Abstract
c-JUN is a major component of heterodimer transcription factor AP-1 (Activator Protein-1) that activates gene transcription in cell proliferation, inflammation and stress responses. SIRT1 (Sirtuin 1) is a histone deacetylase that controls gene transcription through modification of chromatin structure. However, it is not clear if SIRT1 regulates c-JUN activity in the control of gene transcription. Here, we show that SIRT1 associated with c-JUN in co-immunoprecipitation of whole cell lysate, and inhibited the transcriptional activity of c-JUN in the mammalian two hybridization system. SIRT1 was found in the AP-1 response element in the matrix metalloproteinase-9 (MMP9) promoter DNA leading to inhibition of histone 3 acetylation as shown in a ChIP assay. The SIRT1 signal was reduced by the AP-1 activator PMA, and induced by the SIRT1 activator Resveratrol in the promoter DNA. SIRT1-mediaetd inhibition of AP-1 was demonstrated in the MMP9 gene expression at the gene promoter, mRNA and protein levels. In mouse embryonic fibroblast (MEF) with SIRT1 deficiency (SIRT1−/−), mRNA and protein of MMP9 were increased in the basal condition, and the inhibitory activity of Resveratrol was significantly attenuated. Glucose-induced MMP9 expression was also inhibited by SIRT1 in response to Resveratrol. These data consistently suggest that SIRT1 directly inhibits the transcriptional activity of AP-1 by targeting c-JUN.
Keywords: SIRT1, AP-1, MMP9, HDAC, Glucose
Introduction
AP-1 is a transcription factor formed by c-JUN and c-FOS in most cases. Matrix metalloproteinase 9 (MMP9) is a target gene of AP-1 [1], and plays a critical role in tissue remodeling, tumor invasion, and metastasis [2]. In diabetic patients, the increase in plasma MMP9 is associated with hyperglycemia [3]. High glucose is able to induce expression of MMP9 in cell culture [4]. The mechanism is related to activation of c-JUN N-terminal kinase 1 (JNK1) that phosphorylates and activates c-JUN [5]. As a subunit of AP-1, c-JUN mediated JNK signals in the control of MMP9 transcription [1]. SIRT1 activity is reduced by high glucose [6]. The reduction is correlated to activation of AP-1 activity and MMP9 transcription. It is not clear if SIRT1 reduction contributes to the AP-1 activation by glucose.
SIRT1 (Sirtuin 1) referred as Sir2 (silencing information regulator 2) in yeast, is a nicotinamide adenine dinucleotide (NAD)–dependent histone deacetylase, which is implicated in the regulation of many cellular processes, including apoptosis, cellular senescence, aging, longevity and glucose homeostasis [7–9]. It was reported that Resveratrol (RSV) inhibited phorbol myristate acetate (PMA)-induced matrix metalloproteinase-9 (MMP9) expression by inhibiting JNK [10]. RSV, a polyphenol found in grapes and wine, has variety of biological activities. These include anti-aging in yeast, prevention of cancer, and protection of cardiovascular system. The anti-inflammation activity of RSV may contribute to these beneficial effects. At the molecular level, RSV activates the enzyme activity of SIRT1 (Sir2 in yeast) in vivo and in vitro [11, 12]. In the RSV inhibition of AP-1[10], JNK is proposed a target of RSV to mediate the inhibition. The information about SIRT1 direct regulation of AP-1 is missing.
In this study, we elucidated the molecular mechanism by which c-JUN activity is inhibited by RSV. We demonstrated that: 1) SIRT1 physically interacts with c-JUN; 2) SIRT1 inhibits transcriptional activation of MMP9 by targeting c-JUN; 3) Knockout of SIRT1 led to an increase in MMP9 expression. We concluded that SIRT1 directly interacts with c-JUN and represses transcriptional activity of AP-1. This interaction is involved in regulation of MMP9 expression by glucose and RSV.
Materials and Methods
Cell culture and Reagents
HEK 293 (ATCC) and RAW264.7 cells were maintained in 5% FBS DMEM. PMA (P-1585), Resveratrol (R-5010) were purchased from Sigma (St. Louis, MO). SIRT1−/− MEFs were prepared in our lab by collection of embryo of 13 days from a SIRT1+/− female mouse that was crossed with a SIRT1+/− male mouse. The SIRT1 knockout mouse was a gift of Dr. Frederick W. Alt at the Howard Hughes Medical Institute, Children's Hospital, Center for Blood Research, and Department of Genetics, Harvard University Medical School, Boston, MA 02115, USA [13]. The embryo carcasses was minced and digested with trypsin after removal of the limbs, internal organs and brain. After digestion at 37°C for 10 minutes, the cell suspension was collected and washed with DMEM supplemented with 10% newborn calf serum. The cells were plated in 100 mm cell culture plate in the serum-containing medium, and the medium was changed 24 hrs later. After one passage, the cells were collected as MEFs. The SIRT1−/− MEFs and wild type MEFs were confirmed by genotyping.
Immunoblot
The whole cell lysate protein was extracted with sonication in lysis buffer and used in western blot as described elsewhere[14]. Antibodies to Pol II (sc-899) were purchased from Santa Cruz (California). Beta-Actin (ab6276) and MMP9 (ab16306) were from abcam (Cambridge, MA). Antibodies to SIRT1 (07-131) and Acetyl-histone 3 (07-353) were from Upstate Biotechnology (Lake Placid, NY). To detect multiple signals from one membrane, the membrane was stripped with a stripping buffer.
Immunoprecipitation (IP)
Immunoprecipitation was carried out using whole cell lysate (500 µg of total protein), 2–4 µg of antibody, and 20 µl of protein G-Sepharose beads (Amersham Biosciences) as described elsewhere[14]. The product was resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membrane for immunoblotting.
Plasmids and Transfection
Expression plasmid vectors for Gal4-Luc, Gal4-Jun (Cat.#219000, PathDetect® c-Jun trans-Reporting System) and AP1-Luc were purchased from Stratagene (La Jolla, CA). MMP9-Luc was a kind gift from Dr. Lu Jia at the department of microbiology and immunology, West Virginia University, Morgan Town, WV. The reporter assay was performed in HEK 293 cells by transient transfection. Transient transfection was conducted using LipofectAMINE as reported previously [15]. Expression vector for SIRT1 RNAi was a gift from Dr. Picard (Department of Biology, Massachusetts Institute of Technology, Massachusetts).
Chromatin immunoprecipitation (ChIP)
ChIP assay was conducted as described elsewhere [16]. Cells were maintained in 100 mm cell culture plate, pre-treated with 30 uM of Resveratrol and then 200 nM of PMA for 30 minutes after serum-starvation overnight, and collected after formaldehyde treatment. The chromatin DNA was extracted, broken into fragments of 400–1200bp in length by sonication, and immunoprecipitated with antibodies to the target, such as SIRT1, c-JUN, Pol II and Acetyl-histone 3. IgG was used in IP as a control for non-specific signal. DNA in the IP product was amplified in SYBR green RT-PCR. The ChIP assay primer sequences are as follows: forward GACTGTGGGCAGGGCATAGGG, reverse GCTGGGTGTCCGTG AGGTTGG in the mouse MMP9 gene promoter. The qRT-PCR reaction was conducted in following condition: 2× iTaqTM SYBR Green Supermix with ROX buffer (catalog no. 170-8850; Bio-Rad), 500 nM of each primer, and 5 µl of purified ChIP extract in a 20 µl reaction. 7900 HT Fast real-time PCR System (Applied Biosystems) was used to run the reaction.
Quantative RT-PCR
mRNA level of MMP9 was determined using Taqman quantative RT-PCR. The total RNA was extracted using the Trizol protocol. The PCR reaction was conducted in triplicates using Taqman probe and primers set for MMP9 (Mm00442991_m1) from Applied Biosystems. The mRNA signal was normalized over 18S ribosomal RNA. A mean value of the triplicates was used for relative mRNA level of MMP9.
Statistical Analysis
In this study, all of the experiments were conducted three times at least with consistent results. A mean value and standard error of multiple data points or samples were used to represent the final result. Student’s t test was used in statistical analysis of the data with significance P ≤ 0.05.
Results
Inhibition of c-JUN transcriptional activity by RSV
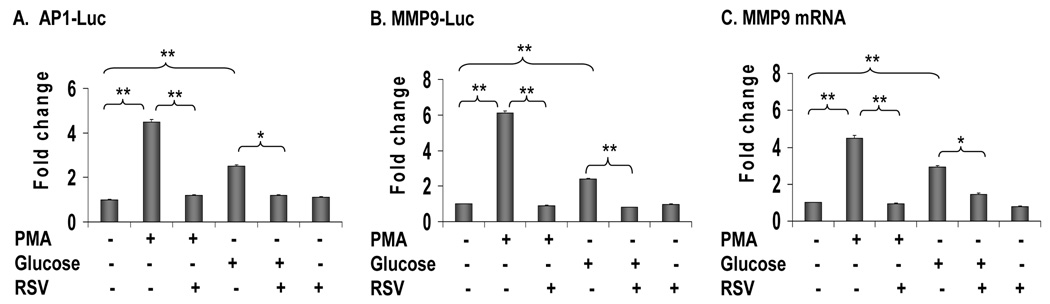
To study regulation of AP-1 by SIRT1, we activated SIRT1 with RSV, and then examined AP-1 activity by quantifying luciferase reporter activities and MMP9 mRNA. In the study, AP-1- or MMP9-specific luciferase reporters were transfected into HEK293 cells, and induced with PMA. Both reporters were induced by PMA, and inhibited by RSV (Fig. 1, A and B). To examine MMP9 gene expression, we used RAW264.7 cells (mouse macrophage cell line) that express a high level of MMP9. In the cells, MMP9 mRNA was induced by PMA, and the induction was inhibited by RSV (Fig. 1C). In these three assay systems, when PMA was replaced with glucose to activate AP-1, similar inhibitory activities were observed for RSV (Fig. 1, A–C). These data suggests that AP-1 activity may be inhibited by SIRT1 in cells treated with RSV. The inhibition was observed in transcription of MMP9 as indicated by MMP9-luciferase reporter and mRNA.
Fig. 1. (Gao) Inhibition of AP1 by SIRT1.
Inhibition of AP-1 by RSV. A–B. Reporter assay. AP-1-luc or MMP9-luc was transfected in HEK293 cells for 24 hours and serum-starved in 0.25% BSA DMEM overnight. The cells were pretreated with 30 uM of RSV for 30 minutes followed by the treatment with 200 nM of PMA or 50 mM of glucose for 4 hours. The luciferase activity was represented in fold changes. C. MMP9 mRNA expression. Raw 264.7 was starved in 0.25% BSA DMEM overnight. The cells were pretreated with 30 uM of RSV for 30 minutes followed by the treatment with 200nM of PMA or 50 mM of glucose for 4 hours. The total RNA were extracted with Tri reagent (Sigma) and MMP9 mRNA were quantified in Taqman real time RT-PCR. All the experiments were repeated 3 times and presented with Mean ± SEM. *, P<0.05, **, P<0.001 by Student’s t-test.
SIRT1 inhibited the transcriptional activity of c-JUN through a direct protein-protein association
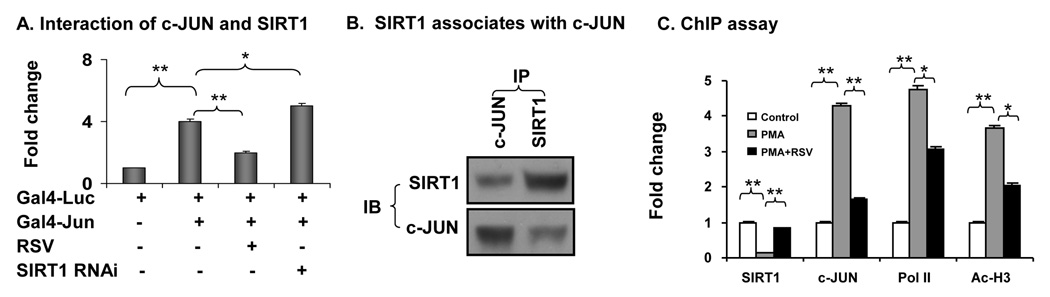
To investigate the SIRT1-JUN interaction, we used Gal4 system, immunoprecipitation and ChIP assay to determine relationship of the two proteins. In the two hybridization system, the reporter is driven by Gal4-JUN. In this system, the transcriptional activity of c-Jun was demonstrated in HEK293 cells through cotransfection of Gal4 luciferase reporter and Gal4-Jun expression vector (Fig. 2A). The c-Jun activity was inhibited by RSV. When SIRT1 was knocked down by RNAi, the inhibition was eliminated. Co-immunoprecipitation was conducted to examine protein-protein interaction for SIRT1 and c-JUN (Fig. 2B). In the precipitate of c-JUN, the SIRT1 protein was detected. In the precipitate of SIRT1, the c-JUN protein was observed. ChIP assay was conducted in the MMP9 gene promoter to determine SIRT1 function in modification of histone acetylation (Fig. 2C). In the basal condition, the SIRT1 protein was detected in the MMP9 promoter DNA. The SIRT1 signal was dramatically reduced by PMA treatment. PMA also increased signals acetylated-histone 3 in the promoter DNA. As expected in the system, c-JUN and Pol II (RNA polymerase II) were increased by PMA. When the cells were pretreated with RSV, this pattern of changes was reversed (Fig. 2C). The signal for SIRT1 was increased, and acetylated histone 3 was decreased together with c-Jun and Pol II. The data suggests that SIRT1 deacetylates histone 3 in the MMP9 gene promoter at the AP-1 response element.
Fig. 2. (Gao) Interaction of c-JUN and SIRT1.
SIRT1 inhibited the transcriptional activity of c-JUN through a direct protein-protein association. A. Yeast two hybrid assay in 293 cells. Expression vectors for Gal4-luc, Gal4-Jun, SIRT1 RNAi and SV40-renilla were transfected into HEK293 cells for 24 hours. The cells were pre-treated with 30 uM of RSV overnight. The luciferase and renilla were measured. The data was normalized with SV40 renilla and presented as fold changes. B. Immunoprecipitation. HEK293 were maintained in 5% FBS DMEM medium for 48 hours then harvested. Whole cell lysate protein (500 ug) was used in IP with anti-JUN or anti-SIRT1 antibodies. The c-JUN and SIRT1 proteins in the IP products were blotted in the immunoblot. C. ChIP assay. 3T3-L1 adipocytes were starved for 48 hours. The cells were pre-treated with 30 uM of RSV then followed by 200
Increased c-JUN activity in SIRT1−/− MEF
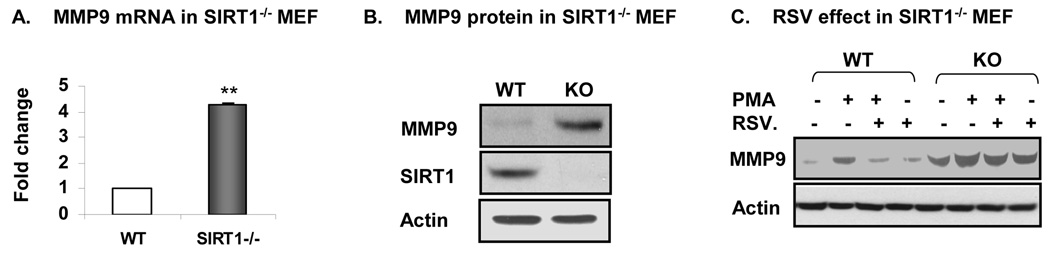
To further confirm the inhibition of c-JUN by SIRT1, we examined MMP9 expression in SIRT1 null cells. To address this question, the SIRT1−/− MEF and control wild type MEF cells were made from embryos. MMP9 expression was determined in mRNA by qRT-PCR (Fig. 3A). In the SIRT1 null cells, MMP9 mRNA was increased by 3 fold. The MMP9 protein was increased in the null cells by about 4 fold as indicated by the Western blot (Fig. 3B). In the blot, absence of SIRT1 was confirmed. When the cells were challenged with PMA, MMP9 protein was increased in the wild type MEFs (Fig. 3C). The induction was inhibited by RSV. In the SIRT1 null cells, the RSV activity was attenuated significantly. This group of data suggests that SIRT1 inhibits MMP9 expression in the wild type cells. This activity of SIRT1 was abolished in the SIRT1 null cells.
Fig. 3. (Gao) Activation of AP1 in absent of SIRT1.
Discussion
The MMP9 promoter has binding site for several transcription factors including AP-1, NF-κB, Sp1 and PEA3/Ets [17, 18]. Using expression cloning strategy, Nair et al. identified several putative regulators on MMP9 promoter such as SM2 and SIRT1. SIRT1 was found as a repressor for MMP9 [19]. However, the molecular mechanism by which SIRT1 inhibits MMP9 is not clear. In the current study, we provide evidence of SIRT1 interaction with c-JUN. The interaction was demonstrated in the gene promoter of MMP9, in which c-JUN induces the gene transcription, and SIRT1 inhibits the c-JUN activity. SIRT1 is able to inhibit NF-kB activity [20], and this relationship may be involved in mechanism of observations about MMP9 in this study. However, our data suggests that the specific interaction of SIRT1 and AP-1 is involved in the MMP9 regulation under PMA stimulation. The specificity is supported by results from the two hybridization, immunoprecipitation, and ChIP assay. In our system, PMA was use to activate c-JUN. The combination of PMA with ChIP assay provides strong support to the SIRT1-JUN interaction in the MMP9 gene promoter.
The current study may provide insight into many physiological or pathophysiological phenomenons. In the physiological conditions, SIRT1 activity is increased by fasting and reduced by high level of glucose [21]. Our study suggests that an increase in SIRT1 may contribute to the reduced AP-1 activity in calorie restriction [22, 23]. Out data also suggests that a reduction in SIRT1 may be involved in the increased AP-1 activity and MMP9 expression in diabetic patients with hyperglycemia [3]. Further, activation of SIRT1 by Resveratrol may be required for inhibition of PMA-induced expression of 11 MMP9 [10]. All these possibilities are supported by the SIRT1-JUN interaction observed in the current study. Our result is different with a report by Swatee Dey et al. in which SIRT1 was found to be essential for the activation of AP-1 in epithelial cells [24]. In that study, SIRT1 activity was determined with SIRT1 RNAi. SIRT1 null cells were not used. In our study, both RNAi and SIRT1 null cells were used. The results from both strategies consistently suggest that SIRT1 interacts with c-JUN leading to inhibition of AP-1 activity.
Acknowledgments
This study was supported by NIH funds (DK68036) and ADA research award (7-07-RA-189) to J. Ye. The qRT-PCR study was conducted in the genomic core that is supported by the CNRU Grant (1P30 DK072476) sponsored by NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1beta. J Biol Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 2.Vu TH, Shipley JM, Bergers G, Berger JE, Helms A, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tayebjee MH, Lim HS, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 in type 2 diabetes: effect of 1 year's cardiovascular risk reduction therapy. Diabetes Care. 2004;27:2049–2051. doi: 10.2337/diacare.27.8.2049. [DOI] [PubMed] [Google Scholar]
- 4.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80:51–57. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Gupta AK, Corry PM, Lee YJ. Hypoglycemia-induced c-Jun phosphorylation is mediated by c-Jun N-terminal kinase 1 and Lyn kinase in drug-resistant human breast carcinoma MCF-7/ADR cells. J Biol Chem. 1997;272:11690–11693. doi: 10.1074/jbc.272.18.11690. [DOI] [PubMed] [Google Scholar]
- 6.Balestrieri ML, Rienzo M, Felice F, Rossiello R, Grimaldi V, Milone L, Casamassimi A, Servillo L, Farzati B, Giovane A, Napoli C. High glucose downregulates endothelial progenitor cell number via SIRT1. Biochim Biophys Acta. 2008;1784:936–945. doi: 10.1016/j.bbapap.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 8.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008;373:341–344. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW, Kwon TK. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 11.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Zabolotny JM, Kim YB. Silencing insulin resistance through SIRT1. Cell Metab. 2007;6:247–249. doi: 10.1016/j.cmet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 15.Ye J, Zeidler P, Young S-h, Martinez A, Robinson VA, Jones W, Baron P, Shi X, Castranova V. Activation of Mitogen-activated Protein Kinase p38 and Extracellular Signal-regulated Kinase Is Involved in Glass Fiber-induced Tumor Necrosis Factor-alpha Production in Macrophages. J. Biol. Chem. 2001;276:5360–5367. doi: 10.1074/jbc.M008814200. [DOI] [PubMed] [Google Scholar]
- 16.Gao Z, Chiao P, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J Biol Chem. 2005;280:21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92-kDa Gelatinase B Promoter Activity by ras Is Mitogen-activated Protein Kinase Kinase 1-independent and Requires Multiple Transcription Factor Binding Sites Including Closely Spaced PEA3/ets and AP-1 Sequences. J. Biol. Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 18.Crowe DL, Brown TN. Transcriptional inhibition of matrix metalloproteinase 9 (MMP-9) activity by a c-fos/estrogen receptor fusion protein is mediated by the proximal AP-1 site of the MMP-9 promoter and correlates with reduced tumor cell invasion. Neoplasia. 1999;1:368–372. doi: 10.1038/sj.neo.7900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair RR, Boyd DD. Expression cloning of novel regulators of 92 kDa type IV collagenase expression. Biochem. Soc. Trans. 2005;33:1135–1136. doi: 10.1042/BST20051135. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 22.Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. Faseb J. 2005;19:1863–1865. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Jung KJ, Yu BP, Cho CG, Chung HY. Influence of aging and calorie restriction on MAPKs activity in rat kidney. Exp Gerontol. 2002;37:1041–1053. doi: 10.1016/s0531-5565(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 24.Dey S, Bakthavatchalu V, Tseng MT, Wu P, Florence R, Grulke EA, Yokel R, Dhar SK, Yang HS, Chen Y, St Clair DK. Interactions between SIRT1 and AP-1 reveal a Mechanistic Insight into the Growth Promoting Properties of Alumina (Al2O3) Nanoparticles in Mouse Skin Epithelial Cells. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn175. [DOI] [PMC free article] [PubMed] [Google Scholar]