Abstract
Background
Rapamycin is an effective immunosuppressant widely used to maintain the renal allograft in pediatric patients. Linear growth may be adversely affected in young children since rapamycin has potent anti-proliferative and anti-angiogenic properties.
Methods
Weanling three week old rats were given rapamycin at 2.5 mg/kg daily by gavage for 2 or 4 weeks and compared to a Control group given equivalent amount of saline. Morphometric measurements and biochemical determinations for serum calcium, phosphate, iPTH, urea nitrogen, creatinine and insulin-growth factor I (IGF-I) were obtained. Histomorphometric analysis of the growth plate cartilage, in-situ hybridization experiments and immunohistochemical studies for various proteins were performed to evaluate for chondrocyte proliferation, chondrocyte differentiation and chondro/osteoclastic resorption.
Results
At the end of the 2 weeks, body and tibia length measurements were shorter after rapamycin therapy associated with an enlargement of the hypertrophic zone in the growth plate cartilage. There was a decrease in chondrocyte proliferation assessed by histone-4 and mammalian target of rapamycin (mTOR) expression. A reduction in parathyroid hormone/parathyroid hormone related peptide (PTH/PTHrP) and an increase in Indian hedgehog (Ihh) expression may explain in part, the increase number of hypertrophic chondrocytes. The number of TRAP positive multinucleated chondro/osteoclasts declined in the chondro-osseous junction with a decrease in the receptor activator of nuclear factor kappa β ligand (RANKL) and vascular endothelial growth factor (VEGF) expression. Although body and tibial length remained short after 4 weeks of rapamycin, changes in the expression of chondrocyte proliferation, chondrocyte differentiation and chondro/osteoclastic resorption which were significant after 2 weeks of rapamycin improved at the end of 4 weeks.
Conclusion
When given to young rats, 2 weeks of rapamycin significantly decreased endochondral bone growth. No catch-up growth was demonstrated at the end of 4 weeks, although markers of chondrocyte proliferation and differentiation improved. Clinical studies need to be done to evaluate these changes in growing children.
Background
Rapamycin is a powerful immunosuppressant widely used in children to maintain the renal allograft. Studies have shown that rapamycin decreases cell proliferation by inhibition of the mammalian target of rapamycin (mTOR), a key regulator in cell growth. In addition, rapamycin has been demonstrated to exert anti-angiogenic properties to control tumor growth by reduction in vascular endothelial growth factor (VEGF) expression [1,2]. Due to its anti-proliferative effects, long-term rapamycin therapy may have adverse effects on linear growth in young children.
Investigators have reported that bone length decreased in young rats with normal renal function treated with rapamycin at 2 mg/kg daily for 14 days accompanied by alterations in growth plate architecture and lower chondrocyte proliferation assessed by bromodeoxyuridine (BrDU) incorporation [3,4]. Changes in trabecular bone modeling and remodeling with decrease in body length have been demonstrated in 10 week old rats after 2 weeks of rapamycin [3]. In contrast, Joffe and coworkers showed that a higher dose of rapamycin at 2.5 mg/kg per day for 14 days transiently lowered serum osteocalcin and calcitriol levels but it did not affect trabecular bone volume or bone formation rate [5]. Rapamycin inhibited osteoclast function, lessened bone resorption, decreased osteoblast proliferation and enhanced osteoblast differentiation in various in-vitro experiments [6-8].
Since rapamycin is now a standard immunosuppressant used to maintain an organ transplant in children, linear growth may be affected if rapamycin is administered long-term to young and growing patients. The aim of the current study is to assess the short and long-term effects of rapamycin on endochondral bone growth in young rats with normal renal function using markers of chondrocyte proliferation, chondrocyte differentiation, chondroclast/osteoclastic resorption and angiogenesis in the tibial growth plate.
Methods
Twenty six male, 3 week old Sprague-Dawley rats with mean weight of 47 ± 4 grams, mean length of 20 ± 1 cm, were obtained from Harlan Laboratories (Indianapolis, IN), housed in individual cages at constant temperature with free access to drinking water. These are the approximate age comparisons between a rat and a child: a three week old weanling rat may be comparable to an infant and a rat between 5 to 7 weeks of age may approximate the age of a child. After 24 hours of acclimatization, the rats were randomly assigned to two groups: Rapamycin, N = 13, or Control, N = 13. Rapamycin was given at 2.5 mg/kg daily by gavage route and equal amount of saline was given to the Control group. The dose of rapamycin was based on previous published studies that demonstrated significant effects on body growth [4] and the length of treatment was adapted from our previous experiments that showed changes in the growth plate after 10 days of treatment [9]. Rapamycin and saline were given either for 2 weeks (Rapamycin-2 wks, N = 6; Control-2 wks, N = 6) or 4 weeks (Rapamycin-4 wks, N = 7; Control-4 wks, N = 7). All procedures were reviewed and approved by the Research Animal Resource Center at the University of Wisconsin and conducted in accordance with the accepted standards of humane animal care.
Rapamycin can lower oral intake which may subsequently affect growth [10]. To ensure equivalent caloric intake in all animals, the Rapamycin group was pair-fed to the Control animals by providing the amount of food each day to Control that had been consumed the previous day by the Rapamycin treated rats using a standard rodent diet (23.4% protein, 0.6% calcium, 0.6% phosphate) (Purina Mills, Indianapolis, IN).
Body weight was obtained weekly and body length was measured at the start and at the end of the 2 weeks or 4 weeks study period under sedation by measuring the distance from the tip of the nose to the end of the tail. At the end of the study period, the rats were anesthetized, killed by exsanguination and underwent trans-cardiac perfusion with 4% paraformaldehyde in phosphate buffered saline. Blood was obtained for determinations of serum calcium, creatinine, phosphate, urea nitrogen, parathyroid hormone (PTH) and insulin-like growth factor-I. Both tibiae from each animal were obtained and tibial length was measured between the proximal and distal articular surfaces using a caliper. Triplicate measurements were obtained for each bone, and the average of these determinations was taken to represent overall tibial length. Bones were decalcified in 15% ethylenediamine tetra-acetic acid in phosphate buffered saline, pH 7.4, at 4°C for approximately two weeks and embedded in paraffin. Five micrometer sections of bone were obtained for morphometric analysis, in-situ hybridization and immunohistochemistry studies.
Serum biochemical determinations
Serum was obtained by centrifugation and samples were stored at -80°C until assays are done. Serum urea nitrogen, creatinine, calcium, and phosphate levels were measured using standard laboratory methods. Parathyroid hormone levels were measured using the Rat Bioactive Intact PTH ELISA assay kit (Immutopics, San Clemente, CA). IGF-I levels were measured using the Rat IGF-I ELISA assay kit (Diagnostic Systems Laboratories Inc, Webster, TX).
Growth plate morphometry
The proximal growth plate of the tibia was selected for the experiments due to its fast growth [11]. For morphometric analysis, three 5-μm sections of bone were obtained from each tibia and stained with hematoxylin and eosin. Sections were viewed by light microscopy at 25× and images were captured onto a computer monitor. The total width of the growth plate cartilage at the proximal end of each tibia was measured at equally spaced intervals along an axis oriented 90° to the transverse plane of the growth plate and parallel to the longitudinal axis of the bone using an image-analysis software (Elektronik 200, Kontron Instruments, Ltd., Hallbergmoos, Germany) [9]. At least ten measurements were obtained from each epiphyseal growth plate. The width of the zones occupied by hypertrophic and proliferative chondrocytes was measured by the same method and the values are expressed as a ratio of the hypertrophic or proliferative zone to the total growth plate width.
In-situ hybridization (radioactive)
For in-situ and immunohistochemistry experiments, individual sections of bone obtained from rats in each study group were mounted together on individual glass slides to permit valid side-by-side comparisons among samples from each group and to minimize differences that could be attributed to slide-to-slide variation during the specimen processing and development. Approximately 70–80 slides are included in each experiment.
In-situ hybridization was performed using methods described elsewhere [9]. Briefly, 35S-labeled sense and antisense riboprobes were generated encoding mouse MMP-9/gelatinase B (provided by Drs. G.V. Segre and K. Lee, MGH, Boston, MA) and rat vascular endothelial growth factor (provided by Dr. Zena Werb, University of California, San Francisco, CA) and labeled to a specific activity of 1–2 × 109 cpm/μg using the Gemini transcription kit (Promega Corp, Madison, WI). After hybridization and post-hybridization washing, the slides were exposed to x-ray film (Kodak Scientific Imaging Systems, Rochester, New York) overnight, and emulsion autoradiography was done using NTB-2 (Eastman Kodak, Rochester, New York) at 4°C.
Slides were viewed at 100× under bright field microscopy and the number of silver grains overlying each chondrocyte profile was counted using an image analysis system (Kontron Instruments, Hallbergmoos, Germany) [9]. In each specimen, fifty to sixty cell profiles (chondrocytes) were assessed in the layer of chondrocytes where mRNA was expressed and the results represent the average of these measurements. Data are expressed as the number of silver grains/1000 μm2 of cell profile. To quantify gelatinase B/MMP-9 expression, the slides were viewed at 65× and the area with the silver grains was measured and expressed as percentage of the total area in the chondro-osseous junction.
Immunohistochemistry experiments
Immunohistochemistry experiments were performed using methods described previously [12]. All primary antibodies were obtained from Santa Cruz Biotechnology unless indicated. Sections were deparaffinized, rehydrated, and immersed in 3% H2O2 and antigen was unmasked using either heat-induced epitope retrieval (10 mmol/L sodium citrate, pH 6.0 at 90°C for 5 min) or microwave for 5 minutes. Blocking was done using 5% goat serum at room temperature. After blocking, the appropriate primary antibody was added and incubated in 4°C overnight. The slides were washed in PBS, incubated with the goat anti-mouse biotin conjugate (Sigma Co, St. Louis, MO), then with extravidin-peroxidase (Sigma) and counterstained with either hematoxylin or 1% methylgreen.
The following primary antibodies were selected to evaluate chondrocyte proliferation: [a]histone-4 at 5 μg/ml, [b] mammalian target of rapamycin (mTOR) at 4 μg/ml, [c] parathyroid hormone/parathyroid hormone related-peptide (PTH/PTHrP) at 4.4 μg/ml (Upstate Biotechnology), [d] Growth Hormone Receptor (GHR) at 4 μg/ml, and [e] type II collagen (Col2a) at 4 μg/ml. Chondrocyte maturation was assessed using: [a] Indian Hedgehog (Ihh) at 10 μg/ml, [b] Insulin-like Growth Factor I (IGF-I) at 10 μg/ml (Upstate Biotechnology], [c] Insulin Growth Factor Binding Protein (IGFBP3) at 10 μg/ml, [d] p57Kip2 at 4 μg/ml, [e] p21Waf1/Cip1 at 8 μg/ml, [f] type × collagen (Col10a) at 8 μg/ml, and [g] Bone Morphogenetic Protein-7 (BMP-7) at 5 μg/ml.
Osteo/chondroclastic activity was evaluated using Receptor Activator for Nuclear Factor Kappa β Ligand (RANKL) at 6 μg/ml and Osteoprotegerin (OPG) at 5 μg/ml. Histochemical staining for tartrate-resistant acid phosphatase (TRAP) and gelatinase B/MMP-9 were done using methods reported previously [9].
For quantification of the protein expression, slides were viewed at 65× by bright field microscopy and images were captured using a CCD video camera control unit. Approximately 50 to 60 cell profiles (chondrocytes) were assessed in the layer of the growth plate where the protein expression was counted and expressed as percentage of the labeled cells over the total number of cells where the expression is localized and the number of positive cells was counted and expressed as percentage of the labeled cells over the total number of cells where the expression is localized (Labeling Index) [12].
Histochemical staining for tartrate-resistant acid phosphatase (TRAP) was done using methods previously reported on sections of bone prepared and mounted in the same manner as for in-situ hybridization and immunohistochemistry experiments [9]. To quantify tartrate resistant acid phosphatase (TRAP), the number of TRAP positive cells in the chondro-osseous junction was counted and expressed as number of cells per area measured in the chondro-osseous junction and in the nearby primary spongiosa [9].
Statistical analysis
All results are expressed as mean values ± 1 SD. Data were evaluated by one-way ANOVA and comparisons among groups were done using Bonferroni/DUNN post-hoc tests using the StatView® statistical software (SAS Institute, Cary, NC). The Pearson product moment correlation coefficient was used to evaluate the relationship between two numerical variables. For all statistical tests, probability values less than 5% were considered to be significant.
Results
Measurements of body weight, body length and food intake
Gain in body weight was 14 percent and 19 percent higher in Control compared to Rapamycin groups after 2 and 4 weeks of treatment (Table 1). Body length measurements declined by 11 percent and 19 percent after 2 and 4 weeks of Rapamycin (Table 1). Tibial length measurements were 6 to 10 percent shorter in both Rapamycin groups (Table 1). Although the total caloric intake was similar in Rapamycin and Control groups, the calculated food efficiency ratio was higher with rapamycin which may suggest that a higher caloric intake may be required for growth or there may be dysregulation in the utilization of calories during rapamycin administration (Table 1).
Table 1.
Morphometric measurements, food intake and food efficiency ratio (FER) in all groups
| Parameters | 2 weeks | 4 weeks | ||
| Rapamycin, N = 6 | Control, N = 6 | Rapamycin, N = 7 | Control, N = 7 | |
| Change in body weighta (grams) | 63 ± 6d, e | 73 ± 8d | 128 ± 7f | 159 ± 11 |
| Change in body lengtha (centimeters) | 7.6 ± 0.6d, e | 8.6 ± 0.5d | 13 ± 1.2f | 16 ± 0.7 |
| Tibial Lengthb (centimeters) | 3.0 ± 0.05d, e | 3.2 ± 0.05d | 3.4 ± 0.09f | 3.7 ± 0.15 |
| Total food intakeb (grams) | 185 ± 1.8 | 183 ± 5.3 | 390 ± 32 | 410 ± 4.8 |
| Food Efficiency Ratioc (FER) | 3.0 ± 0.5e | 2.6 ± 0.6 | 3.0 ± 0.5f | 2.6 ± 0.3 |
aDifference between measurements at 2 or 4 weeks and baseline
bObtained at the time of sacrifice
cTotal food consumed/weight gain during the study period
dp < 0.0001, 2 weeks versus 4 weeks (same group)
ep < 0.001, rapamycin versus control at 2 weeks
fp < 0.0001, rapamycin versus control at 4 weeks
Serum biochemical parameters
Serum parathyroid hormone (PTH) and phosphate levels declined after 4 weeks of rapamycin (Table 2). Serum calcium levels were similar in all groups (Table 2). Serum creatinine levels were comparable in Rapamycin and Control groups at the end of 2 weeks and 4 weeks of treatment (Table 2). Serum IGF-I levels were 18 percent lower in Rapamycin and Control at the end of 2 weeks (Table 2).
Table 2.
Biochemical parameters in all groups
| Serum Parameters | 2 weeks | 4 weeks | ||
| Rapamycin, N = 6 | Control, N = 6 | Rapamycin, N = 7 | Control, N = 7 | |
| Intact PTHa (pg/mL) | 40 ± 22 | 68 ± 34 | 32 ± 21c | 90 ± 37 |
| Calciuma (mg/dL) | 10 ± 0.6 | 10 ± 0.4 | 9.8 ± 0.1 | 10 ± 0.1 |
| Phosphorusa (mg/dL) | 12 ± 0.2b | 12 ± 0.5 | 10 ± 0.5 | 11 ± 0.7 |
| Creatininea (mg/dL) | 0.2 ± 0.04b | 0.2 ± 0.03b | 0.3 ± 0.04 | 0.3 ± 0.07 |
| IGF-Ia (ng/mL) | 708 ± 222b | 867 ± 180b | 1028 ± 256c | 1318 ± 297 |
aObtained at the time of sacrifice
bp < 0.003, 2 weeks versus 4 weeks (same group)
cp < 0.006, rapamycin versus control (4 weeks)
Growth plate measurements
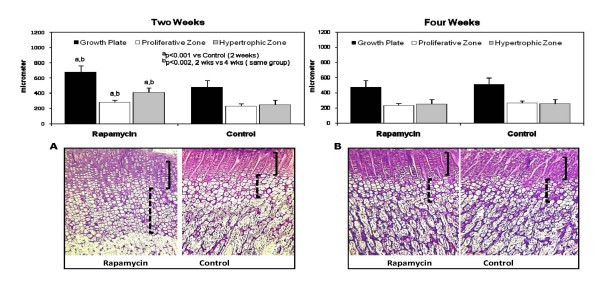
Despite shorter body and tibial length, the growth plate was 26 percent wider compared to Control after two weeks of rapamycin accompanied by an increase in the area occupied by hypertrophic chondrocytes and a decrease in the proliferative zone (Figure 1). At the end of 4 weeks, the growth plate width was similar between the Rapamycin and the Control, 475 ± 89 μm and 509 ± 35 μm, p = NS (Figure 1). There were no obvious abnormalities in the columnar architecture of the growth plate cartilage (Figure 1).
Figure 1.
The upper panels show the measurements of the total growth plate (black square), proliferative zone (white square) and hypertrophic zone (gray square) after 2 weeks (left panel) and after 4 weeks (right panel) in Rapamycin and Control groups, expressed in micrometers. The lower panels show the corresponding photomicrographs of the growth plate cartilage in all groups, 30×. Note the increase in the zone occupied by hypertrophic chondrocytes in the 2 weeks Rapamycin group (left panel). The solid brackets denote the layer of proliferating chondrocytes and the dashed brackets indicate the hypertrophic zone; ap < 0.001 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.002 Rapamycin (2 weeks) vs Rapamycin (4 weeks).
In-situ hybridization and immunohistochemistry studies
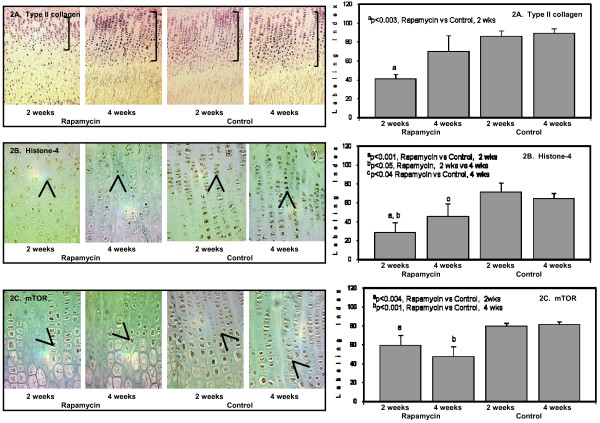
Rapamycin inhibits the mammalian target of rapamycin (mTOR) which is crucial to cell cycle progression and thus, may lower chondrocyte proliferation. In the current study, we evaluated whether the shorter bone growth was primarily due to a decline in chondrocyte proliferation. The protein expression of selected markers associated with chondrocyte proliferation was assessed including PTH/PTHrP receptor, histone-4, mTOR, growth hormone receptor and type II collagen (Col2a1). In the growth plate, Col2a1 is the most abundant collagen which is expressed in all layers of chondrocytes. Rapamycin lowered Col2a1 expression by 40 percent compared to Control at 2 weeks particularly in the hypertrophic chondrocytes (Figure 2A). After 4 weeks of Rapamycin, Col2a1 staining was comparable to Control (Figure 2A).
Figure 2.
The panels on the left show representative photomicrographs of type II collagen protein expression (2A) in the proliferating and hypertrophic chondrocytes, 20× (denoted by the purple color and brackets); ap < 0.003 Rapamycin (2 weeks) vs Control (2 weeks). Histone-4 protein expression (2B, denoted by arrows and brown color), 50×; ap < 0.001 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.05 Rapamycin (2 weeks) vs Rapamycin (4 weeks); cp < 0.04 Rapamycin (4 weeks) vs Control (4 weeks). mTOR protein expression (2C, denoted by arrows and brown color), 50×; ap < 0.004 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.001 Rapamycin (4 weeks) vs Control (4 weeks). The panels on the right show the quantification of the protein expression for type II collagen (upper panel); histone-4 (middle panel); and mTOR (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
Histone-4 localized to the proliferating chondrocytes and declined by 60 percent after 2 weeks of rapamycin compared to Control; 28 ± 11 percent versus 71 ± 10 percent (Figure 2B), p < 0.001. Similar to Col2a1 expression, histone-4 slightly increased after 4 weeks of rapamycin but remained 40 percent lower than Control (Figure 2B), p = < 0.05. Histone and DNA synthesis are initiated at the beginning of S phase of the cell cycle by cyclin/cdk2 activity. Cyclin expression was not evaluated in the current experiment, but our previous results have shown that histone-4 positively correlated with proliferating nuclear staining (PCNA) which is specific to proliferating cells [13].
mTOR expression was demonstrated in both proliferating and upper hypertrophic chondrocytes and declined after 2 and 4 weeks of rapamycin (Figure 2C).
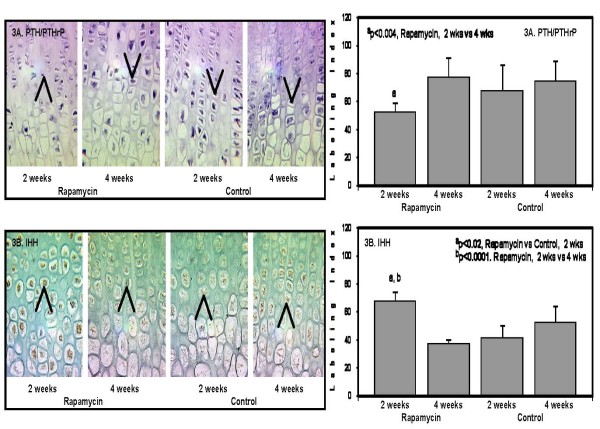
PTH/PTHrP and Ihh are essential in the regulation of chondrocyte proliferation and chondrocyte differentiation in the growth plate cartilage. A feedback loop exists between PTHrP and Ihh which controls the pace of chondrocyte proliferation [14]. Acceleration of chondrocyte differentiation and premature ossification in the growth plate have been reported in PTH/PTHrP null mouse [15,16]. Chondrocyte proliferation declined and the area occupied by hypertrophic chondrocytes increased in targeted deletion of Ihh [14]. After 2 weeks of rapamycin, PTH/PTHrP which localized to the lower proliferating and upper hypertrophic chondrocytes declined by 30 percent compared to Control. In contrast, Ihh expression confined mostly to the hypertrophic chondrocytes increased approximately 2-fold after 2 weeks of rapamycin (Figure 3A). At the end of 4 weeks, PTH/PTHrP and Ihh expression were comparable to the Control group (Figure 3A and 3B). The current results suggest that the widening of the hypertrophic zone and decrease in the proliferative zone may be due in part to enhancement of Ihh and downregulation of PTH/PTHrP.
Figure 3.
The panels on the left show representative photomicrographs of PTH/PTHrP protein expression (3A) in the lower proliferating and upper hypertrophic chondrocytes, 50× (denoted by the purple color and arrows); ap < 0.004 Rapamycin (2 weeks) vs Rapamycin (4 weeks). Indian hedgehog protein expression in the lower proliferating and upper hypertrophic chondrocytes (3B, denoted by arrows and brown color), 50×; ap < 0.02 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.0001 Rapamycin (2 weeks) vs Rapamycin (4 weeks). The panels on the right show the quantification of the protein expression for PTH/PTHrP (upper panel); and Indian hedgehog (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
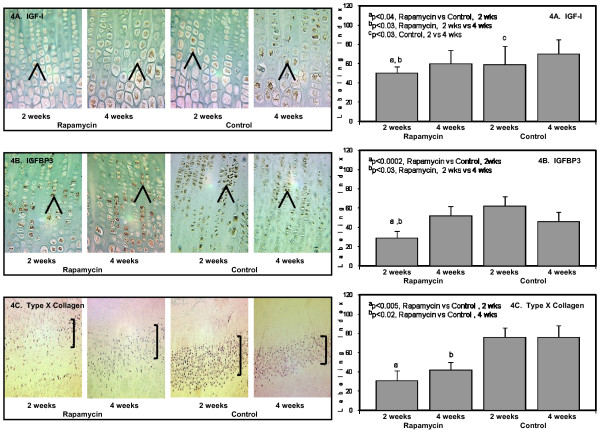
Other markers used in the study to assess chondrocyte maturation include: IGF-I protein, IGF-I binding protein-3 (IGFBP3), type × collagen and bone morphogenetic-7 (BMP-7).
The protein expression of IGF-I which was restricted to the hypertrophic chondrocytes decreased after 2 weeks of rapamycin compared to Control (Figure 4A). In agreement with other published studies, IGF-I staining was 20 percent lower in the 2 weeks Control animals compared to 4 weeks Control (Figure 4A). IGF-II and not IGF-I has been demonstrated to be more abundant in younger animals and that IGF-I may be associated with chondrocyte hypertrophy and mineralization [17]. The expression of IGF-II was not assessed in the current study.
Figure 4.
The panels on the left show representative photomicrographs of IGF-I protein expression (4A), 50× (denoted by the brown color and arrows); ap < 0.04 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.03 Rapamycin (2 weeks) vs Rapamycin (4 weeks); cp < 0.03 Control (2 weeks) vs Control (4 weeks). IGFBP3 protein expression (4B, denoted by arrows and brown color), 50×; ap < 0.002 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.03 Rapamycin (2 weeks) vs Rapamycin (4 weeks). Type × collagen (col10a) protein expression localized only to the hypertrophic chondrocytes (4C, denoted by purple color and brackets), 20×; ap < 0.005 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.02 Rapamycin (4 weeks) vs Control (4 weeks). The panels on the right show the quantification of the protein expression for IGF-I (upper panel); IGFBP3 (middle panel); and type × collagen (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
IGFBP3 protein expression was localized to the proliferating and upper hypertrophic chondrocytes in both 2 weeks and 4 weeks Rapamycin and Control groups (Figure 4B). Two weeks of rapamycin downregulated IGFBP3 by 53 percent compared to the Control group, and by 44 percent compared to the 4 weeks Rapamycin group (Figure 4b). The changes in IGFBP3 were similar to the changes in IGF-I protein expression.
Type × collagen (col10a) is a marker of chondrocyte maturation and solely localized to the hypertrophic chondrocytes. Although the width of the zone occupied by the hypertrophic chondrocytes increased with rapamycin, col10a expression declined 2-fold after 2 and 4 weeks of treatment compared to Control groups (Figure 4C).
It has been demonstrated that the proliferative actions of PTHrP may be mediated by downregulation of cyclin kinase inhibitors p57Kip2 and p27Kip1 [18]. In the current study, there was a 20 to 30 percent reduction in p57Kip2 staining in the hypertrophic chondrocytes of both Rapamycin groups (2 and 4 weeks) compared to Control accompanied by lower histone-4 expression (Figure 5A). There were no changes in p21Cip-1/SDI-1/WAF-1 expression in all groups (Figure 5B). The expression of bone morphogenetic protein-7 (BMP-7) and growth hormone receptor did not differ among groups (pictures not included).
Figure 5.
The panels on the left show representative photomicrographs of p57Kip2 protein expression in the hypertrophic chondrocytes (5A, denoted by arrows and brown color), 50×; ap < 0.05 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.05 Rapamycin (4 weeks) vs Control (4 weeks). p21Cip-1/SDI-1/WAF-1 protein expression localized only to the terminal hypertrophic chondrocytes (5B, denoted by arrows and brown color), 50×. The panels on the right show the quantification of the protein expression for p57Kip2 (upper panel); and p21Cip-1/SDI-1/WAF-1 (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
Vascular invasion and cartilage resorption are crucial steps in endochondral bone growth. Rapamycin did not affect the expression of gelatinase B or matrix metalloproteinase-9 (MMP-9) mRNA after 2 or 4 weeks compared to the Control groups, although the expression was relatively higher in the growth plate of younger animals (Figure 6A).
Figure 6.
The panels on the left show representative photomicrographs of gelatinase B/MMP-9 mRNA expression in the chondro-osseous junction (6A), denoted by the black color and arrows, 20×); ap < 0.02 Rapamycin (2 weeks) vs Rapamycin (4 weeks). RANKL protein expression in the hypertrophic chondrocytes (6B, denoted by arrows and brown color), 50×; ap < 0.008 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.002 Rapamycin (2 weeks) vs Rapamycin (4 weeks). OPG protein expression localized to the hypertrophic chondrocytes (6C, denoted by arrows and brown color), 50×. The panels on the right show the quantification of the protein expression for gelatinase B/MMP-9 (upper panel); RANKL (middle panel); and OPG (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
Receptor activator of nuclear factor-kappa β ligand (RANKL) and osteoprotegerin (OPG) participate in the regulation of osteo/chondroclastogenesis. We have previously demonstrated that RANKL and OPG expression were localized to the hypertrophic chondrocytes and the ratio between RANKL:OPG has been used to estimate the presence of osteo/chondroclast differentiation [19]. There was a 40 percent decrease in RANKL expression after 2 weeks of rapamycin compared to Control (Figure 6B); this change was not evident after 4 weeks of rapamycin (Figure 6B). Since OPG expression did not change in all groups (Figure 6C), the RANKL:OPG ratio was lower in the 2 week rapamycin group which may suggest decline in osteo/chondroclastogenesis (Figure 6C).
Vascular endothelial growth factor (VEGF) was demonstrated in the mature hypertrophic chondrocytes and the expression was 30 percent less after 2 and 4 weeks of rapamycin compared to control (Figure 7A). Histochemical staining for tartrate resistant acid phosphatase (TRAP) was considerably reduced in both rapamycin groups (Figure 7B).
Figure 7.
The panels on the left show representative photomicrographs of VEGF mRNA expression in the hypertrophic chondrocytes (7A), denoted by the black color and arrows, 50×; ap < 0.03 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.03 Rapamycin (4 weeks) vs Control (4 weeks). TRAP positive chondro/osteoclasts (7B), denoted by arrows and red color, 50×; ap < 0.01 Rapamycin (2 weeks) vs Control (2 weeks); bp < 0.03 Rapamycin (4 weeks) vs Control (4 weeks). The panels on the right show the quantification of the protein expression for VEGF (upper panel) and TRAP (lower panel) after 2 weeks and 4 weeks in Rapamycin and Control groups expressed as number of labeled cells to the total number of cells in the appropriate zone (Labeling Index).
Discussion
Rapamycin is a potent immunosuppressant which can inhibit endochondral bone growth in young rats. Our study suggests that rapamycin may decrease chondrocyte proliferation, alter maturation of hypertrophic chondrocytes, delay vascular invasion and reduce TRAP activity in the chondro-osseous junction of the growth plate cartilage.
Currently, there are no available studies that have evaluated the effects of rapamycin in young and growing children. The implications of our findings on linear growth need further evaluation in young children who are maintained on long-term immunosuppressant treatment with rapamycin. The rapamycin dose used in the current study was higher than the currently prescribed amount in pediatric patients, but similar doses were previously utilized in published animal studies [3,4].
The adverse effects of rapamycin on the growth plate were more evident in younger animals. It was expected that the smaller animals which were treated with 2 weeks of rapamycin will have smaller growth plate cartilage however, our findings demonstrated an increase rather than decrease in the total growth plate with widening of the layer occupied by hypertrophic chondrocytes. Although there was a significant increase in hypertrophic zone, the columnar architecture was preserved. The enlargement of the hypertrophic zone may be due in part, to a reduction in the number of proliferating chondrocytes, lower cartilage resorption in the chondro-osseous junction due to a decline in TRAP and there may be a delay in vascular invasion. Although the changes in the growth plate which were evident after 2 weeks improved at the end of 4 weeks of rapamycin, body length and tibial length measurements remained short. Longer follow-up needs to be done in future studies to assess whether catch-up growth will occur in the rapamycin treated animals.
The immunosuppressive effects of rapamycin are based on its ability to inhibit cell cycle progression from G1 to S phase and hinder DNA synthesis by restraining the phosphorylation of p70S6 kinase leading to inactivation of the mammalian target of rapamycin (mTOR) [20,21]. The mammalian target of rapamycin (mTOR) integrates signals from nutrition and growth factors to coordinate cell growth and cell proliferation [22]. Rapamycin can also decrease cyclin D and cyclin E protein expression including downstream effectors involved in cell cycle progression [23]. In the present study, chondrocyte proliferation assessed by histone-4 and mTOR expression was significantly decreased. Although the markers of chondrocyte proliferation improved in older rats treated with rapamycin, bone length remained short after 7 weeks of study period. These findings suggest that the inhibitory effects of rapamycin on chondrocyte proliferation may be more significant in young animals due to rapid growth which may be a concern during long-term rapamycin therapy in young pediatric patients. The reduction in histone-4 and mTOR was also accompanied by a decline in type II (col2a) collagen expression, another marker of chondrocyte proliferation and important in the extracellular matrix support of chondrocytes.
The present study showed a downregulation of PTH/PTHrP accompanied by enhancement of Ihh after 2 weeks of rapamycin; such changes were not significant at the end of 4 weeks. The PTH/PTHrP and Indian hedgehog (Ihh) feedback loop plays an important role in chondrocyte proliferation and differentiation. The increase in the zone occupied by the hypertrophic chondrocytes may be a combination of the decline in PTH/PTHrP and upregulation of Ihh expression. Our current findings show that the downregulation of PTH/PTHrP during rapamycin therapy was not due to the enhancement of cyclin kinase inhibitor p57Kip2.
Chondrocyte proliferation, chondrocyte maturation and apoptosis of the terminal hypertrophic chondrocytes must be precisely coordinated and any delay in each stage can lead to shorter bone growth as shown in the current experiment. Markers of chondrocyte differentiation that were evaluated in the current paper including IGF-I and IGF binding protein-3 were downregulated after 2 weeks but improved at the end of 4 weeks. Only type × collagen (col10) and p57Kip2 expression remained low after 4 weeks of rapamycin treatment. Type × collagen has been demonstrated to play an essential role in the initiation of matrix mineralization in the chondro-osseous junction and in the maintenance of progenitor cells for osteo/chondrogenesis and hematopoiesis [24]. The alterations in proliferation and differentiation of chondrocytes in the growth plate during rapamycin therapy may delay mineralization and vascularization in the appendicular skeleton and consequently, may affect the production of bone marrow progenitor cells. These findings will require further evaluation.
Alvarez and colleagues have demonstrated that 14 days of intraperitoneal rapamycin led to smaller tibial bones associated with decreased body weight and lower food efficiency ratio [4]. Our findings agree with previous reports and may suggest that during rapamycin treatment, animals may require higher amount of calories per day in order to grow. Since mTOR is an important modulator of insulin-mediated glucose metabolism, rapamycin may exert adverse effects on the absorption of nutrients [25]. When given orally as in the current study, rapamycin may lower intestinal absorption of glucose, amino acids and linoleic acids by decreasing the area of the absorptive intestinal mucosa [10].
Rapamycin has been studied as an effective treatment for cancer not only due to its anti-proliferative actions but for its anti-angiogenic properties [26]. Our current findings showed a significant downregulation of vascular endothelial growth factor expression in the hypertrophic chondrocytes of animals treated with rapamycin. Our findings are in agreement with previous reports by Alvarez-Garcia and coworkers [4]. Although there were no changes in gelatinase B/MMP-9 mRNA expression in the chondro-osseous junction, there was a considerable reduction in the number of TRAP positive chondro/osteoclasts suggesting that cartilage resorption may be altered by rapamycin. The delay in cartilage resorption and changes in chondro/osteoclast function may be due to the reduction in RANKL expression as shown in the present experiment and by other investigators [6]. There were no changes in osteoprotegerin (OPG) staining so RANKL/OPG ratio was lower compared to Control. The decrease in RANKL/OPG ratio may reflect a decrease in chondro/osteoclast recruitment and differentiation.
Conclusion
Rapamycin is a novel and powerful immunosuppressant widely used in pediatric renal transplant recipients to maintain the allograft. We have shown in the current study that rapamycin can inhibit endochondral bone growth in a rapidly growing young animal. The shorter bone growth may be due in part, to the decline in chondrocyte proliferation, enhancement of chondrocyte maturation, and alterations in cartilage resorption and vascularization. Our findings have also demonstrated that the 2 week effects of rapamycin on chondrocyte proliferation, chondrocyte maturation and vascular invasion may improve to near normal if rapamycin is administered continuously as the animal matures although, no catch-up growth was demonstrated. The results in the current study may be limited by the semi-quantitative results obtained using in-situ and immunohistochemistry methods, so future experiments should be done using quantitative proteomic and genomic techniques. In addition, clinical studies are needed to assess whether long-term therapy with rapamycin can affect linear growth in young pediatric patients.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CPS conceived the experimental design, drafted the manuscript, analyzed the results and performed the histological and morphometric measurements of the growth plate. YHZ participated in the study design, interpretation of the results and performed most of the in-situ and immunohistochemistry experiments. The authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This paper was supported in part by the Southern Wisconsin National Kidney Foundation Affiliate Grant, NIH/NIDDK Grant No. DK-56688-01 and by the research funds from the Department of Pediatrics, University of Wisconsin School of Medicine & Public Health, Madison, Wisconsin, USA.
Contributor Information
Cheryl P Sanchez, Email: cpsanchez@pediatrics.wisc.edu.
Yu-Zhu He, Email: yhe@wisc.edu.
References
- El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–5177. [PubMed] [Google Scholar]
- Manegold PC, Paringer C, Kulka U, Krimmel K, Eichhorn ME, Wilkowski R, Jauch K-W, Guba M, Bruns CJ. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (Everolimus) increases radiosensitivity in solid cancer. Clin Cancer Res. 2008;14:892–900. doi: 10.1158/1078-0432.CCR-07-0955. [DOI] [PubMed] [Google Scholar]
- Romero DF, Buchinsky FJ, Rucinski B, Cvetkovic M, Bryer HP, Liang XG, Ma YF, Jee WSS, Epstein S. Rapamycin: a bone sparing immunosuppressant? J Bone Miner Res. 1995;10:760–768. doi: 10.1002/jbmr.5650100513. [DOI] [PubMed] [Google Scholar]
- Alvarez-Garcia O, Carbajo-Perez E, Garcia E, Gil H, Molinos I, Rodriguez J, Ordonez F, Santos F. Rapamycin retards growth and causes marked alterations in the growth plate of young rats. Pediatr Nephrol. 2007;22:954–961. doi: 10.1007/s00467-007-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe I, Katz I, Sehgal S, Bex F, Kharode Y, Tamasi J, Epstein S. Lack of change of cancellous bone volume with short-term use of the new immunosuppressant rapamycin in rats. Calcif Tis Int. 1993;53:45–52. doi: 10.1007/BF01352014. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Fisher J, Wesolowski G, Rodan GA, Reszka A. M-CSF, TNF-α and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- Singha U, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103:434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Tokuda M, Tomizawa K, Matsui H, Itano T, Konishi R, Nagahata S, Hatase O. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun. 1998;249:226–230. doi: 10.1006/bbrc.1998.9118. [DOI] [PubMed] [Google Scholar]
- Sanchez CP, Salusky IB, Kuizon BD, Abdella P, Jüppner H, Gales B, Goodman WG. Growth of long bones in renal failure: roles of hyperparathyroidism, growth hormone and calcitriol. Kidney Int. 1998;54:1879–1887. doi: 10.1046/j.1523-1755.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- Dias V, Madsen K, Mulder K, Keelan M, Yatscoff R, Thomson A. Oral administration of rapamycin and cyclosporine differentially alter intestinal function in rabbits. Dig Dis Sci. 1998;43:2227–2236. doi: 10.1023/A:1026610404647. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- Sanchez CP, He YZ. Alterations in the growth plate cartilage of rats with renal failure receiving corticosteroid therapy. Bone. 2002;30:692–698. doi: 10.1016/S8756-3282(02)00696-8. [DOI] [PubMed] [Google Scholar]
- Sanchez C, He Y, Leiferman E, Wilsman N. Bone elongation in rats with renal failure and mild or advanced secondary hyperparathyroidism. Kidney Int. 2004;65:1740–1748. doi: 10.1111/j.1523-1755.2004.00577.x. [DOI] [PubMed] [Google Scholar]
- Karp S, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon A. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biology. 1994;126:1611–1623. doi: 10.1083/jcb.126.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VLJ, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Development. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Shinar DM, Endo N, Halperin D, Rodan GA, Weinreb M. Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology. 1993;132:1158–1167. doi: 10.1210/en.132.3.1158. [DOI] [PubMed] [Google Scholar]
- MacLean HE, Guo J, Knight MC, Zhang P, Cobrinik D, Kronenberg HM. The cyclin-dependent kinase inhibitor p57Kip2 mediates proliferative actions of PTHrP in chondrocytes. J Clin Invest. 2004;113:1334–1343. doi: 10.1172/JCI21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, He YZ. Bone growth during daily or intermittent calcitriol treatment during renal failure with advanced secondary hyperparathyroidism. Kidney Int. 2007;72:582–591. doi: 10.1038/sj.ki.5002375. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270:27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- Jacenko O, Roberts DW, Campbell MR, McManus PM, Gress CJ, Tao Z. Linking hematopoiesis to endochondral skeletogenesis through analysis of mice transgenic for collagen X. Am J Pathol. 2002;160:2019–2034. doi: 10.1016/S0002-9440(10)61152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao Q, Ma S, Yang F, Gong Y, Ke C. Sirolimus inhibits human pancreatic carcinoma cell proliferation by a mechanism linked to the targeting of mTOR/HIF-1 α/VEGF signaling. IUBMB Life. 2007;59:717–721. doi: 10.1080/15216540701646484. [DOI] [PubMed] [Google Scholar]