Abstract
Synthesis and identification of novel phenylalkyl isoselenocyanates (ISCs), isosteric selenium analogs of naturally occurring phenylalkyl isothiocyanates (ITCs), as effective cytotoxic and anti-tumor agents is described. The structure-activity relationship comparison of ISCs with ITCs and effect of the increasing alkyl chain length in inhibiting cancer cell growth was evaluated on melanoma, prostate, breast, glioblastoma, sarcoma, and colon cancer cell lines. IC50 values for ISC compounds were generally lower than their corresponding ITC analogs. Similarly, in UACC 903 human melanoma cells, the inhibition of cell proliferation and induction of apoptosis were more pronounced with ISCs as compared to ITCs. Further, ISCs and ITCs effectively inhibited melanoma tumor growth in mice following intraperitoneal xenograft. A similar reduction in tumor size was observed at three times lower doses of ISCs as compared to corresponding ITCs.
Introduction
Isothiocyanates (ITCs) are naturally occurring compounds that are stored as thioglucoside conjugates, termed glucosinolates, in plants and cruciferous vegetables such as watercress, brussels sprouts, broccoli, cabbage, cauliflower, radish, turnip etc.1–3 They are among the most effective naturally occurring cancer chemopreventive agents,4 which inhibit carcinogenicity in animal models.5 In addition, epidemiological studies have demonstrated that the human consumption of isothiocyanates in vegetables decrease cancer risk.6, 7 This fact is supported by strong literature data that suggests that ITCs are effective chemopreventive agents for specific human cancers. ITCs have been shown to exhibit the anticarcinogenic effects through dual mechanisms occurring at the level of initiation of carcinogenesis by blocking Phase I enzymes (cytochrome P-450) that activate procarcinogens and by inducing Phase II enzymes that detoxify electrophilic metabolites generated by Phase I enzymes.8–13 ITCs are also known to block cell-cycle progression and induce apoptosis in human cancer cells suggesting that these agents act also at the post initiation and progression stages.12–16
The activity of ITCs has been shown to vary with varying alkyl chain length. While some reports show the increase in potency with increasing alkyl chain length,17–19 others have even reported a reverse phenomenon.20 Structure-activity studies have also demonstrated that increased lipophilicity or chain length of ITCs increases inhibitory potency against NNK-induced lung tumorigenesis in A/J mice.21 22 Conaway et al. have shown that synthetic ITCs can be generated with enhanced lipophilicity by increasing alkyl chain length up to 6 carbons.17 In addition, the elimination half-life (T1/2e) of ITCs with longer alkyl chain length has been shown to be higher than those with shorter alkyl chain length.17 Collectively, these data indicate that ITCs with a longer alkyl chain persist longer at the target organ site.
The well established chemopreventive properties of ITCs warranted a structure-activity study of synthetically modified ITC analogs to enhance their chemopreventive and chemotherapeutic efficacy. We hypothesized that isosteric replacement of sulfur in ITCs by selenium would result in more effective anticancer agents. The hypothesis was based on the observation that in comparison to the sulfur structural analogs, selenium compounds are much more active in cancer prevention.23 Furthermore, selenium supplementation is recognized for pharmacological intervention, especially in the clinical domain of cancer chemoprevention, and its use is not limited only to correct nutritional deficiencies. This is evident from the observation that epidemiological studies carried out for last three decades provide evidence of an inverse relationship between selenium intake and cancer mortality, and selenium supplementation in the diet or drinking water has been shown to inhibit neoplasms of the liver, skin, pancreas, colon and mammary glands.24, 25 Furthermore, two-thirds of animal studies showed reduced incidence of tumors triggered by chemical carcinogens or viruses following selenium supplementation.26 The organoselenium compounds have also been reported to inhibit initiation and post-initiation stages of chemical carcinogenesis.27 Mechanistically, organoselenium compounds have been suggested to activate certain pro-apoptotic genes linked to p53, NFκB and stress signal pathways thereby preventing tumorigenesis.28 In addition, selenium supplementation could provide significant therapeutic potential since patients with melanoma, colon, breast, ovary, pancreas, as well as head and neck cancers show decreased levels of selenium in whole blood or serum than do healthy control. Thus, in view of the known anti-cancer properties of ITCs and oraganoselenium coumpounds, we have developed isosteric selenium analogs [the phenylalkyl isoselenocyanates (ISCs), 2] of ITCs (1) (Figure 1). The objectives were to: (a) determine whether replacing sulfur in ITCs with selenium increases the cancer inhibitory potency; and (b) determine the optimal chain length for maximal anticancer activity. The overall goal was to establish whether altered chemical reactivity and lipophilicity, caused by the outlined structural modifications will affect potency of compounds and hence identify structural requirements necessary for optimal activity against multiple cancers. Thus, we synthesized a variety of selenium isostere of naturally occurring and synthetic ITCs and evaluated their effect on in vitro cell viability of melanoma, prostate, colon, glioblastoma, sarcoma, and breast cancers cell lines. The two classes of compounds were also evaluated for their ability to inhibit melanoma cell proliferation and induction of apoptosis in vitro and for inhibiting tumor development in preclinical melanoma xenograft.
Figure 1.
General structures of phenylalkyl isothiocyanates (1) and phenylalkyl isoselenocyanates (2).
Results
Synthesis of isoselenocyanates
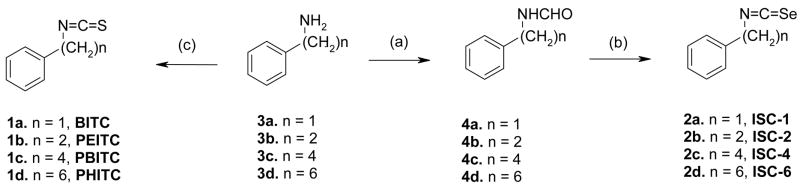
The first synthesis of isoselenocyanates was reported by Barton et al. 29 starting from the corresponding formamides. Several modifications30 in the procedure have been made since then to improve yields and avoid polymerization31 of intermediate compounds.29, 32 We have used the method of Fernandez-Bolanos et al.33 which conveniently uses solid triphosgene, instead of phosgene,29 in a one-pot dehydration of the formamides in refluxing dichloromethane (Scheme 1). The synthetic strategy involved the formylation of phenylalkylamines, followed by treatment with triphosgene and selenium powder in the presence of triethylamine to furnish the desired phenylalkyl isoselenocyanates (2) in good yields. Compounds were purified by silica gel column chromatography and characterized by 1H NMR and high-resolution MS.
Scheme 1.
Synthesis of ITCs 1 and ISCs 2; Reagents and conditions: (a) C2H5OCHO, −20°C to reflux (b) Et3N, triphosgene, Se powder, CH2Cl2, reflux (c) CSCl2, NaOH.
SAR Study on cancer cell lines
The ITCs (1) and the corresponding ISCs (2) were tested for their ability to inhibit cell growth in six cancer cell lines e.g. melanoma (UACC 903), breast (MDA-MB-231), glioblastoma (T98G), fibrosarcoma (HT-1080), colon (Caco-2) and prostate (PC-3) cancer cell lines. The IC50 values for compounds 1 and 2 are depicted in Table 1. The IC50 values consistently decreased with increasing alkyl chain length of ITCs in case of glioblastoma, breast, and prostate cancer cell lines; but showed no particular trend in fibrosarcoma, colon and melanoma cells. In case of melanoma cell line, the IC50 values remained essentially the same for all ITCs while in case of colon cancer cell line the IC50 values actually increased with increasing carbon chain length of ITCs. Among ISC derivatives, ISC-1 was least effective at killing cancer cells compared to higher alkyl chain analogs ISC-2 to ISC-6. The difference was more striking in glioblastoma, prostate and breast cancer cell lines, while in other cancer cell lines, the difference between ISC-1 and higher alkyl chain ISCs was not significant. Among ISC-2, ISC-4 and ISC-6 there was no particular trend. In almost all the cases (except for comparable values of ISC-1 and ISC-2 with BITC and PEITC in UACC 903 cells) the ISC derivatives had lower IC50 values than corresponding ITCs. This suggested superiority of ISC compounds over ITCs and that lower concentrations of selenium analogs would be required for similar therapeutic efficacy.
TABLE 1.
IC50 (μM) of ITC and ISC derivatives on different cancer cells.
| Cancer cell lines IC50 (μM) |
||||||
|---|---|---|---|---|---|---|
| Compounds | Breast MDA-MB-231 | Glioblastoma T98G | Prostate PC-3 | Fibrosarcoma HT-1080 | Colon* Caco-2 | Melanoma UACC 903 |
| 1a (BITC) | 42±3 | >100 | >50 | >50 | 15±2 | 15±3 |
| 1b (PEITC) | 38±6 | >100 | 24±2 | 15±1 | 14±2 | 12±1 |
| 1c (PBITC) | 27±2 | 35±1 | 24±2 | 15±1 | 27±2 | 16±1 |
| 1d (PHITC) | 24±2 | 26±2 | 17±1 | 29±3 | 49±9 | 15±2 |
| 2a (ISC-1) | 29±2 | 43±4 | 24±1 | 13±3 | 13±3 | 16±3 |
| 2b (ISC-2) | 20±3 | 24±1 | 16±1 | 12±3 | 11±1 | 12±4 |
| 2c (ISC-4) | 21±6 | 27±1 | 19±1 | 11±1 | 12±3 | 10±3 |
| 2d (ISC-6) | 22±2 | 23±2 | 14±1 | 12±1 | 10±1 | 10±1 |
Values are mean ± S.E.
Drug treatment for 72 h
In vitro studies on melanoma cells
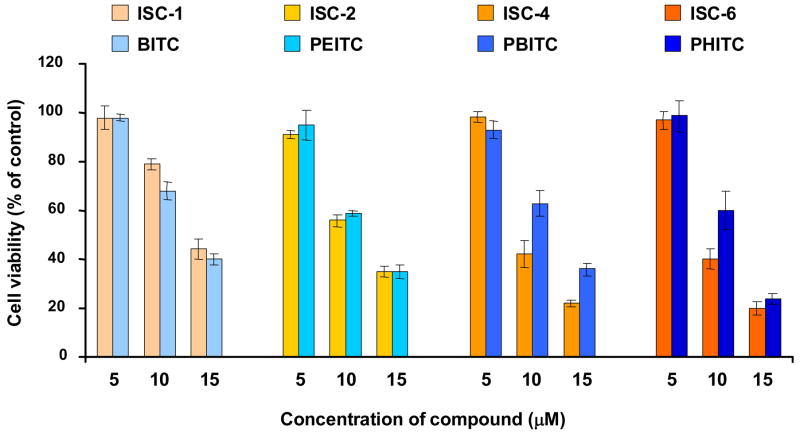
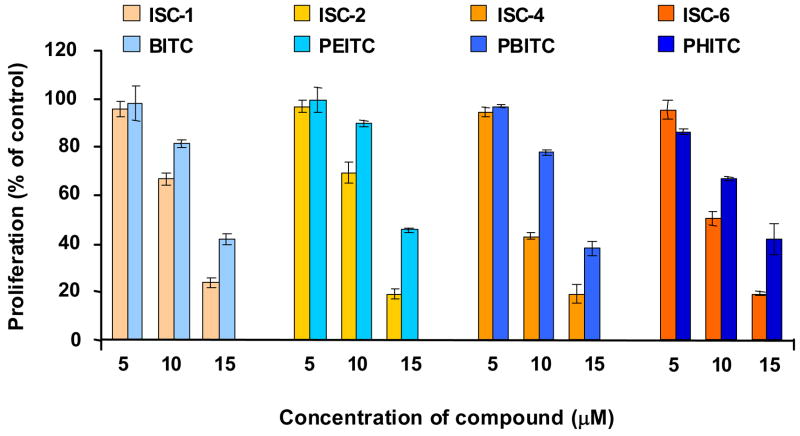
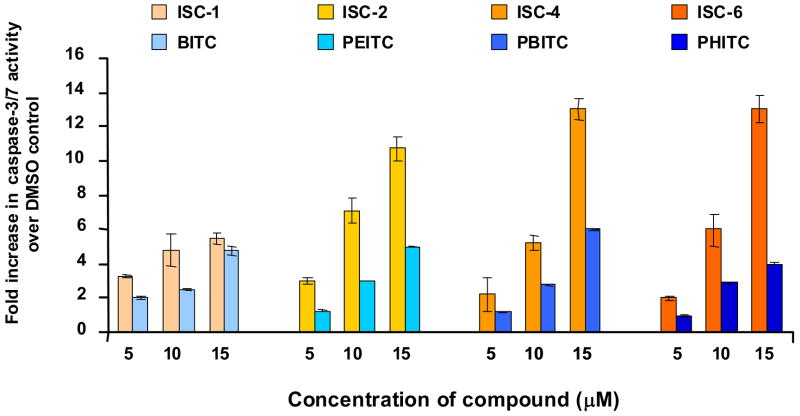
For melanoma cells (UACC 903), increase in chain length or changing from ITCs to ISCs by replacing sulfur with selenium, led to an insignificant change in IC50 values (Table 1) indicating that both ISCs and ITCs are capable of inhibiting melanoma cell growth equally. Cell viability was measured using the MTS assay (Figure 2) which determines the capacity of mitochondrial dehydrogenase in viable cells to transform tetrazolium salt into colorimetric formazan.34 UACC 903 human melanoma cells (5 × 103) were plated in a 96 well plate and 24 hours later exposed to DMSO or increasing concentrations (5μM, 10μM, 15μM) of ITCs (1) or ISCs (2) for 24 hours. MTS assay showed a dose dependent decrease in cell viability for both ISC and ITC series of compounds. There was no significant difference in activities between the two series; longer alkyl chain analogs ISC-4 (at 10 and 15 μM concentrations) and ISC-6 (at 10 μM concentration) were found a little more effective than corresponding ITC derivatives. To establish the potency of ISCs in comparison to ITCs and quantify the effect of increasing chain length of these agents, cell proliferation and apoptosis was carried out in UACC 903 human melanoma cells. Cellular proliferation following ISC and ITC derivatives treatment was measured using bromodeoxyuridine (BrdU) incorporation which is a convenient tool to monitor DNA synthesis within the cells. A dose dependent decrease in number of proliferating cells was observed upon treatment with ITC or ISC derivatives (Figure 3). In general, ISC derivatives were more effective compared to corresponding ITCs with similar alkyl chain length, especially at higher concentrations. The caspase 3/7 activity, which is reflective of apoptosis, was determined using Apo-ONE Homogenous caspase-3/7 Assay kit (Promega Corporation, Madison, WI). A dose dependent increase in caspase-3/7 activity was observed for both ITCs and ISCs. In comparison to ITCs, ISC derivatives exhibited much higher caspase activity. While the difference was not significant between BITC and ISC-1, there was a striking difference between longer alkyl chain derivatives of ITCs and ISCs, especially at higher concentrations of 10 and 15 μM (Figure 4). ISC-4 and ISC-6 were found to be the most effective inducer of apoptosis among all other compounds tested.
Figure 2.
MTS assay showing the viability of UACC 903 cells after treatment with ISC and ITC derivatives. Both ITCs (BITC, PEITC, PBITC and PHITC) and ISCs (ISC-1, ISC-2, ISC-4 and ISC-6) effectively reduced cell viability compared to DMSO control. The average value is represented as the percentage of control DMSO treated cells. The values for all experiments represent mean with bars indicating SEM from three independent experiments.
Figure 3.
Proliferation profile of UACC 903 cells after treatment with ISC and ITC derivatives. 5×103 cells were treated with DMSO or increasing concentrations of ITCs or ISCs for 24 hours and proliferating cells measured using BrdU labeling. The average value represented as the percentage of control DMSO treated cells. The values for all experiments represent mean with bars indicating SEM from three independent experiments.
Figure 4.
Levels of caspase-3/7 activity (an indicator of apoptosis) in cells exposed to ITCs or ISCs were measured using the Apo-ONE homogeneous caspase-3/7 assay kit. Results show fold increase in caspase-3/7 activity relative to DMSO vehicle treated cells. Results represent average of three independent experiments; bars, SEM. Compared to DMSO control, increasing concentrations of ISCs or ITCs elevated caspase-3/7 activity in a dose dependent manner. ISC-2, ISC-4 and ISC-6 showed significantly higher caspase-3/7 activity compared to their corresponding ITCs.
ISCs reduce melanoma tumor development more effectively than ITCs
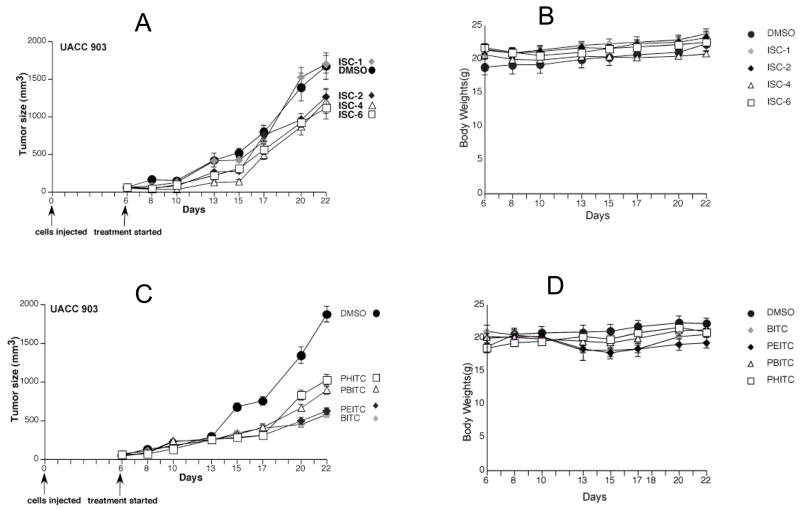
The in vitro studies in general suggested the superiority of ISC compounds over ITC derivatives. To further establish the effect in vivo, the efficacy of ISC and ITC compounds for inhibiting tumor development were evaluated in preclinical mouse model of melanoma. Nude mice were subcutaneously injected with UACC 903 cells and tumor development was allowed to occur for six days, by which time tumors have undergone vascularization (angiogenesis). Mice were then treated i.p with ISC and ITC derivatives three times a week on Mondays, Wednesdays and Fridays. At a dose of 0.76 μM (3 ppm of selenium), ISC-2, ISC-4 and ISC-6 showed a 30–45% reduction in tumor size (Figure 5A). ISC-1 had no effect at this concentration. Notably, none of the ITC derivatives were effective in reducing tumor size at 0.76 μM (data not shown). However, at a dose of 2.5 μM (~3 times that used for ISC compounds) a 40–60% reduction in tumor size was observed. No evidence of systemic toxicity was observed at the doses used for any of the ISC or ITC derivatives (Figure 5B and 5D). Interestingly, a reverse trend of chain length effect was observed i.e. the potency actually decreased with increasing alkyl chain length in ITCs, with BITC being the most effective. A similar trend in behavior was observed by Xiao et al.20 in study where they observed BITC to be more effective compared to PEITC in breast cancer cell lines.
Figure 5. In vivo melanoma tumor inhibition using ISC and ITC derivatives.
Six days after subcutaneous injection of UACC 903 cells, mice were treated i. p. with ISC or ITC derivatives thrice per week. Both ISC and ITC derivatives significantly reduced tumor development, however, the concentration of ISC (0.76 μmol) was 3 fold less than ITCs (2.5 μmol) (Figures 5A and 5C). At a dose of 0.76 μmoles, ISC-2, ISC-4 and ISC-6 showed about 30–45% reduction in tumor size (Figure 5A). ISC-1 failed to show any effect at this concentration. ITC derivatives were also effective in reducing the tumor size at 2.5 μmoles (~3 times higher than ISC compound) (Figure 5C). Figures 5B and 5D show the body weights of mice treated with ISC and ITC derivatives, respectively, compared to the control DMSO vehicle treated mice. No significant difference in weights was detected between groups, demonstrating negligible systemic toxicity.
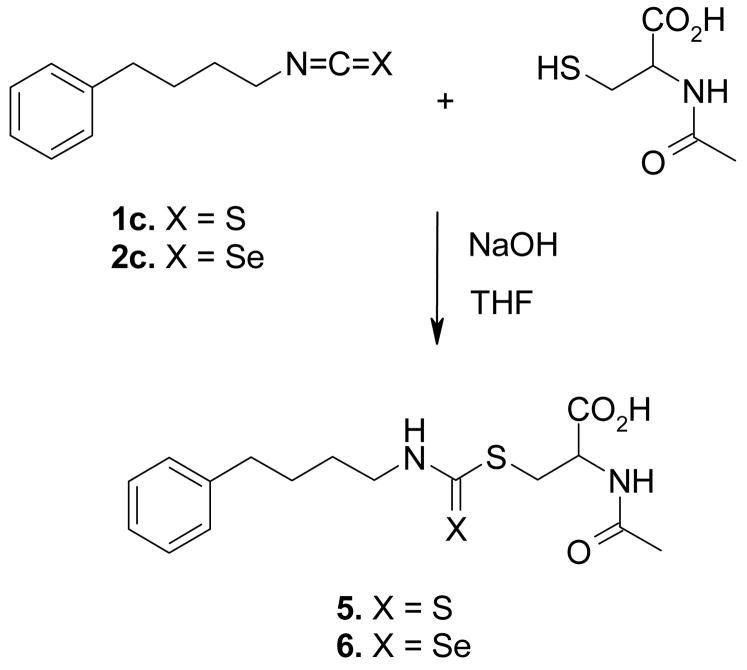
Synthesis and cell viability of N-acetylcysteine (NAC) conjugates
The activity of ITCs is reported to be through the formation of NAC conjugates, and presumably the ISCs may follow a similar mechanism of action. To determine that we synthesized NAC conjugate of representative ISC-4 and the corresponding PBITC by treating them with NAC in THF in the presence of NaOH (Scheme 2). The conjugates were characterized on the basis of 1H NMR and high resolution MS spectra. The cell viability measurements on UACC 903 cells using MTS assay revealed an IC50 value of 17 ± 0.5 μM for the ISC-4-NAC conjugate (6) compared to 10 ± 3 μM for ISC-4, and 24 ± 1 μM for PBITC-conjugate (5) compared to 16 ± 1 μM for PBITC.
Scheme 2.
Synthesis of NAC conjugates of PBITC and ISC-4.
Discussion
ITCs (R-N=C=S) are electrophilic compounds and are known to react predominantly with thiols, and to a much lesser extent with NH2 and OH groups.35 Therefore, the major route of metabolism and elimination of ITCs36 from the body is the mercapturic acid pathway i.e. by formation of nonenzymatic and enzymatic conjugation with glutathione (GSH) to give thiol conjugates. Stepwise enzymatic hydrolysis of GSH conjugates of ITCs yields L-cysteine (Cys) conjugate (ITC-Cys), which on subsequent acetylation gives N-acetyl-L-cysteine (NAC) conjugates of ITCs (NAC-ITCs).37, 38 NAC conjugates of ITCs have been detected as the main metabolite in the urine of rodents and humans.38 NAC and thiol conjugate of ITCs have shown a chemopreventive efficacy against TSNA and PAH-induced lung tumorigenesis in rodents.39–41 Conaway et al. have also shown that ITC conjugates have longer half-life than the parent isothiocyanates.39 ISCs, being structurally similar to ITCs, are expected to follow a similar metabolic and elimination pathway. Thus, like ITCs, the activity of ISCs may also be due to the formation of NAC-ISC conjugate metabolites. Our results have indicated the IC50 value of ISC-4- NAC conjugate (6) (17 ± 0.5 μM) compared to ISC-4 (10 ± 3 μM) and IC50 value of PBITC-NAC conjugate (5) (24 ± 1 μM) compared to PBITC (16 ± 1 μM), were consistent with the trend reported in the literature for ITC and their corresponding conjugates,42 where NAC conjugates have been shown to be slightly less effective than the parent ITC. However, the comparable activity of ISC-4 and the corresponding NAC conjugate indicates that, similar to ITCs, the main route of metabolism and hence the in vivo activity of ISCs may be mediated by metabolic transformation to GSH conjugates.
Literature reports have attributed the increased potency with the increasing chain length of ITCs, to the increase in lipophilicity.17 It has been suggested that increasing lipophilicity decreases the reactivity of ITCs with glutathione (GSH) resulting in slowing down of excretion and thus enhancing the potency of the compound.43 In an attempt to correlate ITC and ISC data to their lipophilicity, we calculated the LogP value of both ITC and ISC series of compounds using ChemDraw 9.0 Ultra. The LogP values increased with increasing chain length in both the series with ISC compounds in general having a slightly higher value than the corresponding ITC analogs (Table 2). The observed reverse trend of tumor inhibition decreasing with increasing alkyl chain length in melanoma model (Figure 5C) suggests that lipophilicity is not the only determining factor in case of ITCs. This reverse trend is in agreement with the Xiao et al. results in breast cancer cells.20 ISC compounds however, showed the increased efficacy both in vitro and in vivo with increasing lipophilicity. Overall the results suggest the participation of other pharmacokinetic and pharmacodynamic factors that influence the efficacy of these compounds. Both ISC-4 and ISC-6 were equally effective in vitro and in inhibiting tumor growth. However, the higher lipophilicity of ISC-6 (LogP, 5.472), just like PHITC, makes it less suitable as a probable chemo-preventive/therapeutic agent. Higher lipophilicity of PHITC has been linked to the observed induction of esophageal carcinogenesis in Fisher 344 rats.44 In view of this, ISC-4 is the best suitable agent for evaluation as a drug.
TABLE 2.
Calculated LogPa (CLogP) values of ITC and ISC compounds.
| ITCs | CLogP | ISCs | CLogP |
|---|---|---|---|
| 1a (BITC) | 3.204 | 2a (ISC-1) | 3.177 |
| 1b (PEITC) | 3.263 | 2b (ISC-2) | 3.506 |
| 1c (PBITC) | 4.171 | 2c (ISC-4) | 4.414 |
| 1d (PHITC) | 5.229 | 2d (ISC-6) | 5.472 |
LogP was estimated using ChemDraw 9.0 Ultra
Conclusion
In summary, ISCs, the isosteric selenium analogs of well known naturally occurring and synthetic anticancer agents ITCs, have been developed. We have demonstrated that by isosterically replacing sulfur with selenium, we were able to significantly improve antitumor activity of ITCs. The ISC compounds were more efficient in inhibiting cell growth in melanoma, glioblastoma, fibrosarcoma, colon, breast, and prostate cancers cells as compared to corresponding ITC analogs. Furthermore, in general, the efficacy increased with increasing alkyl chain length both in case of ITC and ISC compounds. In preclinical melanoma xenograft model, the ISC compounds showed similar tumor inhibition at three times lower doses as compared to ITC derivatives without any measurable systemic toxicity. Interestingly, tumor inhibitory effect decreased with increasing chain length in ITCs while it increased with increasing chain length in case of ISCs. Collectively, based on both in vitro and in vivo experiments, ISC-4 proved to be the most effective agent among the series of ISC and ITC analogs tested. ISC-4 thus holds promise of being an effective chemopreventive/chemotherapeutic agent.
Experimental Section
General
Melting points were recorded on a Fischer-Johns melting point apparatus and are uncorrected. NMR spectra were recorded using a Varian 300 MHz NMR spectrometer or a Bruker Avance 500 MHz spectrometer. Chemical shifts (δ) were reported in parts per million downfield from the internal standard. The signals are quoted as s (singlet), d (doublet), t (triplet), m (multiplet) and dt (doublet of triplet). HRMS were determined at the Chemistry Instrumentation Center, State University of New York at Buffalo, NY. Thin-layer chromatography (TLC) was developed on aluminum-supported pre-coated silica gel plates (EM industries, Gibbstown, NJ). Column chromatography was conducted on silica gel (60–200 mesh). Benzyl isothiocyanate (BITC, 1a), phenethyl isothiocyanate (PEITC, 1b) were obtained from commercial sources. Phenylbutyl isothiocyanate (PBITC, 1c) and phenylhexyl isothiocyanate (PHITC, 1d) were synthesized according to a literature method.21
General method for synthesis of phenylalkyl formamides
Formamides (4b–d) were synthesized following a literature method.45 Briefly, ethyl formate (120 mmol) was added dropwise to phenylalkylamine (40 mmol) at room temperature and the resulting mixture was refluxed for 4–6 h. The excess ethyl formate was removed under reduced pressure to yield the corresponding phenylakylformamide as colorless viscous oils.
Phenylethyl formamide (4b)
Yield, 96%; viscous oil; 1H NMR (CDCl3, 300 MHz) δ 2.84 (t, 2H, J =6.9 Hz), 3.57 (dt, 2H, J = 6.9 Hz and 6.6 Hz), 5.68 (br d, 1H, NH), 7.15–7.35 (m, 5H), 8.12 (s, 1H, CHO); HRMS (EI) calcd for C9H11NO, 149.0835; found, 149.0839.
Phenylbutyl formamide (4c)
Yield, 98%; viscous oil; 1H NMR (CDCl3, 500 MHz) δ 1.56–1.62 (m, 2H), 1.66–1.72 (m, 2H), 2.66 (t, 2H, J = 6.5 Hz), 3.35 (dt, 2H, J = 7.0 and 6.5 Hz), 5.92 (br s, 1H), 7.18–7.24 (m, 3H), 7.29–7.33 (m, 2H), 8.19 (s, 1H); HRMS (EI) calcd for C11H15NO, 177.1148; found, 177.1149.
Phenylhexyl formamide (4d)
Yield, 91%; viscous oil; 1H NMR (CDCl3, 500 MHz) δ 1.36–1.41 (m, 4H), 1.52– 1.58 (m, 2H), 1.61–1.67 (m, 2H), 2.63 (t, 2H, J = 7.5 Hz), 3.30 (dt, 2H, J = 7.0 and 6.5 Hz), 5.58 (br s, 1H), 7.18–7.21 (m, 3H), 7.28–7.31 (m, 2H), 8.19 (s, 1H); HRMS (EI) calcd for C13H19NO, 205.1461; found, 205.1462.
Benzyl isoselenocyanate (ISC-1, 2a)
To a refluxing mixture of the benzyl formamide (1.35 g, 10.0 mmol), triethylamine (4.35 g, 6.0 mL, 43.0 mmol) in CH2Cl2 (35 mL) and 4 Å molecular sieves was added dropwise a solution of triphosgene (1.48 g, 5.0 mmol) in CH2Cl2 (15 mL) for a period of 1 h. After the addition was complete, the mixture was refluxed for an additional 2.5 h. Selenium powder (1.58 g, 20 mmol) was then added and the resulting mixture was refluxed for additional 7 h. The mixture was cooled, filtered, and the solvent was evaporated to yield the crude mixture, which was purified by silica gel column chromatography (EtOAc/hexanes 3:97) to afford 1.21 g (62 %) of 2a as a viscous oil. 1H NMR (CDCl3, 300 MHz) δ 4.81 (s, 2H, CH2), 7.30–7.44 (m, 5H); HRMS (EI) calcd for C8H7NSe, 196.9738; found, 196.9741.
Phenylethyl isoselenocyanate (ISC-2, 2b)
A mixture of phenethyl formamide (0.67 g, 4.5 mmol), triethylamine (1.94 g, 2.67 mL, 19.2 mmol), 4 Å molecular sieves, triphosgene (0.65 g, 2.2 mmol), and selenium powder (0.71 g, 9.0 mmol) in CH2Cl2 (35 mL) was refluxed and worked up as mentioned above for 2a. The crude residue thus obtained was purified by silica gel column chromatography (EtOAc/hexanes 5:95) to give 0.62 g (65%) of 2b as an oil. 1H NMR (CDCl3, 300 MHz) δ 3.03 (t, 2H, J = 6.9 Hz), 3.81 (t, 2H, J = 6.9 Hz) 7.20–7.38 (m, 5H); HRMS (EI) calcd for C9H9NSe, 210.9895; found, 210.9892.
Phenylbutyl isoselenocyanate (ISC-4, 2c)
A mixture of phenylbutyl formamide (1.77 g, 10.0 mmol), triethylamine (4.35 g, 6.0 mL, 43 mmol), 4 Å molecular sieves, triphosgene (1.48 g, 5.0 mmol), and selenium powder (1.58 g, 20.0 mmol) in CH2Cl2 (50 mL) was refluxed and worked up as mentioned above for 2a. The crude residue thus obtained was purified by silica gel column chromatography (EtOAc/hexanes 5:95) to give 1.7 g (71%) of 2c as an oil. 1H NMR (CDCl3, 300 MHz) δ 1.74–1.76 (m, 4H), 2.66 (t, 2H, J = 6.6 Hz), 3.60 (t, 2H, J = 5.6 Hz), 7.15–7.32 (m, 5H); HRMS (EI) calcd for C11H13NSe, 239.0208; found, 239.0211.
Phenylhexyl isoselenocyanate (ISC-6, 2d)
A mixture of phenylbutyl formamide (1.64 g, 8.0 mmol), triethylamine (3.49 g, 4.8 mL, 34.5 mmol), 4 Å molecular sieves, triphosgene (1.19 g, 4.0 mmol), and selenium powder (1.26 g, 16.0 mmol) in CH2Cl2 (40 mL) was refluxed and worked up as mentioned for 2a. The crude residue thus obtained was purified by silica gel column chromatography (EtOAc/hexanes 3:97) to give 1.35 g (63%) of 2d as an oil. 1H NMR (CDCl3, 500 MHz) δ 1.37–1.43 (m, 2H), 1.45–1.51 (m, 2H), 1.64–170 (m, 2H), 1.72–1.78 (m, 2H), 2.64 (t, 2H, J = 7.6 Hz), 3.60 (t, 2H, J = 6.7 Hz), 7.19–7.25 (m, 3H), 7.32–7.40 (m, 2H); HRMS (EI) calcd for C13H17NSe, 267.0521; found, 267.0529.
PBITC-NAC conjugate (5)
To a solution of 1c (382 mg, 2.0 mmol) in THF (25 mL) at 0 °C was added a solution of N-acetylcysteine (326 mg, 2.0 mmol) in water (15 mL) and to this mixture was added 2% NaOH (0.1 mL). The reaction mixture was allowed to warm to room temperature and stirred for 12 h. The mixture was washed with hexane (2 × 5 mL), the aqueous layer was acidified 2N HCl and extracted with ethyl acetate. Organic layer was separated, dried (MgSO4) and concentrated in vaccuo to give 5 as a viscous oil in quantitative yield. 1H NMR (CD3OD, 500 MHz) δ 1.67–1.70 (m, 4H), 1.96 (s, 3H), 2.01–2.03 (m, 2H), 2.64–2.67 (m, 2H), 3.69–3.74 (m, 2H), 4.67 (dd, 1H, J = 8.5 and 4.5 Hz), 7.14–7.22 (m, 3H), 7.25–7.28 (m, 2H); HRMS (ESI) calcd for C16H22N2O3S2.H+, 355.1145; found, 354.1144.
ISC-4-NAC conjugate (6)
To a solution of 2c (238 mg, 1.0 mmol) in THF (15 mL) at 0 °C was added a solution of N-acetylcysteine (163 mg, 1.0 mmol) in water (7.0 mL) and to this mixture was added 2% NaOH (50 μL). Following similar reaction conditions and work up as mentioned for 5, gave 6 as a viscous oil in quantitative yield. 1H NMR (CD3OD, 500 MHz) δ 1.73–1.78 (m, 2H), 1.80–1.84 (m, 4H), 1.98 (s, 3H), 2.69 (t, 2H, J = 8.0 Hz), 3.63–3.67 (m, 2H), 4.69–4.73 (m, 1H), 7.17–7.30 (m, 5H), 7.67 (d, 1H, J = 7.0 Hz), 9.92 (br s, 1H); HRMS (ESI) calcd for C16H22N2O3SSe.H, 403.0589; found, 403.0576.
Cell lines and culture conditions
Colon adenocarcinoma cell line (Caco-2, ATCC No. HTB-37) was grown in Advanced DMEM supplemented with 10% heat treated (56°C for 30 minutes) FBS and L-glutamine. Fibrosarcoma (HT-1080; ATCC No. CCL-121), prostate adenocarcinoma (PC-3; ATCC No. CRL-1435), breast adenocarcinoma cell line (MDA-MB-231; ATCC No. HTB-26), glioblastoma cell line (T98G; ATCC No. CRL-1690) and human melanoma cell line UACC 903 were grown in DMEM supplemented with 10% FBS.
Cell viability, proliferation, and apoptosis determination
In vitro inhibitory efficacy of cancer cell lines representing different cancer types following treatment with ITC and ISC was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, WI). In brief, 2.5 – 5 × 103 cells per well in 100 μL DMEM containing 10% FBS were grown in a 96-well plate for 24 h and treated with either control DMSO vehicle or increasing concentrations (2.5 –100 μM) of ITC and ISC for 24 hours. The percentages of viable cells compared to control DMSO treated cells were determined using MTS assay and IC50 values calculated using GraphPad Prism version 4.01 (GraphPad software, San Diego, CA). IC50 value for each compound was determined by at least three independent experiments and represented with a standard error (Table 1).
Cellular proliferation and apoptosis rates were measured by seeding 5 × 103 human melanoma cell line UACC 903 in 96-well plate, followed by treatment for 24 hours with ITCs or ISCs. Proliferation and apoptosis rates were measured using a BrdU ELISA kit (Roche Applied Sciences, Indianapolis, IN) or Apo-ONE Homogenous caspase-3/7 Assay kit (Promega Corporation, Madison, WI), respectively.
Tumorigenicity assessment
Animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University College of Medicine. Tumor kinetics were measured by subcutaneous injection of 5×106 UACC 903 melanoma cells in 0.2 ml of DMEM supplemented with 10% FBS above both left and right rib cages of 4–6 week old female athymic nude mice (Harlan Sprague Dawley, Indianapolis, IN). Six days later mice were randomly divided in to control (DMSO) and experimental (ISC-1, ISC-2, ISC-4, ISC-6, BITC, PEITC, PBITC, PHITC) groups (5 mice/group; 2 tumors/mouse). Six days after subcutaneous injection of UACC 903 melanoma cells, mice were treated i. p. with ITC (2.5 μmoles) or ISC (0.76 μmoles, equivalent to 3 ppm selenium) three times per week. (Monday, Wednesday and Friday). Control mice received an equal volume of the vehicle. The Dimensions of the developing tumors (using calipers) and body weight were measured three times a week (Monday, Wednesday and Friday) and the size estimated in cubic millimeters.
Statistical analysis
Statistical analysis was undertaken using the One-way ANOVA followed by an appropriate post hoc test. Results were considered significant at a P-value of <0.05.
Supplementary Material
Copies of the 1H NMR spectra for compounds 1c, 1d, 2a, 2b, 2c, 2d, 4b, 4c, 4d, 5, and 6; and 1H NMR data for compounds 1c and 1d. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This study was supported by the Elsa U. Pardee Foundation, Melanoma Research Foundation and NIH (CA-127892-01A) Grants. The project was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The authors thank Dr. Jyh-Ming Lin, Solution Phase NMR Facility at Core Research Facilities of the Penn State Hershey College of Medicine, for recording of NMR spectra.
References
- 1.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 2.Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr. 2000;72(6):1424–35. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- 3.Cinciripini PM, Hecht SS, Henningfield JE, Manley MW, Kramer BS. Tobacco addiction: implications for treatment and cancer prevention. J Natl Cancer Inst. 1997;89(24):1852–67. doi: 10.1093/jnci/89.24.1852. [DOI] [PubMed] [Google Scholar]
- 4.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555(1–2):191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Bianchini F, Vainio H. Isothiocyanates in cancer prevention. Drug Metab Rev. 2004;36(3–4):655–67. doi: 10.1081/dmr-200033468. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733–48. [PubMed] [Google Scholar]
- 7.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54(7 Suppl):1976s–1981s. [PubMed] [Google Scholar]
- 10.Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57(17):3649–52. [PubMed] [Google Scholar]
- 11.Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273(3):1769–75. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 12.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58(3):402–8. [PubMed] [Google Scholar]
- 13.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. 1997;10(11):1228–33. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 14.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38(2):168–78. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 15.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21(12):2287–91. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 16.Chung FL, Jiao D, Conaway CC, Smith TJ, Yang CS, Yu MC. Chemopreventive potential of thiol conjugates of isothiocyanates for lung cancer and a urinary biomarker of dietary isothiocyanates. J Cell Biochem Suppl. 1997;27:76–85. [PubMed] [Google Scholar]
- 17.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab Dispos. 1999;27(1):13–20. [PubMed] [Google Scholar]
- 18.Morse MA, Eklind KI, Amin SG, Hecht SS, Chung FL. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989;10(9):1757–9. doi: 10.1093/carcin/10.9.1757. [DOI] [PubMed] [Google Scholar]
- 19.Lui VW, Wentzel AL, Xiao D, Lew KL, Singh SV, Grandis JR. Requirement of a carbon spacer in benzyl isothiocyanate-mediated cytotoxicity and MAPK activation in head and neck squamous cell carcinoma. Carcinogenesis. 2003;24(10):1705–12. doi: 10.1093/carcin/bgg127. [DOI] [PubMed] [Google Scholar]
- 20.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5(11):2931–45. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 21.Morse MA, Eklind KI, Hecht SS, Jordan KG, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991;51(7):1846–50. [PubMed] [Google Scholar]
- 22.Jiao D, Eklind KI, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54(16):4327–33. [PubMed] [Google Scholar]
- 23.Ip C, Ganther HE. Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis. 1992;13:1167–1170. doi: 10.1093/carcin/13.7.1167. [DOI] [PubMed] [Google Scholar]
- 24.El-Bayoumy K. Cancer Principles and Practice of Oncology. 4. J.B. Lippincott; Philadelphia: 1991. The role of selenium in cancer prevention; pp. 1–15. [Google Scholar]
- 25.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79(3):179–92. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 26.Combs GF, Jr, Lu J. Selenium as a cancer preventive agent Selenium: Its Molecular Biology and Role in Human Health. 2001:205–218. [Google Scholar]
- 27.El-Bayoumy K. Overview: the late Larry C. Clark showed the bright side of the moon element (selenium) in a clinical cancer prevention trial. Nutr Cancer. 2001;40(1):4–5. doi: 10.1207/S15327914NC401_3. [DOI] [PubMed] [Google Scholar]
- 28.Zeng H, Davis CD, Finley JW. Effect of selenium-enriched broccoli diet on differential gene expression in min mouse liver(1,2) J Nutr Biochem. 2003;14(4):227–31. doi: 10.1016/s0955-2863(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 29.Barton DHR, Parekh SI, Tajbakhsh M, Theodorakis EA, Tse CL. A convenient and high yielding procedure for the preparation of isoselenocyanates. Synthesis and reactivity of -alkylselenocarbamates. Tetrahedron. 1994;50:639–654. [Google Scholar]
- 30.Garud DR, Koketsu M, Ishihara H. Isoselenocyanates: a powerful tool for the synthesis of selenium-containing heterocycles. Molecules. 2007;12(3):504–35. doi: 10.3390/12030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel G, Marchand E, Sinbandhit S, Carlier R. α-Thioxothioamides: A Formal [4+1] Cycloaddition Reaction with Isocyanides and Diisocyanides and its Application to a New Straightforward Formation of Extended Tetrathiafulvalenes. Eur J Org Chem. 2001:655–662. [Google Scholar]
- 32.Xu WK, Liang XR. An efficient and convenient route to some isoselenocyanates via reaction of formamides with bis(trichloromethyl)carbonate and selenium. J Indian Chem Soc. 2003;80:645–647. [Google Scholar]
- 33.Fernández-Bolaños JG, López O, Ulgar V, Maya I, Fuentes J. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett. 2004;45:4081–4084. [Google Scholar]
- 34.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3(7):207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 35.Drobinica L, Augustine J. Reaction of isothiocyanate with amino acids, peptides and proteins. I. Kinetics of the reaction of aromatic isothiocyanates and glycines. Chem Commun. 1965;30:99104. [Google Scholar]
- 36.Brusewitz G, Cameron BD, Chasseaud LF, Gorler K, Hawkins DR, Koch H, Mennicke WH. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem J. 1977;162(1):99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev. 1992;1(5):383–8. [PubMed] [Google Scholar]
- 38.Mennicke WH, Gorler K, Krumbiegel G, Lorenz D, Rittmann N. Studies on the metabolism and excretion of benzyl isothiocyanate in man. Xenobiotica. 1988;18(4):441–7. doi: 10.3109/00498258809041680. [DOI] [PubMed] [Google Scholar]
- 39.Conaway CC, Krzeminski J, Amin S, Chung FL. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem Res Toxicol. 2001;14(9):1170–6. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 40.Jiao D, Smith TJ, Yang CS, Pittman B, Desai D, Amin S, Chung FL. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis. 1997;18(11):2143–7. doi: 10.1093/carcin/18.11.2143. [DOI] [PubMed] [Google Scholar]
- 41.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62(1):2–7. [PubMed] [Google Scholar]
- 42.Conaway CC, Jiao D, Chung FL. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: a structure-activity relationship study. Carcinogenesis. 1996;17(11):2423–7. doi: 10.1093/carcin/17.11.2423. [DOI] [PubMed] [Google Scholar]
- 43.Jiao D, Conaway CC, Wang MH, Yang CS, Koehl W, Chung FL. Inhibition of N-nitrosodimethylamine demethylase in rat and human liver microsomes by isothiocyanates and their glutathione, L-cysteine, and N-acetyl-L-cysteine conjugates. Chem Res Toxicol. 1996;9(6):932–8. doi: 10.1021/tx9502094. [DOI] [PubMed] [Google Scholar]
- 44.Stoner GD, Siglin JC, Morse MA, Desai DH, Amin SG, Kresty LA, Toburen AL, Heffner EM, Francis DJ. Enhancement of esophageal carcinogenesis in male F344 rats by dietary phenylhexyl isothiocyanate. Carcinogenesis. 1995;16(10):2473–6. doi: 10.1093/carcin/16.10.2473. [DOI] [PubMed] [Google Scholar]
- 45.Elliott MC, Williams E. Synthesis and reactions of partially reduced biisoquinolines. Org Biomol Chem. 2003;1(17):3038–47. doi: 10.1039/b306159k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Copies of the 1H NMR spectra for compounds 1c, 1d, 2a, 2b, 2c, 2d, 4b, 4c, 4d, 5, and 6; and 1H NMR data for compounds 1c and 1d. This material is available free of charge via the internet at http://pubs.acs.org.