechanism of action
The presumption that the hydraphile compounds alter the osmotic balance is supported by the obvious correlation between toxicity and length. Additional support for this inference was obtained by studying membrane depolarization. This was done by using the membrane dye, 3,3′-dipropylthiadicarbocyanine [DiSC3(5)], DiSC, is sensitive to polarized versus depolarized bilayers as reflected in its fluorescence emission [70]. Thus, membrane depolarization was observed when both the hydraphile and a source of cations were present but not when one of these elements was missing.
4. Hydraphiles as an analytical tool
Protein ion channels are large, complex, and sophisticated molecules that function with remarkable efficiency and selectivity. Their inherent complexity makes it difficult to make modifications that permit extending their functionality. The hydraphiles are certainly not trivial to prepare but their syntheses are less daunting than are large scale modifications of proteins. It is therefore possible to modify hydraphiles to form, for example, the fluorescent, dansyl-substituted ionophore 13. We have prepared a family of hydraphiles that have benzyl sidearms and that vary in overall length. We have used these and other derivatives as analytical tools as described in the following sections.
4.1. Dynamic measurement of bilayer thickness
Although values are published that estimate bilayer thickness, precise values are not known. This is so for at least two reasons. First, the thickness of a bilayer presumably depends, at least in part, on its composition. Second, natural bilayers are complex and dynamic structures [71] that defy precise assessment by current analytical tools. Certainly, much is known about membrane structure and composition as a result of many arduous and excellent biophysical efforts [5,7,25,72,8]. Notwithstanding, the structural versatility of the hydraphiles permit them to be used to directly assay certain membrane properties.
We prepared a series of compounds having the general formula PhCH2<N18N>(CH2)n<N18N>(CH2)n<N18N>CH2Ph in which the value of n was varied in two-carbon increments from 8 to 20. This resulted in compounds 15, 16, 4, 17, 18, 20, and 21, respectively. By estimating from molecular models, we concluded that the approximate span from n = 8 to n = 20 was about 30 to 60 Å. We prepared phospholipid vesicles from three different phospholipids: 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and 1,2-dierucoyl-sn-glycero-3-phosphocholine (DEPC). The fatty acids in each case are cis-unsaturated and designated 14:1, 18:1, or 22:1, respectively. Intuition suggests that liposomes formed from DMPC should have the thinnest bilayer membrane and that those formed from DEPC will be thickest.
Liposomes were prepared in the presence of NaCl from each of the three types of phospholipid monomers. In each case, the ionophore was added to the vesicular suspension and sodium cation release was monitored by ion selective electrode methods. After an hour, Triton X-100 was added to the system to lyse the vesicles. The final Na+ reading was then used to normalize the other data points. Each experiment was repeated at least three times. The method was found to be reproducible over a 10-fold concentration range. The release rate was then recorded as the fractional release at the arbitrarily chosen time point of 1500 s.
Fig. 12 shows release data for the range of hydraphiles from C8 to C20 with DMPC (14:1, filled squares) and DEPC (22:1, open squares) vesicles. Data are not shown in Fig. 11 for the DOPC vesicles (18:1). On this graph, that trace would fall between DMPC and DEPC. Since the results correlated well with the presumed membrane thickness, we asked what effect cholesterol, a putative membrane thickening agent, would have on the system. Cholesterol was therefore added (20 mol%) to DMPC vesicles. The open circles show that the membrane did, indeed, behave as if it was thicker than in the absence of the steroid. To our knowledge, this is the only dynamic membrane thickness assay that has been reported.
Fig. 12.
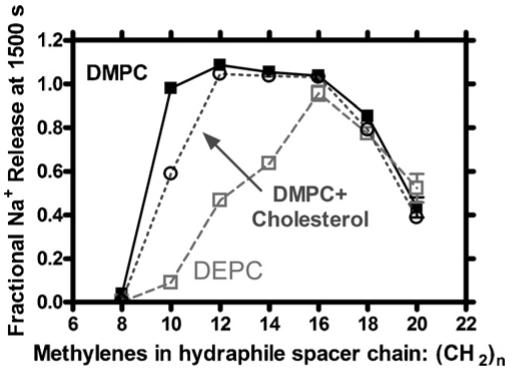
Sodium cation release mediated by C8 to C20 hydraphiles from DMPC (14:1, filled squares) and DEPC (22:1, open squares) vesicles. Addition of 20 mol% cholesterol on DMPC vesicles is plotted in open circles.
4.2. Cation-pi effects on transport efficacy
A striking feature of the KcsA voltage gated K+ channel structure noted in Section 2 is the fact that tryptophan occurs only at the boundaries of the insulator regime. This is illustrated in Fig. 6, above. Our presumption from this and a wealth of other information on tryptophan is that this amino acid acts as an anchor to stabilize the channel within the bilayer. This is likely to take advantage of such typical weak force interactions as hydrogen bonding and salt bridge formation. One type of interaction for which considerable evidence has recently been developed is the cation-pi interaction. This is especially plausible with tryptophan because it is so electron rich.
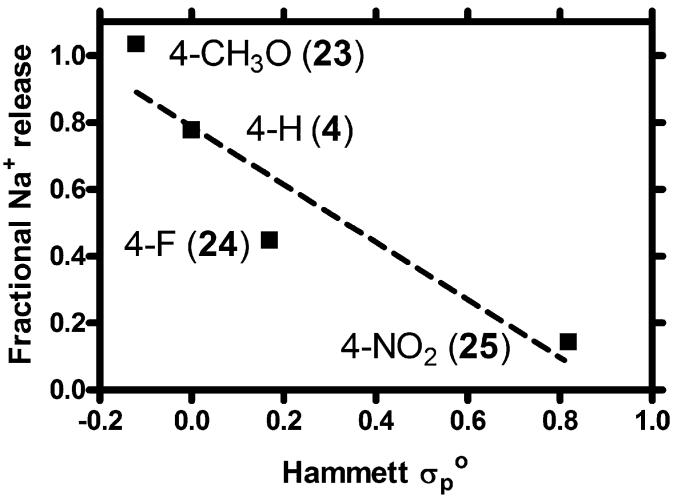
In order to assess this interaction, we prepared a suite of hydraphiles having benzyl sidearms. These included benzyl channel 4 and three analogs (23-25) in which the benzyl group was substituted in the 4-position. The substituents are methoxy, fluoro, and nitro. In order to confirm that some difference in transport efficacy resulted from these substitutions, we used the ISE method to measure Na+ release from DOPC vesicles, mediated by 4, 23, 24, or 25. The structures of 23-25 are shown.
The observed transport rates did, indeed, differ. In order to determine if the variation was systematic, fractional release of Na+, compared at the arbitrarily selected time point of 1500 s, was plotted as a function of the substituent’s Hammett constant. Because the arene is separated from the nitrogen heteroatom by a methylene group, we used the Hammett parameter initially developed for phenylacetic acid derivatives. As shown in Fig. 13, the correlation between transport rate and substituent constant is good. The slope (reaction constant ρ) of the line is -0.86 and the correlation coefficient (R2) is also 0.86 [73]. A negative slope is expected for the interaction of a cation with a Lewis basic donor such as tertiary nitrogen. In a Hammett correlation study involving dibenzyldiaza crowns and sodium cation, a slope of -1.00 was obtained [74]. The transient nature of the Na+···:N(amine) “complexation” interaction may account for the lower reaction constant but the difference is small in any event.
Fig. 13.
Hammett plot for sodium cation transport through DOPC bilayers mediated by 4, 23, 24, and 25.
Having established that some difference in Na+ transport was expected based on a substituent effect, we assessed the effect of phospholipid headgroup interactions on transport. In principle, we expected that electron rich substituents such as p-methoxyphenyl (23) would interact with the quaternary ammonium ion of the DOPC headgroup. Such a cation-pi interaction should stabilize the hydraphile’s position within the bilayer and thus the conduction pathway. The rate of Na+ transport is expected to be better when the conduction pathway is stable than otherwise. In order to diminish the stabilization, we mixed 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) with DOPC in a 3:7 (w/w) proportion. The negative phosphate partially neutralizes DOPC’s positive charge. Compounds such as 23 should be stabilized less in the lipid mixture than in DOPC alone. The result should be lower Na+ transport.
The transport of Na+ was determined by using the ISE method described above. In each case, a minimum of three data sets was combined to obtain each value. Fig. 14 shows the fractional sodium release detected at 1500 s in either DOPC or a DOPC:DOPA mixture. The extent of sidechain interaction with the DOPC headgroup should reflect its electron richness. We therefore expect Na+ transport in DOPC to be in the order 23 > 4 > 24 > 25. In fact, the order is 23 ∼ 4 > 24 > 25. Since the interaction of the arene with the ammonium headgroup of DOPC is not significant when the arene is electron poor, we do not expect much change in the transport rates of 24 and 25 when DOPA is added to DOPC. The graph of Fig. 14 clearly shows that transport by 24 or 25 is about the same in either lipid system.
Fig. 14.
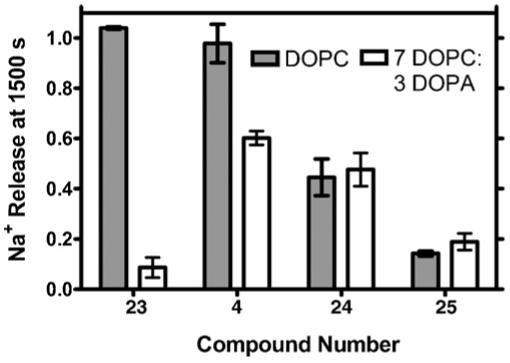
Comparison of sodium cation transport through DOPC or DOPC:DOPA vesicular membranes mediated by 4, 23, 24, and 25.
A remarkable finding from this study is that those hydraphile headgroups that are electron rich and could participate in cation-pi stabilization are dramatically affected by the lipid composition. For p-methoxyphenyl hydraphile 23, transport falls 90% when DOPA is added to pure DOPC. For phenyl hydraphile 4, which is less electron rich, activity falls only 40%. In striking contrast, electron poor 24 or 25 both show lower transport activity than 4, but neither is significantly affected by the change in lipids.
Two important lessons emerge from these findings. First, cation-pi interactions may play an important role in channel protein mediated transport. Second, differences in model liposomal systems may dramatically affect the results of experiments conducted with them. Results obtained in different liposomal systems may not be directly comparable.
5. Conclusions
The transport of alkali metal cations through bilayers presents a challenge that nature has met through millions of years of evolution. Modern protein ion channels are highly selective and efficient. The complexity that imparts their remarkable properties also obscures the mechanism or mechanisms by which they function. Model channels can help to understand the influence of certain structural features on the transport properties of such proteins. The basic challenge for the chemist is to devise a structure that will insert into the bilayer and permit the passage of ions. Once such a functional structure is in hand, the questions of ion selectivity, rectification, gating, etc., can be addressed.
We have shown in this article that relatively low molecular weight structures can exhibit some of the complex properties of protein ion channels. In this case, as in several other channel systems that have been developed, the improvement of selectivity and rectification remain important targets. Still, the compounds that were designed to be model channels are, in fact, channels. They function in liposomal bilayers and in vital mammalian cells. In addition, they exhibit toxicity to certain microbes. Finally, a family of structures can be prepared around the basic design theme and this permits their use as an analytical tool. Taken together, the emerging group of synthetic ion channel presents an exciting prospect in the development of novel structures that have useful and interesting properties.
Acknowledgments
We thank the NIH (GM-36262) and the NSF (CHE-0415586) for grants that supported the aspects of the work from our laboratory that are described herein.
References
- [1].Cairns-Smith AG. Seven Clues to the Origin of Life. Cambridge University Press; Cambridge: 1985. [Google Scholar]
- [2].Dyson F. Origins of Life. 2nd ed. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- [3].Gokel GW, Medina JC, Li C. Synlett. 1991:677. [Google Scholar]
- [4].Hanahan DJ. A Guide to Phospholipid Chemistry. Oxford University Press; Oxford: 1997. p. 214. [Google Scholar]
- [5].Yeagle P. The Membranes of Cells. 2nd ed. Academic Press; London: 1993. p. 349. [Google Scholar]
- [6].Vieth WR. Membrane Systems Analysis and Design: Applications in Biotechnology, Biomedicine, and Polymer Science. John Wiley & Sons; New York: 1994. p. 360. [Google Scholar]
- [7].Vance DE, Vance JE. Biochemistry of Lipids, Lipoproteins, and Membranes. Elsevier; Amsterdam: 1996. p. 551. [Google Scholar]
- [8].Merz KM Jr., Roux B, editors. Biological Membranes: A Molecular Perspective from Computation to Experiment. Birkḧauser; Boston: 1996. p. 593. [Google Scholar]
- [9].Petrache HI, Tristram-Nagle S, Gawrisch K, Harries D, Parsegian VA, Nagle JF. Biophys. J. 2004;86:1574. doi: 10.1016/S0006-3495(04)74225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Graham JM, Higgins JA. Membrane Analysis. Springer Verlag; New York: 1997. p. 208. [Google Scholar]
- [11].Simons K, Vaz WLC. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- [12].Al-Awqati Q. Nat. Cell Biol. 1999;1:E201. doi: 10.1038/70230. [DOI] [PubMed] [Google Scholar]
- [13].Shannon RD. Acta Crystallogr., Sect. A. 1976;A32:751. [Google Scholar]
- [14].Zhou J, Lu X, Wang Y, Shi J. Fluid Phase Equilibria. 2002;194-197:257. [Google Scholar]
- [15].Inoue Y, Gokel GW, editors. Cation Binding by Macrocycles. Marcel Dekker; New York: 1990. [Google Scholar]
- [16].Cox BG, Schneider H. Coordination and Transport Properties of Macrocyclic Compounds in Solution. Elsevier; Amsterdam: 1992. p. 420. [Google Scholar]
- [17].Stein WD. Transport and Diffusion Across Cell Membranes. Academic Press; San Diego: 1986. p. 685. [Google Scholar]
- [18].Hernandez JC, Trafton JE, Gokel GW. Tetrahedron Lett. 1991:6269. [Google Scholar]
- [19].Gokel GW. Chem. Soc. Rev. 1992;21:39. [Google Scholar]
- [20].Xie Q, Gokel GW, Hernandez JC, Echegoyen L. J. Am. Chem. Soc. 1994;116:690. [Google Scholar]
- [21].Dietrich B, Viout P, Lehn J-M. Macrocyclic Chemistry: Aspects of Organic and Inorganic Supramolecular Chemistry. VCH; Weinheim: 1993. p. 384. [Google Scholar]
- [22].Stein WD. Channels, Carriers, and Pumps. Academic Press; New York: 1990. [Google Scholar]
- [23].Nagle JF, Tristram-Nagle S. Curr. Opin. Struct. Biol. 2000;10:474. doi: 10.1016/s0959-440x(00)00117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nagle JF, Tristram-Nagle S. Biochim. Biophys. Acta. 2000;1469:159. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hille B. Ionic Channels of Excitable Membranes. 3rd ed. Sinauer Associates; Sunderland, MA: 2001. p. 814. [Google Scholar]
- [26].Gokel GW, Murillo O. Acc. Chem. Res. 1996;29:425. [Google Scholar]
- [27].Sakai N, Mareda J, Matile S. Acc. Chem. Res. 2005;38:79. doi: 10.1021/ar0400802. [DOI] [PubMed] [Google Scholar]
- [28].Pliska V, Testa B, Waterbeemd H.v.d., editors. Lipophilicity in Drug Action and Toxicology. Vol. 4. VCH; Weinheim: 1996. [Google Scholar]
- [29].Tabushi I, Kuroda Y, Yokota K. Tetrahedron Lett. 1982:4601. [Google Scholar]
- [30].Riddell FG, Hayer MK. Biochem. Biophys. Acta. 1985;817:313. doi: 10.1016/0005-2736(85)90033-1. [DOI] [PubMed] [Google Scholar]
- [31].Riddell FG, Tompsett SJ. Biochim. Biophys. Acta. 1990;990:193. doi: 10.1016/0005-2736(90)90225-d. [DOI] [PubMed] [Google Scholar]
- [32].Weber ME, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2005;126:636. doi: 10.1021/ja044936+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Segel I. Enzyme Kinetics. Behavior and Analysis of Rapid Equilibrium and Steady-state Enzyme Systems. John Wiley & Sons; New York: 1975. p. 371. Wiley Classics Edition, 1993. [Google Scholar]
- [34].Schlesinger PH, Ferdani R, Pajewska J, Pajewski R, Gokel GW. New J. Chem. 2003;27:60. doi: 10.1039/b417808b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McNally BA, Koulov AV, Smith BD, Joos JB, Davis AP. Chem. Commun. 2005:1087. doi: 10.1039/b414589e. [DOI] [PubMed] [Google Scholar]
- [36].Seganish JL, Fettinger JC, Davis JT. Supramol. Chem. 2006;18:257. [Google Scholar]
- [37].Kano K, Fendler JH. Biochim. Biophys. Acta. 1978;509:289. doi: 10.1016/0005-2736(78)90048-2. [DOI] [PubMed] [Google Scholar]
- [38].Menger FM, Davis DS, Persichetti RA, Lee J-J. J. Am. Chem. Soc. 1990;112:2451. [Google Scholar]
- [39].Hervé M, Cybulska B, Gary-Bobo CM. Eur. Biophys. J. 1985;12:121. [Google Scholar]
- [40].Fyles TM, James TD, Kaye KC. J. Am. Chem. Soc. 1993;115:12315. [Google Scholar]
- [41].Sakmann B, Neher E. Single-channel Recording. Kluwer Academic Publishers; 1995. p. 700. [Google Scholar]
- [42].Benz R, Froehlich O, Laeuger P, Montal M. Biochim. Biophys. Acta, Biomembranes. 1975;394:323. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- [43].Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 2nd ed. Garland Publishing, Inc.; New York: 1989. p. 275. [Google Scholar]
- [44].Ohki S, Arnold K. J. Membrane Biol. 1990;114:195. doi: 10.1007/BF01869214. [DOI] [PubMed] [Google Scholar]
- [45].Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. Science. 1998;280:69. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- [46].Schiffer M, Chang C-H, Stevens FJ. Protein Eng. 1992;5:213. doi: 10.1093/protein/5.3.213. [DOI] [PubMed] [Google Scholar]
- [47].Abel E, Fedders MF, Gokel GW. J. Am. Chem. Soc. 1995;117:1265. [Google Scholar]
- [48].Abel E, De Wall SL, Edwards WB, Lalitha S, Covey DF, Gokel GW. J. Org. Chem. 2000;65:5901. doi: 10.1021/jo000040l. [DOI] [PubMed] [Google Scholar]
- [49].Gokel GW, Dishong DM, Diamond CJ. J. Chem. Soc., Chem. Commun. 1980:1053. [Google Scholar]
- [50].Echegoyen LE, Hernandez JC, Kaifer A, Gokel GW, Echegoyen L. J. Chem. Soc., Chem. Commun. 1988;1988:836. [Google Scholar]
- [51].Muñoz S, Mallén J, Nakano A, Chen Z, Gay I, Echegoyen L, Gokel GW. J. Am. Chem. Soc. 1993;115:1705. [Google Scholar]
- [52].Arnold KA, Hernandez JC, Li C, Mallen JV, Nakano A, Schall OF, Trafton JE, Tsesarskaja M, White BD, Gokel GW. Supramol. Chem. 1995;5:45. [Google Scholar]
- [53].Allen TW, Andersen OS, Roux B. Proc. Natl. Acad. Sci. U.S.A. 2004;101:117. doi: 10.1073/pnas.2635314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nakano A, Xie Q, Mallen JV, Echegoyen L, Gokel GW. J. Am. Chem. Soc. 1990;112:1287. [Google Scholar]
- [55].Murillo O, Watanabe S, Nakano A, Gokel GW. J. Am. Chem. Soc. 1995;117:7665. [Google Scholar]
- [56].Meadows ES, Santos EA, Frankel DC, Gokel GW. Tetrahedron Lett. 2000;41:1871. [Google Scholar]
- [57].Pechulis AD, Thompson RJ, Fojtik JP, Schwartz HM, Lisek CA, Frye LL. Bioorg. Med. Chem. 1997;5:1893. doi: 10.1016/s0968-0896(97)00085-0. [DOI] [PubMed] [Google Scholar]
- [58].Murray CL, Shabany H, Gokel GW. Chem. Commun. 2000:2371. [Google Scholar]
- [59].Abel E, Maguire GEM, Murillo O, Suzuki I, Gokel GW. J. Am. Chem. Soc. 1999;121:9043. [Google Scholar]
- [60].Chattopadhyay A, London E. Biochemistry. 1987;26:29. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- [61].Gokel GW, Ferdani R, Liu J, Pajewski R, Shabany H, Uetrecht P. Chem. Eur. J. 2001;7:33. doi: 10.1002/1521-3765(20010105)7:1<33::aid-chem33>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [62].Chung LA, Lear JD, deGrado WF. Biochemistry. 1992;31:6608. doi: 10.1021/bi00143a035. [DOI] [PubMed] [Google Scholar]
- [63].Murray CL, Gokel GW. Chem. Commun. 1998:2477. [Google Scholar]
- [64].Shabany H, Gokel GW. Chem. Commun. 2000:2373. [Google Scholar]
- [65].Leevy WM, Donato GM, Ferdani R, Goldman WE, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2002;124:9022. doi: 10.1021/ja017052o. [DOI] [PubMed] [Google Scholar]
- [66].Leevy WM, Huettner JE, Pajewski R, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2004;126:15747. doi: 10.1021/ja046626x. [DOI] [PubMed] [Google Scholar]
- [67].Leevy WM, Gokel MR, Hughes-Strange G, Schlesinger PH, Gokel GW. New J. Chem. 2005;29:205. [Google Scholar]
- [68].Leevy WM, Gammon ST, Levchenko T, Daranciang DD, Murillo O, Torchilin V, Piwnica-Worms D, Huettner JE, Gokel GW. Org. Biomol. Chem. 2005;3:3544. doi: 10.1039/b508157b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Leevy WM, Weber ME, Schlesinger PH, Gokel GW. Chem. Commun. 2005:89. doi: 10.1039/b413588a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu M, Maier E, Benz R, Hancock REW. Biochemistry. 1999;38:7235. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- [71].Tristram-Nagle S, Nagle JF. Chem. Phys. Lipids. 2004;127:3. doi: 10.1016/j.chemphyslip.2003.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yeagle P, editor. The Structure of Biological Membranes. CRC Press; Boca Raton: 1992. [Google Scholar]
- [73].Weber ME, Elliott EK, Gokel GW. Org. Biomol. Chem. 2006;4:83. doi: 10.1039/b513179k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gustowski DA, Gatto VJ, Mallén J, Echegoyen L, Gokel GW. J. Org. Chem. 1987;52:5172. [Google Scholar]