Abstract
Genetic variants in the gene encoding integrin α2 (ITGA2) have been reported to be associated with an increased risk for ischemic stroke. The purpose of this study was to investigate the association between haplotype-tagging single-nucleotide polymorphisms (tSNPs) in ITGA2 and risk of ischemic stroke in a collection of North American stroke cases and controls. The study included 484 cases and 263 controls. Thirteen tSNPs were genotyped. Association tests at and across each tSNP were performed, including haplotype association analysis. Secondary analyses considered stroke subtypes on the basis of Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria. We observed significant association between tSNP rs3756541 (additive model, odds ratio (OR), 1.49; 95% confidence interval (CI), 1.11 to 2.04; P = 0.009) and disease and a trend toward association at rs2303124 (recessive model, OR, 1.56; 95% CI, 1.05 to 2.33; P= 0.03). These associations remained significant in the haplotype analyses. The associated tSNPs did not distinguish stroke etiology after application of TOAST criteria. Our results suggest that genetic variability within ITGA2 may confer risk for ischemic stroke independent of conventional risk factors. These results provide additional support for a role for platelet receptor genes in the pathogenesis of ischemic stroke of diverse subtypes.
Keywords: atherosclerosis, cerebral infarction, genetics, integrin α2, stroke
Introduction
Platelet receptor genes are good candidates for stroke susceptibility studies, given the key role that platelet–collagen interactions play in mechanisms of ischemic stroke. Integrins are heterodimeric cell surface glycoproteins (GPs) consisting of α and β subunits. The platelet integrin α2β1, also known as GP Ia-IIa (where GP Ia represents the α2 component and GP IIa represents the β1 common subunit), is one of the major platelet collagen receptors (Santoro and Zutter, 1995; Santoro, 1999; Clemetson and Clemetson, 2001; Kunicki, 2002). Via GP Ia-IIa, platelets adhere to collagen exposed in subendothelial structures and subsequently become activated (Nieuwenhuis et al, 1985; Kehrel et al, 1988; Handa et al, 1995), leading to thrombus formation (Santoro and Zutter, 1995). In addition to adhesion, integrins are thought to participate in cell surface-mediated signaling (Inoue et al, 2003). The gene for the integrin α2 component, ITGA2, is located on chromosome 5q23-31, contains 30 exons, and spans approximately 110 kb.
This study comprised three groups of ischemic stroke cases and controls—probands from the Siblings With Ischemic Stroke Study (SWISS) and cases and controls from the Ischemic Stroke Genetics Study (ISGS) and the Mayo Stroke Genetics Databank (MSGD). We have characterized the ITGA2 gene, including the pattern of linkage disequilibrium (LD) across this locus, and examined the role of haplotype-tagging single-nucleotide polymorphisms (tSNPs) in ITGA2 with respect to ischemic stroke susceptibility, demonstrating that variation in ITGA2 is linked to ischemic stroke risk, independent of known stroke risk factors.
Subjects and methods
Study Populations
We evaluated the role of ITGA2 tSNPs and haplotypes in three distinct groups of patients: unrelated probands from SWISS (Meschia et al, 2006) (a sib-pair linkage study) and cases and controls from two case–control association studies, ISGS (Meschia et al, 2003) and MSGD. Eligibility criteria for cases and controls in MSGD and ISGS were identical, except that in MSGD cases included individuals with incident or recurrent ischemic stroke, whereas ISGS limited cases to individuals with incident ischemic stroke. There were 107 probands (from 107 families) used from the SWISS study, 329 cases and 215 controls from ISGS, and 48 cases and 48 controls from MSGD. All study subjects (cases and controls) were collected prospectively under protocols approved by the institutional review boards at participating institutions. All participants gave their written informed consent. Qualifying ischemic stroke events were diagnosed on the basis of medical history, neurologic examination, and imaging (computed tomography or magnetic resonance imaging scan) by experienced neurologists in the participating centers. Stroke was defined according to the World Health Organization criteria (WHO MONICA Project Principal Investigators, 1988). Each case was classified according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (Adams et al, 1993).
Molecular Genetic Analysis
Thirteen tSNPs across ITGA2 were chosen from the International HapMap Project database (http://www.hapmap.org) with use of TagIT version 2.03 (Weale et al, 2003). Median spacing of the tSNPs was 7,500 bp, with a range of 1,645 to 25,566 bp. A total of 13 tSNPs were used to ‘tag’ the LD that was characterized by the HapMap data for ITGA2. For 10 tSNPs, single-tube reagent-based genotyping assays (TaqMan; Applied Biosystems, Foster City, CA, USA) were used (assay details available on request). The tSNP rs3756541 was genotyped by direct DNA sequencing using a genetic analyzer (ABI Prism 3100; Applied Biosystems). Two tSNPs (rs1862639 and rs989073) were analyzed by pyrosequencing (Pyrosequencing Inc., Westborough, MA, USA). (Primers are available on request.) The tSNPs studied and details of probes and primers used are provided in Supplementary Table 1; all genotype assignments were performed masked to clinical data, including stroke-affected status.
Statistical Analysis
Descriptive statistics were reported as frequencies and percentages (categorical data) and means with standard deviations (continuous data). Tests of statistical significance between cases and controls were performed using two-sample tests for binomial proportions (with a χ2 test of independence). The Fisher exact test was used when appropriate. For comparison of age (continuous variable) among case–control groups, a two-sample t-test for independent samples was performed.
Hardy–Weinberg Equilibrium and Linkage Disequilibrium
For each tSNP, tests of deviations from Hardy–Weinberg equilibrium expectations were performed using the methods described by Wigginton et al (2005), with significance considered P < 0.05 under a χ2 goodness-of-fit test. The degree of tSNP–tSNP LD was estimated using the D′ statistic and visualized by haplotype analysis software (Haploview; Broad Institute of MIT and Harvard, Cambridge, MA, USA). Individual tSNP allele frequencies were computed, and differences in allele frequencies were tested by permutation (n = 1000) of the likelihood ratio statistic.
Statistical Genetic Analysis—Haplotype-Tagging Single-Nucleotide Polymorphisms
For tests of association with individual tSNPs between cases and controls, a series of generalized estimating equations (GEEs) were used that permitted inclusion of recognized stroke risk factors as covariates (age, sex, race, hypertension status, presence of atrial fibrillation, history of myocardial infarction, smoking status, presence of diabetes mellitus, and family history of stroke). All GEE models and testing were performed in a hierarchical manner, with a baseline risk model that included only the tSNP of interest as the predictor of outcome (stroke/no stroke). Additional models were tested, with age, sex (age + sex), and race (age + sex + race); additional models were then tested by adding an individual stroke risk factor variable as a covariate. A final, fully saturated model that included stroke risk factors was also used. P values were computed using the 2-degree-of-freedom generalized test of association. When the generalized test of association was significant (P < 0.05), additional models were tested that assumed an underlying mode of inheritance of risk (dominant, additive, recessive) with a 1-degree-of-freedom test. Differences in the distribution of ITGA2 within cases according to TOAST subtype versus control subjects were determined using χ2 tests. Comparisons were made for cases according to large-vessel, cardioembolic, or smallvessel disease and combined phenotype (large-vessel and cardioembolic) categories.
Statistical Genetic Analysis—Haplotypes
Haplotype frequencies and tests for association with stroke risk were computed using the expectation-maximization algorithm. Statistical significance was assessed using a permutation test of the likelihood ratio statistic. For all 2-, 3-, 4-, and 5-tSNP haplotypes, frequencies in cases and controls were estimated by the expectation-maximization algorithm and tested (for all haplotypes with frequencies greater than 10%) by permutation methods. For computation of tests in which stroke risk factors were included, all haplotype–genotype (haplotype pairs) probabilities were determined and used as weights in a weighted GEE1 regression analysis. For both unadjusted and adjusted analyses, the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were computed for each haplotype relative to all other haplotypes.
LD calculations, haplotype block determination, and statistical genetic analysis were performed for white and nonwhite patient groups separately.
Results
There were no significant differences in demographic characteristics among the three cohorts. Thus, cases and controls were pooled across studies. The descriptive statistics associated with cases and controls are shown in Supplementary Table 2. Cases (65.6±14.2 years) were significantly older than controls (60.0±14.7 years; P < 0.001). There were more women in the control group (62.0%) than in the case group (46.7%; P < 0.001). As expected, cases had greater prevalence of risk factors than controls (P < 0.001), including myocardial infarction (17.0% versus 5.7%), atrial fibrillation (14.8% versus 6.9%), smoking history (66.1% versus 47.1%), hypertension (69.5% versus 38.9%), hyperlipidemia (49.7% versus 26.6%), diabetes mellitus (24.6% versus 12.9%), and a positive family history of stroke (52.4% versus 33.5%).
Thirteen tSNPs in ITGA2 were examined with respect to Hardy–Weinberg equilibrium expectations. Significant deviations from expectation could be generated by many factors, but most notably population stratification, genotyping errors, or a true association. In these data, significant (P < 0.05) deviations from the Hardy–Weinberg equilibrium were observed for three tSNPs—rs3756541, rs3212460, and rs2303124—in the overall group (Table 1). The same three tSNPs failed within cases. In the controls, rs3212460 and rs2303124 failed, but rs3756541 did not fail. Two additional tSNPs failed only in the controls (rs1363192 and rs10513009). The tSNP rs3756541 had been assayed by direct sequencing, and reanalysis of these data revealed consistent results. The remaining two tSNPs (rs3212460 and rs2303124) were originally assayed by TaqMan. Dye terminator sequencing of 96 samples for both of these tSNPs showed a genotype discordance rate of 2% (2/96). Thus, errors in genotyping were not believed to be the cause of the deviation from the Hardy–Weinberg equilibrium or a significant confounder in the association analyses. The deviations from Hardy–Weinberg equilibrium were observed across all studies (SWISS, ISGS, MSGD), so the likelihood of population stratification is small. Although these tSNPs deviated from the Hardy–Weinberg equilibrium, they should not be completely eliminated from analyses (Wittke-Thompson et al, 2005).
Table 1.
tSNP association analysis for ischemic stroke adjusted for age, sex, race, myocardial infarction, atrial fibrillation, cigarette smoking, family history, and diabetes status
| tSNP major/minor | Location | Group | No. of genotypesa |
Association (P-value)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 22 | General | Dominant | Additive | Recessive | |||
| rs3212657 T/C | 52324239 | Total | 595 | 135 | 14 | 0.15 | 0.07 | 0.12 | 0.25 |
| Case | 395 | 82 | 6 | ||||||
| Control | 200 | 53 | 8 | ||||||
| rs1645761 A/G | 52332999 | Total | 518 | 198 | 30 | 0.05 | 0.03 | 0.02 | 0.07 |
| Case | 346 | 123 | 15 | ||||||
| Control | 172 | 75 | 15 | ||||||
| rs3756541b C/T | 52338852 | Total | 409 | 237 | 62 | 0.008 | 0.06 | 0.002 | 0.003 |
| Case | 247 | 164 | 46 | ||||||
| Control | 162 | 73 | 16 | ||||||
| rs1862639 C/T | 52343127 | Total | 463 | 252 | 30 | 0.35 | 0.16 | 0.25 | 0.44 |
| Case | 306 | 162 | 15 | ||||||
| Control | 157 | 90 | 15 | ||||||
| rs152088 C/T | 52344772 | Total | 499 | 210 | 34 | 0.35 | 0.19 | 0.19 | 0.31 |
| Case | 335 | 131 | 17 | ||||||
| Control | 164 | 79 | 17 | ||||||
| rs3212418 T/C | 52351929 | Total | 213 | 343 | 184 | 0.12 | 0.05 | 0.04 | 0.17 |
| Case | 150 | 224 | 106 | ||||||
| Control | 63 | 119 | 78 | ||||||
| rs989073 C/A | 52369177 | Total | 328 | 306 | 85 | 0.80 | 0.72 | 0.90 | 0.69 |
| Case | 217 | 199 | 53 | ||||||
| Control | 111 | 107 | 32 | ||||||
| rs1363192 A/C | 52373840 | Total | 380 | 299 | 66 | 0.10 | 0.04 | 0.33 | 0.95 |
| Case | 245 | 207 | 32 | ||||||
| Control | 135 | 92 | 34 | ||||||
| rs3212460b C/T | 52376431 | Total | 687 | 33 | 26 | 0.31 | 0.14 | 0.14 | 0.21 |
| Case | 442 | 23 | 19 | ||||||
| Control | 245 | 10 | 7 | ||||||
| rs3212476 C/G | 52379113 | Total | 379 | 302 | 63 | 0.51 | 0.62 | 0.27 | 0.25 |
| Case | 251 | 194 | 38 | ||||||
| Control | 128 | 108 | 25 | ||||||
| rs984966 T/A | 52404679 | Total | 274 | 355 | 116 | 0.94 | 0.78 | 0.71 | 0.76 |
| Case | 180 | 232 | 71 | ||||||
| Control | 94 | 123 | 45 | ||||||
| rs10513009 C/T | 52407631 | Total | 600 | 132 | 13 | 0.16 | 0.06 | 0.39 | 0.75 |
| Case | 386 | 92 | 5 | ||||||
| Control | 214 | 40 | 8 | ||||||
| rs2303124b C/T | 52414713 | Total | 359 | 245 | 114 | 0.03 | 0.68 | 0.06 | 0.01 |
| Case | 222 | 169 | 76 | ||||||
| Control | 137 | 76 | 38 | ||||||
tSNP, haplotype-tagging single-nucleotide polymorphism.
Genotype 11 indicates the presence of both major alleles; genotype 12, one major allele and one minor allele; and genotype 22, both minor alleles.
Not in Hardy–Weinberg equilibrium.
Each tSNP was tested independently for association with ischemic stroke. After adjustment for stroke risk factors (i.e., age, sex, race, and other stroke risk factors), three tSNPs were significantly associated with ischemic stroke risk (Table 1). The tSNP rs1645761 was significantly associated with ischemic stroke (OR, 2.76; 95% CI, 1.17 to 6.50; P = 0.05, generalized 2-degree-of-freedom test). Adjacent tSNP rs3756541 showed significant association in the unadjusted model (OR, 1.89; 95% CI, 1.03 to 3.45; P = 0.02) with increased significance (OR, 2.27; 95% CI, 1.15 to 4.55; P = 0.008) after adjustment for covariates. The tSNP rs2303124 was also significantly associated with risk of ischemic stroke in the fully adjusted model (OR, 1.43; 95% CI, 0.84 to 2.44; P = 0.03). The tSNP rs1645761 showed a significant association in the dominant (P = 0.03) and additive (P = 0.02) fully adjusted models. The tSNP rs3756541 showed a significant association in the additive (P = 0.002) and recessive (P = 0.003) fully adjusted models. The tSNP rs2303124 showed a significant association in the recessive (P = 0.01) fully adjusted model.
The association between tSNPs rs3756541 and rs2303124 with disease in the total group was largely driven by association at these loci in the group of white patients (Supplementary Table 3). Analyzing white patients, we observed significant association between tSNP rs3756541 (additive model, OR, 1.49; 95% CI, 1.11 to 2.04; P = 0.009) and disease and a trend toward association at rs2303124 (recessive model, OR, 1.56; 95% CI, 1.05 to 2.33; P = 0.03). Comparisons in the nonwhite group did not reveal any significant differences (Supplementary Table 4).
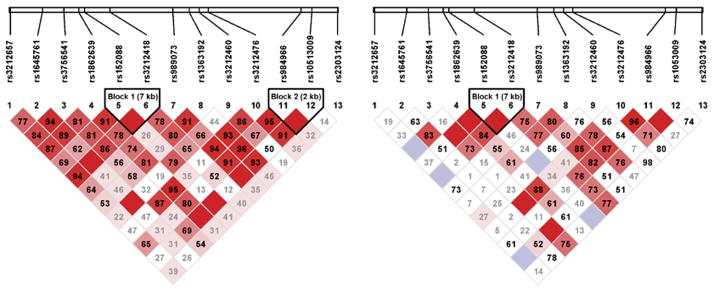
Pairwise LD between the 13 polymorphisms was evaluated using study population data for white and nonwhite patients separately (Figure 1). There was variation across these two groups. In white patients, these analyses showed two LD blocks in ITGA2. The first LD block (block 1) contained two tSNPs (rs152088, rs3212418) of 7 kb. The second LD block (block 2) contained two tSNPs (rs984966, rs10513009) of 2 kb. The tSNP that was associated with ischemic stroke in the adjusted model (rs3756541) does not reside in either of the LD blocks. Analysis of the nonwhite group showed an LD block containing two tSNPs (rs152088, rs3212418).
Figure 1.
Linkage equilibrium blocks in the white (left) and nonwhite (right) patient groups. Numbers within the diamonds are D′ values for the respective SNP pairs. Solid red diamonds represent absolute LD (D′ =1), blue diamonds represent strong LD with low level of significance. Numbers in gray within white diamonds represent a high probability or evidence of historical recombination.
Haplotype analyses were undertaken across the 13 tSNP panels using a 2-, 3-, 4-, and 5-tSNP moving window. Empirical P values were calculated using permutation testing. In the overall group, a majority of the 2-, 3-, and 4-tSNP haplotypes that included the first six tSNPs showed a significant difference between the distributions seen between cases and controls. We saw a similar pattern in the nonwhite group with the first four tSNPs. For the white group, we saw only two 2-tSNP haplotypes that had a significant empirical P value. Haplotype analysis in white and nonwhite subjects separately are summarized in Supplementary Tables 5 and 6.
With the empirical analyses indicating haplotypic differences between cases and controls, specific haplotypes were examined for significance and risk. The overall group had significant haplotypes and ORs evident in all haplotype windows through the first six tSNPs, even when removing tSNP rs3756541 (not in Hardy–Weinberg equilibrium). The white group had significance in a couple of four-marker haplotypes, one involving tSNPs 1 through 4 and the other involving tSNPs 10 through 13. Both of these were not evident when tSNPs not in Hardy–Weinberg equilibrium were removed. In the nonwhite group, significance was seen only after removing the tSNPs not in Hardy–Weinberg equilibrium. This evidence was seen in haplotypes involving the first six tSNPs. In both the white and nonwhite groups, the ORs maintained a significant consistent risk comparable to the overall group. Using weighted GEE1 analyses, almost all the analyses involving a significant haplotype using the full model showed a significant result in both the additive and dominant models. Results for significant individual haplotypes and ORs are listed in Supplementary Tables 7, 8, and 9.
Finally, there was no differential association of any individual tSNP or haplotype with ischemic stroke by TOAST subtype (data not shown), which suggests that the effect of ITGA2 variation may apply to a broad range of ischemic stroke etiologies.
Discussion
The aim of this study was to determine, using a tSNP polymorphism approach, whether common variability in ITGA2 alters risk for ischemic stroke in a large number of North American subjects. Association studies addressing the relationship between ITGA2 polymorphisms and risk of cerebrovascular disease have been performed, with many evaluating only three tSNPs (C807T, G873A, and A1648G) that define three major ITGA2 haplotypes. In particular, the T807 and A873 alleles within exons 8 and 9 have been associated with increased GPIa-IIa expression (Kunicki et al, 1997) and with risk for arterial thrombosis in younger men with a history of myocardial infarction (Santoso et al, 1999), women who smoke cigarettes (Roest et al, 2000), men and women with diabetic retinopathy (Matsubara et al, 2000), and younger men and women with stroke (Carlsson et al, 1999; Reiner et al, 2000). Also the C807T polymorphism has been involved in modeling individual response to antithrombotic treatment (Angiolillo et al, 2005). Another polymorphism, G1649A (Glu534Lys) at codon 534, exon 14, has also been associated with coronary artery disease (Kroll et al, 2000).
Several studies that have evaluated the relationship between the C807T polymorphism and ischemic stroke do not support a genetic association. The results of these studies may be inconsistent because the evaluated tSNPs may not be causal but rather in LD with disease-causing tSNPs and the level of LD across ITGA2 may vary by population. Alternatively, an interaction with another risk factor—genetic or environmental—may be required to lead to an increased risk of stroke. The independence shown in our study of the various ITGA2 tSNPs indicates that selection of only one tSNP as a marker for the entire gene is inadequate to tag diversity across the locus.
Our cohort exhibited racial diversity. Haplotype patterns are known to vary by race, especially in populations with marked genetic diversity. This makes the study of nonhomogenous populations problematic. However, we designed our model to take racial and ethnic diversity into account. To strengthen the generalizability of our results, thereby contributing to the biologic plausibility of our findings, and to provide additional statistical power, we pooled all races in our analysis, but we then adjusted for race. Additionally, we specifically looked for genetic associations in each ethnic group.
The adjusted analysis showed three tSNPs associated with ischemic stroke risk in the total group. Among the white group, only the tSNP rs3756541 was significantly associated with the disease, whereas no tSNP was associated with ischemic stroke in the nonwhite group. Although we cannot ignore the possibility that these discrepancies could be caused by differences in population genetic structure, it is also likely that these differences and our failure to find association in nonwhite patients were affected by the sample size of the nonwhite group. Only 21% of our subjects were nonwhite.
We also need to consider the possibility that the positive findings we obtained in the entire study group were spurious or the result of type I error. However, even considering only white patients, the association with tSNP rs3756541 suggests that this tSNP could be associated with ischemic stroke.
Another factor to take into account is patient age. Although most positive associations have been obtained from studies of younger cohorts (Carlsson et al, 1999; Reiner et al, 2000; Sacchi et al, 2000), in our study, the age of patients and other known risk factors were also used as covariates, suggesting that these are unlikely to significantly modulate the genetic risk associated with ITGA2.
It should be noted that the associated tSNPs identify potential regions in the genome that contribute to genetic risk of ischemic stroke. As these tSNPs account for the majority, but not all, of the information within a haplotype block, any association should be considered as preliminary and serve as a rationale for more in-depth analysis of the region. Thus, association of a tSNP would warrant denser genotyping of tSNPs and other variants within the identified haplotype block as well as consideration of both common and rare variants as they relate to the genetic risk of ischemic stroke.
To date, many of the polymorphisms associated with increased risk of complex disorders are not amino-acid polymorphisms, and several lines of evidence support the functional importance of intronic polymorphisms in the etiology of complex disorders (Cox et al, 2004). In this study, significant association was observed for tSNPs rs1645761 and rs3756541 within intron 1 and rs2303124 within intron 26. According to FastSNP(http://fastsnp.ibms.sinica.edu.tw), a Web server that allows identification and prioritization of high-risk tSNPs, the tSNP 230324 could have a possible functional effect as intronic enhancer, whereas the functions of the other two intronic tSNPs are not known. Very little is known about the function that specific intronic sequences have with regard to the secondary structure, protein binding, stability, and splicing efficiency of heterogeneous nuclear RNA. Thus, any of these associated tSNPs may affect expression, which could be investigated directly in future studies by testing for case–control differences in transcript structures, quantity, spatial distribution, and developmental distribution.
In conclusion, our results suggest that ITGA2 may be a susceptibility region for ischemic stroke in the North American population and can be equally important in all forms of ischemic stroke. Further studies with larger samples, different patient groups, and tSNPs that capture a reasonable amount of variation are needed to corroborate these findings.
Supplementary Material
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Acknowledgments
SWISS is funded by a grant from the NINDS (R01 NS39987; JF Meschia, principal investigator). ISGS is funded by a grant from the National Institute of Neurological Disorders and Stroke (R01 NS42733; JF Meschia, principal investigator). The MSGD was funded by a grant from the Mayo Foundation for Medical Education and Research.
ISGS is a contributor of data and DNA to the National Institute of Neurological Disorders and Stroke Human Genetics Resource Center, Coriell Institute for Medical Research, Camden, New Jersey. Editing, proofreading, and reference verification were provided by the Section of Scientific Publications, Mayo Clinic.
Appendix. Study Participants
SWISS Investigative Team
Executive Committee: James F Meschia, chair; Thomas G Brott, Robert D Brown, Jr, Brett Kissela, John Hardy, Stephen S Rich.
Statistical Center: Wake Forest University School of Medicine, Winston-Salem, North Carolina. Stephen S Rich, director; W Mark Brown.
DNA Repository: Coriell Institute for Medical Research, Camden, New Jersey. Roderick A Corriveau, PhD, scientific program manager.
Data and Site Management: Mayo Clinical Trials Services, Rochester, Minnesota. Hattie M Hanert, RN, project manager; Karen Hurtis, clinical research associate; Lindsey DeGoey, clinical trails assistant; Karen Johnson, regulatory affairs manager.
SWISS Sites and Investigators Listed by Proband Enrollment as of September 2006
Mayo Clinic, Rochester, Minnesota (probands enrolled, 61): Principal investigator (PI): Robert D Brown, Jr, MD; coordinator: Colleen S Albers, RN.
Mayo Clinic, Jacksonville, Florida (probands enrolled, 51): PI: Thomas G Brott, MD; coordinators: Alexa Richie, CRC, Dale Gamble, CRC, Sothear Luke, CRC.
University of Cincinnati, Cincinnati, Ohio (probands enrolled, 47): PI: Brett Kissela, MD; coordinator: Kathleen Alwell, RN.
Neurological Associates, Inc, Richmond, Virginia (probands enrolled, 47): PI: Francis McGee, Jr, MD; coordinator: Cherie Wampler.
University of Virginia Health System, Charlottesville, Virginia (probands enrolled, 45): PI: Bradford Worrall, MD, MSc; coordinator: David Chernawsky.
Shands HealthCare, affiliated with the University of Florida, Jacksonville, Florida (probands enrolled, 43): PI: Scott Silliman, MD; coordinators: Yvonne Douglas, Raam Sambandam.
Mercy Ruan Neurology Clinic, Des Moines, Iowa (probands enrolled, 36): PI: Michael Jacoby, MD; coordinator: Judi Greene, RN.
Main Line Health Stroke Program, Bryn Mawr, Pennsylvania (probands enrolled, 18): PI: Gary Friday, MD; coordinator: Angela Whittington-Smith, RN.
University of Maryland, Baltimore, Maryland (probands enrolled, 17): PI: Steven Kittner, MD; coordinator: Mary J Sparks.
Kaleida Stroke Care Center, Millard Fillmore GatesCircle Hospital, Buffalo, New York (probands enrolled, 15): PI: R Ferguson, MD; coordinator: Kathleen Wrest, MLS.
Centre Hospitalier Affilie Universitaire de Quebec, Quebec City, Province of Quebec, Canada (probands enrolled, 15): PI: Ariane Mackey, MD; coordinators: Annette Hache, RN, Sophie Dube, RN.
Luther Midelfort Hospital, Eau Claire, Wisconsin (probands enrolled, 14): PI: Felix Chukwudelunzu, MD; coordinator: Karen Snobl, RN.
Mayo Clinic, Scottsdale, Arizona (probands enrolled, 14): PI: David W. Dodick, MD; coordinator: Erica L Boyd, RN.
Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania (probands enrolled, 12): PI: Scott Kasner, MD; coordinator: Mary Liz Desanto.
University of South Alabama, Mobile, Alabama (probands enrolled, 12): PI: Richard Zweifler, MD; coordinator: Kelli Boots.
Maine Medical Center, Portland, Maine (probands enrolled, 12): PI: John Belden, MD; coordinator: Diane Diconzo-Fanning, RN.
Ohio State University, Columbus, Ohio (probands enrolled, 12): PI: Andrew Slivka, MD; coordinator: Peggy Notestine, CCRC.
Metro Health Medical Center, Cleveland, Ohio (probands enrolled, 12): PI: Joseph Hanna, MD; coordinators: Alice Liskay, RN, Bonni Kurtz.
Wake Forest University School of Medicine, Winston-Salem, North Carolina (probands enrolled, 11): PI: David Lefkowitz, MD; coordinator: Emily Smith.
Cleveland Clinic Florida, Weston, Florida (probands enrolled, 10): PI: Virgilio Salanga, MD; coordinator: Mercy Lois-Valdez.
University of Iowa Hospital, Iowa City, Iowa (probands enrolled, 10): PI: Patricia Davis, MD; coordinator: Jeri Sieren, RN.
Hôpital Charles LeMoyne, Greenfield Park, Province of Quebec, Canada (probands enrolled, 10): PI: Leo Berger, MD; coordinators: Denise Racicot, Joanna Wai Ling Ma.
Stroke Prevention and Atherosclerosis Research Centre, London, Ontario, Canada (probands enrolled, 10): PI: David Spence, MD; coordinator: Rose Freitas.
University of Texas Southwestern Medical Center at Dallas, Dallas, Texas (probands enrolled, 9): PI: Mark Johnson, MD; coordinator: J. Greggory Wright, BS.
University of California, Davis School of Medicine, Sacramento, California (probands enrolled, 8): PI: PieroVerro, MD; coordinator: Jacqueline Wright.
Indiana University School of Medicine, Indianapolis, Indiana (probands enrolled, 8): PI: Linda Williams, MD; coordinator: Michael Girdler.
Helen Hayes Hospital, West Haverstraw, New York (probands enrolled, 8): PI: Jason P Greenberg, MD; coordinator: Laura Tenteromano, RN.
Washington University School of Medicine, St. Louis, Missouri (probands enrolled, 8): PI: Jin-Moo Lee, MD, PhD; coordinators: Denise Shearrer, RMA, BS, Julie Ruckmann.
University of Wisconsin, Madison, Wisconsin (probands enrolled, 7): PI: Robert Dempsey, MD; coordinator: MJ Washburn, RRT, BSRT.
Marshfield Clinic, Marshfield, Wisconsin (probands enrolled, 7): PI: Percy Karanjia, MD; coordinator: Kathy Mancl, CCRC, Rob Bohl, NP.
Thomas Jefferson University Hospital, Philadelphia, Pennsylvania (probands enrolled, 6): PI: Rodney Bell, MD; coordinator: Lisa Bowman.
University of California Los Angeles Stroke Center, Los Angeles, California (probands enrolled, 6): PI: Jeffery Saver, MD; coordinator: Jill Haines.
East Bay Region Associates in Neurology, Berkeley, California (probands enrolled, 6): PI: Brian Richardson, MD; coordinator: Lauren McCormick.
University of Utah Medical Center, Salt Lake City, Utah (probands enrolled, 5). PI: Elaine J Skalabrin, MD; coordinator: Jolee Mougey, MPH.
University of Kentucky, Lexington, Kentucky (probands enrolled, 5): PI: L Creed Pettigrew, MD; coordinator: Deborah Taylor, MS.
Florida Neurovascular Institute, Tampa, Florida (probands enrolled, 5): PI: Erfan Albakri, MD; coordinators: Mary Katherine Taylor, ARNP; Anessa Hohns.
Mercy Hospital, Sioux City, Iowa (probands enrolled, 5): PI: Jennifer Pary, MD; coordinator: Deb Motz.
Rochester General Hospital, Rochester, New York (probands enrolled, 5): PI: Joshua Hollander, MD: coordinator: Cheryl Weber.
OSF Stroke Center, Peoria, Illinois (probands enrolled, 4): PI: David Wang, DO; coordinator: Mary Buttice.
Scripps Clinic, La Jolla, California (probands enrolled, 4): PI: Mary Kalafut, MD; coordinators: Krista Greiner, CCRC; Ileanna Iedelson, RN, BSN.
Morton Plant Hospital–Neuroscience Institute, Clearwater, Florida (probands enrolled, 3): PI: Ajay Arora, MD; coordinators: Jo Simpson, Teresa Jones, Victoria Bernsee.
Massachusetts General Hospital, Boston, Massachusetts (probands enrolled, 3): PI: Karen Furie, MD; coordinator: Donna Defilippo.
Vanderbilt University Medical Center, Nashville, Tennessee (probands enrolled, 2): PI: Anne E O’Duffy, MD; coordinator: Diane Brown.
Tri-State Neurology, Huntington, West Virginia (probands enrolled, 1): PI: Carl McComas, MD; coordinators: Jenny Edwards, RN, Christy Franklin, RN.
The Stroke Center at Hartford Hospital, Hartford, Connecticut (probands enrolled, 1): PI: Isaac Silverman, MD; coordinator: Martha Ahlquist, LPN, CCRP.
Charles R Drew University of Medicine and Science, Los Angeles, California (probands enrolled, 1): PI: Lowell Nelson, MD; coordinator: Marcia Montenegro, RN.
Virginia MasonMedical Center, Seattle, Washington (probands enrolled, 1): PI: Michael Elliott, MD; coordinator: Allison Singer.
ISGS Investigative Team
Study principal investigator (PI): James F Meschia, MD.
Executive committee: James F Meschia, chair; Thomas G Brott, Robert D Brown, Jr, Michael Frankel, John Hardy, Stephen S Rich, Scott Silliman, and Bradford B Worrall.
Data Management Center: Wake Forest University Medical Center: L Douglas Case, director; Laurie Russell, Carolyn Bell, Darrin Harris, and Wes Roberson.
Clinical Coordinating Center: Mayo Clinic, Jacksonville, Florida: James F Meschia, Alexa Richie, Dale Gamble, and Sothear Luke.
DNA Repository: Coriell Institute for Medical Research, Camden, New Jersey: Roderick A Corriveau, PhD.
Genetics Laboratory: National Institute on Aging, Bethesda, Maryland: John Hardy and Andrew Singleton.
Statistical Genetics Core: University of Virginia, Charlottesville, Virginia: Stephen S Rich; Wake Forest University Medical Center, Winston-Salem, North Carolina: W Mark Brown.
ISGS Sites and Investigators as of September 26, 2006
Mayo Clinic, Jacksonville, Florida (subjects enrolled, 271)—PI: James F Meschia, MD; coordinators: Alexa Richie, CRC, Dale Gamble, CRC, Sothear Luke, CRC; subinvestigators (SI): Thomas G Brott, MD, Benjamin H Eidelman, MD.
University of Florida/Shands Hospital, Jacksonville, Florida (subjects enrolled, 216)—PI: Scott Silliman, MD; coordinators: Yvonne Douglas, Rahm Sambandam; SI: Nader Antonios, MD.
Emory University School of Medicine, Atlanta, Georgia (subjects enrolled, 237)—PI: Michael Frankel, MD, MSc; coordinator: Sharion Sailor- Smith, RN.
Mayo Clinic, Rochester, Minnesota (subjects enrolled, 266)—PI: Robert D Brown, Jr, MD; coordinator: Colleen S Albers, RN.
University of Virginia, Charlottesville, Virginia (subjects enrolled, 231)—PI: Bradford Worrall, MD, MSc; coordinator: Daniel Chernavvsky.
The MSGD Investigative Team
PI: James F Meschia, MD
Clinical investigator: Thomas G Brott, MD
Clinical coordinator: Marc Lojacono
Data management and statistics: Elizabeth J
Atkinson, MS
Geneticists: Richard Crook, John Hardy, PhD
References
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE TOAST Investigators. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Cavallari U, Trabetti E, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Pignatti PF, Macaya C. Variability in platelet aggregation following sustained aspirin and clopidogrel treatment in patients with coronary heart disease and influence of the 807 C/T polymorphism of the glycoprotein Ia gene. Am J Cardiol. 2005;96:1095–9. doi: 10.1016/j.amjcard.2005.06.039. Epub 2005 Aug 29. [DOI] [PubMed] [Google Scholar]
- Carlsson LE, Santoso S, Spitzer C, Kessler C, Greinacher A. The α2 gene coding sequence T807/A873 of the platelet collagen receptor integrin α2β1 might be a genetic risk factor for the development of stroke in younger patients. Blood. 1999;93:3583–6. [PubMed] [Google Scholar]
- Clemetson KJ, Clemetson JM. Platelet collagen receptors. Thromb Haemost. 2001;86:189–97. [PubMed] [Google Scholar]
- Cox NJ, Hayes MG, Roe CA, Tsuchiya T, Bell GI. Linkage of calpain 10 to type 2 diabetes: the biological rationale. Diabetes. 2004;53(Suppl):S19–25. doi: 10.2337/diabetes.53.2007.s19. [DOI] [PubMed] [Google Scholar]
- FastSNP [database on the Internet] Nankang. Taiwan: Institute of Biomedical Sciences and Institute of Information Science, Academia Sinica; 2006. Available from: http://fastsnp.ibms.sinica.edu.tw. [Google Scholar]
- Handa M, Watanabe K, Kawai Y, Kamata T, Koyama T, Nagai H, Ikeda Y. Platelet unresponsiveness to collagen: involvement of glycoprotein Ia-IIa (α2β1 integrin) deficiency associated with a myeloproliferative disorder. Thromb Haemost. 1995;73:521–8. [PubMed] [Google Scholar]
- Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLC gamma2. J Cell Biol. 2003;160:769–80. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Project [homepage on the Internet] Available from: http://www.hapmap.org.
- Kehrel B, Balleisen L, Kokott R, Mesters R, Stenzinger W, Clemetson KJ, van de Loo J. Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen-induced aggregation and spontaneous loss of disorder. Blood. 1988;71:1074–8. [PubMed] [Google Scholar]
- Kroll H, Gardemann A, Fechter A, Haberbosch W, Santoso S. The impact of the glycoprotein Ia collagen receptor subunit A1648G gene polymorphism on coronary artery disease and acute myocardial infarction. Thromb Haemost. 2000;83:392–6. [PubMed] [Google Scholar]
- Kunicki TJ. The influence of platelet collagen receptor polymorphisms in hemostasis and thrombotic disease. Arterioscler Thromb Vasc Biol. 2002;22:14–20. doi: 10.1161/hq0102.100458. [DOI] [PubMed] [Google Scholar]
- Kunicki TJ, Kritzik M, Annis DS, Nugent DJ. Hereditary variation in platelet integrin α2β1 density is associated with two silent polymorphisms in the α2 gene coding sequence. Blood. 1997;89:1939–43. [PubMed] [Google Scholar]
- Matsubara Y, Murata M, Maruyama T, Handa M, Yamagata N, Watanabe G, Saruta T, Ikeda Y. Association between diabetic retinopathy and genetic variations in α2β1 integrin, a platelet receptor for collagen. Blood. 2000;95:1560–4. [PubMed] [Google Scholar]
- Meschia JF, Brott TG, Brown RD, Jr, Crook RJ, Frankel M, Hardy J, Merino JG, Rich SS, Silliman S, Worrall BB. The Ischemic Stroke Genetics Study (ISGS) Protocol. BMC Neurol. 2003;3:4. doi: 10.1186/1471-2377-3-4. Epub 2003 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia JF, Kissela BM, Brott TG, Brown RD, Jr, Worrall BB, Beck J, Skarp AN. The Siblings With Ischemic Stroke Study (SWISS): a progress report. Clin Med Res. 2006;4:12–21. doi: 10.3121/cmr.4.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–2. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- Reiner AP, Kumar PN, Schwartz SM, Longstreth WT, Jr, Pearce RM, Rosendaal FR, Psaty BM, Siscovick DS. Genetic variants of platelet glycoprotein receptors and risk of stroke in young women. Stroke. 2000;31:1628–33. doi: 10.1161/01.str.31.7.1628. [DOI] [PubMed] [Google Scholar]
- Roest M, Banga JD, Grobbee DE, de Groot PG, Sixma JJ, Tempelman MJ, van der Schouw YT. Homozygosity for 807T polymorphism in α2 subunit of platelet α2β1 is associated with increased risk of cardiovascular mortality in high-risk women. Circulation. 2000;102:1645–50. doi: 10.1161/01.cir.102.14.1645. [DOI] [PubMed] [Google Scholar]
- Sacchi E, Tagliabue L, Duca F, Landi G, Merlini PA, Mannucci PM. A C 807T substitution in the platelet collagen receptor integrin α2β1 gene is a genetic risk factor for stroke at a young age but not for myocardial infarction [abstract] Haematologica. 2000;85(Suppl):10–1. [Google Scholar]
- Santoro SA. Platelet surface collagen receptor polymorphisms: variable receptor expression and thrombotic/hemorrhagic risk. Blood. 1999;93:3575–7. [PubMed] [Google Scholar]
- Santoro SA, Zutter MM. The α2β1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost. 1995;74:813–21. [PubMed] [Google Scholar]
- Santoso S, Kunicki TJ, Kroll H, Haberbosch W, Gardemann A. Association of the platelet glycoprotein Ia C807T gene polymorphism with nonfatal myocardial infarction in younger patients. Blood. 1999;93:2449–53. [PubMed] [Google Scholar]
- Weale ME, Depondt C, Macdonald SJ, Smith A, Lai PS, Shorvon SD, Wood NW, Goldstein DB. Selection and evaluation of tagging SNPs in the neuronalsodium-channel gene SCN1A: implications for linkage- disequilibrium gene mapping. Am J Hum Genet. 2003;73:551–65. doi: 10.1086/378098. Epub 2003-Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–14. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy–Weinberg equilibrium. Am J Hum Genet. 2005;76:887–93. doi: 10.1086/429864. Epub 2005 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rational inferences about departures from Hardy– Weinberg equilibrium. Am J Hum Genet. 2005;76:967–86. doi: 10.1086/430507. Epub 2005 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)