Abstract
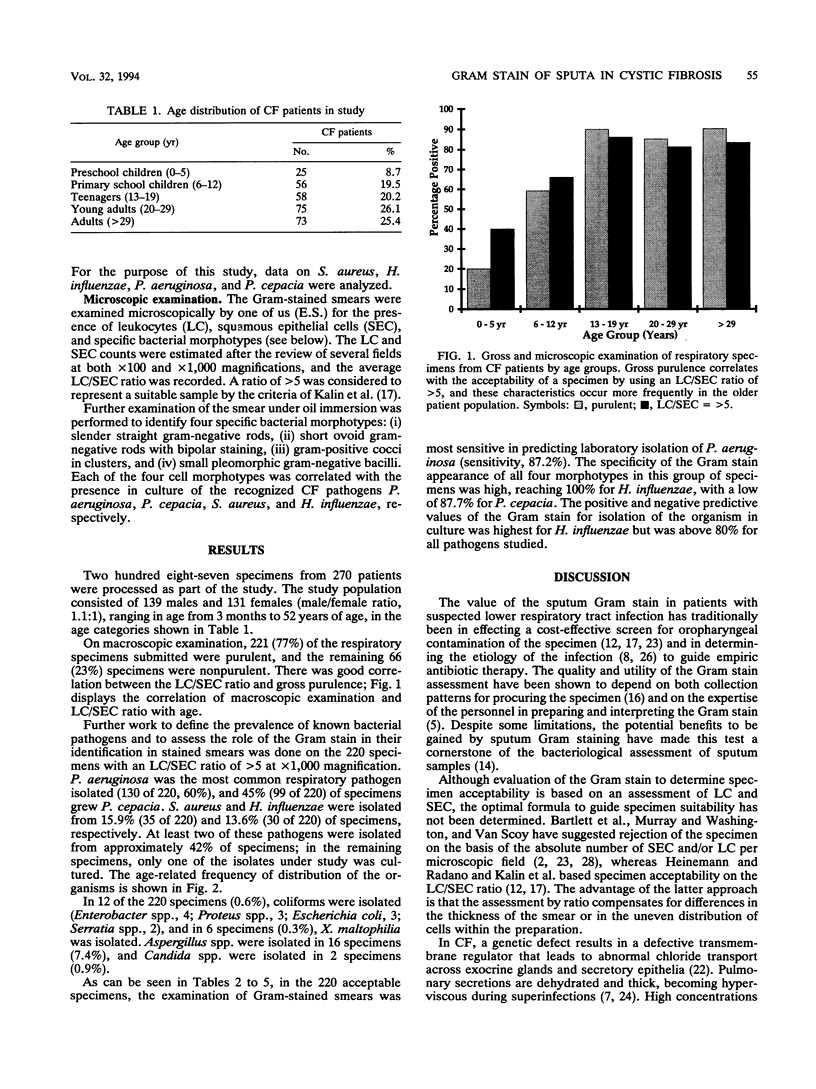
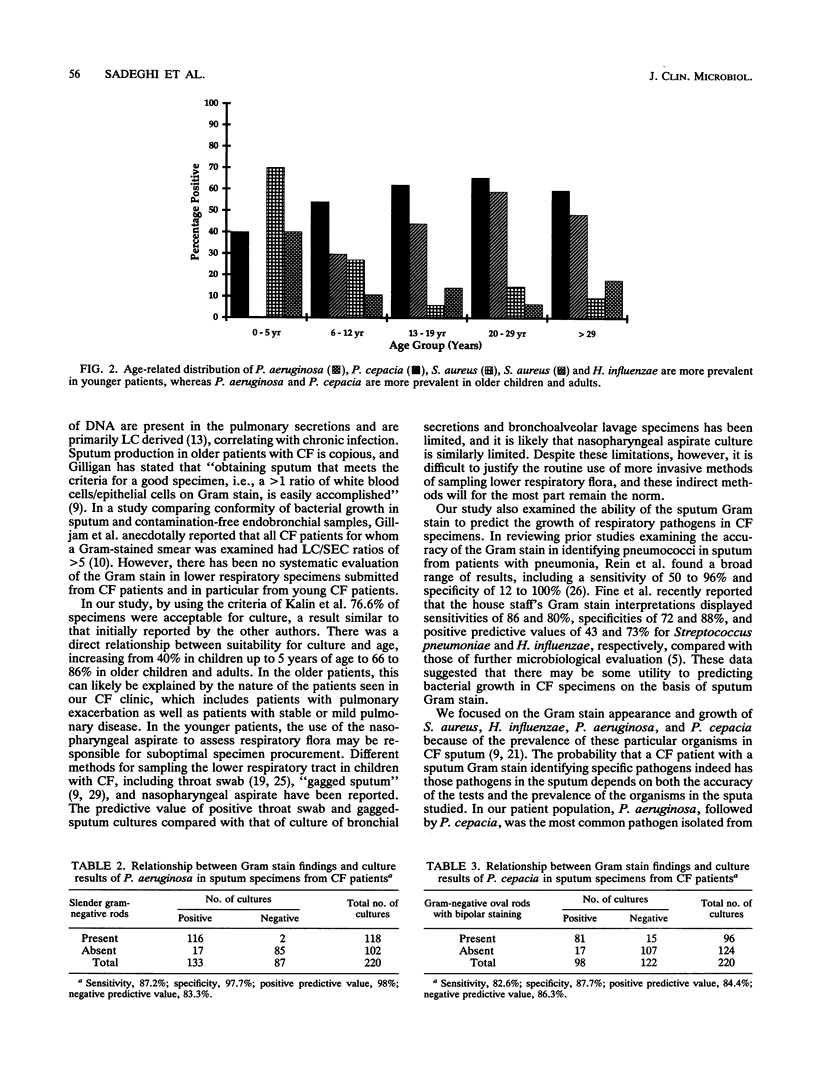
The utility of sputum Gram stain in assessing salivary contamination and in predicting the presence of pathogens on the basis of morphology was investigated in 287 respiratory specimens from patients with cystic fibrosis. Where acceptability for culture was defined as a leukocyte/squamous epithelial cell ratio of > 5, 76.6% (220 of 287) of respiratory specimens received in the laboratory were considered acceptable. Unacceptable specimens were more common in younger patients. The positive predictive value of the Gram stain for growth from acceptable sputum samples was 98% for Pseudomonas aeruginosa, 84.4% for Pseudomonas cepacia, 86.3% for Staphylococcus aureus, and 100% for Haemophilus influenzae. In cystic fibrosis patients, as has been reported for respiratory specimens in general, Gram stain of respiratory specimens in helpful for interpreting culture results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abman S. H., Ogle J. W., Harbeck R. J., Butler-Simon N., Hammond K. B., Accurso F. J. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J Pediatr. 1991 Aug;119(2):211–217. doi: 10.1016/s0022-3476(05)80729-2. [DOI] [PubMed] [Google Scholar]
- Christenson J. C., Welch D. F., Mukwaya G., Muszynski M. J., Weaver R. E., Brenner D. J. Recovery of Pseudomonas gladioli from respiratory tract specimens of patients with cystic fibrosis. J Clin Microbiol. 1989 Feb;27(2):270–273. doi: 10.1128/jcm.27.2.270-273.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey M., Allison L., Prober C., Levison H. Sputum bacteriology in patients with cystic fibrosis in a Toronto hospital during 1970-1981. J Infect Dis. 1984 Feb;149(2):283–283. doi: 10.1093/infdis/149.2.283. [DOI] [PubMed] [Google Scholar]
- Fine M. J., Orloff J. J., Rihs J. D., Vickers R. M., Kominos S., Kapoor W. N., Arena V. C., Yu V. L. Evaluation of housestaff physicians' preparation and interpretation of sputum Gram stains for community-acquired pneumonia. J Gen Intern Med. 1991 May-Jun;6(3):189–198. doi: 10.1007/BF02598958. [DOI] [PubMed] [Google Scholar]
- Galabert C., Jacquot J., Zahm J. M., Puchelle E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clin Chim Acta. 1987 Apr 30;164(2):139–149. doi: 10.1016/0009-8981(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Geckler R. W., Gremillion D. H., McAllister C. K., Ellenbogen C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J Clin Microbiol. 1977 Oct;6(4):396–399. doi: 10.1128/jcm.6.4.396-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilljam H., Malmborg A. S., Strandvik B. Conformity of bacterial growth in sputum and contamination free endobronchial samples in patients with cystic fibrosis. Thorax. 1986 Aug;41(8):641–646. doi: 10.1136/thx.41.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Harding L., Macone A., Smith A. L., Goldmann D. A. Bacteriology of sputum in cystic fibrosis: evaluation of dithiothreitol as a mucolytic agent. J Clin Microbiol. 1980 Jun;11(6):552–557. doi: 10.1128/jcm.11.6.552-557.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman H. S., Radano R. R. Acceptability and cost savings of selective sputum microbiology in a community teaching hospital. J Clin Microbiol. 1979 Oct;10(4):567–573. doi: 10.1128/jcm.10.4.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. C., McElvaney N. G., Birrer P., Shak S., Robinson W. W., Jolley C., Wu M., Chernick M. S., Crystal R. G. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. N Engl J Med. 1992 Mar 19;326(12):812–815. doi: 10.1056/NEJM199203193261207. [DOI] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Jacobson J. T., Burke J. P., Jacobson J. A. Ordering patterns, collection, transport, and screening of sputum cultures in a community hospital: evaluation of methods to improve results. Infect Control. 1981 Jul-Aug;2(4):307–311. doi: 10.1017/s019594170005534x. [DOI] [PubMed] [Google Scholar]
- Kalin M., Lindberg A. A., Tunevall G. Etiological diagnosis of bacterial pneumonia by gram stain and quantitative culture of expectorates. Leukocytes or alveolar macrophages as indicators of sample representativity. Scand J Infect Dis. 1983;15(2):153–160. doi: 10.3109/inf.1983.15.issue-2.05. [DOI] [PubMed] [Google Scholar]
- Kerem E., Corey M., Gold R., Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990 May;116(5):714–719. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- Lorian V., Waluschka A., Kim Y. Abnormal morphology of bacteria in the sputa of patients treated with antibiotics. J Clin Microbiol. 1982 Aug;16(2):382–386. doi: 10.1128/jcm.16.2.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I. Clinical significance of Staphylococcus aureus in cystic fibrosis. Infection. 1990 Jan-Feb;18(1):53–56. doi: 10.1007/BF01644186. [DOI] [PubMed] [Google Scholar]
- May T. B., Shinabarger D., Maharaj R., Kato J., Chu L., DeVault J. D., Roychoudhury S., Zielinski N. A., Berry A., Rothmel R. K. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991 Apr;4(2):191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Washington J. A. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975 Jun;50(6):339–344. [PubMed] [Google Scholar]
- Puchelle E., Jacquot J., Beck G., Zahm J. M., Galabert C. Rheological and transport properties of airway secretions in cystic fibrosis--relationships with the degree of infection and severity of the disease. Eur J Clin Invest. 1985 Dec;15(6):389–394. doi: 10.1111/j.1365-2362.1985.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Ramsey B. W., Wentz K. R., Smith A. L., Richardson M., Williams-Warren J., Hedges D. L., Gibson R., Redding G. J., Lent K., Harris K. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am Rev Respir Dis. 1991 Aug;144(2):331–337. doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- Rein M. F., Gwaltney J. M., Jr, O'Brien W. M., Jennings R. H., Mandell G. L. Accuracy of Gram's stain in identifying pneumococci in sputum. JAMA. 1978 Jun 23;239(25):2671–2673. doi: 10.1001/jama.239.25.2671. [DOI] [PubMed] [Google Scholar]
- Van Scoy R. E. Bacterial sputum cultures. A clinician's viewpoint. Mayo Clin Proc. 1977 Jan;52(1):39–41. [PubMed] [Google Scholar]


