Abstract
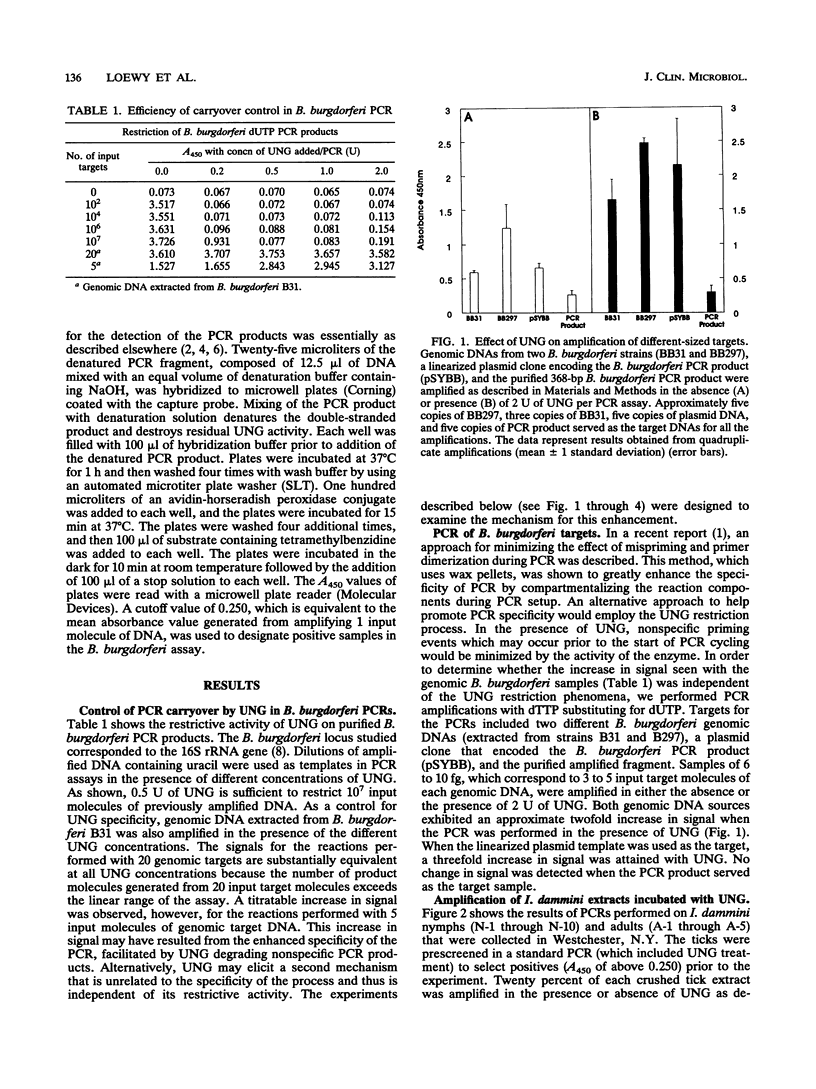
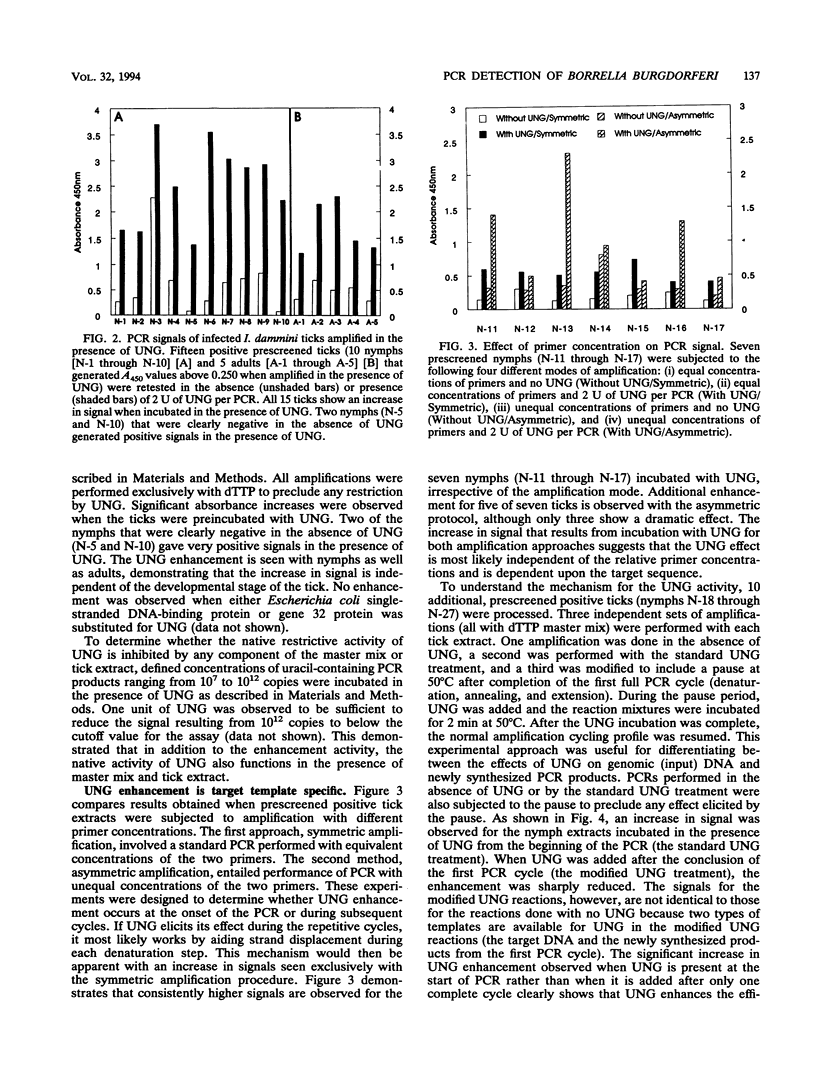
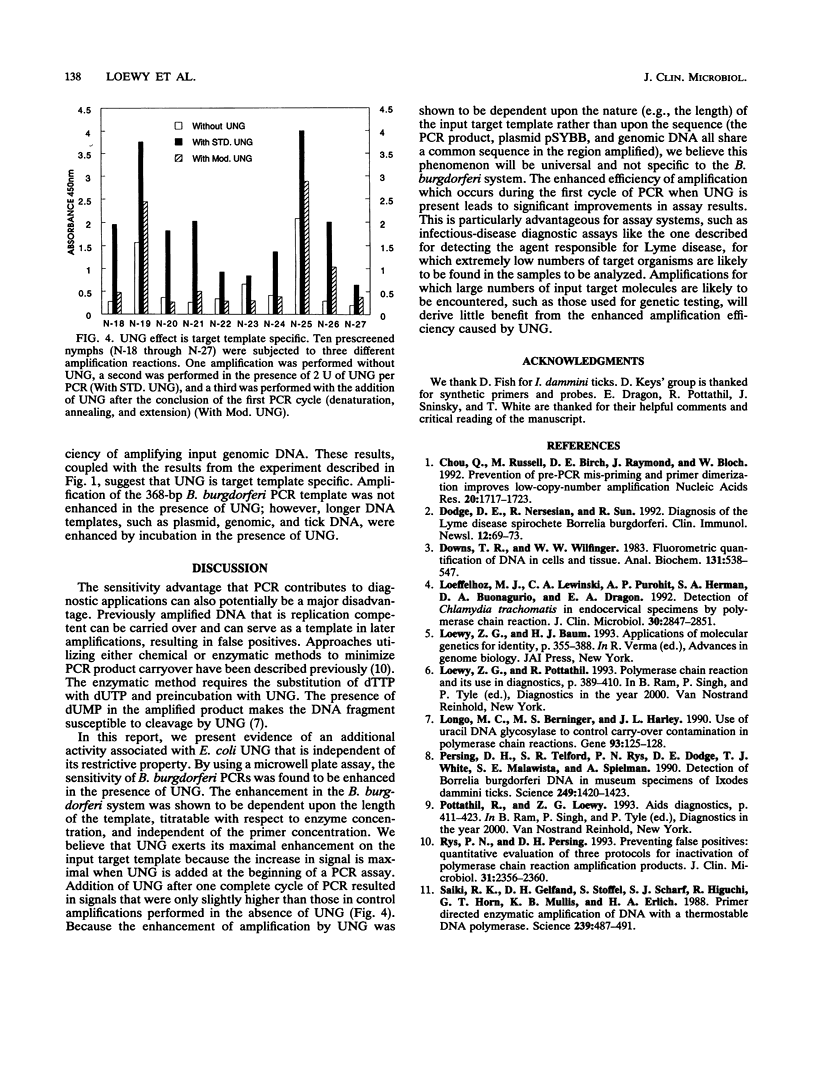
Uracil DNA glycosylases are DNA repair enzymes present in virtually every organism. These enzymes function by excising from DNA uracil residues resulting from either misincorporation of dUMP residues by a DNA polymerase or deamination of cytosine. Recently, the enzyme has been exploited in PCRs as a means for controlling carryover contamination from previously amplified DNA. When the enzyme is used in amplifications of Borrelia burgdorferi target sequences, we have observed an enhancement in signal detected by a microwell plate DNA hybridization assay. This increase in signal is dependent upon the length of the target, is titratable with enzyme concentration, and has been observed with amplifications performed with both symmetric and asymmetric PCR profiles. The enhancement is shown to occur at the level of the target genomic DNA.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs T. R., Wilfinger W. W. Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983 Jun;131(2):538–547. doi: 10.1016/0003-2697(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Loeffelholz M. J., Lewinski C. A., Silver S. R., Purohit A. P., Herman S. A., Buonagurio D. A., Dragon E. A. Detection of Chlamydia trachomatis in endocervical specimens by polymerase chain reaction. J Clin Microbiol. 1992 Nov;30(11):2847–2851. doi: 10.1128/jcm.30.11.2847-2851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M. C., Berninger M. S., Hartley J. L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990 Sep 1;93(1):125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Telford S. R., 3rd, Rys P. N., Dodge D. E., White T. J., Malawista S. E., Spielman A. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science. 1990 Sep 21;249(4975):1420–1423. doi: 10.1126/science.2402635. [DOI] [PubMed] [Google Scholar]
- Rys P. N., Persing D. H. Preventing false positives: quantitative evaluation of three protocols for inactivation of polymerase chain reaction amplification products. J Clin Microbiol. 1993 Sep;31(9):2356–2360. doi: 10.1128/jcm.31.9.2356-2360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]


