Abstract
Background
We explored the heterogeneity of philadelphia chromosome-positive acute lymphoblastic leukemia (Ph1-ALL) in a study of the effect of early features on prognosis in children. Here we report the long-term results of the FRALLE 93 study conducted in the era before the use of tyrosine kinase inhibitors.
Methods
Between 1993 and 1999, 36 children with Ph1-ALL were enrolled into the FRALLE 93 protocol. After conventional four-drug induction, children were stratified by availability of an HLA-matched sibling.
Results
Complete remission (CR) was observed in 26 children (72%), of which 13 underwent allogeneic bone marrow transplantation (BMT). Thirty-one children were good responders to prednisone, defined on day 8, and 21 were good responders to chemotherapy, defined by day-21 bone marrow (M1). Overall five-year disease-free survival (DFS) was 42 ± 9.7%. Based on multivariate analysis, two groups showed marked differences in five-year outcome: children with age<10, leukocyte count <100,000/mm3 and day-21 M1 marrow had a more favorable prognosis (14 pts: 100% CR, event free survival [EFS]: 57%, overall survival [OS]: 79%), than the high-risk group (22 patients: 55% CR, EFS: 18%, OS: 27%) (p < 0.005). We also observed a non statistically significant difference (p = 0.14) in outcome between these groups for transplanted patients (5-year DFS: 83 ± 14% and 33 ± 15%, respectively).
Conclusion
Age, leukocyte count and early response to treatment defined by the D21 bone marrow response provide an accurate model for outcome prediction. The combination of available tools such as minimal residual disease assessment with determination of these simple factors could be useful for refining indications for BMT in the current era of tyrosine-kinase inhibitor-based therapy.
Background
The philadelphia chromosome (Ph1) is detectable in 2% to 5% of children with acute lymphoblastic leukemia (ALL) [1,2]. The detection of a philadelphia chromosome remains a major prognostic factor of induction failure. Despite the steady improvement in the management of ALL in children, Ph1-ALL is associated with high rates of relapse or resistance to treatment [3-5]. This disease is heterogeneous in terms of clinical parameters such as leukocyte count, age at diagnosis, and initial steroid response [4,6]. A slow early response to conventional therapy has also been reported as indicative of a poor prognosis [2].
Recent gene expression studies have identified a heterogeneous pattern of expression associated with BCR-ABL status, which may be useful for developing novel prognostic markers and future patient stratification procedures [7-9]. New therapeutic agents such as tyrosine kinase inhibitors (imatinib and dasatinib) have been developed and yield good results in adults with Ph1-ALL [10-12]. Little information on the use of these drugs in children has been reported; the identification of predictors of responsiveness to early conventional treatment may thus be beneficial for the accurate stratification of children and for improving outcome [13,14].
We studied the impact of the National Cancer Institute (NCI) risk factors and steroid and early chemotherapy responses in 36 children with untreated Ph1-ALL enrolled in the FRALLE 93 trial between 1993 and 1999.
Methods
The FRALLE 93 trial was open to children aged 0 to 20 years with untreated ALL, not including those with L3 ALL or Down's syndrome. Between June 1, 1993, and December 31, 1999, 1395 children were enrolled onto the FRALLE 93 trial in 18 French pediatric centers and one Belgian pediatric center. This study was approved by the ethics committee of the hôpital Saint Louis, France (accepted April 29, 1993). All patients, or their parents, provided informed consent in accordance with the Declaration of Helsinki. The diagnosis of ALL was based on morphological, immunophenotypic and cytogenetic analyses of bone marrow samples. From 1994, children were systematically screened for four fusion transcripts (TEL-AML1, BCR-ABL, E2A-PBX1, MLL-AF4).
Stratification and treatment
Patients carrying t(9,22) or BCR-ABL were assigned to the very high risk group of the FRALLE 93 trial (Table 1) [5]. Patients received initial treatment comprising a prednisone prophase and a triple-drug intrathecal injection. Induction treatment then included prednisone, vincristine, L-asparaginase, a 120 mg/m2 cumulative dose of daunorubicin (increased to 160 mg/m2 after July 1996) and two more triple-drug intrathecal injections. Treatment was then stratified according to availability of an HLA-matched sibling. Children with an HLA-matched sibling received alternating courses of R3 (Cytarabine, Etoposide, Dexamethasone) and COPADM (Vincristine, Methotrexate, Doxorubicin, Cyclophosphamide, Prednisone) therapy (for a total of 3 courses of treatment) before an allogeneic bone marrow transplantation (Table 1). The remaining children with no sibling donors were eligible for either autologous transplantation after six courses of treatment (with graft harvesting carried out after the fifth course of chemotherapy) or non genoidentical allogeneic transplantation.
Table 1.
FRALLE 93 protocol schedule for very high risk patients
| Phase | Treatment | Dose |
|---|---|---|
| Induction | Vincristine | 1.5 mg/m2 IV (max, 2 mg) on D8, D15, D22, D29 |
| Prednisone | 60 mg/m2/d PO on D1–8, 40 mg/m2/d on D8–28 | |
| Daunorubicin§ | 40 mg/m2 IV on D8, D9, D10, D15 | |
| L-Asparaginase§ | 10,000 U/m2 IM or IV on D20, D22, D24, D26, D29, D31, D33, D35 | |
| intrathecal therapy* | before D4, D8, and D15 | |
| R3 course | Cytarabine | 2 g/m2 IV bid on D1–D2 |
| Etoposide | 150 mg/m2 IV on D3, D4, D5 | |
| Dexamethasone | 20 mg/m2 PO on D2–D5 | |
| intrathecal therapy* | D5 | |
| COPADM course | Vincristine | 1.5 mg/m2 (max, 2 mg) IV on D1 |
| Methotrexate | 8000 mg/m2 (24-hour IV infusion with leucovorin rescue) on D1 | |
| Doxorubicin | 60 mg/m2 IV on D2 | |
| Cyclophosphamide | 375 mg/m2 bid on D2–D3 | |
| Prednisone | 60 mg/m2 PO on D1–D5 | |
| Stem-cell transplantation | age >4 years: TBI | 2 Gy bid on D-9 to D-7 |
| age <4 years: Busulfan | 30 mg/m2/6 hours PO on D-10 to D-7 | |
| All patients: Cytarabine | 3 g/m2 bid on D-5 to D-4 | |
| Melphalan | 140 mg/m2IV on D-2 | |
| Transplantation | IV day 0 | |
| Maintenance after autologous transplantation | 6-mercaptopurine Vincristine | 75 mg/m2/d 2 years after complete remission 1.5 mg/m2 monthly for 12 months |
Abbreviations: PO, per os; IV, intravenous; IM, intramuscular; max, maximum; TBI, total body irradiation
§before July 1996: daunorubicin on D8, D15, and D22 and L-Asparaginase on D22, D24, D26, D29, D31, D33.
*Methotrexate, Cytarabine, and Depomedrol in doses based on patient age
Other post-induction regimen: no R3 or COPADM but 1 consolidation, 2 intensifications, and an interim phase over 36 weeks before 18 months of maintenance therapy
Treatment outcome was analyzed according to indicators of early response to therapy. The prognostic value of persistent lymphoblasts in blood sampled at day 8 and in bone marrow at day 21–22 has been demonstrated in several previous studies [15-17]. We also recently showed that the persistence of lymphoblasts in bone marrow on day 21 of four-drug induction was associated with a higher risk of induction failure [5]. A good prednisone response was defined as a blast count <1000/μl blood after the first seven days on prednisone therapy (i.e. on day 8) and one triple-drug intrathecal injection. A poor prednisone response was defined by a blast count ≥ 1000/μl on day 8. A good early response to chemotherapy was defined by a blast count fewer than 5% in bone marrow smears on day 21 (M1) and a poor early chemotherapy response by a blast count equal to or more than 5% (with two categories: M2 = 5% to 25% and M3 = more than 25%). Complete remission (CR) was defined by no physical evidence of disease, no detectable leukemic blasts on blood smears and less than 5% blasts on bone marrow smears, active hematopoiesis and normal cerebrospinal fluid.
Statistical analysis
Analysis was based on an intent-to-treat principle. CR rates were compared using Fisher's exact test. Censored endpoints were estimated by the non parametric Kaplan-Meier method, and then compared by the log-rank test. Multivariate analyses were carried out to define the set of informative prognostic factors, using regression models adapted to the endpoint, namely the logistic model for CR rates, and Cox model for overall survival (OS) and event free survival (EFS).
Type I error was fixed at 5%. All tests were two-tailed. Statistical analysis was performed using SAS 9.1 (SAS, Inc, Cary, NC).
Results
Ph1 was detected by conventional cytogenetic analysis (t(9;22) (q34;q11)) or a BCR-ABL rearrangement was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in 3% of the B-lineage ALL. Of the 36 Ph1-ALL children, 30 had detectable t(9;22) and six were identified solely by the presence of the BCR-ABL fusion transcript. Thus, fusion transcript analysis was able to detect nearly 17% more Ph1 patients than conventional karyotype. The m-BCR breakpoint was detected in 30 patients and the M-BCR breakpoint in three others (3 patients were identified solely by the detection of t(9;22)). At baseline, median age was eight years [range: 0.70–19.5; Q1–Q3: 4.35 to 12.35], male-to-female ratio was 1.1, median leukocyte count was 29.1 × 109/l [Q1–Q3: 12.5–137] and median hemoglobin was 9 g/dl [2.5–15]; four patients displayed central nervous system defects (11.1%).
Complete remission was observed in 26 children (72%), after a median of 40 days [range 32–51] consistent with previous findings [2,4]. We did not find any differences between the effects of including patients at two different time periods (before or after July 1996) and the addition of one dose of daunorubicin and two doses of asparaginase (but the numbers of children within each group are small) (Table 2).
Table 2.
Outcome of the 36 children with philadelphia chromosome-positive acute lymphoblastic leukemia as a function of early characteristics
| No patients | No CR (%) | p | 5 yr-EFS | p | 5 yr-OS | p | |
|---|---|---|---|---|---|---|---|
| Whole cohort | N = 36 | 26 (72%) | 33.3 ± 7.9 | 47.2 ± 8.3 | |||
| Inclusion period | |||||||
| < July, 1996 | 15 | 9 (60%) | 20 ± 10 | 33 ± 12 | |||
| > July, 1996 | 21 | 17 (81%) | 0.26 | 43 ± 11 | 0.13 | 57 ± 11 | 0.31 |
| Age (years) | |||||||
| <10 | 23 | 20 (87%) | 48 ± 10 | 61 ± 10 | |||
| ≥ 10 | 13 | 6 (46%) | 0.018 | 15 ± 10 | 0.01 | 23 ± 12 | 0.006 |
| WBC (/mm3) | |||||||
| <50,000 | 21 | 17 (81%) | 33 ± 10 | ||||
| ≥ 50,000 | 15 | 9 (60%) | 0.26 | 33 ± 12 | 0.54 | 0.91 | |
| <100,000 | 26 | 22 (85%) | 42 ± 10 | 58 ± 10 | |||
| ≥ 100,000 | 10 | 4 (40%) | 0.014 | 10 ± 9 | 0.003 | 20 ± 13 | 0.03 |
| Response to steroid (+3 drug intra-thecal injection) at D8 | |||||||
| poor (≥ 1000 blasts/mm3) | 5 | 2 (40%) | 20 ± 18 | 20 ± 18 | |||
| good(<1000 blasts/mm3) | 31 | 24 (77%) | 0.12 | 35 ± 9 | 0.33 | 52 ± 9 | 0.19 |
| Response to chemotherapy evaluated on D21 | |||||||
| M1(≤ 5% blasts) | 21 | 20 (95%) | 43 ± 11 | 62 ± 11 | |||
| M2+M3 (>5%–25% and >25% blasts) | 15 | 6 (40%) | 0.0004 | 20 ± 10 | 0.18 | 27 ± 11 | 0.06 |
| Age, D21 response and WBC | |||||||
| Age<10 and D21 M1 and WBC count<100,000 | 14 | 14 (100%) | 57 ± 13 | 79 ± 11 | |||
| Others | 22 | 12 (55%) | 0.003 | 18 ± 8 | 0.002 | 27 ± 9 | 0.003 |
WBC = white blood cell; D = day; Response to steroid is evaluated in peripheral blood on day 8; Response to chemotherapy is evaluated in bone marrow on day 21
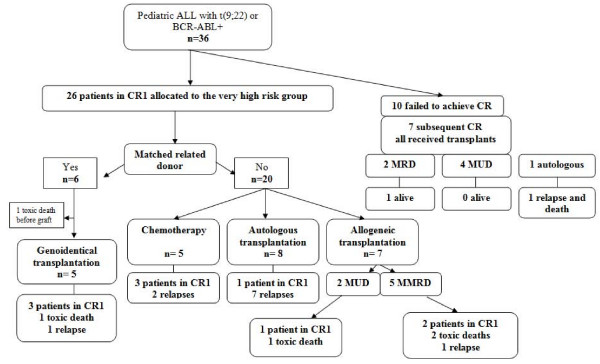
Ten patients did not achieve CR after induction therapy, but CR was observed in seven of them after one further course of chemotherapy. The dexamethasone, cytarabine, cyclophosphamide, etoposide, and idarubicin or daunorubicin (CAZED) scheme was recommended as salvage treatment in the FRALLE 93 protocol and thus was used in five of these seven patients (amsacrine and cytarabine for the 2 others) [5]. All seven children were then transplanted (2 matched related transplants, 4 matched unrelated transplants and one autologous transplantation); outcomes are described in Figure 1. Of the other children with CR observed after induction, five of the six children with an HLA-matched sibling (MRD) received alternate courses of R3 and COPADM treatment before allogeneic bone marrow transplantation (one toxic death occurred before graft). Eight children received an autologous transplant, five a mismatched related transplant and two others a matched unrelated transplant (MUD) (Figure 1). Five other CR patients received a different post-induction regimen with intensified chemotherapy, including one consolidation, a double delayed intensification, and an interim phase over 36 weeks before 18 months of maintenance therapy.
Figure 1.
flowchart of the FRALLE 93 trial for philadelphia chromosome-positive ALL. Children with ALL were allocated to very high risk group as soon as a t(9;22) or BCR-ABL rearrangement was detected. Post-induction treatment was then stratified according to availability of an HLA-matched sibling. CR1 = first complete remission; MRD = matched related donor; MUD = matched unrelated donor; MMRD = mismatched related donor.
Patient follow-up data were updated in February, 2007. In an intention-to-treat analysis, 10 children remained in CR1, giving a five-year disease-free survival (DFS) rate of 42.3% ± 9.7% and a five-year OS rate of 47.2% ± 8.3. Leukemic relapse was the most common cause of adverse events. Eleven children relapsed (no late relapse after 5 years) and five toxic deaths occurred (including one late death from infection). No secondary malignancy was observed.
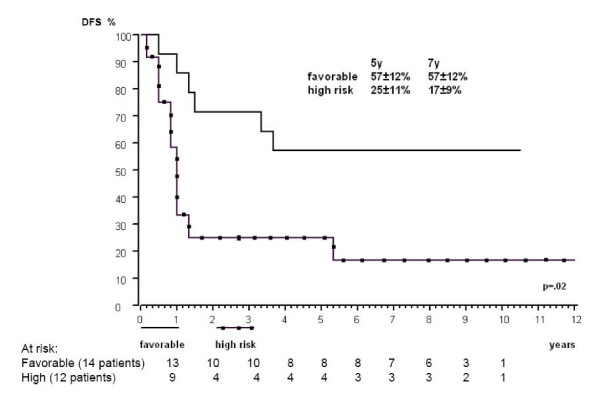
Table 2 summarizes the main endpoints – CR, EFS and survival – overall and as a function of the main patient characteristics. Based on univariate analyses using a 5% significance level for all endpoints, we identified two predictors of poor outcome: age ≥ 10 and white blood cells (WBC) count >100,000/mm3. Response to chemotherapy based on day 21 bone marrow was only predictive of induction response (p = 0.0004). When incorporated simultaneously in multivariate regression models, only one predictive factor retained significant prognostic value for a particular endpoint: M1 for CR, WBC count >100,000/mm3 for EFS and age ≥ 10 for OS. Combining the information of these three binary variables defined two groups differing widely in terms of outcome: the 14 children with age<10, WBC<100,000/mm3 and M1 defined a group with a favorable prognosis (100% CR, 5-yr EFS: 57%, 5-yr OS: 79%), whereas the remaining 22 children had poorer outcomes (55% CR, 5-yr EFS: 18%, 5-yr OS: 27%) (p = 0.003, 0.002, and 0.003, respectively) (Figure 2). These prognostic factors remain important in the transplanted patients (equally divided in both groups), even if significance is not reached due to the small number of patients (5-yr DFS: 83 ± 14% for patients with age<10, WBC<100,000/mm3 and M1 and 33 ± 15% for the remaining patients) (p = 0.14).
Figure 2.
Kaplan-Meier five- and seven-year DFS analysis based on risk group (n = 26). The multivariable regression model revealed the independent prognostic value of age<10 years, M1 bone marrow and WBC count<100,000/mm3, defining the group with favorable outcome.
Discussion and conclusion
This long-term study, carried out before the introduction of imatinib mesylate, confirmed the prognostic value of two major clinical factors (age, WBC count at diagnosis) and identified the D21 marrow response as a powerful complementary tool. We did not find prednisone response to have statistically significant predictive value for any endpoint studied, in contrast with earlier reports [4,6]. This could be explained by the small sample size, and the small number of poor prednisone responders (5 of 36; 14%). However, in our protocol the combination of a three-drug intrathecal injection (methotrexate, cytarabin and depomedrol) on day 1 with the 7 day prednisone prophase prevents the comparison of our steroid-resistance data to data in other studies using intrathecal methotrexate.
Based on these three easily available indicators (age, WBC at diagnosis and D21 response to chemotherapy), a predictive model can be built to define two subsets of children that differ widely in terms of outcome. Such a model should be further investigated in larger samples and in ongoing pediatric trials integrating tyrosine-kinase inhibitors. The improved early responses observed with imatinib or dasatinib in adult studies, and similar, but very preliminarily, results obtained in one pediatric study with imatinib, now question the appropriate use of allogeneic stem-cell transplantation [14,18]. Indeed, the COG AALL0031 study showed that continuous administration of imatinib given in combination with intensive chemotherapy backbone resulted in a significant improvement in early EFS. More specifically cohort 5, who received 340 mg/m2 of imatinib for 280 days with chemotherapy only, had a similar two years EFS as compared to the cohort of patients who underwent stem cell transplantation (32 patients) either according to the protocol (MRD-21 patients) or from MUD (11 patients)[14]. The combination of available tools, including minimal residual disease assessment, with these easily measured predictive features could be useful for refining the indications for bone marrow transplantation [19-22]. Thus, if the long term follow-up of the AALL0031 non randomized study was confirmed, good risk patients could be spared by transplantation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All the authors have substantially contributed to the conception and design or acquisition of data or analysis and interpretation of the data in this multicentric study, and participated to drafting and revising the article. SC and MFA performed statistical analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Virginie Gandemer, Email: virginie.gandemer@chu-rennes.fr.
Marie-Francoise Auclerc, Email: marie-francoise.auclerc@paris7.jussieu.fr.
Yves Perel, Email: yves.perel@chu-bordeaux.fr.
Jean-Pierre Vannier, Email: Jean-Pierre.Vannier@chu-rouen.fr.
Edouard Le Gall, Email: edouard.le.gall@chu-rennes.fr.
Francois Demeocq, Email: fdemeocq@chu-clermontferrand.fr.
Claudine Schmitt, Email: c.schmitt@chu-nancy.fr.
Christophe Piguet, Email: piguet@unilim.fr.
Jean-Louis Stephan, Email: j.louis.stephan@chu-st-etienne.fr.
Odile Lejars, Email: odile.lejars@chu-tours.fr.
Marianne Debre, Email: marianne.debre@nck.ap-hop-paris.fr.
Philippe Jonveaux, Email: p.jonveaux@chu-nancy.fr.
Jean-Michel Cayuela, Email: jean-michel.cayuela@sls.ap-hop-paris.fr.
Sylvie Chevret, Email: sylvie.chevret@paris7.jussieu.fr.
Guy Leverger, Email: guy.leverger@trs.aphp.fr.
Andre Baruchel, Email: andre.baruchel@sls.aphp.fr.
the FRALLE group, Email: andre.baruchel@sls.aphp.fr.
Acknowledgements
Supported by: Délégation à la Recherche Clinique (Assistance Publique-Hôpitaux de Paris), a public non profit organization.
Other members of the FRALLE 93 group:
France
Amiens, B Pautard
Bayonne, JF Bauduer
Brest, JF Abgrall, C Berthou
Clermont-Ferrand, C Paillard, J Kanold
Dijon, G Couillault
Le Mans, M Damay
Limoges, L de Lumley
Marseille, G Michel, I Thuret, H Chambost
Nancy, P Bordigoni, D Sommelet
Paris Saint Louis, T Leblanc, G Schaison
Paris Trousseau, MD Tabone, J Donadieu, J Landman-Parker, A Auvrignon
Paris Necker, C Thomas, A Fisher
Paris Bicêtre, JP Dommergues, B Bader-Meunier
Paris Créteil, F Bernaudin, S Lemerle
Pau, J Choulot, V Doireau
Rennes, C Edan, C Bergeron
Rouen, P Schneider
Tours, JP Lamagnere
Saint Etienne, C Berger
Belgium: Brussels, G Cornu, C Vermylen
References
- Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig W-D, Henze G, Gadner H, Odenwald E, Riehm H. Long term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- Roy A, Bradburn M, Moorman A, Burrett J. Early response to induction is predictive of survival in chilhood philadelphia chromosome positive acute lymphoblastic leukemia: results of the Medical Research Council ALL 97 trial. Br J Haematol. 2005;129:35–44. doi: 10.1111/j.1365-2141.2005.05425.x. [DOI] [PubMed] [Google Scholar]
- Schultz K, Pullen D, Sather H, Shuster J, Devidas M, Borowitz M, Carroll A, Heerema N, Rubnitz J, Loh M, Raetz E, Winick N, Hunger S, Carroll W, Gaynon P, Camitta B. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico M, Valsecchi M, Camitta B, Schrappe M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G, Kamps W, Pui C-H, Masera G. Outcome of treatment in children with philadelphia chromosome positive childhood acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- Oudot C, Auclerc MF, Levy M, Porcher R, Piguet C, Perel Y, Gandemer V, Debre M, Vermylen C, Pautard B, Berger C, Schmitt C, Leblanc T, Cayuela JM, Socie G, Michel G, Leverger G, Baruchel A. Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: the FRALLE 93 study. J Clin Oncol. 2008;26(9):1496–1503. doi: 10.1200/JCO.2007.12.2820. [DOI] [PubMed] [Google Scholar]
- Ribeiro RC, Broniscer A, Rivera GK, Hancock ML, Raimondi SC, Sandlund JT, Evans WE, Pui C-H. Philadelphia chromosome positive acute lymphoblastic leukemia in children: durable responses to chemotherapy associated with low initial white blood cell counts. Leukemia. 1997;11:1493–1496. doi: 10.1038/sj.leu.2400749. [DOI] [PubMed] [Google Scholar]
- Fine B, Stanulla M, Schrappe M, Ho M, Viehmann S, Harbott J, Boxer L. Gene expression patterns associated with recurrent chromosomal translocations in acute lymphoblastic leukemia. Blood. 2004;103:1043–1049. doi: 10.1182/blood-2003-05-1518. [DOI] [PubMed] [Google Scholar]
- Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F, Foà R, Ritz J. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res. 2005;11(20):7209–19. doi: 10.1158/1078-0432.CCR-04-2165. [DOI] [PubMed] [Google Scholar]
- Juric D, Lacayo NJ, Ramsey MC, Racevskis J, Wiernik PH, Rowe JM, Goldstone AH, O'Dwyer PJ, Paietta E, Sikic BI. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25(11):1341–9. doi: 10.1200/JCO.2006.09.3534. [DOI] [PubMed] [Google Scholar]
- de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, Réa D, Cayuela JM, Vekemans MC, Reman O, Buzyn A, Pigneux Ar, Escoffre M, Chalandon Y, MacIntyre E, Lhéritier V, Vernant JP, Thomas X, Ifrah N, Dombret H. Imatinib combined with induction or consolidation chemotherapy in patients with de novo philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–13. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, Kobayashi T, Ueda Y, Takeuchi M, Miyawaki S, Maruta A, Emi N, Miyazaki Y, Ohtake S, Jinnai I, Matsuo K, Naoe T, Ohno R. Japan Adult Leukemia Study Group. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–6. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- Wassman B, Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M, Bornhäuser M, Reichle A, Perz J, Haas R, Ganser A, Schmid M, Kanz L, Lenz G, Kaufmann M, Binckebanck A, Brück P, Reutzel R, Gschaidmeier H, Schwartz S, Hoelzer D, Ottmann OG. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108(5):1469–77. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- Fuster JL, Bermudez M, Galera A, Llinares ME, Calle D, Ortuño FJ. Imatinib mesylate in combination with chemotherapy in four children with de novo and advanced stage philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2007;92:1723–1724. doi: 10.3324/haematol.11525. [DOI] [PubMed] [Google Scholar]
- Schultz KR Bowman WP Slayton W Aledo A Devida M Sather H Borowitz MJ Davies SM Trigg M Pasut B Jorstad D Eslinger T Burden LE Wang C Rutledge R Gaynon PS Carroll AJ Heerema NA Winick N Hunger S Carroll WL Camitta B Improve early event free survival in children with philadelphia chromosome-positive acute lymphoblastic leukemia with intensive imatinib in combination with high dose chemotherapy: children's oncology group study AALL0031 Blood 200711017429008 [Google Scholar]
- Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995;86(4):1292–5. [PubMed] [Google Scholar]
- Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84(9):3122–33. [PubMed] [Google Scholar]
- Sandlund JT, Harrison PL, Rivera G, Behm FG, Head D, Boyett J, Rubnitz JE, Gajjar A, Raimondi S, Ribeiro R, Hudson M, Relling M, Evans W, Pui CH. Persistence of lymphoblasts in bone marrow on day 15 and days 22 to 25 of remission induction predicts a dismal treatment outcome in children with acute lymphoblastic leukemia. Blood. 2002;100(1):43–7. doi: 10.1182/blood.V100.1.43. [DOI] [PubMed] [Google Scholar]
- Sharathkumar A, Saunders EF, Dror Y, Greenberg M, Weitzman S, Chan H, Calderwood S, Freedman MH, Doyle J. Allogeneic bone marrow transplantation vs chemotherapy for children with philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2004;33:39–45. doi: 10.1038/sj.bmt.1704319. [DOI] [PubMed] [Google Scholar]
- Scheuring UJ, Pfeifer H, Wassmann B, Bruck P, Gehrke B, Petershofen EK, Gschaidmeier H, Hoelzer D, Ottmann O. Minimal residual disease analysis as a predictor of response duration in philadelphia chromosome-positive acute lymphoblastic leukemia during imatinib treatment. Leukemia. 2003;17:1700–1706. doi: 10.1038/sj.leu.2403062. [DOI] [PubMed] [Google Scholar]
- Pane F, Cimino G, Izzo B, Camera A, Vitale A, Quintarelli C, Picardi M, Specchia G, Mancini M, Cuneo A, Mecucci C, Martinelli G, Saglio G, Rotoli B, Mandelli F, Salvatore F, Foà R. GIMEMA group. Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2005;19(4):628–35. doi: 10.1038/sj.leu.2403683. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Carpenter PA, Snyder DS, Flowers ME, Sanders JE, Gooley TA, Martin PJ, Appelbaum FR, Radich JP. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791–2793. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in philadelphia-positive acute lymphoblastic leukaemia. Bone Marrow Transplant. 2008;41(5):447–53. doi: 10.1038/sj.bmt.1705904. [DOI] [PubMed] [Google Scholar]