Abstract
The ubiquitin-proteasome system has recently emerged as a major target for drug development in cancer therapy. The proteasome inhibitor bortezomib has clinical activity in multiple myeloma and mantle cell lymphoma. Here we report that Eeyarestatin I (EerI), a chemical inhibitor that blocks endoplasmic reticulum (ER)-associated protein degradation, has antitumor and biologic activities similar to bortezomib and can synergize with bortezomib. Like bortezomib, EerI-induced cytotoxicity requires the up-regulation of the Bcl-2 homology3 (BH3)-only pro-apoptotic protein NOXA. We further demonstrate that both EerI and bortezomib activate NOXA via an unanticipated mechanism that requires cooperation between two processes. First, these agents elicit an integrated stress response program at the ER to activate the CREB/ATF transcription factors ATF3 and ATF4. We show that ATF3 and ATF4 form a complex capable of binding to the NOXA promoter, which is required for NOXA activation. Second, EerI and bortezomib also block ubiquitination of histone H2A to relieve its inhibition on NOXA transcription. Our results identify a class of anticancer agents that integrate ER stress response with an epigenetic mechanism to induce cell death.
Keywords: bortezomib, cancer therapy, ER stress/UPR, histone H2A, ubiquitin
The ubiquitin proteasome system (UPS) constitutes a major disposal pathway for misfolded proteins at the endoplasmic reticulum (ER) and therefore promotes protein homeostasis. It has been shown that misfolded ER proteins are exported from the ER into the cytosol by a process termed ER-associated protein degradation (ERAD) or retrotranslocation (1, 2). This process requires a cytosolic ATPase named p97, which acts as a “dislocase” to extract misfolded proteins from the ER membranes once substrates have undergone polyubiquitination. p97 subsequently hands substrates over to the proteasome for degradation (3). Defects in ERAD cause accumulation of misfolded proteins in the ER and thus trigger ER stress (also called Unfolded Protein Response, UPR).
The functional integrity of the UPS is also essential for the survival of cancer cells because their uncontrolled proliferation requires proteasome mediated degradation of many cell cycle factors (4). Accordingly, the proteasome inhibitor bortezomib (VelcadeTM) has cytotoxic activities against a broad range of cancer cell lines and is now used in the clinic to treat multiple myeloma and mantle cell lymphoma (MCL) (5). The precise mechanism by which bortezomib induces cytotoxicity in cancer cells remains elusive. Recent studies showed that the induction of the Bcl-2 homology3 (BH3)-only pro-apoptotic protein NOXA plays an important role (6–12). On a separate note, several other studies suggested that ER stress elicited by bortezomib may account for its cytotoxicity (8, 13). It is currently unclear whether NOXA activation and ER stress are mechanistically connected or independently triggered by bortezomib as a result of stabilization of distinct proteasomal substrates. In addition, although the tumor suppressor p53 was previously implicated in NOXA regulation in response to DNA damage (14), bortezomib can activate NOXA in cancer cells lacking p53 (9, 11). How bortezomib activates NOXA independently of p53 is unclear.
Given the critical role played by the UPS and ER homeostasis in cancer, we hypothesize that agents causing UPS deficiency and ER stress such as those targeting the ERAD pathway may possess anticancer activities similar to bortezomib. To date, the only known ERAD specific inhibitor is a class of compound named Eeyarestatins (15). We previously showed that Eeyarestatin I (EerI) targets a p97 complex to inhibit deubiquitination of p97-associated ERAD substrates, which is required for their turnover (16, 17).
Here we report that EerI indeed has cytotoxic activity preferentially against cancer cells. As hypothesized, EerI and bortezomib have similar characteristics: both agents activate an integrated stress response at the ER, and both induce cell death via the BH3-only protein NOXA. Most importantly, we identify a transcription factor complex comprising two ER stress-induced transcription factors, ATF3 and ATF4, that regulate NOXA expression independently of p53. We show that these transcription factors cooperate with an ubiquitin dependent epigenetic mechanism to activate NOXA. These results establish a connection between ER stress and NOXA activation and thus implicate ER stress as a primary mechanism underlying the anticancer activity of ERAD inhibitors.
Results
EerI Induces Cell Death in Hematological Cancer Cells.
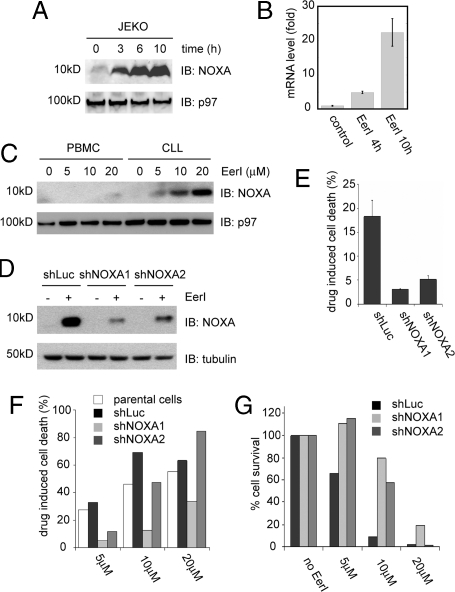
We first tested whether EerI could induce cell death in the MCL cell lines HBL-2 and JEKO-1. The viability of cells treated with EerI at different concentrations was measured by a colorimetric assay using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole). As a control, we treated each of these cell lines with bortezomib, which resulted in cytotoxic activity, as expected (Fig. 1A). EerI also induced cell death in these cells (Fig. 1B), as well as in four additional lymphoid cell lines tested: MINO (MCL), Jurkat (T cell leukemia), KMS-12 (multiple myeloma), and BJAB (Burkitt's lymphoma) (Fig. S1). Interestingly, like bortezomib, EerI was also cytotoxic in primary leukemia cells isolated from patients with chronic lymphocytic leukemia (CLL), whereas peripheral blood mononuclear cells (PBMC) from healthy donors were considerably less sensitive to EerI (Fig. 1D). This resembled the antitumor activity of bortezomib (Fig. 1C). Accordingly, EerI could also synergize with bortezomib in causing cytotoxicity (Fig. 1E). Thus, similar to bortezomib, EerI has cytotoxic activity preferentially against cancer cells.
Fig. 1.
EerI has anticancer activity and can synergize with bortezomib. (A and B) Cytotoxic activities of bortezomib (BZM) and EerI in HBL-2 and JEKO-1 cells. Cells were treated as indicated for 48 h, and cell viability was measured by a MTT assay. Error bar, SD (n = 3). (C) As in (A) and (D) as in (B) except that primary cells isolated from CLL patients and healthy donors (PMBC) were used. (E) Synergistic action of EerI and bortezomib. HBL-2 cells were treated with the indicated concentrations of EerI, bortezomib, or a combination of the two drugs (EerI, Bottom; BZM, Top). The combination index at IC50 is < 0.1, suggesting a strong synergy between the two agents.
EerI and Bortezomib Induce an Integrated Stress Response at the ER.
To dissect the mechanistic link between EerI and bortezomib, we analyzed the gene expression profiles of cells treated with EerI or bortezomib at a concentration twice the respective IC50 by array hybridization. Among 873 genes up-regulated by at least a factor of 1.5-fold after exposure to EerI, 352 were also induced by bortezomib (Fig. S2). Likewise, approximately 30% of the genes downregulated by EerI were also downregulated by bortezomib. These results indicate that EerI and bortezomib have overlapping activities.
Genes up-regulated in response to both EerI and bortezomib include those involved in ER stress, ERAD, and amino acid import, and metabolism (Table S1). These data indicated that an integrated ER stress response is commonly elicited by these compounds. Quantitative RT-PCR (qRT-PCR) using a select set of probes confirmed this conclusion (Fig. 2A).
Fig. 2.
EerI and bortezomib induce ER stress. (A) The mRNA levels of the indicated genes in JEKO-1 cells after exposure to either EerI (10 μM) or bortezomib (BZM, 20 nM) were determined by qRT-PCR. Error bar, SD (n = 3). (B) EerI induces ATF3 expression. Cell extracts from JEKO-1 cells were analyzed by immunoblotting. (C) EerI induces the expression of ATF4. Cell extracts of WT or ATF4 deficient (ATF4−/−) MEF cells, treated as indicated, were analyzed by immunoblotting. (D) EerI induces phosphorylation of eIF2α. Protein extracts from JEKO-1 cells untreated or treated with EerI (8 h) were analyzed by immunoblotting. As a control, we treated cells with Salubrinal (Sal), a phosphatase inhibitor that promotes eIF2α phosphorylation (36). (E and F) EerI induces Ire1 dependent splicing of Xbp1 mRNA. (E) RNA from JEKO-1 cells treated with EerI (10 μM) was analyzed by RT-PCR. (H) RNA from EerI-treated WT and Ire1α−/− MEF cells (8 h) was analyzed by RT-PCR.
Since bortezomib can activate the PERK and Ire1 branches of ER stress (8, 13), we examined the effect of EerI on these two processes. In EerI-treated cells, the most highly up-regulated genes included CHOP, GADD34, and ATF3 (Fig. 2 A and B). The latter encodes a transcription factor of the ATF/CREB family. A rapid accumulation of another ATF protein (ATF4) was also observed in cell lines treated with EerI (Fig. 2C). The above mentioned proteins constitute a pathway downstream of the ER-associated protein kinase PERK to mediate responses to stress signals at the ER (18). Thus, the PERK branch of the UPR appeared to be activated by EerI. This conclusion was further supported by the observation that EerI-induced eIF2α phosphorylation (Fig. 2D), an event directly mediated by PERK.
We next assayed the splicing of Xbp1 mRNA, which gives rise to an Xbp1 variant lacking a small intron. This process is a direct indicator of Ire1 activation in response to ER stress (19–21). RT-PCR showed that EerI-induced Xbp1 splicing in an Ire1 dependent manner (Fig. 2 E and F), suggesting that EerI activates the Ire1 branch of the UPR.
EerI Induces Cell Death via the BH3 only Protein NOXA.
Since previous studies demonstrated that bortezomib induces cytotoxicity via the Bcl-2 antagonist protein NOXA, which was also induced by EerI according to our array experiment (Table S1), we tested whether NOXA contributes to EerI-induced cytotoxicity.
We first validated the activation of NOXA in EerI-treated cells. Immunoblotting showed that NOXA protein was rapidly increased in a variety of cancer cell lines following EerI treatment (Fig. 3A, data not shown). qRT-PCR revealed a parallel increase in NOXA mRNA levels in EerI-treated JEKO-1 cells (Fig. 3B). Thus, the increase in NOXA protein level appears to be at least in part due to enhanced NOXA transcription. Interestingly, EerI only induced NOXA expression in CLL cells, not in healthy PBMC cells (Fig. 3C), supporting a role of NOXA in the selective killing of cancer cells by EerI. To further test this idea, we stably expressed short hairpin RNA (shRNA) targeting NOXA in JEKO-1 (Fig. 3D). Parental JEKO-1 cells or cells expressing an irrelevant shRNA (shLuc) were used as controls. The expression of NOXA specific shRNAs (shNOXA1 and shNOXA2) did not affect cell viability in untreated cells (data not shown), but it effectively protected cells from EerI-induced cell death, particularly at low concentrations (Fig. 3 E-G). These data indicate that similar to bortezomib, EerI induces apoptosis in hematological cancer cells by acting through NOXA and its pro-apoptotic effect on Bcl family members. Consistently, we found that EerI also enhanced the cytotoxicity of BH3i-1 (Fig. S3), a chemical antagonist of the Bcl-2 protein (22).
Fig. 3.
NOXA is required for EerI-induced cell death. (A and B) Induction of NOXA expression by EerI. (A) Cell extracts from JEKO-1 cells treated with EerI (10 μM) were analyzed by immunoblotting. (B) NOXA mRNA level in EerI-treated JEKO-1 cells (10 μM) was determined by qRT-PCR. Error bar, SD (n = 3) (C) NOXA is selectively activated by EerI in CLL cells. The NOXA protein level in CLL and PBMC cells treated with the indicated concentration of EerI was determined by immunoblotting. (D) NOXA knock-down cells. JEKO-1 cells stably expressing two different NOXA shRNAs or a luciferase shRNA (shLuc) were treated with EerI 5 μM (+) or with DMSO (−) as a control (24 h). Protein extracts were analyzed by immunoblotting. (E-G) NOXA knock-down protects cells from EerI-induced cell death. (E) JEKO-1 cells expressing the indicated shRNA were treated with EerI (5 μM) for 24 h. Cell death was determined by an annexin V-labeling apoptotic assay. Shown are drug-induced cell death normalized using untreated cells as a control. Error bars indicate the mean of two independent experiments. (F) As in (E) except that cells were treated with the indicated concentrations of EerI and the parental JEKO-1 line was also included. (G) JEKO-1 cells expressing the indicated shRNA were treated with EerI for 24 h. Cell viability was measured by a MTT assay.
ATF3 and ATF4 Form a Complex Capable of Binding to the NOXA Promoter.
ER stress has been implicated in bortezomib-induced apoptosis. However, whether there is a direct connection between NOXA activation and ER stress has not been defined. To understand the regulation of NOXA expression in response to EerI and bortezomib, we generated a reporter construct that expressed luciferase under the control of a DNA segment upstream of the NOXA gene (N1-Luc) (Fig. 4A). Because our microarray data indicated that several CREB/ATF transcription factors were activated by EerI and bortezomib, we used a luciferase assay to test whether this putative NOXA promoter could be activated by any of the several CREB/ATF transcription factors that had been previously implicated in ER stress regulation. It turned out that ATF3, ATF4, and CEBPβ each could moderately activate the NOXA promoter, whereas CHOP, ATF6, and CEBPα had no or minimal effects (Fig. S4A). Interestingly, when ATF3 and ATF4 were coexpressed, the expression of luciferase was induced to a much greater extent (Fig. 4B and Fig. S4). By contrast, other transcription factors tested could not significantly enhance the activity of either ATF3 or ATF4 (Fig. S4). The synergistic activation of the NOXA promoter by ATF3 and ATF4 required both the C-terminal bZIP DNA binding domain and the N terminus of ATF4 (Fig. 4C).
Fig. 4.
ATF3 and ATF4 form a complex to activate the NOXA promoter. (A) Schematic representation of the reporter constructs. A predicted p53 binding site was labeled. (B-C) ATF3 and ATF4 synergistically activate the NOXA promoter. (B) Luciferase activity in the extract of HeLa cells transfected with N1-Luc together with the indicated plasmids was determined. Shown is the mean of 2 independent experiments. A fraction of the extracts was analyzed by immunoblotting to verify the expression of ATF3 and ATF4. (C) As in (B) except that ATF3 was cotransfected with the indicated ATF4 variants. (D) ATF3 and ATF4 regulate NOXA expression in a p53-independent manner. (E) Interaction of ATF4 with ATF3. Extracts from 293T cells expressing His-ATF3 together with the indicated Flag-ATF4 variants were subjected to immunoprecipitation and immunoblotting analyses. (F) ATF3 and ATF4 form a complex on the NOXA promoter. A DNA fragment corresponding to the NOXA regulatory sequence was radiolabeled and incubated with purified ATF3 and ATF4 either singly or in combination. The reaction was analyzed by native gel electrophoresis and autoradiography. (Right) An example of the purified ATF3 and ATF4. (G) ATF3 and ATF4 associate with the endogenous NOXA promoter. JEKO-1 cells were treated with EerI (10 μM, 8 h). Chromatin immunoprecipitated with the indicated antibodies was analyzed by PCR using primers corresponding to the different regions of the NOXA promoter as shown in Fig. 6E.
Although NOXA is known to be regulated by p53 in response to DNA damage (14), p53 could not synergize with ATF3 or ATF4 to activate the NOXA promoter (data not shown), indicating that ATF3 and ATF4 bind the NOXA promoter independently of p53. Consistent with this notion, ATF3 and ATF4 could activate both N1-Luc and a NOXA reporter lacking a predicted p53-binding site (N2-Luc) (Fig. 4 A and D).
We therefore tested whether ATF3 and ATF4 can form a complex that directly interacts with the NOXA promoter. Coimmunoprecipitation showed that ATF3 and ATF4 could be coprecipitated from cell extract by antibodies to ATF4 (Fig. 4E, lane 2). The N-terminal domain of ATF4 was dispensable for interaction with ATF3 (Fig. 4E, lane 3). By contrast, the ATF4 C-terminal DNA binding domain was required for interaction with ATF3 (Fig. 4E, lane 5), although it was insufficient to bind ATF3 on its own (lane 4). Purified ATF3 and ATF4 proteins also interacted with each other in vitro (Fig. S5A), suggesting that these transcription factors may bind directly to each other. Moreover, endogenous ATF3 could interact with ectopically expressed ATF4 in bortezomib-treated HeLa cells and vice versa (Fig. S5 B and C).
To test the association of ATF3 and ATF4 with the NOXA promoter, we performed Electrophoretic Mobility Shift Assays (EMSA) using purified ATF3 and ATF4 proteins and a radiolabeled DNA probe corresponding to bases −78 to + 45 relative to the NOXA transcription start site, because our mapping experiment suggested that this fragment was sufficient to activate luciferase expression in response to overexpression of ATF3 and ATF4 (data not shown). Our EMSA results showed that ATF3 alone, but not ATF4, bound the NOXA promoter (Fig. 4F, lane 2 versus 3). Interestingly, when both ATF3 and ATF4 were present, a new complex with slower mobility was observed (Fig. 4F, lane 4), suggesting the formation of a ternary DNA-protein complex comprising both ATF3 and ATF4 (Fig. 4F, lane 4). Endogenous ATF3 and ATF4 could also be detected in association with the NOXA promoter in JEKO-1 cells, as revealed by chromatin immunoprecipitation (CHIP) (Fig. 4G). Together, these results demonstrate that ATF3 is capable of recruiting ATF4 to the NOXA promoter to regulate its activity.
The ATF3-ATF4 Complex Regulates NOXA Expression.
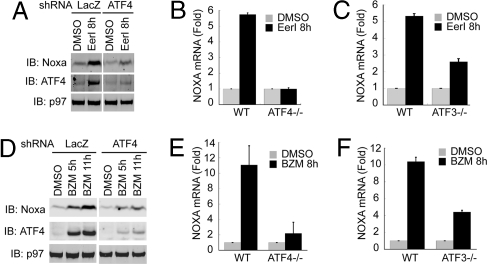
We next tested whether ATF3 and ATF4 are required for the induction of NOXA in response to EerI or bortezomib. We used HeLa and MEF cells because they are amenable to genetic manipulation to alter the level of ATF3 and ATF4. We first used shRNA to knock down ATF4 in HeLa cells. Immunoblotting showed that compared to control cells the induction of NOXA by EerI was attenuated in knock-down cells (Fig. 5A). Likewise, ATF4 knock-down reduced bortezomib-induced NOXA expression (Fig. 5D). Thus, ATF4 is required for NOXA activation in response to these agents. This conclusion was further confirmed using ATF4 deficient MEF cells (Fig. 5 B and E).
Fig. 5.
ATF3 and ATF4 regulate NOXA expression in response to EerI and bortezomib. (A and B) ATF4 is required for NOXA activation by EerI. (A) HeLa cells transfected with constructs expressing either a control shRNA or an ATF4 specific shRNA were treated as indicated (EerI 10 μM). Cell extracts were analyzed by immunoblotting. (B) WT and ATF4 deficient MEF cells were treated as in (A). Shown are normalized NOXA mRNA levels determined by qRT-PCR. Error bar, SD (n = 3). (C) ATF3 regulates NOXA expression in response to EerI. As in (B) except that WT and ATF3 deficient MEF cells (ATF3−/−) were analyzed. (D and E) ATF4 is required for NOXA activation by bortezomib. (D) As in (A) and (E) as in (B) except that cells were treated with bortezomib (BZM 25 nM). (F) ATF3 regulates NOXA expression in response to bortezomib; (F) as in (C), except that cells were treated with bortezomib (25 nM).
To examine the role of ATF3 in NOXA activation, we used ATF3 deficient MEF cells, because ATF3 shRNAs failed to overcome the strong ATF3 induction by EerI and bortezomib (data not shown). Our data showed that compared to WT cells, the induction of NOXA by EerI and bortezomib was partially abolished in ATF3−/− cells (Fig. 5 C and F). These results, together with the observations that endogenous ATF3 is associated with the NOXA promoter upon its activation and that ATF3 can drive the expression of a reporter construct containing the NOXA promoter, implicate ATF3 in NOXA induction in response to EerI and bortezomib. Since our in vitro study suggests that ATF4 on its own does not bind NOXA promoter, and yet the lack of ATF3 only leads to partial defect in NOXA activation, ATF4 may have other partners that facilitate its recruitment to the NOXA promoter during its activation.
Downregulation of Ub-H2A Contributes to NOXA Activation.
Although ATF3 and ATF4 together can activate the NOXA promoter in the reporter construct, surprisingly, these factors, when ectopically expressed or induced by the canonical ER stressor tunicamycin, were insufficient to activate NOXA expression from its endogenous promoter (Fig. 6A; data not shown). We suspected that the expression of NOXA from its endogenous promoter might be subject to additional regulation by epigenetically marked nucleosomes, which are often not associated with transfected reporter DNA.
Fig. 6.
EerI and bortezomib down-regulate Ub-H2A to activate NOXA expression. (A) NOXA activation correlates inversely with downregulation of Ub-H2A. Whole cell extracts from JEKO-1 cells exposed to the indicated agents were subjected to immunoblotting analyses. For H2A blot, chromatin extracts from a portion of the cells were used. (B) Specificity of the anti-H2A antibody. The color panel shows a merged image (red, anti-Ub-H2A; green, anti-H2A). (C and D) Knock-down of RING2 activates NOXA expression. (C) Whole cell extracts from untransfected 293T cells or cells expressing the indicated shRNA for 48 h were analyzed by immunoblotting. The numbers indicate the normalized levels of Ub-H2A. (D) As in (C), except that HeLa cells transfected with the indicated constructs for 36 h were analyzed. (E) Schematic representation of the NOXA locus and the primers used in CHIP experiments. (F) Ub-H2A is associated with the NOXA promoter. (G) EerI and bortezomib reduce the presence of Ub-H2A in the NOXA promoter. Cells were treated for 8 h with EerI (10 μM) or bortezomib (BZM, 25 nM). (H-I) Bortezomib and EerI increase the association of Pol II with the body of the NOXA gene. (H) JEKO-1 cells exposed to bortezomib (BZM, 25 nM, 8 h) were subjected to CHIP analysis. (I) As in (H), except that cells were treated with EerI (10 μM, 8 h).
Given the critical role played by ubiquitinated histone H2A (Ub-H2A) in gene silencing (23, 24) and the observation that the proteasome inhibitor MG132 can block histone H2A ubiquitination (25), we investigated the level of Ub-H2A in cells treated with bortezomib, EerI or the ER stressor tunicamycin. Indeed, both bortezomib and EerI treatment reduced the level of Ub-H2A without affecting unmodified H2A, whereas tunicamycin had little effect (Fig. 6 A and B). Interestingly, the induction of NOXA by EerI and bortezomib correlated inversely with the level of Ub-H2A (Fig. 6A), suggesting that Ub-H2A may inhibit NOXA expression.
We therefore used shRNA to reduce the expression of the polycomb group (PcG) gene RING2, the catalytic subunit of an ubiquitin ligase complex that ubiquitinates H2A (26). We found that downregulation of RING2 by approximately 80% (judged by qRT-PCR, data not shown) reduced the level of Ub-H2A, but increased NOXA expression (Fig. 6C). This phenotype could be further enhanced by coexpression of ATF3 and ATF4 (Fig. 6D), suggesting that the level of NOXA induction by RING2 depletion might be limited by the abundance of transcription factors. Together, these results indicate that downregulation of Ub-H2A cooperates with ER stress-induced transcription factors ATF3 and ATF4 to activate NOXA expression.
To test whether Ub-H2A directly regulates NOXA expression, we examined the association of Ub-H2A with the NOXA locus by a previously established sequential CHIP assay (27). Our results show that Ub-H2A was indeed enriched at the NOXA transcription start site but was absent from the gene body (Fig. 6 E-G). As anticipated, treatment of cells with EerI, bortezomib, or RING2 shRNA all reduced the association of Ub-H2A with the NOXA locus (Fig. 6G and Fig. S6). These data indicate that Ub-H2A may inhibit NOXA expression by binding to its promoter.
To further understand the mechanism by which Ub-H2A inhibits NOXA expression, we analyzed the effect of EerI and bortezomib on the association of the RNA polymerase II (Pol II) with the NOXA locus. In untreated cells, very few Pol II molecules could be detected in the body of the NOXA gene despite its abundant presence in the promoter region (Fig. 6H). Both bortezomib and EerI treatment significantly enhanced the presence of Pol II in the NOXA gene body (Fig. 6 H-I, primer 4), which correlated with NOXA activation. These results suggest that the repression of NOXA expression is not due to lack of Pol II recruitment. Instead, Ub-H2A may cause Pol II polymerase to pause at the NOXA transcription initiation site, resulting in the inhibition of NOXA expression.
Discussion
The ERAD Inhibitor EerI Possesses Anticancer Activities.
In this study, we demonstrate that EerI, a potent ERAD specific inhibitor, has anticancer activity similar to the proteasome inhibitor bortezomib. EerI can induce cell death in cell lines derived from various lymphoid malignancies as well as in primary leukemia cells from CLL patients. Similar to bortezomib, EerI-induced cytotoxicity is mediated by the anti-Bcl-2 protein NOXA. Accordingly, EerI can synergize with a chemical inhibitor of Bcl-2. This also resembles bortezomib, which has been shown to synergize with the Bcl-2 family inhibitor otaboclax (28). Importantly, we show that EerI also synergizes with bortezomib in inducing cytotoxicity. At the molecular level, this synergism reflects a corporation between these agents in activating NOXA expression by a sophisticated mechanism that involves both ER stress induction and epigenetic derepression (Fig. S7 and also see below). Together, these results suggest that ERAD specific inhibitors may be useful as a novel class of anticancer agents either alone or in combination with existing treatments.
ER Stress and the Anticancer Mechanism of Bortezomib.
We identify ER stress-induced transcription factors ATF3 and ATF4 as a key mediator of NOXA activation in response to EerI and bortezomib. This newly established connection between ER stress and NOXA activation explains why these two seemingly unrelated events have been implicated separately in the antitumor action of bortezomib and therefore highlights ER stress as a major mechanism of bortezomib action. Interestingly, it was shown recently that ER stress can also activate another BH3-only protein, Bim, to induce apoptosis (29). However, EerI and bortezomib likely induce cell death independently of Bim: first, biallelic deletions of Bim are common in MCL cell lines that are highly sensitive to bortezomib and EerI, including JEKO-1 cells. Second, even in cells that express Bim, bortezomib does not increase Bim expression (12, 30).
Regulation of NOXA Expression.
Our results reveal a p53 independent mechanism of NOXA transcriptional regulation in response to EerI and bortezomib, which involves cooperation between the ER stress-activated transcription factor complex ATF3-ATF4 and the relief of Ub-H2A-mediated transcriptional repression (Fig. S8). Consistent with this model, we demonstrate that Ub-H2A is associated with the NOXA locus, and downregulation of Ub-H2A by depletion of RING2 activates NOXA expression, which could be further enhanced by ATF3 and ATF4. It is worth noting that deletion of Bmi1, another PcG protein, can also induce NOXA expression, as demonstrated recently in mice. However, whether Bmi1 act through Ub-H2A to inhibit NOXA expression was not examined (31). In further support of our model, we show that both EerI and bortezomib treatments activate ATF3 and ATF4 and at the same time block ubiquitination of histone H2A. These changes correlate with the activation of NOXA expression. Downregulation of Ub-H2A by a proteasome inhibitor was previously reported and was attributed to ubiquitin stress caused by global impairment of the UPS (25). Given that EerI also induces accumulation of polyubiquitinated proteins to a similar degree as the proteasome inhibitor MG132 (17), the downregulation of Ub-H2A in EerI-treated cells may also be a secondary defect caused by ubiquitin deficiency.
Ubiquitinated H2A plays important roles in silencing the polycomb target genes and in X-chromosome inactivation. A recent study reported that Ub-H2A may cause the Pol II RNA polymerase to pause at the initiation sites of genes that are poised for transcription activation and thereby inhibit their expression (32). Our data suggest that Ub-H2A can silence NOXA in untreated cells by a similar mechanism, as Pol II accumulates at the NOXA transcription initiation site but is largely absent from the body of the NOXA gene. Inhibition of H2A ubiquitination by EerI and bortezomib allows Pol II to move into the NOXA gene body to activate its expression.
Since NOXA is a key mediator of apoptosis, it is anticipated that its expression is subject to sophisticated regulation. NOXA was previously found to be regulated by p53 under certain conditions (14, 33). However, the induction of NOXA in many cancer cells is apparently p53-independent. Recent studies suggested that the c-Myc oncogene may participate in global chromatin activation to modulate the expression of genes such as NOXA (11, 34). Although further studies will be needed to elucidate the mechanistic connection between Ub-H2A and Myc dependent chromatin modulation during NOXA activation, our work has revealed a p53 independent mechanism that governs NOXA expression. Moreover, our study also suggests that agents capable of causing both ER stress and ubiquitin deficiency may offer new therapeutic possibilities for certain types of cancer.
Materials and Methods
Mammalian Cell Culture, Transient Gene Expression, Co-Immunoprecipitation.
HeLa and 293T cells from ATCC were maintained according to standard procedures. JEKO-1 and HBL-2 cell lines were kept in RPMI, 10% FCS as previously described (35). NOXA and control knock-down JEKO cells were generated by retroviral transfection followed by puromycin selection. MEF cells were cultured in complete DMEM supplemented with 10% FCS, 1X Pen/Strep, 55 μm β-mercaptoethanol, and non-essential amino acids (Invitrogen). PBMC from normal volunteers were obtained from the Department of Transfusion Medicine, National Institutes of Health and PBMC from CLL patients were obtained under National Institutes of Health protocol 97-C-0178 with informed consent. Transfection was done with TransIT293 reagent for 293T cells and HeLa Monster for HeLa cells (Mirus). For coimmunoprecipitation experiments, cells were extracted with buffer N (50 mM Tris/HCl pH 7.4, 150 mM sodium chloride, 1 mM EDTA, 1% Nonidet P-40, and a protease inhibitor mixture).
Additional methods are available in SI Text.
Supplementary Material
Acknowledgments.
We thank H. Wang (University of Alabama, Birmingham, AL), L. Staudt (National Cancer Institute, Bethesda, MD), H. Puthalakath, and A. Strasser (The Walter and Eliza Hall Institute of Medical Research, Melbourne), R. Prywes (Columbia University, New York), F. Urano (University of Massachusetts, Worcester, MA), and S. Fang (University of Maryland, Baltimore) for reagents; G. Felsenfeld (National Institute of Diabetes and Digestive and Kidney Diseases), C. Jin (National Institute of Diabetes and Digestive and Kidney Diseases), Z. Wang, and G. Wei (National Heart, Lung, and Blood Institute) for helpful discussions; F. Gibellini, and C. Chapman for help with cell culture; and M. Krause, M. Gellert, G. Felsenfeld, and A. Shaffer (National Cancer Institute) for critical reading of the manuscript. The research is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, and the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, and by National Institutes of Health grants DK47119 and ES08681 to D. Ron, and CA118306 and DK064938 to T. Hai.
Footnotes
Conflict of interest statement: Based on the work presented here, the National Institutes of Health have submitted a patent application for EerI as anticancer agent. Several of the authors (Q.W., H.M.-J., A.W., W.T., and Y.Y.) are named as inventors on this patent application.
This article is a PNAS Direct Submission.
Data depostion: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE14003).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807611106/DCSupplemental.
References
- 1.McCracken AA, Brodsky JL. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD) BioEssays. 2003;25:868–877. doi: 10.1002/bies.10320. [DOI] [PubMed] [Google Scholar]
- 2.Kostova Z, Wolf DH. For whom the bell tolls: Protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 2003;22:2309–2317. doi: 10.1093/emboj/cdg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Y. Diverse functions with a common regulator: Ubiquitin takes command of an AAA ATPase. J Struct Biol. 2006;156:29–40. doi: 10.1016/j.jsb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Adams J. The proteasome: A suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 5.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cells. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez Y, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 7.Qin JZ, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 8.Fribley AM, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Galan P, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Bougie P, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67:5418–5424. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov MA, et al. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzatti EG, et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma. 2008;49:798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- 13.Obeng EA, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda E, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 15.Fiebiger E, et al. Dissection of the dislocation pathway for type I membrane proteins with a new small molecule inhibitor, eeyarestatin. Mol Biol Cell. 2004;15:1635–1646. doi: 10.1091/mbc.E03-07-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Li L, Ye Y. Regulation of retrotranslocation by p97-associated deubiquitinating enzyme ataxin-3. J Cell Biol. 2006;174:963–971. doi: 10.1083/jcb.200605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem. 2008;283:7445–7454. doi: 10.1074/jbc.M708347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 19.Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 21.Calfon M, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 22.Degterev A, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 23.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 24.Weake VM, Workman JL. Histone ubiquitination: Triggering gene activity. Mol Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Dantuma NP, Groothuis TA, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 27.Joo HY, et al. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15–070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 29.Puthalakath H, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Mestre-Escorihuela C, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109:271–280. doi: 10.1182/blood-2006-06-026500. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita M, et al. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J Exp Med. 2008;205:1109–1120. doi: 10.1084/jem.20072000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stock JK, et al. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: Multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 34.Knoepfler PS, et al. Myc influences global chromatin structure. EMBO J. 2006;25:2723–2734. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mora-Jensen H, Rizzatti EG, Wiestner A. Resistance to bortezomib develops slowly in MCL cells, extends to the class of proteasome inhibitors, and is associated with decreased proliferation of the resistant cells. Blood. 2006;108:4397a. [Google Scholar]
- 36.Boyce M, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.