Abstract
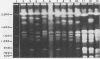
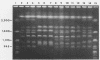
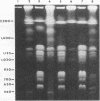
DNA subtyping by pulsed-field gel electrophoresis and in vitro susceptibility testing were used to study strain variation and fluconazole resistance in Candida albicans isolates from patients with AIDS undergoing azole (fluconazole and clotrimazole) therapy for oropharyngeal candidiasis. A total of 29 patients suffered 71 episodes of oropharyngeal candidiasis. Overall, 121 isolates of C. albicans recovered throughout the course of treatment of each infection were available for further characterization. DNA subtyping revealed a total of 61 different DNA subtypes. In vitro susceptibility testing of the 121 isolates by using proposed standard methods of the National Committee for Clinical Laboratory Standards revealed MICs of fluconazole ranging from < or = 0.125 to > 64 micrograms/ml. The MIC for 50% of isolates tested was 0.25 microgram/ml, and the MIC for 90% of isolates tested was 8.0 micrograms/ml. MICs were > or = 64 micrograms/ml for only 7.4% of the isolates tested. The majority (62%) of the patients with oropharyngeal candidiasis and undergoing azole therapy were infected or colonized with more than one DNA subtype, and the introduction or selection of strains with a more resistant DNA subtype during the course of fluconazole therapy was not uncommon. With one exception, this did not appear to have an adverse effect on clinical outcome. In contrast, for patients with AIDS and oropharyngeal candidiasis infected with a single DNA subtype of C. albicans, an increase in fluconazole MICs for the infecting strain was rarely demonstrated over the course of therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clift R. A. Candidiasis in the transplant patient. Am J Med. 1984 Oct 30;77(4D):34–38. [PubMed] [Google Scholar]
- Doebbeling B. N., Lehmann P. F., Hollis R. J., Wu L. C., Widmer A. F., Voss A., Pfaller M. A. Comparison of pulsed-field gel electrophoresis with isoenzyme profiles as a typing system for Candida tropicalis. Clin Infect Dis. 1993 Mar;16(3):377–383. doi: 10.1093/clind/16.3.377. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kish C. W., Jr, Kerkering T. M., Fromtling R. A., Bartizal K., Galgiani J. N., Villareal K., Pfaller M. A., Gerarden T., Rinaldi M. G. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992 Dec;30(12):3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A., Galgiani J. N., Pfaller M. A., Espinel-Ingroff A., Bartizal K. F., Bartlett M. S., Body B. A., Frey C., Hall G., Roberts G. D. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993 Jan;37(1):39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. L., Winston D. J., Greenfield R. A., Chandrasekar P. H., Fox B., Kaizer H., Shadduck R. K., Shea T. C., Stiff P., Friedman D. J. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992 Mar 26;326(13):845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Ollert M., Georgii A., Fröschl M. In vitro susceptibilities and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1988 Dec;26(12):2626–2631. doi: 10.1128/jcm.26.12.2626-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Bowdin L., Staudinger J. Comparison of molecular typing methods for Candida albicans. J Clin Microbiol. 1992 Oct;30(10):2674–2679. doi: 10.1128/jcm.30.10.2674-2679.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Connelly C., Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988 May;26(5):842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier F., Aoun M., Gerard M. Therapy for oropharyngeal candidiasis in the immunocompromised host: a randomized double-blind study of fluconazole vs. ketoconazole. Rev Infect Dis. 1990 Mar-Apr;12 (Suppl 3):S364–S368. doi: 10.1093/clinids/12.supplement_3.s364. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani R., Owens N. J., Quercia R. A., Klimek J. J., Nightingale C. H. Treatment and prevention of oropharyngeal candidiasis. Am J Med. 1984 Oct 30;77(4D):44–48. [PubMed] [Google Scholar]
- Schmid J., Odds F. C., Wiselka M. J., Nicholson K. G., Soll D. R. Genetic similarity and maintenance of Candida albicans strains from a group of AIDS patients, demonstrated by DNA fingerprinting. J Clin Microbiol. 1992 Apr;30(4):935–941. doi: 10.1128/jcm.30.4.935-941.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavitian A., Raufman J. P., Rosenthal L. E. Oral candidiasis as a marker for esophageal candidiasis in the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Jan;104(1):54–55. doi: 10.7326/0003-4819-104-1-54. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Bjorkman D. J. Esophageal, gastric, and intestinal candidiasis. Am J Med. 1984 Oct 30;77(4D):39–43. [PubMed] [Google Scholar]
- Vazquez J. A., Beckley A., Sobel J. D., Zervos M. J. Comparison of restriction enzyme analysis and pulsed-field gradient gel electrophoresis as typing systems for Candida albicans. J Clin Microbiol. 1991 May;29(5):962–967. doi: 10.1128/jcm.29.5.962-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Kirsch D. R., Kwon-Chung K. J., Wahl S. M., Smith P. D. Candida albicans in patients with the acquired immunodeficiency syndrome: absence of a novel of hypervirulent strain. J Infect Dis. 1990 Aug;162(2):513–518. doi: 10.1093/infdis/162.2.513. [DOI] [PubMed] [Google Scholar]
- Willocks L., Leen C. L., Brettle R. P., Urquhart D., Russell T. B., Milne L. J. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991 Dec;28(6):937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]