Abstract
Autism is common in individuals with fragile X syndrome and it is often difficult to diagnose. We compared the diagnostic classifications of two measures for autism diagnosis, the ADOS and the ADI-R, in addition to the DSM-IV-TR in 63 males with FXS. Overall, 30% of the subjects met criteria for Autistic Disorder and 30% met criteria for PDD-NOS. The classifications on the ADOS and DSM-IV-TR were most similar, whereas the ADI-R classified subjects as autistic much more frequently. We further investigated the relationship of both FMRP and FMR1 mRNA to symptoms of autism in this cohort and found that there was no significant relationship between the measures of autism and molecular features, including FMRP, FMR1 mRNA, and CGG repeat number.
Keywords: autism, fragile X syndrome, ADOS, mRNA
Introduction
The behavioral phenotype of children with fragile X syndrome (FXS) can vary greatly among individuals, although there are several “core” behaviors seen in most. Some of the behaviors often associated with autism, such as avoidant eye gaze, hand flapping, repetitive behaviors and speech perseverations have been reported in 60 to 90% of individuals with FXS (see Bailey, Hatton, Skinner, & Mesibov, 2001; Bailey et al., 1998; R. J. Hagerman, 1999; R.J. Hagerman, 2002; Hatton et al., 2006; Kaufmann et al., 2004 for review). It is important to determine whether an individual with FXS meets criteria for autism or PDD-NOS, because these diagnoses will lead to more intensive autism-related treatment endeavors including early intervention programs or school systems that have been specifically designed for children with autism (Ozonoff, 2003). The cause of autism in individuals with FXS is not known, but it is likely multifactorial with both genetic and environmental elements as reviewed below (Belmonte & Bourgeron, 2006).
Below we review the prevalence of FXS in autism, autism in FXS, and possible mechanisms of involvement leading to autism. Brown et al. (1982) first reported the association between FXS and autism, and subsequently summarized the findings of multiple centers (Brown et al., 1986). They reported an overall rate of 6% of individuals with autism who had the FXS full mutation, although this was through cytogenetic diagnosis, rather than the more accurate DNA test which is currently available. Overall, the rate of FXS in those with autism varies from 2 to 8% when DNA testing is utilized (Chudley, Gutierrez, Jocelyn, & Chodirker, 1998; Estecio, Fett-Conte, Varella-Garcia, Fridman, & Silva, 2002; Li, Chen, Lai, Hsu, & Wang, 1993; Wassink, Piven, & Patil, 2001).
Depending on the measures used, reports on the prevalence of autism within the FXS population have ranged from 15 to 33% (Bailey, Hatton, Mesibov, Ament, & Skinner, 2000; Bailey et al., 2001; Baumgardner, Reiss, Freund, & Abrams, 1995; R. J. Hagerman, Jackson, Levitas, Rimland, & Braden, 1986; Reiss & Freund, 1992; Rogers, Wehner, & Hagerman, 2001; Turk & Graham, 1997). Bailey et al. (2001) found that 25% of young boys with FXS met criteria for autistic behavior on the Childhood Autism Rating Scale (CARS), and that their behavior profile was similar to children with autism and no FXS. The use of the CARS demonstrated a rate of autism as high as 47% in a small study (15 children with FXS) by Demark, Feldman and Holden (2003).
Gold standard diagnostic measures for autism, the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994) are now being utilized more consistently in the evaluation of children with FXS. Rogers et al. (2001) found that 33% of two to four year old children with FXS met the full criteria for autism, and that their profile of autistic features was indistinguishable from the children with autism without FXS. Similar rates of autism in individuals with FXS were found by Kaufmann et al. (2004) for which they utilized the ADI-R and DSM-IV autism criteria in a sample of 56 boys with FXS aged three to eight. They found that items on the ADI-R that involve complex social interaction differentiated the group of children with FXS and autism spectrum disorders from those with FXS without ASD.
Several studies have investigated the relationship between factors such as molecular features, IQ, and sensory difficulties, and the presence of autism in those with FXS. Bailey et al. (2000) reported that the level of the fragile X protein (FMRP) did not correlate with the presence or absence of autism, suggesting that autism within FXS may be related to genetic or environmental factors that could be additive to the FMR1 mutation (Feinstein & Reiss, 1998). However, in an expanded sample recently published, FMRP did correlate with the level of autistic behavior as measured by the CARS (Hatton et al., 2006). Hessl et al. (2001) found a correlation between symptoms of autism and environmental factors in boys with FXS, however FMRP was not associated with these symptoms after controlling for IQ. Loesch and colleagues evaluated the largest sample to date (147 males and females with the FXS full mutation) and found that FMRP correlated with the degree of autism on the ADOS but when IQ was controlled this relationship disappeared (Loesch et al., 2006).
The recent reports of autism and autism spectrum disorders (ASD) in boys with the fragile X premutation (a CGG repeat number between 55 and 200) suggests that the premutation itself may be toxic to the brain in development (Aziz et al., 2003; Farzin et al., 2006; Goodlin-Jones, Tassone, Gane, & Hagerman, 2004). A study of an older subgroup of carriers with the premutation showed some individuals with white matter disease, brain atrophy, and some of these individuals eventually develop the fragile X-associated tremor-ataxia syndrome (FXTAS), and this may be related to RNA toxicity (P. J. Hagerman & Hagerman, 2004). Neuroimaging fMRI studies of young adult males with the premutation demonstrate amygdala dysfunction when viewing fearful faces (Hessl et al., 2007), and amygdala dysfunction has been shown to be present in some individuals with autism (Baron-Cohen et al., 2000; Dziobek, Fleck, Rogers, Wolf, & Convit, 2006; Sweeten, Posey, Shekhar, & McDougle, 2002). Elevations of mRNA can occur in males who are mosaic with both a premutation or a full mutation or males who have an unmethylated full mutation (Tassone, Hagerman, Chamberlain, & Hagerman, 2000; Tassone, Hagerman, Loesch et al., 2000). Therefore the current study further investigated whether the level of FMR1 mRNA is associated with the diagnosis of autism or autistic symptoms in males with FXS.
Several studies have found a lower IQ (Bailey et al., 2001; Bailey et al., 1998; Kau et al., 2004; Kaufmann et al., 2004; Rogers et al., 2001), lower adaptive skills (Hatton et al., 2003; Kau et al., 2004), or lower expressive language skills (Philofsky, Hepburn, Hayes, Hagerman, & Rogers, 2004) in those with FXS and autism compared to FXS alone. Roberts, Boccia, Bailey, Hatton and Skinner (2001) found more autonomic dysfunction and hyperarousal in children with both FXS and autism compared to those with FXS alone.
Rogers, Hepburn and Wehner (2003) studied sensory symptoms in toddlers with autism and other developmental disorders. They reported that 7 of the 20 children (35%) with FXS had autism, and these children had sensory impairments similar to those with autism without FXS. In another study by Rogers, Hepburn, Stackhouse and Wehner (2003), the imitation skills of the children with FXS were strongly related to whether or not they also had autism. Kau et al. (2004) compared social behavior profiles of young males with FXS, and found that those boys who had both FXS and autism showed more impairment than those with FXS alone in several areas, including cognition, adaptive behavior, and problem behaviors. They also reported that the boys with FXS and autism were less impaired on the reciprocal social interaction domain on the ADI-R than the comparison groups of boys with autism and boys with developmental language delay and autism. This observation suggests that individuals with autism and FXS may have greater social strengths than those with autism without FXS.
Diagnosing autism in those with FXS is difficult due to overlapping symptoms of social anxiety, autism, ADHD, language deficits and overall mental impairment. In our clinical experience, even the gold standard measures often do not provide a clear consensus. The current study examines the profile of autism in boys with FXS using current gold standard diagnostic measures, including the ADI-R, and ADOS and the relationship of the molecular measures to the diagnosis of autism and autism symptoms. It expands upon the previous study by Rogers et al. (2001) by analyzing a broader age range, and expands upon the Kaufmann et al. (2004) study by utilizing both the ADI-R and the ADOS to examine features of autism in boys with FXS syndrome aged 2 to 19 years. We also examine the measures used to diagnose autism, and discuss some of the variability in results seen between them.
Our hypotheses were as follows:
A significant correlation would emerge between one or more molecular variables of the FMR1 gene and the autism status of the subjects in our sample.
FMR1-mRNA levels would be associated with autism status
The ADI-R would “overcall” autism in our sample, such that the ADOS and DSM-IV-TR ratings for our sample would show a closer correlation in diagnostic classification than the ADI-R.
The Vineland Adaptive Behavior Composite scores would correlate with the autism status of the subjects in our sample, such that those with lower VABC scores would be those more likely to have autism.
Methods
Subjects
We assessed 63 males aged 2.8 to19.5 years (mean=7.9 ± 4.3 years) at the M.I.N.D. Institute between 2001 and 2005 and confirmed to have the FMR1 mutation by DNA studies (described below). Of the 63 study participants, 39 had the full mutation of the FMR1 gene, meaning that they have a FMR1 allele with 200 or more CGG repeats, and 24 had the full mutation with size mosaicism, whereby they had some cells showing a FMR1 full mutation, and some cells with a FMR1 premutation. The Full Scale IQ (FSIQ) scores for the group ranged from 25 to 87 (mean=56 ± 13). All study participants and/or their caregivers signed a consent form approved by our institutional review board to participate in research studies at the M.I.N.D. Institute. See Table 1 for a summary of subject demographics.
Table 1. Subject Demographics.
| Overall Autism Classification | Subjects N (%) | Age range (mean, SD) | IQ range (mean, SD) | FMRP range (mean, SD) | mRNA range (mean, SD) |
|---|---|---|---|---|---|
| Total Sample | 63 | 2.8-19.5 (7.9 ± 4.3) |
25-87 (56 ± 13) |
0-55 (11 ± 13) |
0-8.05 (0.36 ± 0.66) |
| Autism | 19 (30%) | 3.2-19.4 (8.3 ± 4.9) |
25-87 (50 ± 15) |
0-55 (13 ± 13) |
0-8.05 (0.74 ± 1.89) |
| PDD | 19 (30%) | 3.0-19.6 (8.1 ± 4.9) |
40-77 (56 ± 12) |
0-33 (7 ± 9) |
0-1.66 (0.28 ± 0.56) |
| Non-ASD | 25 (40%) | 2.8-12.8 (7.1 ± 3.3) |
42-76 (60 ± 15) |
0-46 (12 ± 14) |
0-5.35 (0.70 ± 1.34) |
Procedures
All 63 subjects were assessed with the ADOS, ADI-R, and DSM-IV-TR criteria. The ADOS and ADI were administered by clinicians who have achieved research reliability through training at the University of Michigan. The ADI-R was scored for each individual using the ‘diagnostic’ algorithm appropriate to the subjects’ current age (either the 2 years 0 months to 3 years 11 months, or 4 years 0 months or more algorithm). These tests were carried out independently of each other and typically by different clinicians. The DSM-IV-TR rating was completed by a licensed psychologist and developmental pediatrician who had spent a significant amount of time assessing the child and it was completed after a team consensus discussion.
A blood sample was obtained for the CGG repeat status and other molecular factors, including FMR1 protein level (FMRP), and messenger RNA (mRNA) level. Cognitive levels (full scale IQ) for all participants were assessed with one of the Wechsler Intelligence Scales appropriate for age, including the WPPSI-III, WISC-III, and WAIS-III, or with the Kaufman Assessment Battery for Children (K-ABC). Although these cognitive measures are used routinely for individuals with FXS, to our knowledge there have been no validity or reliability studies done regarding use of these measures with individuals who have FXS. Fifty-six of the participants were also assessed with the Vineland Adaptive Behavior Scale. The assessments were completed over a one to two day period of time.
Behavioral Measures
The Autism Diagnostic Observation Schedule (ADOS) is a standardized assessment developed by Lord and colleagues (Lord, Rutter, DiLavore, & Risi, 1999; Rutter, Le Couteur, & Lord, 2003). It is a play-based assessment of the child’s current behavior, and utilizes “presses” to elicit behaviors from the individual being assessed. One of four modules is administered to the client, and the choice of module administered is based on the individual’s expressive language level and overall developmental functioning. The scoring of the ADOS is based on an algorithm of several of the items that are coded for the entire battery, and includes domains of Communication, Reciprocal Social Interaction, Imagination/Creativity, and Stereotyped Behaviors and Restricted Interests. The Communication and Reciprocal Social Interaction domain scores are used together for the determination of the overall ADOS classification, which includes a cut-off for Autism Spectrum and Autism.
The Autism Diagnostic Interview - Revised (ADI-R) is a semi-structured parent interview used in the assessment of autism (Lord et al., 1994; Rutter et al., 2003). It is administered to the primary caregiver(s) of the individual being assessed, and includes questions encompassing abnormalities in reciprocal social interaction; abnormalities in communication; restricted, repetitive, and stereotyped patterns of behavior; and abnormality of development evident at or before 36 months. The interview includes questions regarding the current functioning of the individual being assessed, as well as questions about the individual at the 4-5 year age period, although the algorithm used to score and rate the individual is based primarily on coding of the 4-5 year age period. This is an important distinction between the ADI-R and the ADOS, because these ratings may be based on very different behaviors (current behaviors for the ADOS or age 4-5 for the ADI-R) depending on the current age of the child being assessed. In order to meet the criteria for autism on the ADI-R, scores must be at or above the cutoff level for each of the three domains, and there must be at least one positive indicator in the child’s developmental history, such as age when symptoms were first noted by parents. Another important distinction between the ADI-R and the ADOS is that the ADI-R does not include a cutoff for PDD-NOS, whereas the ADOS does.
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000) criteria for autism were also used in the determination of autism spectrum disorder classification. Briefly, the DSM-IV-TR criteria for autism include domains of social function, communication, and repetitive behaviors. We used a checklist-type format for assessing the DSM-IV-TR criteria, whereby each characteristic within the three domains (social, communication, repetitive behaviors) were rated on a yes, partial or none basis. In order to meet criteria for autism according to this DSM-IV-TR checklist, an individual must show “significant impairment” in all three domains. For the social domain, significant impairment is defined as at least two ratings of yes; for the communication and behavior domains, significant impairment is defined as at least one rating of yes. In order to meet criteria for PDD on the checklist, an individual must show “significant impairment for PDD” in the social domain (defined as at least 1 rating of yes and 1 rating of partial), and significant impairment in either the communication or behavior domain (at least one rating of yes in either domain). Both the developmental and behavioral pediatrician and the psychologist contributed to the scoring of the DSM-IV-TR. The final scores were completed after a team discussion and consensus of the findings of all the clinicians who worked with the child.
The Vineland Adaptive Behavior Scales (VABS) (Sparrow, Balla, & Cicchetti, 1984) is a measure of adaptive functioning which can be used with individuals from birth through 18 years of age, and also with adults above the age of 18 who have mental retardation. The VABS has been used in many previous studies of individuals with FXS (Dykens et al., 1996; Fisch et al., 1999), and Glaser et al. reported on interrater reliability of this measure when used with both boys and girls with FXS (Glaser et al., 2003). This measure provides an overall Adaptive Behavior Composite (ABC) score, and it also provides scores for individual domains of adaptive functioning, including Communication, Daily Living Skills, and Socialization.
Molecular Measures
Each of the participants underwent genomic DNA assessment to assess the CGG repeat number of the FMR1 gene. Both Southern Blot and PCR analysis were performed on DNA isolated (Puregene kits, Gentra) from peripheral blood leukocytes. Southern blot analysis was performed using the FMR1-specific probe StB12.3 as described in Tassone et al. (2004). PCR analysis was utilized to determine the number of CGG trinucleotide repeats within the normal-premutation range, by using primer c and f as described in Saluto et al. (2005).
Because we wanted to also compare the CGG repeat number with autism ratings, we calculated an ‘average’ CGG repeat number, which was the average of the low and high CGG repeat for those with multiple alleles. We did this because we had subjects with several different sized alleles in the full mutation range, as well as subjects that showed size mosaicism, with some alleles in the premutation range. This methodology is imperfect, but allows for a single repeat number to be used in the comparisons.
The mRNA levels were carried out with RT-PCR technology that has been reported previously (Tassone, Hagerman, Taylor et al., 2000).
Results
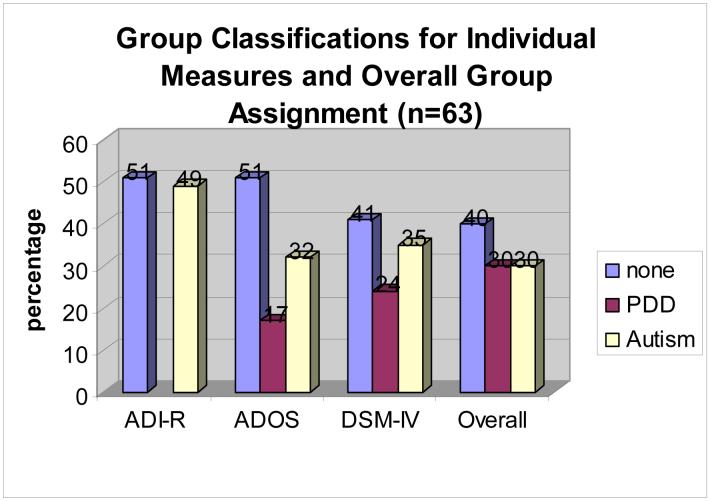
An overall autism classification rating was assigned to each study participant based on the ADOS, ADI-R, and DSM-IV-TR results (see Figure 1). Fifteen of 63 (24%) met criteria for autism on all three measures; 2 of 63 (3%) met criteria for PDD-NOS on the DSM-IV-TR and ADOS, and met criteria for autism on the ADI-R, and were assigned an overall rating of PDD-NOS; and 18 of 63 (29%) did not meet criteria for either PDD-NOS or autism on all three measures. The remaining 28 participants (44%) showed some kind of discrepancy between the ratings of the three measures, and were assigned ratings of none, PDD-NOS, or autism, utilizing clinical judgment. To be rated as PDD-NOS or autism, subjects had to meet criteria on at least one standardized measure and also by clinical judgment. See Figure 1 and Table 2 for a summary of overall ASD ratings.
Figure 1. Autism Classification of sample: ADI-R, ADOS, and DSM-IV-TR.
Table 2. Overall Autism Classification Assignments for Total Sample (n=63).
| Number of Subjects (of 63 total) | DSM-IV-TR class | ADOS class | ADI-R class | Overall Autism Classification Assigned |
|---|---|---|---|---|
| 18 | Normal | Normal | Did not meet all cutoffs | none |
| 2 | PDD-NOS | PDD-NOS | Autism | PDD-NOS |
| 15 | Autism | Autism | Autism | Autism |
| 5 | None | Normal | Autism | None |
| 3 | None | PDD-NOS | Did not meet all cutoffs | PDD-NOS |
| 3 | PDD-NOS | Normal | Did not meet all cutoffs | none (n=2) PDD-NOS (n=1) |
| 3 | PDD-NOS | PDD-NOS | Did not meet all cutoffs | PDD-NOS |
| 4 | PDD-NOS | Normal | Autism | PDD-NOS |
| 2 | PDD-NOS | Autism | Did not meet all cutoffs | PDD-NOS |
| 1 | PDD-NOS | Autism | Autism | PDD-NOS |
| 1 | Autism | Normal | Did not meet all cutoffs | PDD-NOS |
| 1 | Autism | Normal | Autism | PDD-NOS |
| 3 | Autism | PDD-NOS | Autism | Autism |
| 2 | Autism | Autism | Did not meet all cutoffs | PDD-NOS (n=1) Autism (n=1) |
Based on the ADOS, ADI-R, and DSM-IV-TR criteria, the overall ratings of autism for our sample of 63 boys showed that 30% had autism and 30% had Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), for an overall autistic spectrum disorder rate of 60%.
When utilizing only the ADI-R, 49% (n=31) of the 63 participants in our sample met criteria for autism. When utilizing only the ADOS, 32% (n=20) of the 63 participants met criteria for autism, and an additional 17% (n=11) met criteria for PDD-NOS, for a total of 49% meeting criteria for classification of autistic spectrum disorder (ASD). When utilizing only the TSM-IV-TR criteria, 35% (n=22) of the 63 participants met criteria for autism, and 24% (n=15) met criteria for PDD-NOS, for a total of 59% meeting criteria for classification of autistic spectrum disorder (ASD).
We compared molecular features (CGG repeat number, FMRP, mRNA) to overall ratings of autism and scores on the measures of autism used in this study using Spearman’s correlations. We found that the ADOS communication-social total score did not correlate significantly with mRNA (p=.38), FMRP (p=.65), or CGG repeat number (p=.47). We also found no significant correlation between the overall rating of autism and molecular measures. We used a one-way analysis of variance (ANOVA) to determine if the Vineland Adaptive Behavior Composite (VABC) score. would show group differences based on autism classifications for the sample. We found a significant autism group difference for the VABC (F (1, 47)= 5.49, p=0.007), showing that those with autism or PDD-NOS had a lower VABC than those without autism or PDD.
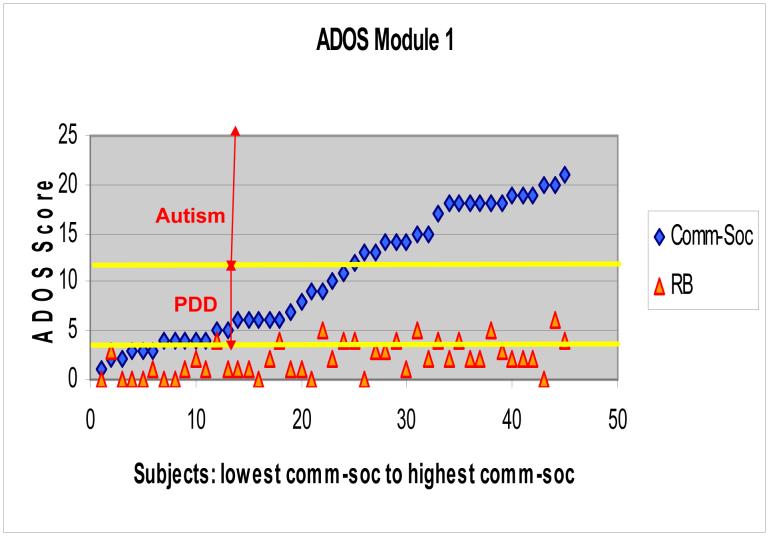
The current data show that on Module 1 of the ADOS, scores are continuously distributed throughout the PDD-NOS range into autism and there is no clear cutoff in behavior other than what is set by the ADOS measure (see Figure 2). Similar continuous distributions were seen for Module 2 and 3 (data not shown).
Figure 2. ADOS Module 1.
Module 1 Communication + Social Total (Comm-Soc) can range from 0 to 24. Module 1 Autism cutoff=12, autism spectrum cutoff=4. Module 1 Stereotyped Behaviors and Restricted Interests Score (RB) can range from 0 to 6 (this score, however, is not used in the algorithm used to determine the overall autism classification of the ADOS).
Discussion
Our results demonstrate that a significant percentage of young boys with FXS met criteria for autism spectrum disorders. We found that the overall rates of autism classification with the ADI-R (49%) and ADOS (32%) were quite disparate. It is important to note that because the ADI-R does not provide a cutoff level for classification of PDD-NOS, it should always be used in conjunction with additional measures, such as the ADOS, which can help to elucidate this distinction. There is a great deal of clinical variation seen in those with FXS, and the ADI-R may overcall autism in those with FXS based on behaviors that were seen at four years of age, when autistic-like behaviors including stereotypies are most common in children with FXS. An important distinction between the ADI-R and ADOS is that the ADI-R scoring algorithm includes a domain for restricted, repetitive, and stereotyped patterns of behavior. Although this domain is also coded on the ADOS, it is not included in the scoring algorithm, and may not help to distinguish those with FXS and autism. The ADI-R may also over-identify the presence of autism in other organic subgroups, including those with 15q duplication as was seen by Bolton et al. (2001). Both of these measures are considered gold standard diagnostic measures for autism, but the majority of items used in the scoring criteria of the diagnostic algorithm of the ADI-R focuses on the period between the fourth and fifth birthday of the individual being assessed. In our study, 14% of the participants (n=9) met criteria on the ADI-R according to what symptoms they had in early childhood but did not meet criteria on the ADOS for either autism or PDD-NOS. Notable also, is that four of these nine participants met DSM-IV-TR criteria for PDD-NOS, and five of the nine participants did not meet DSM-IV-TR criteria for any autism spectrum disorder.
It is also important to note that the ADI-R assessment involves parent/caregiver history and recollection over time, whereas the ADOS and DSM-IV-TR coding is based on the current behaviors of the individual as rated by a clinician. There are some parents that may not recollect symptoms well (especially, perhaps, for those parents whose children are older, and therefore are asked to remember qualities of their child’s play and behaviors back several years) or that may downplay autistic features so that on occasion the ADI-R is not in the autism range, whereas the ADOS and DSM-IV-TR do meet autism criteria. We observed this in two of the 63 cases (see Table 2). It is possible that autistic behaviors present at age four to five are outgrown by some children with FXS as they age and undergo treatments for autism, so that when the ADOS is done later in childhood they do not meet criteria for autism. Conversely, the ADOS is a short assessment (30-45 minutes) with the individual, and some behaviors that may commonly be displayed by an individual in other settings may not be elicited from the individual during this brief period of time. Therefore some behaviors which might be missed on the ADOS may be more accurately captured on the ADI-R and DSM-IV-TR, and this could explain some of the disparity seen in the classifications between these measures. In our clinical experience, the ADOS is a more accurate measure to judge the current presence or absence of autism in a child with FXS in clinic. There is evidence from Hatton et al. (2006) utilizing the CARS that demonstrates that autism may become worse over time through childhood into adulthood. Such progression of symptoms would be best assessed prospectively with the ADOS and the DSM-IV-TR by the clinician.
Although FMRP has been found to correlate with IQ (Loesch, Huggins, & Hagerman, 2004; Tassone et al., 1999) our data does not confirm an association between FMRP levels and autism. In a recent study by Hatton et al. (2006) an inverse correlation was reported between the CARS scale as a measure of autism and FMRP levels, although this study also included females with FXS, whereas our current study includes only males, so the FMRP range is more restricted. Another recent study by Loesch et al. (2006) found that the FMRP correlation with autism disappeared with correction for IQ level. The association between FMRP and IQ or cognitive or adaptive scores is strong and was also seen in our study in that the VABS correlated with the ADOS score.
It is likely that other background gene effects beyond FMRP are associated with autism in FXS. One example of this is the association between lowered CYFIP levels (a gene located at the 15q region associated with Prader-Willi Syndrome) and the Prader-Willi phenotype in FXS which has a high rate of ASD (Nowicki et al., 2007). Hessl et al. (2007) found that individuals with FXS who have two copies of the long allele of the serotonin transporter polymorphism were more likely to show stereotypic behaviors and aggression. Therefore it is likely that the presence of autism in FXS is related to additional effects beyond just FMRP levels, including other genetic and environmental effects that are independent of, or interact with, the FXS genotype and phenotype.
An additional genetic effect we assessed is the elevation of FMR1 mRNA. This can be elevated in individuals with FXS and a mosaic status or those with a lack of methylation (Tassone, Hagerman, Chamberlain et al., 2000; Tassone, Hagerman, Loesch et al., 2000). Since elevated FMR1 mRNA can lead to CNS toxicity in FXTAS and may be related to autism in boys with the premutation (Aziz et al., 2003; Farzin et al., 2006; Goodlin-Jones et al., 2004), it could have an additive effect for ASD in boys with FXS. The range of FMR1 mRNA in our subjects was 0 to 8.05, and 13 of the 63 subjects (20%) had an elevated level of mRNA (>1.0). We did not see a correlation between autism and FMR1 mRNA levels, however, the boy with the most elevated mRNA (8.05), had autism. Further studies regarding this relationship are warranted.
As Figure 2 shows, the ADOS scores are continuously distributed throughout the PDD-NOS range into autism and there is no clear cutoff in behavior other than what is set by the ADOS measure. This suggests that there is not one additive effect but multiple effects, likely both genetic and environmental, that contribute to this continuous variation.
As can be seen from Table 3, repetitive or perseverative speech and stereotypic mannerisms are common in children with FXS both with and without ASD. It seems that the differentiating features between these groups may be mainly in the social domain regarding eye contact and non-verbal behavior, social/emotional reciprocity, and ability to make friends, which is similar to the findings of Kaufmann et al. (2004). It would be beneficial to distinguish these differences in a future study with a larger sample size. The degree of these abnormalities is what the standardized testing for autism helps to differentiate, but no test is perfect and a variety of emotional and environmental factors may influence how a child performs on the ADOS. The diagnosis of autism or PDD-NOS is a clinical diagnosis based on behavior during the time that the standardized assessments are utilized. A limitation to this study is the fact that behaviors captured on a measure like the ADOS can vary day to day, such that some behaviors may be missed during the assessment. Also, performance on the ADOS could be affected by the individual being anxious or shutting down, reciprocity/comfort level with the examiner, or a child being over-tired. A team evaluation involving sessions on different days with different examiners and a clinical consensus regarding the diagnosis of autism or PDD-NOS with the help of standardized measures has been most beneficial in our experience. This type of an evaluation for all children with FXS is recommended because clarification of the diagnosis of autism or ASD can dramatically impact the intensity and type of intervention that a child with FXS receives.
Table 3. DSM-IV-TR items for each group: percentage positive (rated yes) for each item.
| Total Sample N=63 | Overall class=Non-spectrum n=25 | Overall class=PDD-NOS n=19 | Overall class=Autism n=19 | ||
|---|---|---|---|---|---|
| Social Domain | Impairments in reciprocal social interaction | 60% 38/63 |
20% 5/25 |
79% 15/19 |
95% 18/19 |
| Impairment in use of multiple nonverbal behaviors | 42% 25/60* |
4% 1/23* |
39% 7/18* |
89% 17/19 |
|
| Failure to develop peer relationships | 19% 12/63 |
0% 0/25 |
5% 1/19 |
58% 11/19 |
|
| Lack of social or emotional reciprocity | 29% 18/63 |
8% 2/25 |
5% 1/19 |
79% 15/19 |
|
| Communication Domain | Delay in or lack of spoken language | 40% 21/53* |
15% 3/20* |
14% 2/14* |
84% 16/19 |
| Impairment in conversation | 36% 14/39* |
20% 4/20* |
43% 6/14* |
80% 4/5* |
|
| Stereotyped & repetitive use of language or idiosyncratic language | 69% 36/52* |
68% 15/22* |
72% 13/18* |
67% 8/12* |
|
| Lack of spontaneous make believe play or imitative play | 40% 25/62* |
16% 4/25 |
39% 7/18* |
74% 14/19 |
|
| Repetitive Behaviors | Preoccupation with stereotyped patterns of interest | 35% 22/63 |
12% 3/25 |
32% 6/19 |
68% 13/19 |
| Adherence to nonfunctional routines or rituals | 37% 22/60* |
24% 6/25 |
31% 5/16* |
58% 11/19 |
|
| Stereotyped and repetitive motor mannerisms | 89% 56/63 |
84% 21/25 |
84% 16/19 |
100% 19/19 |
|
| Preoccupation with parts of objects | 43% 27/63 |
32% 8/25 |
32% 6/19 |
68% 13/19 |
Note. The asterisk (*) indicates that the sample size for this item is different from the overall category, because some of the responses were “not applicable or unknown”
Acknowledgments
This research was supported by NICHD grant numbers HD036071 and HD02274, and Autism Speaks. We are grateful to the many families who have participated in our fragile X research studies at the M.I.N.D. Institute.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, et al. Clinical features of boys with fragile X premutations & intermediate alleles. American Journal of Medical Genetics. 2003;121B(1):119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Hatton DD, Mesibov GB, Ament N, Skinner M. Early development, temperament and functional impairment in autism and fragile X syndrome. J Autism Dev Disord. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Hatton DD, Skinner M, Mesibov GB. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28(6):499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, Abrams MT. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics. 1995;95(5):744–752. [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9(10):1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Bolton P, Dennis NR, Browne CE, Thomas NS, Veltman MWM, Thompson RJ, et al. The Phenotypic Manifestations of Interstitial Duplications of Proximal 15q with Special Reference to the Autistic Spectrum Disorders. Am J Med Gen. 2001;105:675–685. doi: 10.1002/ajmg.1551. [DOI] [PubMed] [Google Scholar]
- Brown WT, Jenkins EC, Cohen IL, Fisch GS, Wolf-Schein EG, Gross A, et al. Fragile X and autism: a multicenter survey. American Journal of Medical Genetics. 1986;23(12):341–352. doi: 10.1002/ajmg.1320230126. [DOI] [PubMed] [Google Scholar]
- Brown WT, Jenkins EC, Friedman E, Brooks J, Wisniewski K, Raguthu S, et al. Autism is associated with the fragile-X syndrome. J Autism Dev Disord. 1982;12(3):303–308. doi: 10.1007/BF01531375. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Gutierrez E, Jocelyn LJ, Chodirker BN. Outcomes of genetic evaluation in children with pervasive developmental disorder. J Dev Behav Pediatr. 1998;19(5):321–325. doi: 10.1097/00004703-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Demark JL, Feldman MA, Holden JJA. Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation. 2003;108(5):314–326. doi: 10.1352/0895-8017(2003)108<314:BRBAAF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dykens E, Ort S, Cohen I, Finucane B, Spiridigliozzi G, Lachiewicz A, et al. Trajectories and profiles of adaptive behavior in males with fragile X syndrome: multicenter studies. J Autism Dev Disord. 1996;26(3):287–301. doi: 10.1007/BF02172475. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Fleck S, Rogers K, Wolf OT, Convit A. The ‘amygdala theory of autism’ revisited: linking structure to behavior. Neuropsychologia. 2006;44(10):1891–1899. doi: 10.1016/j.neuropsychologia.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Estecio M, Fett-Conte AC, Varella-Garcia M, Fridman C, Silva AE. Molecular and cytogenetic analyses on Brazilian youths with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2002;32(1):35–41. doi: 10.1023/a:1017952123258. [DOI] [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(2 Suppl):S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Reiss AL. Autism: the point of view from fragile X studies. Journal of Autism and Developmental Disorders. 1998;28(5):393–405. doi: 10.1023/a:1026000404855. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter NJ, Holden JJ, Simensen R, Howard-Peebles PN, Maddalena A, et al. Longitudinal assessment of adaptive and maladaptive behaviors in fragile X males: growth, development, and profiles. American Journal of Medical Genetics. 1999;83(4):257–263. doi: 10.1002/(sici)1096-8628(19990402)83:4<257::aid-ajmg5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Glaser B, Hessl D, Dyer-Friedman J, Johnston C, Wisbeck J, Taylor A, et al. Biological and environmental contributions to adaptive behavior in fragile X syndrome. Am J Med Genet A. 2003;117(1):21–29. doi: 10.1002/ajmg.a.10549. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones B, Tassone F, Gane LW, Hagerman RJ. Autistic spectrum disorder and the fragile X premutation. Journal of Developmental and Behavioral Pediatrics. 2004;25(6):392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74(5):805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ. Neurodevelopmental Disorders: Diagnosis and Treatment. Oxford University Press; New York: 1999. Fragile X Syndrome; pp. 61–132. [Google Scholar]
- Hagerman RJ. The Physical and Behavioral Phenotype. In: Hagerman PJ, editor. Fragile X Syndrome: Diagnosis, Treatment, and Research. The Johns Hopkins University Press; Baltimore: 2002. pp. 3–110. [Google Scholar]
- Hagerman RJ, Jackson AW, Levitas A, Rimland B, Braden M. An analysis of autism in fifty males with the fragile X syndrome. American Journal of Medical Genetics. 1986;23(12):359–374. doi: 10.1002/ajmg.1320230128. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr., Roberts J, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Wheeler AC, Skinner ML, Bailey DB, Sullivan KM, Roberts JE, et al. Adaptive behavior in children with fragile X syndrome. American Journal on Mental Retardation. 2003;108(6):373–390. doi: 10.1352/0895-8017(2003)108<373:ABICWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, et al. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001;108(5):e88. doi: 10.1542/peds.108.5.e88. electronic. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130(Pt 2):404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Cordeiro L, Koldewyn K, McCormick C, Green C, et al. Brief Report: Aggression and Stereotypic Behavior in Males with Fragile X Syndrome-Moderating Secondary Genes in a “Single Gene” Disorder. J Autism Dev Disord. 2007 doi: 10.1007/s10803-007-0365-5. [DOI] [PubMed] [Google Scholar]
- Kau ASM, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, et al. Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. American Journal of Medical Genetics. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Li SY, Chen YC, Lai TJ, Hsu CY, Wang YC. Molecular and cytogenetic analyses of autism in Taiwan. Hum Genet. 1993;92(5):441–445. doi: 10.1007/BF00216447. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X [Electronic Version] Neuroscience and Biobehavioral Reviews. 2006 doi: 10.1016/j.neubiorev.2006.09.007. doi:10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Nowicki ST, Tassone F, Ono MY, Ferranti J, Croquette MF, Goodlin-Jones B, et al. The Prader-Willi Phenotype of Fragile X Syndrome. Journal of Developmental & Behavioral Pediatrics. 2007;28:133–138. doi: 10.1097/01.DBP.0000267563.18952.c9. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Rogers SJ, Hendren RL. Autism Spectrum Disorders: A research review for practitioners. American Psychiatric Publishing, Inc.; Washington, DC: 2003. [Google Scholar]
- Philofsky A, Hepburn SL, Hayes A, Hagerman RJ, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. American Journal on Mental Retardation. 2004;109(3):208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund L. Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. Am J Med Genet. 1992;43(12):35–46. doi: 10.1002/ajmg.1320430106. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Hatton D, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39(2):107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism & Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner EA, Hagerman RJ. The Behavioral Phenotype in Fragile X: Symptoms of Autism in Very Young Children with Fragile X Syndrome, Idiopathic Autism, and other Developmental Disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Saluto A, Brussino A, Tassone F, Arduino C, Cagnoli C, Pappi P, et al. An enhanced polymerase chain reaction assay to detect pre- and full mutation alleles of the fragile X mental retardation 1 gene. J Mol Diagn. 2005;7(5):605–612. doi: 10.1016/S1525-1578(10)60594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales Survey Form Manual. American Guidance Service; Circle Pines: 1984. [Google Scholar]
- Sweeten TL, Posey DJ, Shekhar A, McDougle CJ. The amygdala and related structures in the pathophysiology of autism. Pharmacol Biochem Behav. 2002;71(3):449–455. doi: 10.1016/s0091-3057(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. American Journal of Medical Genetics (Semin. Med. Genet.) 2000;97(3):195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41(4):e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. American Journal of Medical Genetics. 1999;84(3):250–261. [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. American Journal of Medical Genetics. 2000;94:232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in fragile X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J, Graham P. Fragile X syndrome, autism, and autistic features. Autism. 1997;1(2):175–197. [Google Scholar]
- Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatric Genetics. 2001;11(2):57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]