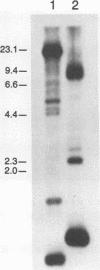
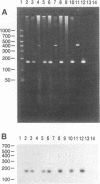
Abstract
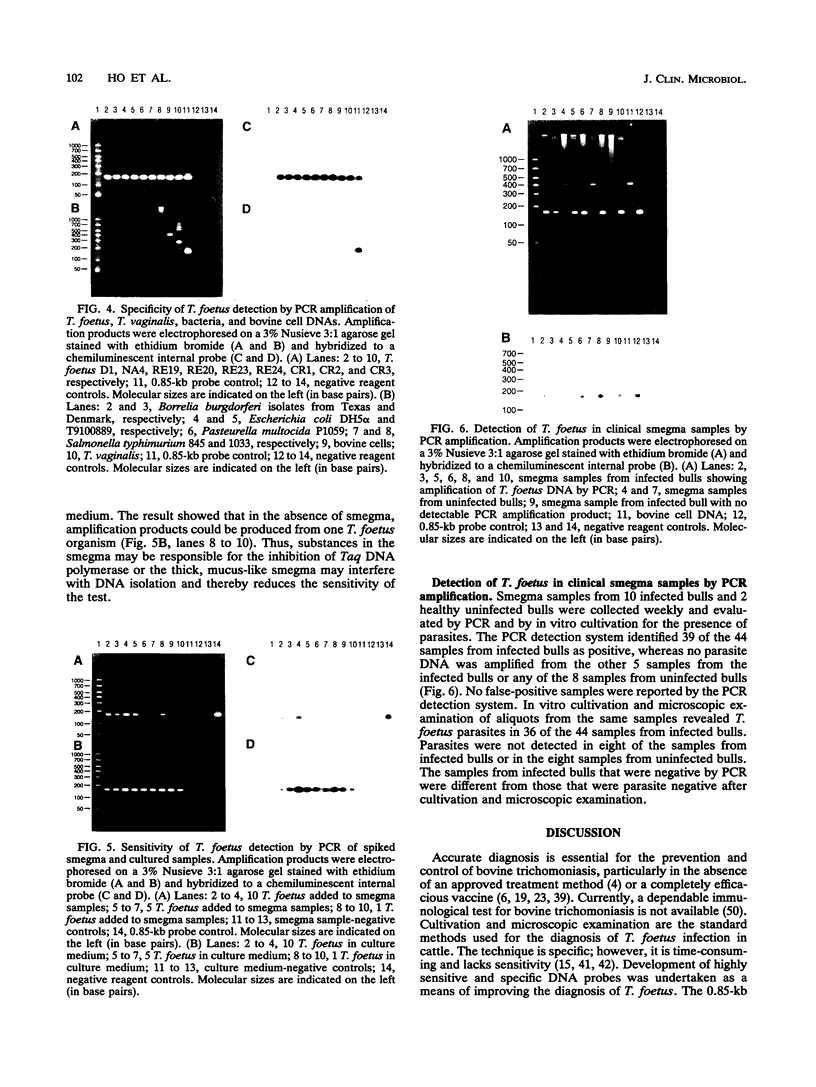
Trichomoniasis is a widespread, economically important venereal disease of cattle which causes infertility and abortion. Effective control of trichomoniasis has been impeded by the insensitivity of traditional diagnostic procedures, which require the isolation and cultivation of the parasite, Tritrichomonas foetus, from infected cattle. We developed a 0.85-kb T. foetus DNA probe by identifying conserved sequences in DNAs from T. foetus that were isolated from cattle in California, Idaho, Nevada, and Costa Rica. The probe hybridized specifically to DNAs of T. foetus isolates from different geographic areas but not to DNA preparations of Trichomonas vaginalis, bovine cells, or a variety of bacteria from cattle. The probe detected DNA from a minimum of 10(5) T. foetus organisms. To improve sensitivity, a partial sequence of the probe was used to identify oligonucleotide primers (TF1 and TF2) which could be used to amplify a 162-bp product from T. foetus DNAs by PCR. A chemiluminescent internal T. foetus sequence probe was hybridized to Southern blots of the amplification product. This system detected as few as one T. foetus organism in culture media or 10 parasites in samples containing bovine preputial smegma. Analysis of 52 clinical samples showed that 47 (90.4%) of the 52 samples were correctly identified, with no false-positive reactions. In comparison, the traditional cultivation method detected 44 (84.6%) of the 52 samples from T. foetus-infected and uninfected bulls. These results indicate that the PCR-based amplification system could be a useful alternative method for the diagnosis of bovine trichomoniasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbitt B., Meyerholz G. W. Trichomonas fetus infection of range bulls in South Florida. Vet Med Small Anim Clin. 1979 Sep;74(9):1339–1342. [PubMed] [Google Scholar]
- Avila H. A., Sigman D. S., Cohen L. M., Millikan R. C., Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease. Mol Biochem Parasitol. 1991 Oct;48(2):211–221. doi: 10.1016/0166-6851(91)90116-n. [DOI] [PubMed] [Google Scholar]
- BonDurant R. H., Anderson M. L., Blanchard P., Hird D., Danaye-Elmi C., Palmer C., Sischo W. M., Suther D., Utterback W., Weigler B. J. Prevalence of trichomoniasis among California beef herds. J Am Vet Med Assoc. 1990 May 15;196(10):1590–1593. [PubMed] [Google Scholar]
- BonDurant R. H., Corbeil R. R., Corbeil L. B. Immunization of virgin cows with surface antigen TF1.17 of Tritrichomonas foetus. Infect Immun. 1993 Apr;61(4):1385–1394. doi: 10.1128/iai.61.4.1385-1394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P. A., Iams K., Brown W. C., Sohanpal B., ole-MoiYoi O. K. DNA probes detect genomic diversity in Theileria parva stocks. Mol Biochem Parasitol. 1987 Oct;25(3):213–226. doi: 10.1016/0166-6851(87)90085-5. [DOI] [PubMed] [Google Scholar]
- Corbeil L. B., Woodward W., Ward A. C., Mickelsen W. D., Paisley L. Bacterial interactions in bovine respiratory and reproductive infections. J Clin Microbiol. 1985 May;21(5):803–807. doi: 10.1128/jcm.21.5.803-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Dennett D. P., Reece R. L., Barasa J. O., Johnson R. H. Observations on the incidence and distribution of serotypes of Tritrichomonas foetus in beef cattle in north-eastern Australia. Aust Vet J. 1974 Oct;50(10):427–431. doi: 10.1111/j.1751-0813.1974.tb06863.x. [DOI] [PubMed] [Google Scholar]
- Ferrari F. A., Ferrari E., Hoch J. A. Chromosomal location of a Bacillus subtilis DNA fragment uniquely transcribed by sigma-28-containing RNA polymerase. J Bacteriol. 1982 Nov;152(2):780–785. doi: 10.1128/jb.152.2.780-785.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. R. Bovine trichomoniasis. Vet Clin North Am Food Anim Pract. 1986 Jul;2(2):277–282. doi: 10.1016/s0749-0720(15)31237-8. [DOI] [PubMed] [Google Scholar]
- Grover C. M., Thulliez P., Remington J. S., Boothroyd J. C. Rapid prenatal diagnosis of congenital Toxoplasma infection by using polymerase chain reaction and amniotic fluid. J Clin Microbiol. 1990 Oct;28(10):2297–2301. doi: 10.1128/jcm.28.10.2297-2301.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. E. INCIDENCE AND DIAGNOSIS OF TRICHOMONIASIS IN WESTERN BEEF BULLS. J Am Vet Med Assoc. 1964 Nov 15;145:1007–1010. [PubMed] [Google Scholar]
- Kvasnicka W. G., Hanks D., Huang J. C., Hall M. R., Sandblom D., Chu H. J., Chavez L., Acree W. M. Clinical evaluation of the efficacy of inoculating cattle with a vaccine containing Tritrichomonas foetus. Am J Vet Res. 1992 Nov;53(11):2023–2027. [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- MANDEL M., HONIGBERG B. M. ISOLATION AND CHARACTERIZATION OF DEOXYRIBONUCLEIC ACID OF TWO SPECIES OF TRICHOMONAS DONNE. J Protozool. 1964 Feb;11:114–116. doi: 10.1111/j.1550-7408.1964.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Müller M. Biochemical cytology of trichomonad flagellates. I. Subcellular localization of hydrolases, dehydrogenases, and catalase in Tritrichomonas foetus. J Cell Biol. 1973 May;57(2):453–474. doi: 10.1083/jcb.57.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., Gallery F., MacConnell P., Becker J., Bloch W. An improved technique for the in situ detection of DNA after polymerase chain reaction amplification. Am J Pathol. 1991 Dec;139(6):1239–1244. [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Mathiesen D., Marshall W. F., Telford S. R., Spielman A., Thomford J. W., Conrad P. A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992 Aug;30(8):2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H. Polymerase chain reaction: trenches to benches. J Clin Microbiol. 1991 Jul;29(7):1281–1285. doi: 10.1128/jcm.29.7.1281-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. E., Krieger J. N. Rapid and practical DNA isolation from Trichomonas vaginalis and other nuclease-rich protozoa. Mol Biochem Parasitol. 1992 Mar;51(1):161–163. doi: 10.1016/0166-6851(92)90212-3. [DOI] [PubMed] [Google Scholar]
- Riley D. E., Roberts M. C., Takayama T., Krieger J. N. Development of a polymerase chain reaction-based diagnosis of Trichomonas vaginalis. J Clin Microbiol. 1992 Feb;30(2):465–472. doi: 10.1128/jcm.30.2.465-472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino S., Muresu R., Rappelli P., Fiori P. L., Rizzu P., Erre G., Cappuccinelli P. Molecular probe for identification of Trichomonas vaginalis DNA. J Clin Microbiol. 1991 Apr;29(4):702–706. doi: 10.1128/jcm.29.4.702-706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow S. Z., BonDurant R. H. Induced Tritrichomonas foetus infection in beef heifers. J Am Vet Med Assoc. 1990 Mar 15;196(6):885–889. [PubMed] [Google Scholar]
- Skirrow S., BonDurant R., Farley J., Correa J. Efficacy of ipronidazole against trichomoniasis in beef bulls. J Am Vet Med Assoc. 1985 Aug 15;187(4):405–407. [PubMed] [Google Scholar]
- Skirrow S. Identification of trichomonad-carrier cows. J Am Vet Med Assoc. 1987 Sep 1;191(5):553–554. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tedesco L. F., Errico F., Del Baglivi L. P. Diagnosis of Tritrichomonas foetus infection in bulls using two sampling methods and a transport medium. Aust Vet J. 1979 Jul;55(7):322–324. doi: 10.1111/j.1751-0813.1979.tb00418.x. [DOI] [PubMed] [Google Scholar]
- Turner G., Müller M. Failure to detect extranuclear DNA in Trichomonas vaginalis and Tritrichomonas foetus. J Parasitol. 1983 Feb;69(1):234–236. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Isolation and characterization of DNA from Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1985 Mar;14(3):323–335. doi: 10.1016/0166-6851(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Yule A., Skirrow S. Z., Staats J., Bondurant R. H. Development and preliminary assessment of a polyclonal antibody-based enzyme immunoassay for the detection of Tritrichomonas foetus antigen in breeding cattle. Vet Parasitol. 1989 May;31(2):115–123. doi: 10.1016/0304-4017(89)90026-5. [DOI] [PubMed] [Google Scholar]