Abstract
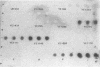
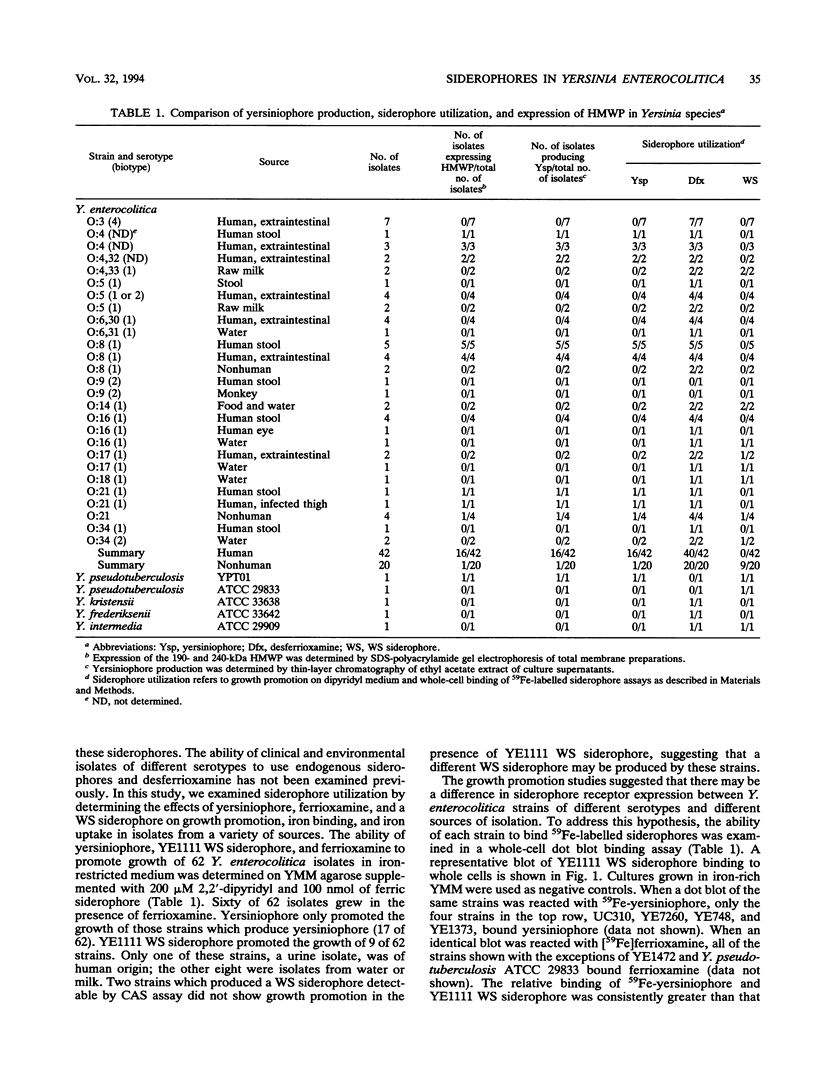
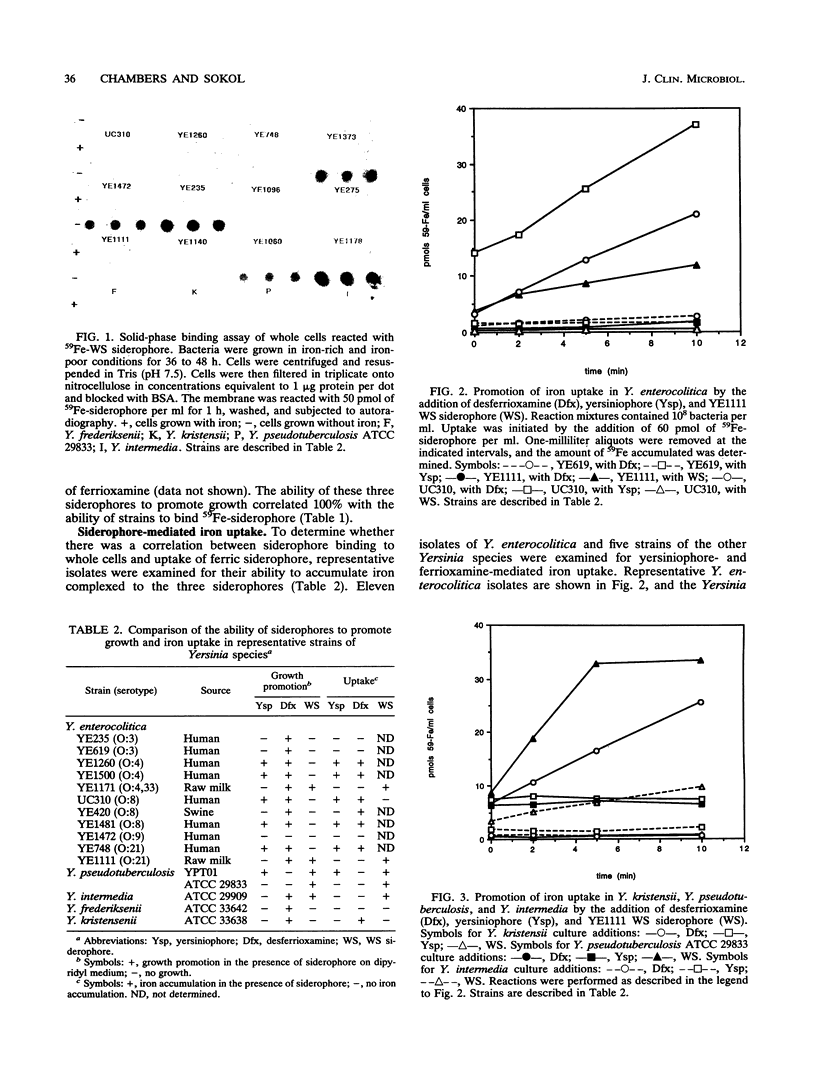
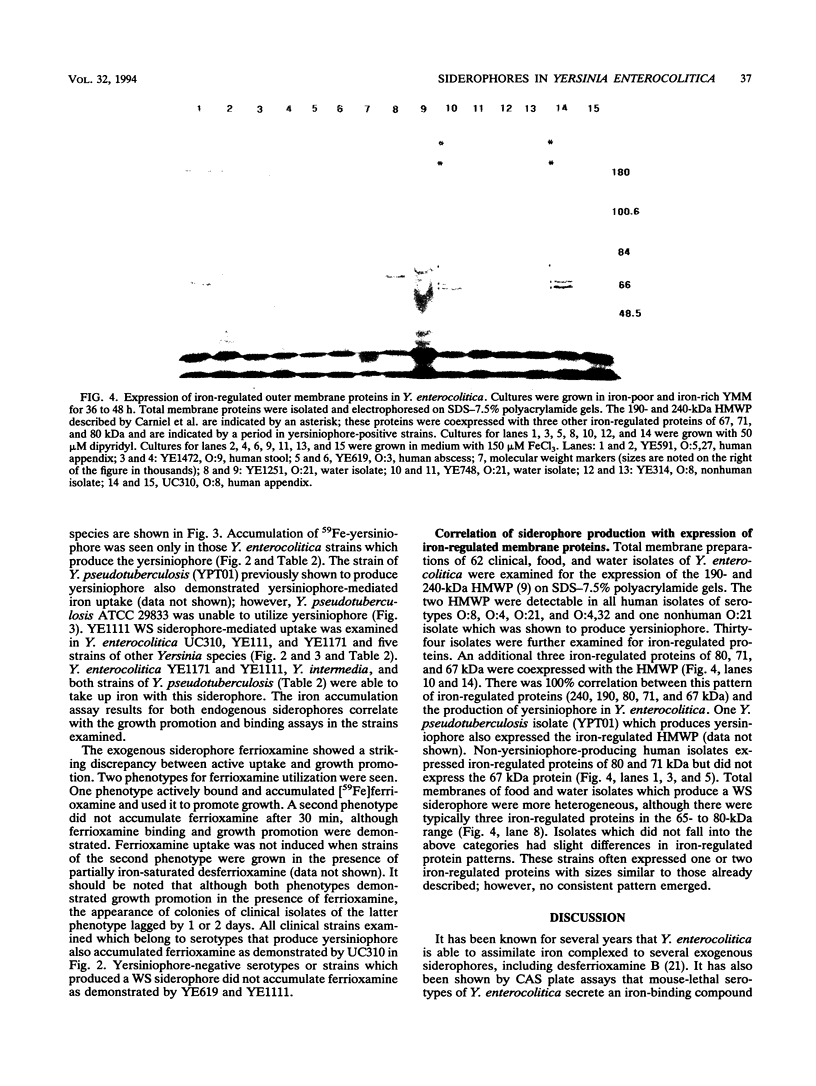
Yersinia enterocolitica strains of serotypes lethal to mice have been reported previously to produce an endogenous siderophore. In this study, an ethyl acetate-extractable siderophore was characterized and given the name yersiniophore. Yersiniophore was produced by 16 of 16 human isolates of serotypes O:4, O:4,32, O:8, O:21, and one nonhuman isolate of serotype O:21. It was not produced by isolates of serotype O:3, O:5, or O:9. One strain of Yersinia pseudotuberculosis produced yersiniophore, but strains of Yersinia kristensenii, Yersinia frederiksenii, and Yersinia intermedia did not produce or utilize yersiniophore. Food and water isolates of Y. enterocolitica produced a water-soluble siderophore but not yersiniophore. Sixty-two strains of Y. enterocolitica including 42 isolates from human infections, 2 animal isolates, and 18 water and food isolates were examined for utilization of yersiniophore, the water-soluble siderophore, and ferrioxamine. Yersiniophore promoted growth rate, iron binding, and uptake in 17 of 62 strains, all of which produced yersiniophore. Ten of 17 food and water isolates and one human isolate were capable of utilizing the water-soluble siderophore. Utilization studies suggest that at least one additional water-soluble siderophore may be produced. Ferrioxamine promoted the growth of 60 of 62 strains examined; however, only the 17 strains which produced yersiniophore actively accumulated [59Fe]ferrioxamine. Yersiniophore production and utilization may be important in clinical infections since all human strains belonging to serotype O:8 produced yersinophore. The water-soluble siderophore was not detected in human isolates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett M. L., Powers C., Abbott S. L., Janda J. M. Epidemiologic investigations of Yersinia enterocolitica and related species: sources, frequency, and serogroup distribution. J Clin Microbiol. 1990 May;28(5):910–912. doi: 10.1128/jcm.28.5.910-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert J. R., van Landuyt H. W., Valcke Y. J., Cantinieaux B., Lornoy W. F., Vanherweghem J. L., Moreillon P., Vandepitte J. M. The role of iron overload in Yersinia enterocolitica and Yersinia pseudotuberculosis bacteremia in hemodialysis patients. J Infect Dis. 1987 Aug;156(2):384–387. doi: 10.1093/infdis/156.2.384. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991 Jul;4(3):309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A. J., Hantke K. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol Microbiol. 1992 May;6(10):1309–1321. doi: 10.1111/j.1365-2958.1992.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mercereau-Puijalon O., Bonnefoy S. The gene coding for the 190,000-dalton iron-regulated protein of Yersinia species is present only in the highly pathogenic strains. Infect Immun. 1989 Apr;57(4):1211–1217. doi: 10.1128/iai.57.4.1211-1217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Kay B. A., Wachsmuth K., Gemski P. New virulence-associated plasmid in Yersinia enterocolitica. J Clin Microbiol. 1982 Jun;15(6):1161–1163. doi: 10.1128/jcm.15.6.1161-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Modell B. Advances in the use of iron-chelating agents for the treatment of iron overload. Prog Hematol. 1979;11:267–312. [PubMed] [Google Scholar]
- Mors V., Pai C. H. Pathogenic properties of Yersinia enterocolitica. Infect Immun. 1980 Apr;28(1):292–294. doi: 10.1128/iai.28.1.292-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux C., Jordan D. C., Rattray J. B. Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem. 1983 Aug;133(1):163–169. doi: 10.1016/0003-2697(83)90238-5. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Prpic J. K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985 Mar;47(3):774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Devenish J. A., Toma S. Characteristics of virulence in human isolates of Yersinia enterocolitica. Infect Immun. 1981 Apr;32(1):400–403. doi: 10.1128/iai.32.1.400-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Prpic J. K., Robins-Browne R. M. Production of aerobactin by some species of the genus Yersinia. J Bacteriol. 1986 Jun;166(3):1131–1133. doi: 10.1128/jb.166.3.1131-1133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake A., Misawa M., Matsui A. Siderochrome production by Yersinia pestis and its relation to virulence. Infect Immun. 1975 Nov;12(5):1211–1213. doi: 10.1128/iai.12.5.1211-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]