Abstract
Background
The Bone Morphogenetic Protein (BMP) genes bmp2 and bmp4 are expressed in highly conserved patterns in the developing vertebrate inner ear. It has, however, proved difficult to elucidate the function of BMPs during ear development as mutations in these genes cause early embryonic lethality. Previous studies using conditional approaches in mouse and chicken have shown that Bmp4 has a role in semicircular canal and crista development, but there is currently no direct evidence for the role of Bmp2 in the developing inner ear.
Methodology/Principal Findings
We have used an RNA rescue strategy to test the role of bmp2b in the zebrafish inner ear directly. Injection of bmp2b or smad5 mRNA into homozygous mutant swirl (bmp2b−/−) embryos rescues the early patterning defects in these mutants and the fish survive to adulthood. As injected RNA will only last, at most, for the first few days of embryogenesis, all later development occurs in the absence of bmp2b function. Although rescued swirl adult fish are viable, they have balance defects suggestive of vestibular dysfunction. Analysis of the inner ears of these fish reveals a total absence of semicircular canal ducts, structures involved in the detection of angular motion. All other regions of the ear, including the ampullae and cristae, are present and appear normal. Early stages of otic development in rescued swirl embryos are also normal.
Conclusions/Significance
Our findings demonstrate a critical late role for bmp2b in the morphogenesis of semicircular canals in the zebrafish inner ear. This is the first demonstration of a developmental role for any gene during post-embryonic stages of otic morphogenesis in the zebrafish. Despite differences in the early stages of semicircular canal formation between zebrafish and amniotes, the role of Bmp2 in semicircular canal duct outgrowth is likely to be conserved between different vertebrate species.
Introduction
The inner ears of all vertebrates detect both auditory and vestibular stimuli to a lesser or greater extent, depending on the species. The region of the ear responsible for detection of rotational motion (angular acceleration) consists of three orthogonally arranged semicircular canals and their associated sensory patches, the cristae. Angular motion stimuli normally cause displacement of sensory hair cells in the cristae due to the inertia of the fluid in the semicircular canals, and compensatory muscle movements (the vestibular righting reflex) allow an animal to maintain postural equilibrium as it turns. The semicircular canal system is well conserved among jawed vertebrates; in the embryo, the developing canal ducts and cristae have been shown to express members of the Bone Morphogenetic Protein (BMP) family of signalling molecule genes in all vertebrate species examined. bmp4 is expressed in the developing cristae of zebrafish, mouse and chick [1]–[4], while in mouse and chick, Bmp2 is expressed in the canal genesis zone, adjacent to the cristae, and in the epithelium of the developing canals [5]. Similarly, in zebrafish, bmp2b is expressed in the developing cristae, and by 72 hours post fertilisation (hpf) expression is also detected in the epithelial projections forming the semicircular canals [1], [6]. Bmp7 is expressed strongly in epithelium of the developing semicircular canal system in both chick [7] and zebrafish (bmp7b) [8].
Despite the conserved expression of Bmp genes in the developing ear, it has been difficult to assess BMP function during inner ear development using a conventional genetic approach, as BMP signalling plays a vital role in establishing ventral identity in the early embryo. Thus mutations in genes encoding BMP pathway components often cause early embryonic lethality [9]–[11]. To circumvent this early requirement for BMP signalling in the embryo, it is therefore necessary to use a conditional approach to restrict BMP disruption either to later developmental stages or to tissues of the developing ear. Ectopic application of the BMP antagonists Noggin or DAN to the developing chick inner ear showed that BMPs have a role in the formation of cristae and semicircular canal ducts: both structures are variably absent in treated embryos [12]–[14]. These experiments did not, however, indicate which BMPs are involved. More recently, Wu and colleagues [15] have used Cre-lox technology and electroporation techniques to knock down Bmp4 function specifically in the inner ears and cristae of mouse and chick embryos, respectively. This work clearly demonstrates an important role for Bmp4 in semicircular canal and crista development, with the most severely affected embryos having no canal ducts or cristae together with malformed saccules and utricles [15].
The study by Chang et al. suggests that the effect of Bmp4 on canal development in the mouse and chick is mediated by Bmp2, as Bmp2 expression is down-regulated when Bmp4 is knocked down [15]. This follows from previous work by the same group implicating Bmp2 in canal development [5], [7]. In the chick, a canal genesis zone adjacent to the crista expresses Bmp2 under the control of FGF signalling. Ectopic FGF treatments resulted in ectopic Bmp2 expression and a failure of resorption at the canal fusion plate (and thus excess canal duct tissue), while reduced FGF signalling resulted in reduced Bmp2 expression and a lack of semicircular canal ducts, although the crus commune and some ampullae were still formed [5]. Crucially, application of Noggin alongside FGF rescued the canal duct phenotype, suggesting that the promotion of canal duct outgrowth by FGF is mediated through Bmp2. While these data strongly suggest that Bmp2 is required for semicircular canal development, they do not, however, provide direct evidence.
We have used an alternative approach in the zebrafish to test the role of bmp2b directly, exploiting the ability to rescue the early defects in BMP pathway mutants by mRNA injection. Homozygous zebrafish swirl (swr/bmp2b) mutant embryos are severely dorsalised and lyse at the 13–14 somite stage (around 16 hpf) due to pressure on the yolk [9], [11], [16]. This corresponds to the otic placode stage in the developing embryo, but as a result of the dorsalisation, otic placodal tissue is severely reduced or absent from these embryos [11]. However, injection of in vitro-synthesised bmp2b or smad5 mRNA at the 1–2 cell stage results in complete rescue of the dorsoventral patterning defects, allowing the homozygous mutant fish to reach adulthood [9], [11]. As the injected mRNA is only expected to last for the first few days of embryogenesis, all subsequent developmental stages are completed in the absence of bmp2b function. As we show here, rescued homozygous swr/bmp2b−/− adults have severe structural abnormalities of the inner ear and concomitant balance defects, revealing a critical late requirement for bmp2b during semicircular canal morphogenesis in the zebrafish.
Results
Rescued homozygous swr/bmp2b adult fish have an abnormal swimming behaviour indicative of vestibular dysfunction
Rescued homozygous swr/bmp2b mutant fish are adult viable and fertile, and appear grossly morphologically normal, but display a very specific and fully penetrant abnormal swimming behaviour (data not shown). They swim normally except when making sudden changes in direction: instead of making a neat turn they lose their dorsoventral orientation momentarily and perform a messy somersault. This is particularly evident when the fish are excited or disturbed, for example when they are about to be fed. This indicates a failure or delay in the detection of angular motion stimuli, or in the vestibular righting reflex that maintains normal postural control as the fish turns. The abnormal swimming behaviour is based on independent observations of several batches of rescued swr/bmp2b mutant fish (>100 individuals); 100% of rescued adults swam abnormally. Such behaviour is similar to, but not as severe as, that of fish that cannot balance correctly due to defects in the sensory hair cells of the inner ear hair [17]. It is worth noting, however, that bmp2b-rescued swrta72 fish (n = 5) show a wild-type dorsal light reflex, indicating that they retain the ability to sense, and orient themselves with respect to, gravity (see Materials and Methods; data not shown).
The inner ears of adult rescued swr/bmp2b fish lack semicircular canal ducts, but all sensory epithelia are present
To investigate the causes of the behavioural defect further, we analysed inner ear morphology in the rescued swr adults by histological sectioning. We cut resin sections at 10 µm through the heads of six rescued homozygous adult swr fish (three bmp2b-rescued swrta72, two smad5-rescued swrtdc24 and one smad5-rescued swrtc300) and three age-matched wild-type fish. Examination of these sections indicated that the semicircular canal ducts were absent in all six rescued swr mutant specimens (Fig. 1). The ampullae, utricule, saccule and lagena were all present, however, and contained sensory patches (cristae, utricular macula, saccular macula and lagenar macula, respectively) of apparently normal morphology (Fig. 1).
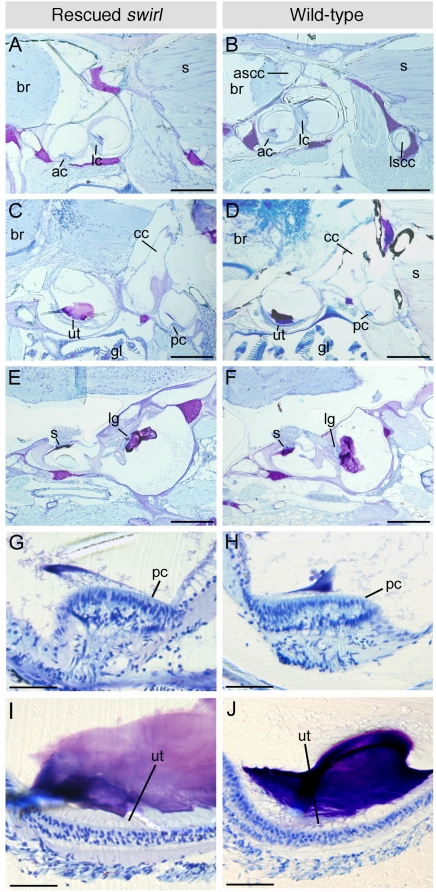
Figure 1. Semicircular canal ducts are absent, but sensory patches are present, in the inner ears of adult rescued swr zebrafish.
(A–F) 10 µm resin parasagittal sections through the inner ears of adult rescued swr zebrafish and age-matched wild-types (anterior to the left, dorsal to the top). Note the absence of semicircular canal ducts in the rescued swr ear (A), which are clearly present in the wild-type ear in an equivalent section (B, showing lumens of anterior and posterior canal ducts). All sensory patches detected in the wild-type ears are also present in the rescued swr ears. Differences in the orientation of the lagenar macula (E, F) reflect a slight difference in the position of the wild-type and swr sections for this pair of panels. (G–J) Higher magnification views of the posterior crista (G, H) and utricular macula (I, J). The structure of the sensory patches is similar in both rescued swr and wild-type ears. Abbreviations: ac, anterior crista; lc, lateral crista; pc, posterior crista; ascc, anterior semicircular canal duct; lscc, lateral semicircular canal duct; cc, crus commune; ut, utricular macula; s, saccular macula; lg, lagenar macula; br, brain; s, somite; gl, gill. Scale bar, (A–F) 400 µm; (G–J) 50 µm.
To obtain a clearer picture of the morphological defects, we used the sections to generate three-dimensional reconstructions of the inner ears of three rescued swr fish (two bmp2b-rescued swrta72 and one smad5-rescued swrtdc24) and two wild-type fish. We obtained full reconstructions of three rescued swr ears from two fish and two partial reconstructions from a single fish. We also obtained three fully reconstructed ears from the two wild-type fish (Fig. 2 and Supplementary Videos S1, S2). The reconstructions confirmed that all three rescued swr specimens (five ears examined) lacked semicircular canal ducts. The ampullae, crus commune and the posterior end of the lateral canal were present in all cases, but ended blindly. In the three rescued swr ears in which the vestibular chambers were reconstructed, the utricule, saccule and lagena were all present and grossly normal in size and morphology (Fig. 2 and Supplementary Videos S1, S2).
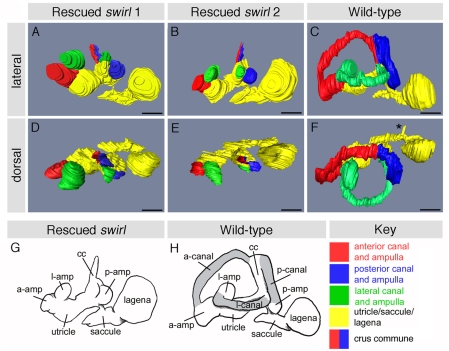
Figure 2. Three-dimensional reconstructions of adult rescued swr inner ears.
(A–F) Three-dimensional reconstructions of the inner ears of two adult rescued swr fish (swirl 1 (A, D) and swirl 2 (B, E)) and an age-matched wild-type fish (C, F). Semicircular canals are absent in the rescued swr fish ears, while the ampullae, crus commune, utricle, saccule and lagena are present and appear normal. Note that the pars superior (ampullae, crus commune and utricle) of swirl 1 is twisted relative to the pars inferior (saccule and lagena), about the point marked with the arrow (we are unable to tell whether or not this is an experimental artefact). The kink in the lateral canal of the wild-type ear (C) is an artefact. The asterisk marks the transverse canal that links the two saccules across the midline. (G, H) Sketch of the inner ears of adult rescued swr and wild-type fish. Shading in the wild-type ear indicates the regions missing from rescued swr ears. Abbrevations: a-amp, anterior ampulla; l-amp, lateral ampulla; p-amp, posterior ampulla; a-canal, anterior semicircular canal; l-canal, lateral semicircular canal; p-canal, posterior semicircular canal; cc, crus commune. swirl 1 is a smad5-rescued swrdc24 fish; swirl 2 is a bmp2b-rescued swrta72 fish. A–C, G and H are lateral views of left hand ears, with anterior to the left; D–F are dorsal views. Scale bar, 500 µm.
Sensory patch and semicircular canal development are normal in the inner ears of rescued swr/bmp2b fish during embryonic stages
The absence of canal duct tissue in adult fish led us to examine otic development in rescued swr mutants at embryonic and early larval stages. To analyse the development of sensory patches and semicircular canals, we stained smad5-RNA rescued swrta72 and swrtdc24 embryos and wild-type controls at 48 hpf to 7 dpf (days post fertilisation) with FITC-conjugated phalloidin. This marks both cortical actin and the actin-rich stereocilia of sensory hair cell bundles, allowing both general ear morphology and the sensory patches to be visualised (see, for example, [18]). In the wild-type ear at 48 hpf, hair cells have differentiated in two sensory patches, the utricular and saccular maculae. At the same stage, projections of epithelium that will form the hubs of the semicircular canals begin to ingress into the vesicle, meeting at a fusion plate to form pillars by approximately 72 hpf. Hair cells in the cristae are present by 60 hpf [19]. At all stages examined, both sensory patch morphology and semicircular canal development appeared normal in the rescued swr ears, including the formation of semicircular canal fusion plates (Fig. 3), although we cannot rule out the presence of any subtle defects. Of thirteen rescued swr embryos examined (four at 48 hpf, two at 72 hpf, five at 5 dpf and two at 7 dpf) only one of the 5 dpf fish did not show a wild-type phenotype. In the ears of this fish, all sensory patches and semicircular canal pillars were present, but the posterior macula was disorganised (data not shown). As the balance defect in rescued swr adults is fully penetrant, and all six sets of adult sections examined show a consistent phenotype, the posterior macula defect in this individual is likely to be non-specific rather than due to a specific lack of bmp2b. Overall, our data demonstrate a late requirement for bmp2b in the development of semicircular canals in the zebrafish that becomes manifest after 7 dpf.
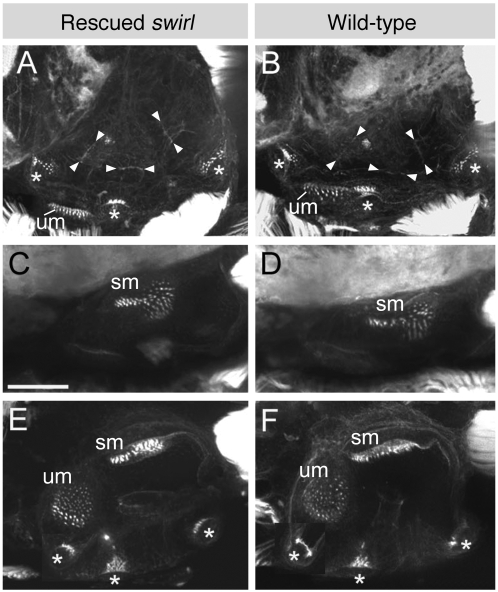
Figure 3. Sensory patches and fusion plates are present in the inner ears of 5 dpf rescued swr embryos.
Projected confocal z-stacks through inner ears of 5 dpf embryos stained with FITC-conjugated phalloidin, revealing the actin-rich stereociliary bundles of the sensory hair cells and the cortical actin in every cell. (A–D) Lateral views; anterior to left, dorsal to top. (A, B) Lateral plane of focus, showing the cristae, utricular macula and the fusion plates (arrowheads) between the semicircular canal projections. (C, D) More medial plane of focus showing the saccular macula. (E, F) Dorsal views; anterior to left, medial to top. Images shown are composites of two sets of projected z-stacks as the anterior crista is in a more dorsal plane of focus than the remaining sensory patches. Abbreviations: sm, saccular macula; um, utricular macula. Cristae are indicated with an asterisk. Scale bar, 50 µm.
Discussion
Morphological defects in the ears of rescued swr/bmp2b mutant fish are consistent with their behavioural abnormalities
Several adult viable zebrafish lines have previously been described that exhibit abnormal swimming behaviour similar to, but more severe than, that of the rescued swr fish described here. These ‘circler’ mutants have defects in the fine structure of the sensory hair cells, and identification of the causative mutations in these lines has revealed genes with roles in hair cell structure and function [17], [20]–[26]. All hair cells (of the maculae and cristae in the ear, and neuromasts in the lateral line system) are affected in the circler mutant lines, and homozygous viable adult fish exhibit a potentiated dorsal light reflex, indicating a loss of gravistatic postural control [17]. In contrast, in the adult rescued swr fish, we see a gross structural abnormality in the inner ear that is robust, highly specific and consistent with the observed behavioural defects. Although ampullae and cristae are present in the ears of rescued swr adults, the associated non-sensory epithelium forming the semicircular canal ducts is absent. The cristae would therefore be unable to receive correct angular motion stimuli, accounting for the failure of the vestibular righting reflex and loss of postural control whilst turning. The sensory patches responsible for detection of gravitoinertial stimuli—the lagenar, saccular and utricular maculae—are all present in grossly normal vestibular chambers, accounting for the normal dorsal light reflex and gravistatic postural control.
bmp2b has a late, highly specific role in morphogenesis of the semicircular canal ducts of the zebrafish inner ear
The requirement for bmp2b function for correct morphogenesis of the semicircular ducts is the first demonstration of a developmental role for any gene during post-embryonic stages of otic morphogenesis in the zebrafish. We see a very consistent phenotype in all six fish examined, in contrast to the variable phenotypes obtained by conditional approaches in other species. Previous studies in other species implicate BMPs, in particular Bmp4, in both crista and semicircular canal development during embryogenesis: both structures are affected when Bmp4 is knocked down specifically in mouse and chicken inner ears [15]. However, it has been suggested that the effect of Bmp4, Fgf3 and Fgf10 (all expressed in the cristae) on semicircular canal development may be mediated by Bmp2, which is expressed in a canal genesis zone adjacent to the crista in mouse and chicken [5], [15]. Thus non-sensory development may require the prior specification of sensory tissue. Our data, which demonstrate that zebrafish bmp2b has a highly specific late role in semicircular canal morphogenesis, are consistent with this model.
Several other factors, including Wnt signalling from the dorsal hindbrain and Hmx, Dlx, Prx and Netrin1 genes in the ear, are also implicated in vestibular morphogenesis in mouse and chick [27]–[32]. Mutations in several of these genes, however, have widespread effects on both sensory and non-sensory epithelia in the ear. It is worth noting that some of the effects of these genes may be mediated by BMP signalling; down-regulation of Wnt, Hmx or Dlx genes results in down-regulation or disorganisation of Bmp4 expression in the developing cristae [27]–[29], [33]. Equally, however, some are affected by manipulation of Bmp4 levels; in particular, Dlx5 is down-regulated when Bmp4 function is absent from the ear [15].
BMPs are likely to signal through Alk8 in the zebrafish ear
Several other homozygous mutants of the BMP signalling pathway have been rescued to adulthood by mRNA injection, including snh/bmp7a [34], [35], laf/alk8 [36] and sbn/smad5 [37], [38], but few other late (post-embryonic) phenotypes affecting organ systems have been revealed by mRNA rescue. One example is provided by the rescued laf/alk8 adult fish, which—unlike rescued swr/bmp2b adults—have stunted growth and an enlarged heart [36]. Interestingly, these fish also display a very similar abnormal swimming behaviour to that described here for the rescued swr/bmp2b adults [36] (K. Mintzer and MCM, unpublished observation), suggesting that they may have similar inner ear defects. Rescued homozygous smad5 and bmp7a mutant adults, however, appear to swim and balance normally (MCM and MH, unpublished observations).
alk8 codes for a type 1 BMP receptor, through which Bmp2b and other BMPs act; the similar behavioural phenotype in rescued swr/bmp2b and laf/alk8 fish suggests that semicircular canal duct outgrowth requires BMP signalling through Alk8. smad5 codes for an activator Smad, a downstream component of the BMP signal transduction cascade: normal vestibular function in rescued sbn/smad5 mutants suggests that other activator Smads (Smad1 and Smad8) can compensate for the loss of Smad5 function during semicircular canal morphogenesis.
Bmp2 has a conserved role in semicircular canal morphogenesis
Details of the early stages of semicircular canal morphogenesis differ between vertebrate species. In mammals and birds, a canal pouch (a flattened outpocketing of the otic vesicle) is formed first. The sides of the pouch then come together to form a fusion plate, and cell death, epithelial resorption or epithelial-to-mesenchymal transition (or a combination of these processes) at the fusion plate results in the formation of the canal [39]–[44]. In zebrafish, there are no pouches; instead, epithelial projections (topologically equivalent to the flattened sides of the canal pouches in amniotes) move towards the centre of the otic vesicle, and meet at a small fusion plate to form a pillar, around which the lumen of the semicircular canal runs [19], [45]. Fusion plates form in the zebrafish between 60 and 72 hpf [19], although a concentration of actin at the fusion plate is still evident at 24 dpf (CM, unpublished observation).
The initial arrangement of semicircular canals in the zebrafish, compared to that in amniotes, is very compact; much further outgrowth is required—to expand both the lumen and circuit radii of each canal, and to form the ampullae—before the canals attain their final shape and become functional [46], [47]. The ears of rescued swr fish appear normal during the stages of epithelial projection outgrowth and fusion plate formation (up to 7 dpf). We cannot exclude a role for bmp2b during these early stages, as protein from the rescuing RNA may persist during this time. However, our results reveal a definite requirement during the later stages of semicircular canal outgrowth and remodelling. Interestingly, in rescued laf/alk8 mutants, the heart appears normal during embryonic and larval stages, and both the heart defect and behavioural phenotype appear together at about 2–4 weeks of age (K. Mintzer and MCM, unpublished observations). The zebrafish is known to undergo a larval to juvenile metamorphosis at this stage, involving substantial changes to the morphology of several organ systems, together with alterations to physiology and behaviour [48] (and references within). It is therefore possible that BMP signalling is redeployed in the ear during this metamorphosis to mediate the growth and maturation of the semicircular canal system.
Despite the differences between zebrafish and avian ear morphogenesis, and the fact that the ears of rescued swr fish appear normal during embryonic stages, it is interesting that the phenotype of rescued adult swr inner ears is similar to that observed when the FGF inhibitor SU5402 was applied to chicken inner ears prior to canal pouch formation, which resulted in the down-regulation of both FGF signalling and Bmp2 expression [5]. Our data therefore suggest a conserved role for Bmp2 in semicircular canal development across the species from mammals and birds to zebrafish.
Materials and Methods
Ethics Statement
All animal work was conducted according to relevant national and international guidelines.
Zebrafish stocks
Wild-type fish used were the Tübingen strain (Tü); swr alleles used were swrta72, swrtdc24 and swrtc300. All adults used were at least 6 months old and of normal size. Embryonic stages are given as hours post-fertilisation (hpf) at 28.5°C and as somite stages for embryos younger than 24 hpf [49], [50].
mRNA Injections
5-methylguanosine-capped full length sense mouse smad5 mRNA or bmp2b mRNA was injected into 1–2 cell embryos from a swr+/−×swr+/− mating as previously described [9], [11], [49], [51]. Alternatively, for swrtdc24, the progeny of two adult rescued swr−/− homozygotes were injected [52]. pCS2+-smad5 template was linearised with Acc651, p64T-bmp2b was linearised with Xba1 and RNA for both was transcribed using SP6 polymerase according to standard protocols [53]. For bmp2b rescue, 94% of injected mutant embryos are rescued to a wild-type or weakly dorsalised phenotype (∼50%) or a ventralised phenotype (∼50%) [11]. For smad5 rescue, efficiency is similar to that already described for smad1 [11]: about 80% of injected mutant embryos are rescued to a wild-type or a weakly dorsalised (viable) phenotype. Only those animals rescued to a wild-type phenotype were analysed here. Injected embryos were processed for phalloidin staining (see below) or raised to adulthood and rescued swr−/− individuals identified by PCR genotyping as previously described (swrta72: [37]; swrtc300: [54]). DNA was obtained from fin clips according to standard procedures [49].
Dorsal light reflex
In fish, the tendency to orient dorsal side up relies primarily on vestibular inputs, but visual inputs can compensate for a loss of vestibular function [55]. When a fish tank is illuminated from the side in a darkened room, fish that cannot detect gravitoinertial stimuli will orient their dorsal side towards the light source (the dorsal light reflex), while there is no immediate effect on wild-type fish. The dorsal light reflex of five adult bmp2b-rescued swrta72 fish was assessed as described [17]. The test was repeated three times for each fish.
Histological analysis
Histology was carried out on swrta72 fish rescued with bmp2b RNA and swrtdc24 and swrtc300 rescued with smad5 RNA. Heads of adult fish were fixed for 1 to 2 days at 4° C in 4% paraformaldehyde, treated with 0.12 M EDTA for three days to remove otoliths, dehydrated through an ethanol series, and embedded in JB4 resin (Polysciences), before sectioning at 10 µm using a steel knife. Sections were stained with toluidine blue and mounted in DePeX (Sigma) before photography using a Camedia (C-3030ZOOM) camera, AnalySIS software and a BX51 compound microscope (Olympus). Images were assembled and painted using Adobe Photoshop and reconstructions produced using Amira 4.0 software (Visage Imaging).
FITC-Phalloidin staining
Embryos were fixed overnight in 4% paraformaldehyde, rinsed in PBS, and whole-mount stained with FITC-conjugated phalloidin as described previously [19], mounted in Vectashield (Vector laboratories) and imaged using a Leica SP confocal microscope. Ears were dissected for dorsal views.
Supporting Information
360° rotation of the reconstructed adult swr/bmp2b mutant ear shown in Fig. 2B
(2.70 MB MOV)
Acknowledgments
We would like to thank all the aquaria staff at Sheffield, Freiburg and the University of Pennsylvania for care of the zebrafish.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by grants from the Wellcome Trust (057101) and the MRC (G78/5898) to TW, from the NIH (R01-GM56326) to MCM, and from the British Heart Foundation to BC. Funding for the confocal imaging in Fig. 3 was provided by Yorkshire Cancer Research. The CDBG zebrafish aquaria were supported by the MRC (G0400100, G0700091). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mowbray C, Hammerschmidt M, Whitfield TT. Expression of BMP signalling pathway members in the developing zebrafish inner ear and lateral line. Mech Dev. 2001;108:179–184. doi: 10.1016/s0925-4773(01)00479-8. [DOI] [PubMed] [Google Scholar]
- 2.Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu DK, Oh S-H. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh S-H, Johnson R, Wu DK. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J Neurosci. 1996;16:6463–6475. doi: 10.1523/JNEUROSCI.16-20-06463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- 6.Mowbray C. 2002. Bone morphogenetic proteins and zebrafish inner ear development: PhD thesis, University of Sheffield.
- 7.Chang W, ten Dijke P, Wu DK. BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev Biol. 2002;251:380–394. doi: 10.1006/dbio.2002.0822. [DOI] [PubMed] [Google Scholar]
- 8.Shawi M, Serluca FC. Identification of a BMP7 homolog in zebrafish expressed in developing organ systems. Gene Exp Patt. 2008;8:369–375. doi: 10.1016/j.gep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto Y, Lee K-H, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 10.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- 12.Gerlach LM, Hutson MR, Germiller JA, Nguyen-Luu D, Victor JC, et al. Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development. 2000;127:45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Chang W, Nunes FD, De Jesus-Escobar JM, Harland R, Wu DK. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol. 1999;216:369–381. doi: 10.1006/dbio.1999.9457. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach-Bank LM, Cleveland AR, Barald KF. DAN directs endolymphatic sac and duct outgrowth in the avian inner ear. Dev Dyn. 2004;229:219–230. doi: 10.1002/dvdy.10414. [DOI] [PubMed] [Google Scholar]
- 15.Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, et al. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral speicifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, et al. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 18.Hammond KL, Loynes HE, Folarin AA, Smith J, Whitfield TT. Hedgehog signalling is required for correct anteroposterior patterning of the zebrafish otic vesicle. Development. 2003;130:1403–1417. doi: 10.1242/dev.00360. [DOI] [PubMed] [Google Scholar]
- 19.Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–123. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Ernest S, Rauch G-J, Haffter P, Geisler R, Petit C, et al. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 21.Söllner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 22.Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–4223. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, et al. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–338. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Seiler C, Finger-Baier KC, Rinner O, Makhankov YV, Schwarz H, et al. Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development. 2005;132:615–623. doi: 10.1242/dev.01591. [DOI] [PubMed] [Google Scholar]
- 25.Kappler JA, Starr CJ, Chan DK, Kollmar R, Hudspeth AJ. A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc Natl Acad Sci USA. 2004;101:13056–13061. doi: 10.1073/pnas.0405224101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robledo RF, Lufkin T. Dlx5 and Dlx6 homeobox genes are required for specification of the mammalian vestibular apparatus. Genesis. 2006;44:425–437. doi: 10.1002/dvg.20233. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Grimmer JF, Van De Water TR, T L. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev Cell. 2004;7:439–453. doi: 10.1016/j.devcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Salminen M, Meyer BI, Bober E, Gruss P. netrin 1 is required for semicircular canal formation in the mouse inner ear. Development. 2000;127:13–22. doi: 10.1242/dev.127.1.13. [DOI] [PubMed] [Google Scholar]
- 31.ten Berge D, Brouwer A, Korving J, Martin JF, Meijlink F. Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development. 1998;125:3831–3842. doi: 10.1242/dev.125.19.3831. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Chan EK, Baron S, Van de Water T, Lufkin T. Hmx2 homeobox gene control of murine vestibular morphogenesis. Development. 2001;128:5017–5029. doi: 10.1242/dev.128.24.5017. [DOI] [PubMed] [Google Scholar]
- 33.Merlo GR, Paleari L, Mantero S, Zerega B, Adamska M, et al. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev Biol. 2002;248:157–169. doi: 10.1006/dbio.2002.0713. [DOI] [PubMed] [Google Scholar]
- 34.Dick A, Hild M, Bauer H, Imai Y, Maifeld H, et al. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- 35.Schmid B, Fürthauer M, Connors SA, Trout J, Thisse B, et al. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- 36.Mintzer KA, Lee MA, Runke G, Trout J, Whitman M, et al. lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 37.Hild M, Dick A, Rauch G-J, Meier A, Bouwmeester T, et al. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–2159. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- 38.Kramer C, Mayr T, Nowak M, Schumacher J, Runke G, et al. Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev Biol. 2002;250:263–279. [PubMed] [Google Scholar]
- 39.Fekete DM, Homburger SA, Waring MT, Riedl AE, Garcia LF. Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development. 1997;124:2451–2461. doi: 10.1242/dev.124.12.2451. [DOI] [PubMed] [Google Scholar]
- 40.Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- 41.Bissonnette JP, Fekete DM. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J Comp Neurol. 1996;368:620–630. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Lang H, Bever MM, Fekete DM. Cell proliferation and cell death in the developing chick inner ear: spatial and temporal patterns. J Comp Neurol. 2000;417:205–220. doi: 10.1002/(sici)1096-9861(20000207)417:2<205::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi Y, Nakamura H, Funahashi J-I. Epithelial-mesenchymal transition as a possible mechanism of semicircular canal morphogenesis in chick inner ear. Tohoku J Exp Med. 2008;215:207–217. doi: 10.1620/tjem.215.207. [DOI] [PubMed] [Google Scholar]
- 44.Cecconi F, Roth KA, Dolgov O, Munarriz E, Anokhin K, et al. Apaf1-dependent programmed cell death is required for inner ear morphogenesis and growth. Development. 2004;131:2125–2135. doi: 10.1242/dev.01082. [DOI] [PubMed] [Google Scholar]
- 45.Waterman RE, Bell DH. Epithelial fusion during early semicircular canal formation in the embryonic zebrafish, Brachydanio rerio. Anat Rec. 1984;210:101–114. doi: 10.1002/ar.1092100113. [DOI] [PubMed] [Google Scholar]
- 46.Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev Dyn. 2002;223:536–543. doi: 10.1002/dvdy.10062. [DOI] [PubMed] [Google Scholar]
- 47.Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci. 2008;28:8086–8095. doi: 10.1523/JNEUROSCI.1288-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westerfield M. The Zebrafish Book: a guide for the laboratory use of zebrafish (Danio rerio) Oregon: University of Oregon Press; 1995. [Google Scholar]
- 50.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 51.Bauer H, Lele Z, Rauch G-J, Geisler R, Hammerschmidt M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development. 2001;128:849–858. doi: 10.1242/dev.128.6.849. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, et al. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- 53.Krieg PA, Melton DA. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nuc Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner DS, Mullins MC. Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev Biol. 2002;245:109–123. doi: 10.1006/dbio.2002.0614. [DOI] [PubMed] [Google Scholar]
- 55.Orlovsky GN. Gravistatic postural control in simpler systems. Curr Op Neurobiol. 1991;1:621–627. doi: 10.1016/s0959-4388(05)80039-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
360° rotation of the reconstructed adult swr/bmp2b mutant ear shown in Fig. 2B
(2.70 MB MOV)