Abstract
Attention increases the gain of visual neurons, which improves visual performance. How attention is controlled, however, remains unknown. Clear correlations between attention and saccade planning indicate that the control of attention is mediated through mechanisms housed in the oculomotor network. Here, we used event-related fMRI to compare overt and covert attention shifts. Subjects covertly or overtly shifted attention based on an endogenous cue, and maintained attention throughout a long and variable delay. To insure continued attention, subjects counted when the attended target dimmed at near-threshold contrast levels. Overt and covert tasks used identical stimuli and required identical motor responses. Additionally, a staircase procedure that adjusted the target-dimming contrast separately for covert and overt trials equated the difficulty between conditions and across subjects. We found that the same regions along the precentral and intraparietal sulci were active during shifts of covert and overt attention. We also found sustained activation in the hemisphere contralateral to the attended visual field. We conclude that maps of prioritized locations are represented in areas classically associated with oculomotor control. The read-out of these spatial maps by posterior visual areas directs spatial attention just as the read-out by downstream saccade generators directs saccades.
Keywords: attention, control, saccade, salience, spatial, eye field, fMRI
INTRODUCTION
Our ability to shift attention has evolved to offset the capacity limitations of our visual system. Eye movements are the primary mechanism by which we explore space. Through a series of gaze shifts we use foveal vision to construct a higher fidelity representation of the world than if we relied on extrafoveal vision. Moreover, even in the absence of overt eye movements visual analysis can be enhanced by covertly shifting our attention (Carrasco et al. 2004; Egeth and Yantis 1997; Liu et al. 2006; Posner 1980; Posner et al. 1982). At the neuronal level, visual signals are augmented in neurons that have receptive fields that overlap with the locus of covert attention (Kastner and Ungerleider 2000; 2001; Luck et al. 1997; Motter 1993; Reynolds and Chelazzi 2004; Reynolds and Desimone 2003). Therefore, we use two key mechanisms, overt saccades and covert attention shifts, to select targets for further visual processing.
Interestingly, there are strong links between the preparation of saccades and visuospatial attention. For instance, visual perception is enhanced at the locus of the saccade goal, and separating the saccade goal from the locus of attention impairs visual or saccade performance (Deubel and Schneider 1996; Hoffman and Subramaniam 1995; Kowler et al. 1995; Rizzolatti et al. 1987; Sheliga et al. 1995; Sheliga et al. 1994; Van der Stigchel et al. 2006). In humans, neuroimaging studies of spatial attention (for reviews see (Corbetta and Shulman 2002; Serences and Yantis 2006) and saccade planning (Connolly et al. 2002; Curtis et al. 2005; Curtis and D'Esposito 2003) appear to activate similar frontal and parietal areas. In monkeys, spike rate in frontal eye field (FEF) neurons is correlated with target selection even when a saccade is never made to the target (Thompson and Bichot 2005). Neurons in monkey lateral intraparietal area (LIP) show delay activity that is correlated with spatially directed attention and saccade planning (Andersen and Buneo 2002; Colby et al. 1996; Goldberg et al. 2002; Sereno and Amador 2006). Inactivation of monkey FEF impairs both saccade planning (Dias and Segraves 1999; Sommer and Tehovnik 1997) and covert attention (Wardak et al. 2006). Transcranial magnetic stimulation of the putative human FEF not only disrupts saccade execution (Ro et al. 2002), but also impairs performance in attention demanding tasks (Muggleton et al. 2003; O'Shea et al. 2004). Together, the link between saccade planning and the locus of attention appears to be tightly coupled and may even be supported by overlapping cortical areas.
Here, we tested the hypothesis that the exact same frontal and parietal cortical areas support overt and covert shifts of attention. We compared human brain activity during shifts and maintenance of covert and overt attention. Several methodological precautions ensured a rigorous comparison. First, the experimental stimuli, attention cueing, and task demands were identical between the two tasks. Most importantly, we used psychophysical procedures to equate the difficulty between covert and overt tasks. Second, we used event-related functional magnetic resonance imaging (fMRI) to separate transient activation time-locked to the shift of attention from the sustained activation that persisted throughout the maintenance of attention. To date, only blocked design studies have compared overt and covert shifts of attention (Beauchamp et al. 2001; Corbetta et al. 1998; Nobre et al. 2000) and it remains unknown whether shifting attention or maintaining attention or even factors unrelated to attention may have contributed to the results. Third, since we wanted to compare the anatomical overlap of overt and covert activation maps in frontal and parietal cortex, areas that have extensive individual variability in folding patterns that could lead to misregistration across subjects, we used anatomical landmarks that constrained the registration of subjects to one another (Van Essen 2005). With the same goal in mind, we analyzed time-series data derived from each subject's statistical maps to accommodated inter-subject variability in activation. Fourth, we ensured that we were indeed measuring the maintenance of attention by using long, variable, and unpredictable maintenance durations during which subjects' attention was verified with a contrast change detection task. These methodological advances allowed for a rigorous test of our hypotheses and we were able to carefully measure and characterize the evoked responses during the shift and maintenance of visual attention.
METHODS
Subjects
Fourteen neurologically healthy subjects (8 males, 12 right handed, 2 left handed, age between 21 and 35) were recruited for participation and were paid for their time. All subjects had normal or corrected to normal vision. Subjects gave written informed consent and all procedures were in compliance with the safety guidelines for fMRI research and approved by the human subjects Institutional Review Board at New York University.
Behavioral procedures
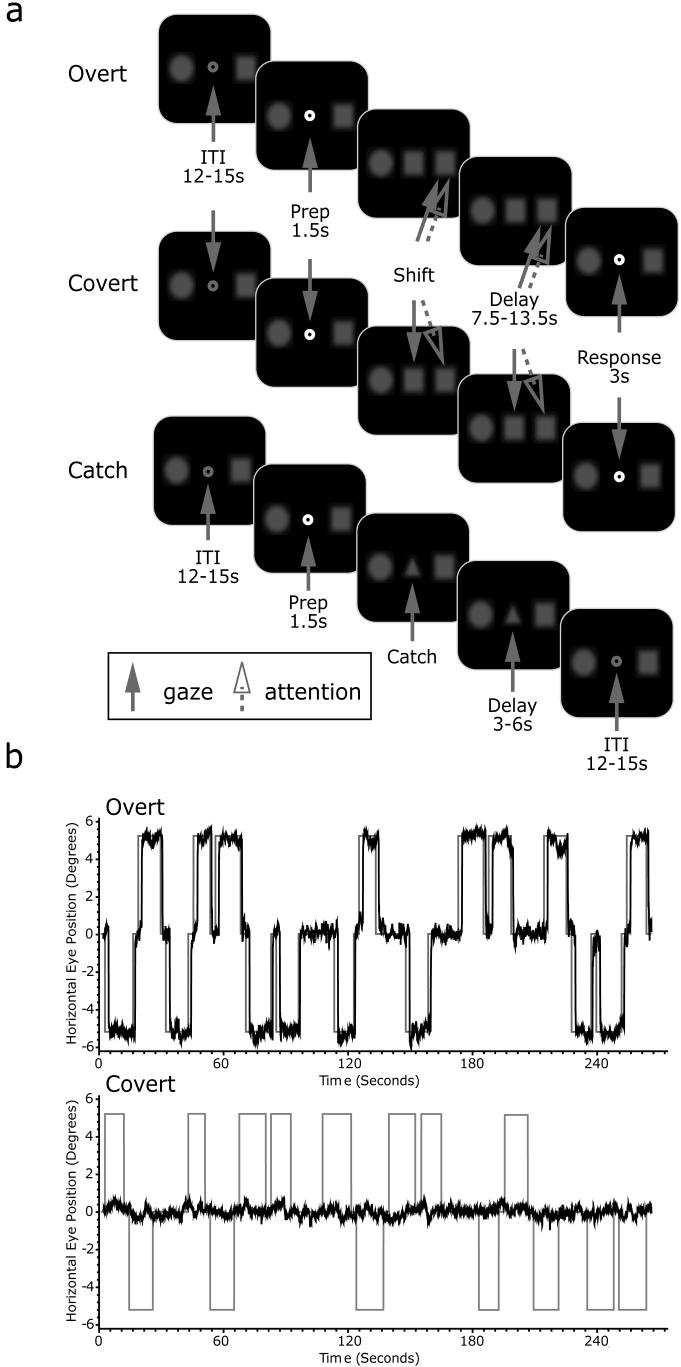
The experimental stimuli were controlled by E-Prime (Psychology Software Tools, Inc., PA) and projected (Eiki LC-XG100) into the bore of the scanner on a screen that was viewed by the subjects through an angled mirror. The schematic of the experiment is illustrated in Figure 1a. Subjects initially fixated a centrally presented gray fixation point that was flanked by a circle and a square (both 1 degree of visual angle) on a black background. The flanking shapes were blurred slightly with a Gaussian filter to reduce high spatial frequencies that could be used to easily detect luminance changes even when the stimuli were not actively attended. These stimuli were always present throughout the experiment at ± 5.5 degrees away from the center of the screen on the horizontal meridian. Luminance of both shapes was set at 40% of pure white. The fixation point changed to white for 1.5 seconds to signal the beginning of a new trial and then was replaced to match one of the two flanking shapes, instructing subjects to shift their attention to the matching shape. Subject did not have to use peripheral vision or shift their attention to detect which flanking shape matched the cue because the shapes were on the same side of fixation throughout the entire experiment (e.g., square always on left and circle always on right). Subjects maintained attention at the cued location for a long, variable, and unpredictable duration (7.5, 9, 10.5, 12, or 13.5 sec). During this interval, the attended target occasionally and unpredictably dimmed in luminance, resulting in a contrast change that was near-threshold for 70% performance accuracy. We had subjects count the number of times the target dimmed in order to ensure that they were actively maintaining their attention at the cued location throughout the entire interval. After the delay, the fixation point turned to green (3 sec) instructing subjects to report the number of target dims by pressing a corresponding button. The attended target dimmed between 1 to 4 times during the delay for 100ms. Subjects were explicitly informed of the validity of the cue. Trials were separated by an intertrial interval (ITI) between 12~15 sec to allow hemodynamic response to return to baseline.
Figure 1.
(a). Schematic of trials. Overt and covert trials were performed in blocks (4 blocks each). During the catch trials, subjects were instructed to simply fixate on the triangle. Solid arrow represents locus of the gaze whereas the dotted arrow represents the location of attention. Fixation point became green at the beginning of the response phase, and became white at the beginning of ITI. (b). Examples of eye-tracking data for an overt (top) and covert (bottom) block, corrected for blinks and ITI removed. Each block had 20 trials, with 4 catch trials. During the overt trials, subjects made eye movement (black traces) to the peripheral target (gray traces) and kept the gaze on the target for the duration of delay. In a catch trial, gaze was kept at the center. Negative and positive values on the y-axis indicate left and right target, respectively. During the covert trials, subject's gaze was always on the center of display.
There were two main types of trials: overt and covert trials. In an overt trial, subjects made a saccade to the cued target and fixated it throughout the delay. In a covert trial, subjects maintained central fixation at all times. We also included partial catch trials, where a triangle, not circle or square, cue appeared for 3~6 sec and then the trial aborted. Subjects, aware of these catch trials, simply maintained central fixation and did not shift attention. The partial catch trials were included to help in deconvolution and allowed us to examine the effects of simply orienting to an instructional cue. Each scan consisted of 4 overt blocks and 4 covert blocks. Within each block, there were 20 trials, 4 of which were catch trials. Overall, there were 64 covert, 64 overt, and 32 catch trials. Overt and covert blocks were performed alternately with the order of blocks counterbalanced.
We controlled task difficulty with psychophysical staircases that changed the contrast level with which the target dimmed. Separate staircases were used for the overt and covert tasks in order to maintain a desired accuracy of about 70% for each task. At the end of each trial, the cumulative accuracy was calculated, and if it was above or below 70%, the next dimming was decreased or increased by 2.5%, respectively. The overt task began with a dimming by 5% of the original luminance whereas the covert task began with a dimming by 12.5%. The range of possible dimming was between 37.5% and 2.5%. The short dimming duration (100ms), the use of blurred targets, and the near-threshold change in contrast all made detecting the target dimming extremely difficult unless one carefully attended the cued target.
Oculomotor procedures
Eye position was monitored in the scanner at 60 Hz with an infrared videographic camera equipped with a telephoto lens (ASL 504LRO; Applied Sciences Laboratories, Bedford, MA; modified with a Sony HAD CCD) that focused on the right eye viewed from the flat surface mirror mounted inside the RF coil. Nine-point calibrations were performed at the beginning of the session and between runs when necessary. Eye-movement data were transformed to degrees of visual angle, calibrated using a third-order polynomial algorithm that fit eye positions to known spatial positions, and scored offline with in-house software (GRAPES). An example of eye-tracking data is shown in Figure 1b. In the overt attention task, subjects made a saccade to target immediately after the onset of the shift cue, and maintained gaze at target for the duration of trial, whereas in the covert attention task, subject's gaze remained at the center of display. Overt trials with failures to shift and/or maintain gaze at the target, and covert trials with failures to maintain central fixation throughout the trial were discarded. Trials with excessive blinks were also discarded. Out of 14 subjects, 12 completed all 8 blocks. Two subjects terminated the experiment after 6 blocks (3 covert and 3 overt blocks). Another subject did not have oculomotor data due to the technical difficulty during the recording. This subject's eye movements were reviewed for task compliance by carefully watching the offline videotape of the session. A total of 67 trials (3.10%) from all subjects were discarded from analyses because of non-compliance.
MRI procedures
All MRI data were collected using a 3T head-only scanner (Allegra, Siemens, Germany) at the Center for Brain Imaging at New York University. Images were acquired using custom radio frequency coils (NM-011 transmit head-coil and NMSC-021 four-channel phased array receive coil; NOVA Medical, Wakefield, MA) placed over lateral frontal and parietal cortices. During each fMRI scan, a series of volumes were acquired using a T2*-sensitive echo planar imaging pulse sequence (repetition time, 1500 ms; echo time, 30 ms; flip angle, 75°; 24 slices; 3 × 3 × 3 mm voxels; 192 × 192 mm FOV). High-resolution (1 mm isotropic voxels) MP-RAGE three-dimensional T1-weighted scans were acquired for anatomical registration, segmentation, and display. To minimize head motion, subjects were stabilized with foam padding around the head.
BOLD analytic procedures
Post hoc image registration was used to correct for residual head motion [MCFLIRT (motion correction using FMRIB's Linear Image Registration Tool)] (Jenkinson et al. 2002). Additional preprocessing of the fMRI data were as follows. First, we bandpass filtered the time series of each voxel (0.01 to 0.33 Hz) to compensate for the slow drift typical in fMRI measurements (Biswal and Hyde 1997; Zarahn et al. 1997), divided the time series of each voxel by its mean intensity to convert to percent signal modulation and compensate for the decrease in mean image intensity with distance from the receive coil.
We modeled each within-trial event (i.e., shift, maintenance, and response) for overt and covert trials separately. The attention shift and motor response were short transient events and were thus modeled with a impulse time-locked to the event convolved with a canonical hemodynamic response function (HRF) (Polonsky et al. 2000). The maintenance of attention delay spanned 7.5 - 13.5 seconds and was modeled very well by the linear combination of a zero-order polynomial (i.e., boxcar) and a first-order polynomial (i.e., linear ramp). Both delay regressors spanned the delay period and were time-shifted by 4000 ms to account for the hemodynamic lag. The parameter estimates from the first-order polynomial was used to estimate delay period activity at the group level because at the individual subject level it predicted significant delay period activity, confirmed by plotting the time series of the voxels identified by this parameter. Each of the independent variable regressors were entered into a modified general linear model (GLM; (Worsley and Friston 1995)) for statistical analysis using VoxBo (http://www.voxbo.org).
For each subject, we used Caret (http://brainmap.wustl.edu/caret) for anatomical segmentation, gray-white matter surface generation, flattening, and multi-fiducial deformation mapping to the PALS atlas (Van Essen 2005). Registering subjects in a surface space using anatomical landmark constraints (e.g., central sulcus, sylvian fissure, etc.) results in greater spatial precision of the alignment compared to standard volumetric normalization methods (Van Essen 2005). Further, statistical maps for contrasts of interest were created using the beta-weights estimated from each subject's GLM. We used a nonparametric statistical approach based on permutation tests to help address the problem of multiple statistical comparisons (Holmes et al. 1996; Nichols and Holmes 2002). First, we constructed a permuted distribution of clusters of neighboring surface nodes with t-values > 3.0. We chose a primary t-statistic cutoff of 3.0 because it is strict enough that intense focal clusters of activity would pass but not so strict that diffuse large clusters of activity are lost. In the case of a one-sample comparison, where measured values are compared to the test value of 0, the signs of the beta values for each node were randomly permuted for each subject's surface, prior to computing the statistic. One thousand iterations, N, of this procedure were performed to compute a permutation distribution for each statistical test performed. Then, we ranked the resulting suprathreshold clusters by their area. Finally, corrected p-values at α = 0.05 for each suprathreshold cluster were obtained by comparing their area to the area of the top 5% of the clusters in the permuted distribution, where the critical suprathreshold cluster size, C, at a t-score threshold of t > 3.0 is C = Nα+1. The permutation tests controlled for Type I error by allowing us to formally compute the probability that an activation of a given magnitude could cluster together by chance.
Region-of-Interest (ROI) time series procedures
We used ROI-based analyses of the time courses of BOLD signal change. First, on each subject's high resolution anatomical scans, we traced around grey matter of several a priori ROIs including the superior precentral sulcus (sPCS), inferior precentral sulcus (iPCS), posterior portion of inferior frontal sulcus (pIFS), and intraparietal sulcus (IPS). The sPCS was defined as the dorsal segment of the precentral sulcus at the junction of the superior frontal sulcus. sPCS were further divided into dorsolateral (dl) sPCS and dorsomedial (dm) sPCS segments depending on whether it was lateral or medial to the junction. iPCS was ventral segment of precentral sulcus extended to the junction of the inferior frontal sulcus, and pIFS was defined as the portion of inferior frontal sulcus just anterior to that junction. IPS was defined as the sulcus that divides the superior and inferior parietal lobules. IPS was divided into an anterior (aIPS) segment that extended from the junction with postcentral sulcus to the junction with parieto-occipital sulcus, and a posterior (pIPS) segment that extended from the POS to the junction with transverse-occipital sulcus. Example ROIs are illustrated in Figure 2 projected on a subject's inflated surface. Next, within each ROI, we selected the 20 voxels (540 mm3) with the strongest main effect of the linear combination of all the task covariates. These voxels showed some consistent deviation from baseline during the task without being biased by any task component. Using combined structural-functional criteria to select voxels for study is similar to the way electrophysiologists first identify neurons that respond to the task and then examine those neurons for further study.
Figure 2.
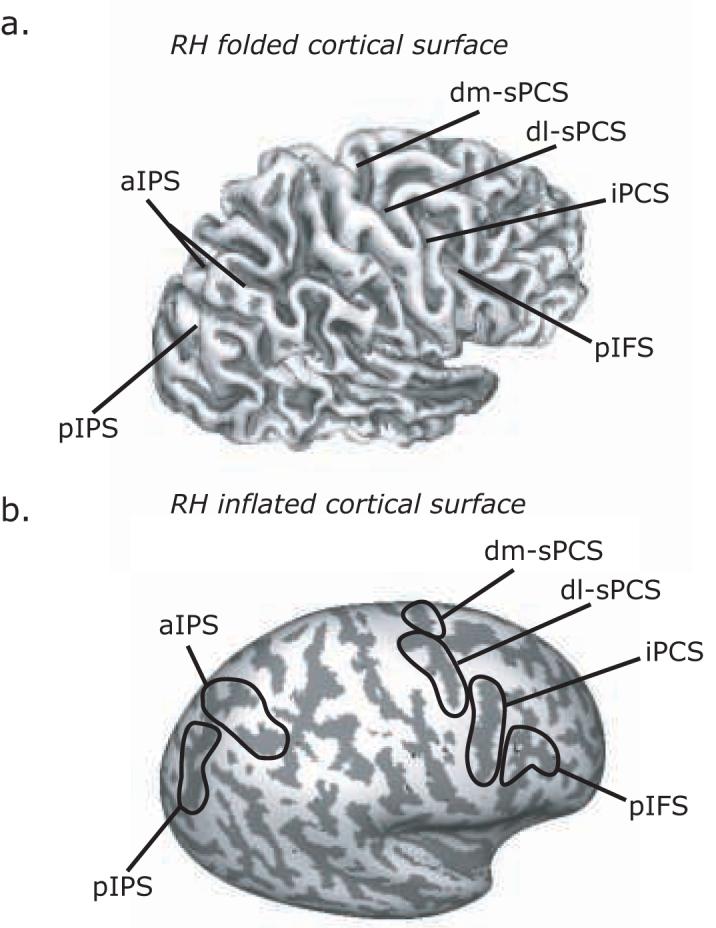
Key regions of interest (ROI) are denoted on the grey-white matter boundary of the right lateral hemisphere folded (a) and inflated (b). Dark grey overlay indicates sulci, while light grey indicates gyri. Abbreviations: dm-sPCS = dorsal medial superior precentral sulcus; dl-sPCS = dorsal lateral superior precentral sulcus; iPCS = inferior precentral sulcus; pIFS = posterior inferior frontal sulcus; aIPS = anterior intraparietal sulcus; pIPS = posterior intraparietal sulcus.
We plotted the time series of BOLD responses, averaged across voxels within an ROI and averaged across subjects from analogous ROIs, time-locked to the presentation of the attention shift cue. The average signal was baselined against the average response of the last two TRs before the trial began. Since the delays varied in length, contributions to the average plot only included data from TRs up to the end of the delay so as not to contaminate the estimation with activity evoked by the motor response after the delay. The error bands were computed by taking the average of each individual's standard error, which appropriately estimates the mean of the within-subject variance.
In order to quantitatively evaluate the time course data, we created separate shift and maintenance indices for each ROI. Activity related to shifting attention was defined as the average of the time points in the epoch between 1.5 and 6 seconds following the cue to shift attention. Activity related to maintaining attention was defined as the average of the time points in the delay period, specifically, between 7.5 seconds following the shift cue until the end of the maintenance of attention, which was variable. Since we found very similar patterns of activation in the left and right hemispheres for our sPCS and IPS ROIs, we combined data from left and right homologous ROIs for these regions. This procedure doubled the number of observations and increased our power to test for effects of laterality of responses with regard to the direction of attention shifts. Contralateral activation was defined as activation in the left ROIs when the selected target fell in the right visual field, plus activation in the right ROIs when the selected target fell in the left visual field. Ipsilateral activation was defined as activation in the left ROIs to the left visual field target, plus activation in the right ROI to the right visual field target. The shift and maintenance indices were plotted against each other with contralateral values on the y-axis and ipsilateral values on the x-axis and fitted with a linear function. Further, we calculated a laterality index for each subject as the contrast ratio between contralateral and ipsilateral activity [(contra-ipsi)/(contra+ipsi)], where negative activations were rounded to zero so as to exclude the possibility that the index could reflect a difference of deactivations.
RESULTS
Behavioral results
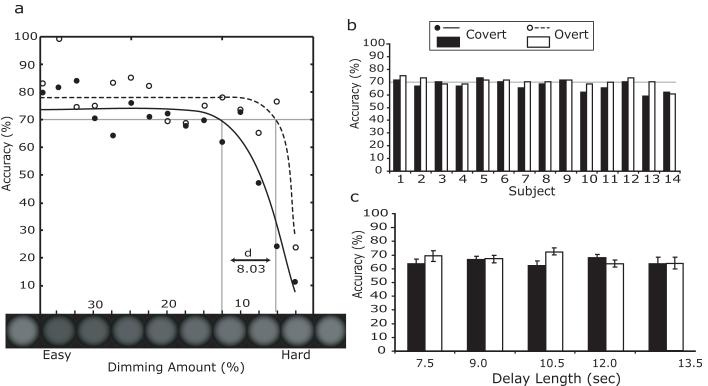
First, we compared the overall accuracies of covert and overt trials. Figure 3a illustrates the psychometric functions for accuracy during the covert and overt conditions as a function of the relative dimming of the target stimulus. As the relative dimming level decreased, subjects' performance worsened. Because vision is superior at the fovea, overt task performance was better at any given dimming level than covert task performance. At the target accuracy (~70%), the difference in average contrast dimming was about 8%, which reflects the benefit of detecting a change in contrast of a foveated compared to peripheral target. The performance of each subject was near the target accuracy (Figure 3b) and there were no significant differences between covert and overt task performance for any subject (Fisher's exact test, all p's >0.05.). By using separate adaptive dimming levels for the covert and overt tasks, we successfully equated the difficulty of the two conditions, which is a necessary prerequisite for comparison. Next, we confirmed that the duration of the maintenance of attention did not affect behavioral accuracy (Figure 3c). Using a repeated measures ANOVA, we found no significant main effect of the duration of attention on accuracy, F(4,52)<1, and no significant interaction between duration and attention condition (i.e., overt, covert), F(4,52)=1.46, p>0.05. This allowed us to collapse the data across the different maintenance durations in further analyses. Overall, the behavioral results clearly demonstrate that both conditions at all delay lengths were equally challenging for all subjects. Therefore, any brain activation differences between covert and overt attention cannot be attributed to differences in task difficulty.
Figure 3.
Behavioral data for overt (unfilled) and covert (filled) trials. (a). Psychometric functions for each trial type. Blurred circles below the x-axis depict the dimmed target, which can be compared to the original luminance depicted at both ends of the x-axis). Difficulty is greater toward the right side of the graph (smaller dimming amount). (b). Behavioral accuracy by subject. (c). Accuracy as a function of delay length. Error bars represent the standard error of mean.
Imaging results: surface statistical analyses
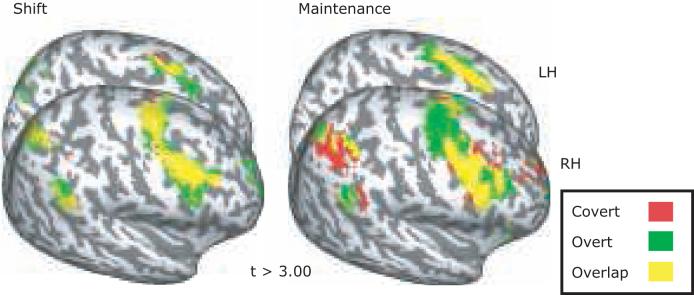
We first quantified the cortical activations evoked by shifting and maintaining attention separately for the overt and covert tasks (Figure 4; Table 1). Overall, we found a strikingly similar pattern of activity time-locked to covert and overt shifts of attention. There were significant activations bilaterally along the superior and inferior PCS into IFS, as well as the IPS. Particularly strong activations were observed in dl-sPCS, iPCS, pIFS, and aIPS for both covert and overt shifts (all p's <0.05 corrected). Additionally, we found significant clusters of shift-related activity on the frontal medial wall in the dorsal anterior cingulate cortex (dACC), and in paracentral suclus, presumably the supplementary eye field (SEF) (left hemisphere: p=0.068 corrected, right hemisphere: p<0.05 corrected), as well as in the right superior temporal gyrus (STG, p<0.05 corrected). We also found a smaller cluster of activity in the frontal poles during overt shifts of attention, but it did not surpass the correction for multiple comparisons. Again, the most striking feature of the data is the remarkable degree of cortical overlap evoked by overt and covert shifts of attention. A paired t-test comparing the two types of attention shifts was not significant in any of these regions. The only significant difference was in the calcarine sulcus in both hemispheres, where overt shifts of attention evoked larger response than covert shifts of attention (left: p < 0.05 corrected, right: p < 0.01 corrected). When we lowered threshold of a paired t-test to an uncorrected level, left frontal pole and STG were significantly more active during the covert than overt shift, and left transverse parietal sulcus (tPS), right SEF, iPCS, and STG were more active during the overt than covert shift additionally.
Figure 4.
Surface statistics displaying activations time-locked to the shift (left) and maintenance (right) of attention are overlaid onto an inflated surface brain. Medial surface of left hemisphere and the lateral surface of right hemisphere are shown. All activations displayed are significant (p<0.05, corrected for multiple comparisons). Peak coordinates are shown in Table 1.
Table 1.
Peak MNI volumetric coordinates.
| Peak MNI Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Covert Shift |
Overt Shift |
||||||
| Region | Hemi | x | y | z | x | y | z |
| dm-sPCS | L | -23.8 | -11.7 | 57.6 | -24.2 | -12.2 | 56.0 |
| R | 18.4 | -11.4 | 60.8 | 17.9 | -11.0 | 61.3 | |
| dl-sPCS | L | -36.6 | -12.0 | 50.8 | -35.7 | -12.8 | 52.5 |
| R | 46.5 | -2.2 | 45.7 | 37.4 | -7.0 | 49.0 | |
| iPCS | L | -45.1 | -1.5 | 32.6 | -43.3 | -0.8 | 34.0 |
| R | 38.8 | 2.4 | 33.3 | 43.8 | 0.6 | 34.8 | |
| pIFS | L | -39.4 | 9.6 | 31.7 | -41.1 | 11.3 | 27.0 |
| R | 41.5 | 14.6 | 37.8 | 36.4 | 4.7 | 34.5 | |
| SEF | L | -6.1 | -18.8 | 53.6 | -7.8 | -11.1 | 52.0 |
| R | 2.7 | -11.3 | 55.0 | 2.7 | -11.3 | 55.0 | |
| aIPS | L | -36.4 | -47.1 | 45.5 | -33.4 | 55.8 | 46.8 |
| R | 26.6 | -61.0 | 52.3 | 24.3 | -62.9 | 49.8 | |
| pIPS | L | -27.3 | -74.0 | 28.1 | -27.8 | -80.6 | 21.3 |
| R | 26.7 | -78.3 | 24.0 | 30.5 | -80.0 | 22.7 | |
| STG | L | -52.2 | -53.5 | 15.0 | -59.1 | -50.2 | 28.1 |
| R | 55.0 | -44.2 | 27.8 | 54.7 | -44.7 | 29.0 | |
| Covert Maintenance |
Overt Maintenance |
||||||
|---|---|---|---|---|---|---|---|
| Region | Hemi | x | Y | Z | X | Y | z |
| dm-sPCS | L | -23.4 | -8.2 | 59.3 | -21.3 | -12.6 | 62.4 |
| R | 21.2 | -11.8 | 54.2 | 18.1 | -10.1 | 61.5 | |
| dl-sPCS | L | -41.2 | -9.9 | 48.8 | -33.8 | -13.5 | 52.6 |
| R | 43.2 | -0.4 | 48.0 | 35.6 | -5.6 | 47.5 | |
| iPCS | L | -48.1 | 1.0 | 28.9 | -44.6 | -1.0 | 32.9 |
| R | 41.9 | 1.2 | 33.8 | 39.1 | 2.0 | 33.8 | |
| pIFS | L | -47.0 | 9.2 | 21.9 | -39.7 | 11.6 | 28.5 |
| R | 43.0 | 8.6 | 24.7 | 39.9 | 11.9 | 37.3 | |
| SEF | L | -7.4 | -12.2 | 52.4 | -7.3 | -12.3 | 53.0 |
| R | 5.3 | 0.4 | 54.1 | 2.7 | -9.4 | 54.5 | |
| aIPS | L | -36.1 | -52.9 | 49.6 | -35.8 | -53.3 | 48.7 |
| R | 35.4 | -46.4 | 42.4 | 30.2 | -51.8 | 45.2 | |
| pIPS | L | -30.6 | -76.8 | 24.2 | -29.8 | -75.0 | 26.5 |
| R | 29.1 | -79.2 | 22.4 | 25.6 | -78.2 | 28.5 | |
| STG | L | -58.0 | -51.2 | 15.0 | -57.5 | -51.0 | 14.2 |
| R | 56.2 | -41.4 | 34.9 | 49.7 | -47.3 | 23.3 | |
Bold numbers indicate that the cluster was significant at p<0.05 corrected. Non-bold coordinates were significant at p<0.05 uncorrected. See figure 2 for abbreviations.
Surprisingly, we found a very similar pattern of activation during the maintenance of attention at a foveated target (i.e., following an overt shift of attention) compared to a peripheral target (i.e., following a covert shift of attention). There were significant bilateral activations along the superior and inferior PCS, IFS, IPS, and SEF/dACC (all p's <0.05 corrected). The STG activation did not reach significance in either hemisphere. A paired t-test comparing the maintenance of attention during the overt and covert tasks revealed no significant differences, though left aIPS approached significance, where covert maintenance evoked larger activation than overt maintenance (p = 0.056 corrected).
Imaging results: region of interest time-series analyses
Next, we plotted the time series of BOLD responses in the various ROIs to test several hypotheses. Figure 5 plots the mean time course of activation in the hemisphere ipsilateral and contralateral to the attended visual field. We collapsed data in the left and right hemispheres for sPCS and IPS ROIs because there were no differences between the hemispheres. However, activations in iPCS and pIFS showed different patterns of activity in each hemisphere and therefore, were analyzed separately. In each ROI, we found a robust transient response time-locked to the attention shift that peaked at about 4.5 seconds after the shift. Activation persisted throughout the duration of maintained attention in dl-sPCS, aIPS, bilateral iPCS, and right pIFS. Activation persisted in dm-sPCS only during the maintenance of covert attention. In order to quantitatively evaluate the time series data from the ROIs, we calculated separate shift and maintenance indices for each ROI (see Methods). The BOLD signal time-locked to the overt and covert attention shifts were significantly greater than baseline in all ROIs (all t's >2.27, df=13, p<0.05), except for left pIFS, which only approached significance (t[13]=2.06, p=0.06) (Figure 5). During the maintenance of attention, the BOLD signal in the dl-sPCS, aIPS, left and right iPCS, and right pIFS remained significantly above baseline (all t's >2.19, df=13, p<0.05). BOLD signal in the dm-sPCS was significantly larger than baseline only during the covert maintenance of attention, t(13)=2.86, p<0.03. Paired t-test revealed that activations in the sPCS were larger during the maintenance of covert attention than during the maintenance of overt attention, dl-sPCS t(13)=2.42, p<0.05, dm-sPCS t(13) = 3.06, p<0.01. No other ROI showed this difference in activation.
Figure 5.
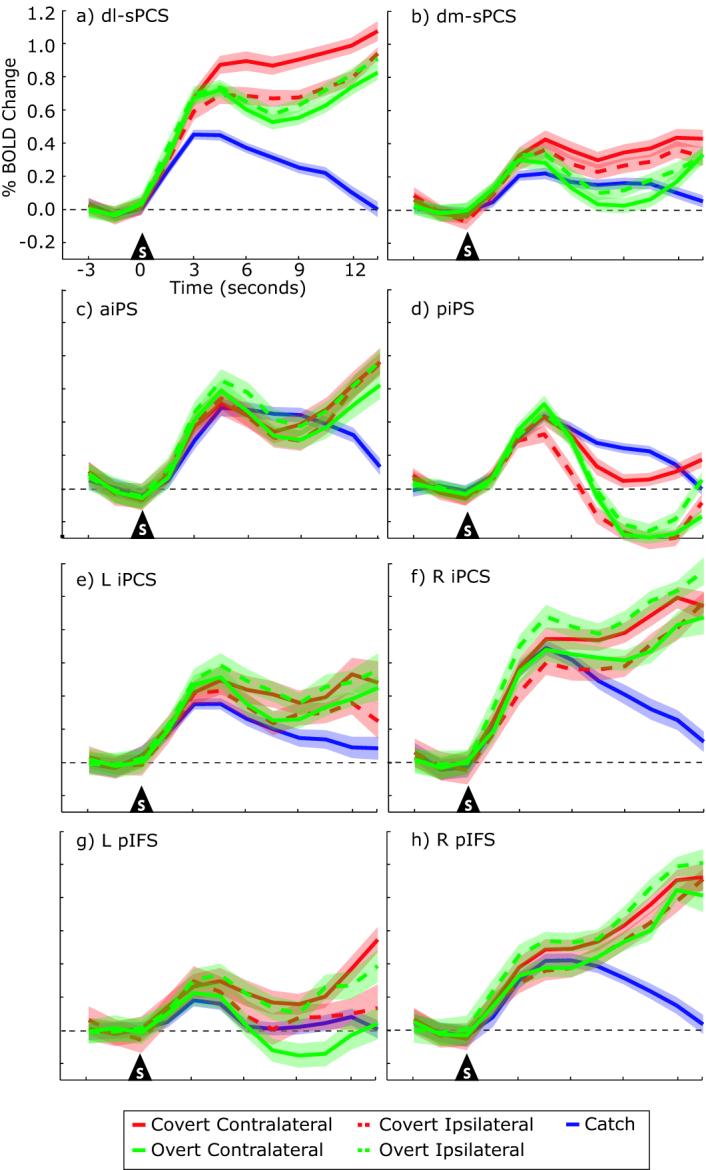
Percent BOLD signal change time-locked to the shift of attention in each ROI. Time series for overt (green), covert (red) and catch (blue) trials are shown separately. Solid lines represent the time series from the hemisphere contralateral to the attended side, while the dashed lines represent the time series from the hemisphere ipsilateral to the attended side. Error bands represent the average standard error of mean for each subject. Both transient responses associated with shifts of attention and sustained responses associated with maintenance of attention can be seen.
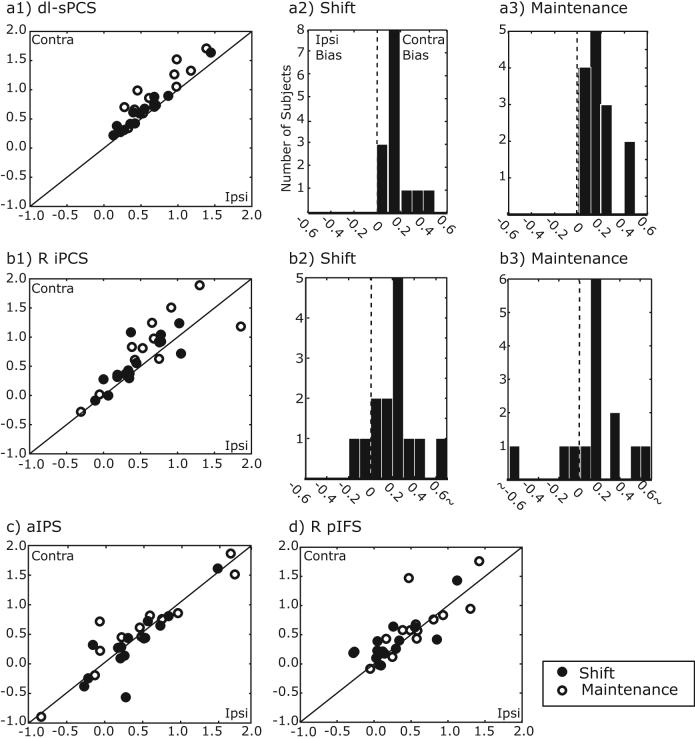
Next, we quantified the degree to which the BOLD signals showed a hemispheric lateralization with regard to the direction of covert attention. The sPCS showed the strongest contralateral bias during the maintenance of covert attention (dl-sPCS: F(1,13)=20.48, p<0.001, dm-sPCS: F(1,13)=9.31, p<0.005). The right iPCS also showed a contralateral bias during the maintenance of covert attention, F(1,13)=4.82, p<0.05. It showed greater activity when subjects covertly attended the left visual field compared to the right visual field, a contralateral bias. Interestingly, activation was greater following an overt shift of attention to the right compared to left visual field (the left iPCS shows the same trend, but reversed). Following the overt shift of attention, subjects were then fixating the peripheral target and therefore, the lateralized difference in iPCS activation may reflect a contralateral bias if we assume that covert attention is deployed towards the fixation point so that the subject will know when to respond. All 14 subjects showed a contralateral bias at the shift and during the maintenance of covert attention in dl-sPCS (Figure 6). In right iPCS, 10/14 subjects showed a contralateral bias time-locked to the covert shift of attention and 10/14 subjects showed a contralateral bias during the maintenance of attention. On the other hand, aIPS and right pIFS showed similar magnitudes of BOLD signals in the hemispheres contralateral and ipsilateral to the attended visual field.
Figure 6.
Lateralized activation during shift (filled circles) and maintenance (open circles) of attention on covert trials. Each dot represents one subject. A contralateral bias can be seen in (a) dl-sPCS and (b) right iPCS. Histograms of the contralateral bias indices for shift (a2 and b2) and maintenance (a3 and b3) of attention. See Methods for details. (c) aIPS and (d) right pIFS showed significant delay activation during the maintenance of attention, but neither region showed contralateral bias.
Critically, we wanted to confirm that the signals we measured during the maintenance interval were evoked by the maintenance of attention. If true, then the signals should persist as long as attention is maintained. Figure 7 shows the evoked response from the dl-sPCS during covert trials for each of the different delay lengths. Following the transient response, sustained activations were observed throughout all delay lengths until the motor response was made. This pattern was observed even at the longest delay, when subjects maintained attention for 13.5 seconds. Additionally, as can be seen in all of the ROIs in Figure 5, BOLD signals during the maintenance of attention were greater than in the catch trials when attention was not allocated (all p's >0.05). This indicates that the persistent neural activity we measured is related to the maintenance of attention and is not due to residual effects of orienting to an instructional cue. Overall, these data provide strong support that the sustained activations reflect the maintenance of attention.
Figure 7.
Percent BOLD signal change for each delay length for dl-sPCS time-locked to the shift of attention. Each triangle at the bottom of the figure represents the end of delay.
DISCUSSION
The present study used event-related fMRI to measure the neural activity evoked when humans redirect their attention with or without eye movements to an endogenously cued location, and we distinguished this activity from that due to the maintenance of attention. In general, we found that the same parts of the precentral and intraparietal sulci are involved both in overt and covert shifts of attention, as well as the maintenance of attention. The superior precentral sulcus, the putative human FEF, showed 1) robust shift related activity, 2) persistent activity during the maintenance of attention, which was greater during the maintenance of attention in the periphery compared to at fixation, and 3) greater activity in the hemisphere contralateral to the attended visual field. Below we interpret these new findings in the context of existing data and theory.
Shifts of Attention
We measured robust (e.g., 0.4~1%) activations time-locked to voluntary overt shifts of attention in frontal and parietal regions, including dorsal and lateral regions of the PCS, IFS, SEF, and IPS. This is consistent with past studies on the frontal-parietal network's role in voluntary eye movements in humans (Connolly et al. 2005; Connolly et al. 2002; Curtis et al. 2005; Curtis and D'Esposito 2006; Curtis et al. 2004; Luna et al. 1998; Medendorp et al. 2005) and monkeys (Andersen and Buneo 2002; Bruce et al. 1985; Goldberg et al. 2002; Goldberg and Bruce 1985; Schall et al. 1995). We observed equally robust activations time-locked to voluntary covert shifts of attention that overlapped perfectly with that from the overt shifts of attention. Because we excluded all trials in which uninstructed eye-movements were made, we can be confident that the evoked activations that we measured are not due to saccade-related activity. These results are consistent with past studies of covert spatial attention that have reported increased frontal and parietal activation in humans (Corbetta et al. 2002; Serences et al. 2005; Serences and Yantis 2007; Yantis et al. 2002).
Interestingly, when we directly compared overt and covert shifts of attention, we found almost perfect spatial alignment of overt and covert shift evoked activity (Figure 4). Past block design studies found that performing blocks of covert attention trials evoked more (Corbetta et al. 1998; Nobre et al. 2000) or less (Beauchamp et al. 2001) activity than overt attention trials. It is likely that the inconsistencies across studies stem from the different methodologies used. The past block design studies are hard to interpret because of the following differences between the overt and covert attention tasks that may have led to potential violations in the assumptions of cognitive subtraction: differences in task difficulty, differences in cuing (e.g., exogenous vs. endogenous), and differences in task requirements besides the shift of attention (e.g., preparatory, visual, and motor processes). Although attention can be deployed to a location based on exogenous or endogenous cues, the type of cueing has different time courses and might rely on different mechanisms (Muller and Rabbitt 1989). To restrict task differences to the type of shift, and not the method for cueing the shift, we used an endogenous cue for both attention tasks that additionally equated any differences in visual stimulation. Under these conditions, only one area showed a difference in activity during shifting. The sPCS showed slightly greater activity evoked by covert than overt shifts of attention. The location of this area in the dorsal portion of the precentral sulcus just below the junction with the superior frontal sulcus is the most likely candidate for the human FEF (Amiez et al. 2006; Paus 1996; Rosano et al. 2003).
In general, we conclude that the same network of brain areas is responsible for shifts of attention with and without eye movements. An implication is that this network houses the neural mechanisms that support both voluntary saccades and covert attention shifts. The premotor theory of attention states that covert attention and saccade programming are supported by the same neural mechanisms (Rizzolatti et al. 1987). Supporting this theory, past behavioral studies found that saccades are affected by attention and vice versa (Deubel and Schneider 1996; Hoffman and Subramaniam 1995; Kowler et al. 1995; Rizzolatti et al. 1987; Sheliga et al. 1995; Sheliga et al. 1994; Van der Stigchel et al. 2006). Moreover, electrical microstimulation of monkey FEF neurons with currents too low to evoke saccades increases the monkey's sensitivity to detect contrast changes in targets that spatially overlap with the neuron's response field (Moore et al. 2003). This effect is similar to the performance advantage with covert attention. Therefore, artificially induced activity in FEF neurons below the threshold that will evoke a saccade causes a shift in covert attention. This subthreshold activity may be analogous to the presaccadic neural activity correlated with saccade planning (Schall 2002). On the other hand, at the single neuron level within the monkey FEF, different neurons appear to make distinct contributions to saccade planning and covert attention (Juan et al. 2004; Sato and Schall 2003; Thompson et al. 2005).
Maintenance of attention
Following a covert shift in the locus of attention, we were also able to measure neural activity that persisted above baseline as long as attention was deployed. We observed sustained activity in the PCS, IFS, SEF, and IPS. These were the same areas in which we observed activity time-locked to shifts of attention and may reflect the initiation and ongoing deployment of attention. In the same frontal and parietal areas, we have observed sustained neural activity during spatial working memory delay periods (Curtis and D'Esposito 2006; Curtis et al. 2004; Srimal and Curtis in press). One possibility is that the activity we observed during working memory delays in these studies arises from the maintenance of covert attention (Awh and Jonides 2001) or the maintenance of a planned saccade to the location of the memoranda (Curtis 2006; Curtis and D'Esposito 2006).
We were able to unambiguously measure signals related to sustained attention in the putative human FEF. We confirmed our statistical maps of delay period activity by plotting the ROI time-courses. The signals following the shift of attention clearly persisted until the end of the attention interval (Figures 5 and 7). Two other imaging studies reported sustained responses in the precentral and intraparietal sulci during the maintenance of covert attention (Corbetta et al. 2002; Serences and Yantis 2007). Corbetta et. al. (2002) used a short (7s) and fixed length delay periods and did not find any statistical evidence for a sustained response in the human FEF (dorsal PCS near junction with SFS). However, these studies did find sustained responses in a more ventral part of the precentral sulcus that was approximately 1-2 cm below the putative human FEF (Paus 1996; Rosano et al. 2003). This area corresponds to our iPCS ROI, which does show a robust sustained signal during the maintenance of attention. In the present study, we clearly demonstrated persistent activity in more dorsal portion of PCS, which is a more likely candidate for human FEF. Additionally, the epochs during which Serences and Yantis (2007) reported sustained activation during the maintenance of spatially directed attention, had high contrast visual displays of 6 letters/numbers that were changing every 100ms and the subjects were making motor responses to indicate the detection of targets. Indeed, motor responses evoke large activations in the same frontal and parietal areas that show sustained responses during the maintenance of attention. See for instance the robust hemodynamic response time-locked to the button press event in the superior precentral sulcus illustrated in Figure 7. The sustained responses that we report here are not contaminated by motor induced activations. Nonetheless, a motor confound cannot explain the greater activation in the hemisphere contralateral to the direction of attention that Serences and Yantis (2007) reported (see Laterality below).
Surprisingly, we observed a remarkable amount of common activation across the cortex during the maintenance of attention at the fovea, following an overt shift, and at the periphery, following a covert shift of attention. Several interesting explanations exist. Persistent activity may reflect the current locus of attention even when it is directed to a location in alignment with gaze. Therefore, such persistant signals may include contributions from head-centered representations in addition to retinotopic, or eye-centered ones, since the signal did not change when gaze changed. Additionally, both tasks involved effortful, controlled fixation, and both required subjects to count the number of times the target dimmed. These factors, although less likely, may have contributed to the similarities in the sustained responses following attentional shifts. Interestingly, only the superior PCS, the putative FEF, showed a difference between foveal and peripheral attention; activation was significantly larger in this ROI when attention and gaze were unyoked (Figure 5). However, this effect may simply reflect the need to suppress saccades to the attended target mediated by the activation of fixation neurons found in the FEF (Hanes et al. 1998).
Laterality
We found that the superior and inferior PCS had larger responses during shifting and maintaining attention to stimuli in the contralateral compared to ipsilateral visual field. Again, we consider the superior PCS, at the junction with the SFS, to be the putative human FEF. The response field (RF) of monkey FEF neurons is often located in the contralateral visual field (Bruce et al. 1985; Marrocco 1978; Schall 1991; Tehovnik and Sommer 1997), and attending to stimuli that fall into the neuron's RF increases neuronal firing rate compared to attending to stimuli that fall outside of the RF (Thompson and Bichot 2005; Thompson et al. 2005). In humans, electrical stimulation of PCS induces saccades to the contralateral visual field (Blanke et al. 1999), and lesions disrupt contraversive saccades (Gaymard et al. 1999; Rivaud et al. 1994).
Recent neuroimaging studies suggest that the human PCS and IPS may also be topographically organized. Attentional shifts that systematically progress in angle, like hands on a clock, evoke a traveling wave of activity at the task's frequency in portions of the human IPS in the hemisphere contralateral to the direction of attention (Schluppeck et al. 2005; Sereno et al. 2001; Silver et al. 2005). Similar results have been recently reported in the human dorsal and ventral PCS (Hagler et al. 2007; Hagler and Sereno 2006; Kastner et al. 2007). Since these phase-encoding experiments could not specify which portion of the task is driving the responses (e.g., visual cues, working memory or attentional delays, or motor responses), the current results nicely compliment these data by providing compelling evidence that persistent activity during the maintenance of attention is topographically biased towards the contralateral visual field in the human PCS.
Also, Serences and Yantis (2007) recently showed a contralateral bias of activity in iPCS, as well as IPS, during shifts of covert attention. We believe that directing attention to the contralateral hemifield caused the bias in neural activity we measured. However, we cannot rule out the possibility that the dimming of the attended target caused the bias. We do not think this is likely because the dimming was near perceptual threshold and in pilot testing went unnoticed when attention was not directed to the target. Recall that the flanking stimuli were visible at all times, dimmed at psychophysical threshold for only 100ms, lacked high spatial frequencies, and were cued by an endogenous stimulus. These features together reduced the likelihood that the bias was driven by sensory rather than attentional factors.
In the parietal cortex, we did not observe a significant contralateral bias during the maintenance of attention, a finding that is contrary to other data (Schluppeck et al. 2006; Serences and Yantis 2007; Sereno et al. 2001; Silver et al. 2005; Vogel and Machizawa 2004; Yantis et al. 2002). Monkey LIP neurons have RFs that are large and often include the fovea and even up to 5 degrees of the ipsilateral visual field (Ben Hamed et al. 2001). Our visual stimuli were only 5.25 degrees in the periphery and therefore may have reduced our sensitivity to detect lateralized responses. Indeed, many of the studies cited above (Schluppeck et al. 2006; Serences and Yantis 2007; Sereno et al. 2001), that have found contralateralized responses in human IPS have used stimuli placed greater than 10 degrees in the periphery.
Conclusions
Given the strong cortical overlap of evoked responses during overt and covert shifts of attention, we conclude that one key mechanism for the voluntary control of attention is mediated within the classic oculomotor system. On the one hand, the ability to attend to locations away from our fovea may have evolved by co-opting eye movement mechanisms within cortical oculomotor centers, like the FEF. The so-called premotor theory of attention posits that sub-threshold pre-saccadic activity in neurons that code for eye movements may be the mechanism by which we shift our attention covertly (Awh et al. 2006). On the other hand, the FEF may be best thought of as an area that contains a map of prioritized locations in the visual environment, not strictly a motor “eye field.” In this case, an ongoing map of prioritized locations could be built by bottom-up inputs from sensory cortices and top-down goals from the PFC (Itti and Koch 2001). A read out of such a map by the superior colliculus or brainstem saccade generator may be used to plan eye movements. Moreover, a read out by posterior visual areas may be used to select or tag portions of space, provide a boost in gain to neurons with matching receptive fields, and bias competition for neural representation (Buschman and Miller 2007; Desimone and Duncan 1995; Thompson and Bichot 2005; Treisman and Gelade 1980; Wolfe et al. 1989). Implicit in this idea is that a unitary mechanism, like a dynamic spatial priority map, could contribute to a variety of cognitive behaviors, like attention, intention, and working memory, depending on the afferents used to construct the map and the efferents that read-out the map.
ACKNOWLEDGMENTS
We thank Marcus Lauer, Riju Srimal, Jason Connolly, Souheil Inati, and Keith Sanzenbach for assistance. This work was supported by grants from the NIH R01 EY016407 and the Seaver Foundation.
REFERENCES
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26:2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: Links, causes and implications for spatial attention. Trends in Cognitive Sciences. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hyde JS. Contour-based registration technique to differentiate between task-activated and head motion-induced signal variations in fMRI. Magn Reson Med. 1997;38:470–476. doi: 10.1002/mrm.1910380315. [DOI] [PubMed] [Google Scholar]
- Blanke O, Morand S, Thut G, Michel CM, Spinelli L, Landis T, Seeck M. Visual activity in the human frontal eye field. Neuroreport. 1999;10:925–930. doi: 10.1097/00001756-199904060-00006. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: an FMRI study of countermanding saccades. Cereb Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol. 1999;81:2191–2214. doi: 10.1152/jn.1999.81.5.2191. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann N Y Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Cerebral cortical activity associated with the orientation of visual attention in the rhesus monkey. Vision Res. 1985;25:471–481. doi: 10.1016/0042-6989(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Desimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic Maps in Human Frontal Cortex Revealed in Memory-Guided Saccade and Spatial Working Memory Tasks. J Neurophysiol. 2007 doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Liu T, Fuller S, Carrasco M. Attention alters the appearance of motion coherence. Psychonomic Bulletin & Review. 2006;13:1091–1096. doi: 10.3758/bf03213931. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysilogy. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Marrocco RT. Saccades Induced by Stimulation of the Frontal Eye Fields: interaction with voluntary and reflexive eye movements. Brain Research. 1978;146:23–34. doi: 10.1016/0006-8993(78)90215-9. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16:1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37:853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Ro T, Farne A, Chang E. Locating the human frontal eye fields with transcranial magnetic stimulation. J Clin Exp Neuropsychol. 2002;24:930–940. doi: 10.1076/jcen.24.7.930.8385. [DOI] [PubMed] [Google Scholar]
- Rosano C, Sweeney JA, Melchitzky DS, Lewis DA. The human precentral sulcus: chemoarchitecture of a region corresponding to the frontal eye fields. Brain Res. 2003;972:16–30. doi: 10.1016/s0006-8993(03)02431-4. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol. 1991;66:559–579. doi: 10.1152/jn.1991.66.2.559. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357:1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Amador SC. Attention and memory-related responses of neurons in the lateral intraparietal area during spatial and shape-delayed match-to-sample tasks. J Neurophysiol. 2006;95:1078–1098. doi: 10.1152/jn.00431.2005. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Craighero L, Rizzolatti G. Spatial attention-determined modifications in saccade trajectories. Neuroreport. 1995;6:585–588. doi: 10.1097/00001756-199502000-00044. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. NeuroImage. doi: 10.1016/j.neuroimage.2007.08.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Electrically evoked saccades from the dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Exp Brain Res. 1997;117:369–378. doi: 10.1007/s002210050231. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Meeter M, Theeuwes J. Top-down influences make saccades deviate away: The case of endogenous cues. Acta Psychol (Amst) 2006 doi: 10.1016/j.actpsy.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL. Guided search: An alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]