Abstract
Staphylococcus aureus is an important human commensal and opportunistic pathogen responsible for a wide range of infections. Long chain unsaturated free fatty acids represent a barrier to colonisation and infection by S. aureus and act as an antimicrobial component of the innate immune system where they are found on epithelial surfaces and in abscesses. Despite many contradictory reports, the precise anti-staphylococcal mode of action of free fatty acids remains undetermined. In this study, transcriptional (microarrays and qRT-PCR) and translational (proteomics) analyses were applied to ascertain the response of S. aureus to a range of free fatty acids. An increase in expression of the σB and CtsR stress response regulons was observed. This included increased expression of genes associated with staphyloxanthin synthesis, which has been linked to membrane stabilisation. Similarly, up-regulation of genes involved in capsule formation was recorded as were significant changes in the expression of genes associated with peptidoglycan synthesis and regulation. Overall, alterations were recorded predominantly in pathways involved in cellular energetics. In addition, sensitivity to linoleic acid of a range of defined (sigB, arcA, sasF, sarA, agr, crtM) and transposon-derived mutants (vraE, SAR2632) was determined. Taken together, these data indicate a common mode of action for long chain unsaturated fatty acids that involves disruption of the cell membrane, leading to interference with energy production within the bacterial cell. Contrary to data reported for other strains, the clinically important EMRSA-16 strain MRSA252 used in this study showed an increase in expression of the important virulence regulator RNAIII following all of the treatment conditions tested. An adaptive response by S. aureus of reducing cell surface hydrophobicity was also observed. Two fatty acid sensitive mutants created during this study were also shown to diplay altered pathogenesis as assessed by a murine arthritis model. Differences in the prevalence and clinical importance of S. aureus strains might partly be explained by their responses to antimicrobial fatty acids.
Introduction
Staphylococcus aureus is the aetiological agent for a wide range of human infections, including abscesses, septicaemia, arthritis and endocarditis. The increased prevalence of meticillin resistant- (MRSA) and vancomycin insensitive-S. aureus strains, and the emergence of community-acquired MRSA make investigations into the pathogenicity of this species imperative. Inevitably, this focuses research into the development of novel antimicrobial agents, which requires a rigorous study of staphylococcal physiology. Long chain unsaturated free fatty acids (LC-uFFAs), typically ≥C16, are known to possess anti-staphylococcal activity and LC-uFFAs are important components of the innate immune system. Individuals with atopic dermatitis exhibit deficient production of the skin-specific LC-uFFA, hexadecenoic acid [C16:1 (n-6)], which is associated with increased carriage of S. aureus and susceptibility to bacterial skin infections [1]–[3]. In human tissue and nasal fluid, the major LC-FFAs are the unsaturated linoleic [C18:2 (n-6,9)], oleic [C18:1 (n-9)] and palmitoleic [C16:1 (n-7)] acids and the saturated palmitic [C16:0] and stearic [C18:0] acids [4]–[7]. Assay of staphylococcal abscess homogenates has revealed the presence of anti-staphylococcal activity comprising a pool of monoglycerides and free fatty acids [8]–[10]. The most abundant compound present in this active pool was identified as linoleic acid and was found at millimolar concentrations.
FFAs of various chain lengths and with different levels of unsaturation are primarily effective against Gram-positive bacteria [11]–[18]. Inhibition of several membrane-enveloped viruses has also been demonstrated [19]–[21]. Although several studies have attempted to pinpoint the specific cellular target(s) of LC-uFFAs, the actual anti-bacterial mechanism has not been unambiguously determined. Conflicting data have proposed that LC-uFFAs inhibit all major bacterial biosynthetic pathways within the cell, or alternatively, that they specifically inhibit FabI, which catalyses the final and rate-limiting step in fatty acid biosynthesis [12], [18], [22], [23]. Oleic acid was proposed by Won et al. [24] to inhibit glucosyltransferases, while other proposed mechanisms for LC-uFFA-mediated growth inhibition include peptidoglycan (PG) precipitation, peroxidative stress, interference with energy metabolism and alteration of the membrane permeability or fluidity [12], [16], [18], [22], [25], [26].
A diversity of mechanisms have been proposed to account for resistance to LC-uFFAs in S. aureus. Enhanced production of the carotenoid staphyloxanthin (giving aureus its golden title) has been proposed as a mechanism to relieve the inhibitory effects of increased membrane fluidity due to insertion of LC-uFFAs into the lipid bilayer in S. aureus [26]–[28]. Increased staphylococcal resistance to LC-uFFAs was positively correlated with pigmentation, although these experiments were performed using non-isogenic strains [28]. A fatty acid modifying enzyme (FAME), which catalyses the esterification of FFAs with cholesterol has also been purified from several S. aureus strains and its production correlated with increased disease severity in an abscess model [29]–[32]. Nonetheless the gene encoding FAME remains unidentified. Furthermore, in Neisseria gonorrhoea, FFA resistance has been linked to the presence of FFA-specific efflux pumps [33] while in S. aureus, the expression of Fur-iron-regulated staphylococcal surface-associated protein IsdA was identified as contributing to FA resistance in iron-limited environments by reducing cellular hydrophobicity [34]. Another proposed mechanism included the increased production of a ‘protective slime’ composed of precipitated PG complexed to fatty acids [25].
Previous studies demonstrated that S. aureus responds to the C12 monoester glycerol monolaurate (GML) and the component FFA lauric acid by reducing levels of expression of alpha toxin (Hla) [35]–[37]. Similarly, Clarke et al. [34] showed that expression of hla was reduced following exposure of S. aureus to the LC-uFFA hexadecenoic acid [C16:1 (n-6)]. More recently, GML was shown to inhibit the synthesis of toxins in several Gram-positive bacteria and also limited the effect of these toxins on eukaryotic cells [38]–[40].
While the biological effects of free fatty acids as antimicrobial compounds have been catalogued, there remains no unequivocal identification of the targets or mechanisms of action in relation to S. aureus. Transcriptomic and proteomic analyses have the potential to elucidate complex cellular and metabolic responses and are applied here for the first time to analyse the reaction of S. aureus to the LC-uFFAs linoleic, oleic and hexadecenoic acid. In addition, an analysis of existing well-characterised mutants and the generation of new allelic replacement mutants based on gene array data coupled to transposon screens was carried out to identify loci important for survival. Finally, a murine arthritis model of infection was used to ascertain whether two of the genes highlighted in this study have a role in pathogenesis.
Results
Comparative resistance of S. aureus strains to unsaturated C18 free fatty acids
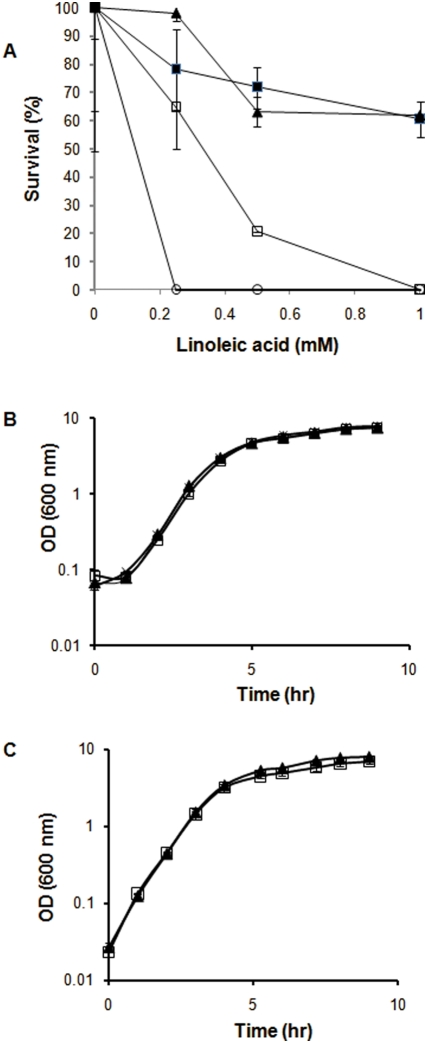
The relative resistances of different strains of S. aureus to the unsaturated C18 free fatty acids linoleic acid [C18:2 (n-6,9)] and oleic acid [C18:1 (n-9)] were compared using a previously described agar plate assay [13]. Many strains, such as MSSA476 and N315, were unable to grow on emulsion agar plates containing 1 mM linoleic acid (Fig. 1A). In contrast MRSA252, an epidemic ERMSA-16 strain, and the laboratory strain SH1000 displayed high levels (>60%) of survival at millimolar concentrations. Consequently, all subsequent experiments were performed using MRSA252 and SH1000 strains of S. aureus, owing to their enhanced growth in the presence of C18 LC-uFFAs.
Figure 1. Inhibition of S. aureus by C18 unsaturated fatty acids.
A Graph showing percentage survival of wild-type strains of S. aureus when these strains were incubated on BHI plates containing 0, 0.25, 0.5 and 1 mM linoleic acid. The strains analysed were SH1000 (closed box), MRSA252 (closed triangle), MSSA476 (open box) and N315 (open circle). This assay was performed in triplicate and is representative of multiple experiments. B Growth of a 0.5% (vol/vol) inoculum of MRSA252 in 100 ml BHI containing 0 mM fatty acid (closed triangle), 0.01 mM oleic acid (cross) or 0.01 mM linoleic acid (open box) at 37°C with shaking at 250 rpm. RNA was extracted from these cells at an OD600 of 3 and analysed in microarray experiments as the growth exposure conditions. C Growth of a 0.5% (vol/vol) inoculum of MRSA252 in 100 ml BHI at 37°C with shaking at 250 rpm with (open box) or without (closed triangle) the addition of 0.1 mM linoleic acid at an OD600 of 3. RNA was extracted from these cells 20 min post-exposure and analysed in microarray experiments as the challenge conditions. The growth curves shown in B and C were performed in biological triplicate. The error bars shown in graphs B and C correspond to standard errors of the mean.
Growth of MRSA252 in the presence of LC-uFFAs
To facilitate analysis of gene transcription and protein expression, a range of different concentrations of linoleic or oleic acid and the timing of their addition were examined during growth (data not shown). Upon inoculation 0.01 mM linoleic acid was determined to be the maximum concentration, which did not retard the aerobic growth of MRSA252 in BHI broth (Fig. 1B). Cells were subsequently grown in the presence of 0.01 mM linoleic or oleic acid with the FFAs being added at the start of growth (growth exposure conditions). To test the response of MRSA252 to LC-uFFAs under slightly different conditions, a higher concentration of linoleic acid (0.1 mM) was added during the late-exponential growth phase (OD600 = 3) where it was observed to reduce subsequent growth (challenge conditions) (Fig. 1C). These culture conditions were repeated for independent samples and cells were harvested to determine the transcriptional and translational responses of the cells to treatment with LC-uFFAs.
The transcriptional response of S. aureus to C18 free fatty acids
A pronounced differential transcriptional response was observed in MRSA252 cells treated with linoleic acid when it was added to a final concentration of 0.1 mM for 20 min during late-exponential growth (linoleic acid challenge) compared to unexposed control cells; 213 genes were up-regulated (Table 1) and 179 genes were down-regulated (Table 2). When transcription was analysed for cells grown in the presence of a lower concentration of linoleic acid (0.01 mM) from the time of inoculation (linoleic acid growth exposure) a correspondingly smaller subset of genes displayed differential transcription; 37 genes were up-regulated (Table 3) and 28 genes were down-regulated (Table 4). Oleic acid differs from linoleic acid in its degree of unsaturation, containing one less double bond in the chain. When cells were grown under the conditions of oleic acid growth exposure, 20 genes were up-regulated (Table 5) and 23 genes were down-regulated (Table 6).
Table 1. MRSA252 genes up-regulated following the addition of linoleic acid (0.1 mM) to exponentially growing cells (linoleic acid challenge).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Up Regulated | P-value |
| Virulence Factors and Regulators | SAR0156 | capF | capsular polysaccharide synthesis enzyme | 2.23 | 4.22E-02 |
| SAR0163 | capM | capsular polysaccharide synthesis enzyme | 2.23 | 3.20E-02 | |
| SAR0164 | capN | capsular polysaccharide synthesis enzyme | 2.83 | 1.79E-02 | |
| SAR0625 | sarA | staphylococcal accessory regulator A | 2.14 | 1.50E-02 | |
| SAR0842 | clfA | clumping factor | 4.12 | 6.58E-03 | |
| SAR2122 | hld | delta-hemolysin precursor | 3.28 | 1.23E-02 | |
| SAR2295 | putative exported MAP/eap domain protein | 3.21 | 8.77E-04 | ||
| SAR2443 | tcaR | MarR family regulatory protein | 3.15 | 1.76E-03 | |
| RNAIII | RNAIII | RNAIII accessory gene regulator (agr) locus | 2.01 | 3.02E-02 | |
| Stress Response | SAR0577 | proP | putative proline/betaine transporter | 8.31 | 5.78E-04 |
| SAR0859 | putative organic hydroperoxide resistance protein | 3.82 | 1.23E-02 | ||
| SAR0938 | clpB | putative ATPase subunit of an ATP-dependent protease | 2.49 | 8.15E-04 | |
| SAR1344 | katA | catalase | 5.71 | 1.86E-03 | |
| SAR1656 | dnaJ | chaperone protein | 2.30 | 4.25E-02 | |
| SAR1657 | dnaK | chaperone protein | 2.41 | 2.17E-03 | |
| SAR2273 | asp23 | alkaline shock protein 23 | 2.06 | 3.86E-02 | |
| SAR2276 | opuD2 | glycine betaine transporter 2 | 4.42 | 6.16E-03 | |
| SAR2561 | alkylhydroperoxidase, AhpD family | 6.83 | 8.77E-04 | ||
| SAR2628 | clpL | putative ATPase subunit of an ATP-dependent protease | 4.06 | 4.79E-03 | |
| Energy Metabolism | SAR0113 | lldP1 | L-lactate permease 1 | 2.15 | 1.07E-03 |
| SAR0188 | putative isochorismatase | 4.91 | 8.03E-04 | ||
| SAR0141 | drm | putative phosphopentomutase | 2.45 | 1.31E-02 | |
| SAR0574 | putative hexulose-6-phosphate synthase | 2.36 | 1.80E-03 | ||
| SAR0575 | putative 6-phospho-3-hexuloisomerase | 2.16 | 5.11E-03 | ||
| SAR0775 | Osmoprotectant ABC transporter | 2.13 | 4.80E-03 | ||
| SAR0776 | Osmoprotectant ABC transporter, permease protein | 2.99 | 3.00E-04 | ||
| SAR0824 | putative malolactic enzyme | 2.59 | 9.27E-03 | ||
| SAR0830 | tpiA | triosephosphate isomerase | 2.22 | 3.39E-02 | |
| SAR0831 | pgm | putative phosphoglycerate mutase | 2.64 | 1.39E-02 | |
| SAR1017 | menD | putative menaquinone biosynthesis bifunctional protein | 2.24 | 1.65E-03 | |
| SAR1018 | putative hydrolase | 2.80 | 1.65E-03 | ||
| SAR2386 | putative NAD-dependent dehydrogenase | 3.73 | 3.00E-04 | ||
| SAR2506 | dpgm | putative phosphoglycerate mutase | 2.06 | 7.33E-04 | |
| SAR2684 | fda | fructose-bisphosphate aldolase class I | 2.02 | 5.85E-03 | |
| SAR2687 | putative AMP-binding enzyme | 2.01 | 9.65E-03 | ||
| SAR2724 | isochorismatase family protein | 3.00 | 8.30E-04 | ||
| DNA Repair and Replication | SAR0363 | ssb | putative single-strand DNA-binding protein | 2.26 | 3.29E-03 |
| SAR0744 | putative DNA photolyase | 3.46 | 6.97E-04 | ||
| SAR0813 | uvrA | excinuclease ABC subunit A | 2.45 | 2.06E-03 | |
| SAR0836 | rnr | putative ribonuclease R | 3.40 | 3.55E-03 | |
| SAR0837 | smpB | putative tmRNA-binding protein | 3.07 | 3.70E-04 | |
| Protein Synthesis | SAR0364 | rpsR | 30S ribosomal protein S18 | 2.40 | 1.99E-02 |
| SAR0552 | fus | translation elongation factor G | 2.10 | 3.39E-02 | |
| SAR1638 | rpoD | RNA polymerase sigma factor | 2.86 | 3.70E-04 | |
| SAR2308 | rplQ | 50S ribosomal protein L17 | 2.60 | 1.99E-02 | |
| SAR2309 | rpoA | DNA-directed RNA polymerase alpha chain | 2.36 | 3.35E-02 | |
| SAR2310 | rpsK | 30S ribosomal protein S11 | 2.46 | 3.77E-02 | |
| SAR2311 | rpsM | 30S ribosomal protein S13 | 2.45 | 3.12E-02 | |
| SAR2313 | infA | translation initiation factor IF-1 | 2.08 | 1.49E-02 | |
| SAR2728 | preprotein translocase SecA subunit-like protein | 3.85 | 6.58E-03 | ||
| Peptidoglycan Synthesis | SAR0878 | csdB | putative selenocysteine lyase | 2.52 | 3.07E-02 |
| SAR1026 | atl | bifunctional autolysin precursor | 2.65 | 6.16E-03 | |
| SAR1158 | mraY | phospho-N-acetylmuramoyl-pentapeptide-transferase | 2.13 | 9.56E-04 | |
| SAR1159 | murD | UDP-N-acetylmuramoylalanine–D-glutamate ligase | 2.39 | 7.64E-03 | |
| SAR1160 | putative cell division protein | 2.10 | 1.31E-02 | ||
| SAR1290 | putative exported CHAP domain protein | 3.17 | 6.97E-04 | ||
| SAR1430 | murG | putative N-acetylglucosamine transferase | 5.26 | 1.86E-03 | |
| SAR1761 | lysP | lysine-specific permease | 2.07 | 3.01E-02 | |
| SAR2109 | dapE | putative succinyl-diaminopimelate desuccinylase | 4.89 | 3.00E-03 | |
| SAR2188 | murA1 | putative carboxyvinyltransferase | 2.94 | 6.54E-03 | |
| SAR2269 | putative alanine racemase | 2.64 | 1.78E-03 | ||
| SAR2346 | fmhB | putative pentaglycine interpeptide biosynthesis protein | 2.49 | 4.22E-03 | |
| SAR2394 | putative protein associated with cell-envelope regulation | 2.34 | 2.55E-03 | ||
| SAR2420 | hutG | arginase family protein | 2.83 | 4.71E-03 | |
| SAR2521 | putative membrane GtrA-like protein | 3.11 | 5.78E-04 | ||
| Fatty Acid Metabolism | SAR1438 | conserved hypothetical protein | 2.64 | 4.94E-03 | |
| SAR2187 | fabZ | putative hydroxymyristoyl-(acyl carrier protein) dehydratase | 2.41 | 4.22E-02 | |
| Carotenoid Biosynthesis | SAR0596 | mvaK1 | mevalonate kinase | 2.32 | 3.00E-04 |
| SAR0597 | mvaD | mevalonate diphosphate decarboxylase | 3.35 | 9.23E-04 | |
| SAR0598 | mvaK2 | phosphomevalonate kinase | 3.18 | 5.09E-04 | |
| SAR2642 | crtN | squalene synthase | 4.95 | 8.03E-04 | |
| SAR2643 | crtM | squalene desaturase | 7.18 | 2.38E-02 | |
| SAR2645 | crtQ | putative glycosyl transferase | 6.07 | 3.00E-03 | |
| SAR2646 | crtP | putative phytoene dehydrogenase related protein | 6.28 | 1.73E-03 | |
| SAR2647 | putative membrane protein | 4.47 | 1.73E-03 | ||
| Antibiotic Resistance | SAR0139 | putative tetracycline resistance protein | 4.06 | 1.59E-03 | |
| SAR1622 | metallo-beta-lactamase superfamily protein | 2.08 | 3.93E-03 | ||
| SAR1785 | metallo-beta-lactamase superfamily protein | 3.05 | 1.08E-03 | ||
| SAR1831 | blaZ | beta-lactamase precursor | 2.02 | 2.72E-02 | |
| SAR2505 | mdeA | putative transport system protein | 3.93 | 7.74E-03 | |
| SAR2558 | conserved hypothetical beta-lactamase-like protein | 8.72 | 3.70E-04 | ||
| SAR2632 | Putative MMPL efflux pump | 2.03 | 4.58E-02 | ||
| SAR2655 | putative glyoxalase | 5.15 | 1.11E-03 | ||
| SAR2668 | hypothetical aminoglycoside phosphotransferase protein | 4.35 | 6.30E-03 | ||
| Miscellaneous | SAR1738 | tnpB2 | transposase B 2 | 2.14 | 1.25E-03 |
| SAR2725 | sasF | putative surface anchored protein | 16.80 | 4.68E-05 | |
| Metabolism | SAR0108 | putative peptidase | 2.98 | 5.22E-03 | |
| SAR0109 | putative transporter protein | 2.37 | 1.52E-02 | ||
| SAR0170 | putative cation efflux system protein | 2.50 | 1.77E-03 | ||
| SAR0306 | ABC transporter ATP-binding protein | 6.10 | 1.68E-03 | ||
| SAR0324 | putative lipoate-protein ligase A | 2.09 | 4.31E-03 | ||
| SAR0325 | putative reductase | 4.80 | 8.17E-04 | ||
| SAR0556 | ThiJ/PfpI family protein | 7.20 | 7.59E-04 | ||
| SAR0589 | putative amino acid permease | 4.19 | 3.75E-03 | ||
| SAR0600 | pyridine nucleotide-disulphide oxidoreductase protein | 2.26 | 2.77E-04 | ||
| SAR0624 | putative esterase | 6.49 | 7.59E-04 | ||
| SAR0729 | putative acetyltransferase | 2.92 | 3.23E-03 | ||
| SAR0732 | putative acetyltransferase | 2.34 | 3.00E-04 | ||
| SAR0756 | aldo/keto reductase family protein | 2.96 | 6.16E-04 | ||
| SAR0757 | putative glucosyl transferase | 3.49 | 7.59E-04 | ||
| SAR0764 | putative 6-pyruvoyl tetrahydropterin synthase | 3.70 | 8.03E-04 | ||
| SAR0841 | putative acetyltransferase | 5.22 | 3.29E-03 | ||
| SAR0883 | putative dioxygenase | 5.40 | 1.75E-03 | ||
| SAR0903 | putative pyridine nucleotide-disulphide oxidoreductase | 2.82 | 3.00E-04 | ||
| SAR0953 | transport system extracellular binding lipoprotein | 2.18 | 6.05E-03 | ||
| SAR1076 | Spermidine/putrescine-binding protein homolog. | 4.46 | 8.77E-04 | ||
| SAR1247 | putative tRNA pseudouridine synthase B | 2.31 | 2.10E-03 | ||
| SAR1340 | thrB | homoserine kinase | 2.20 | 3.71E-02 | |
| SAR1431 | putative acetyltransferase | 4.58 | 1.80E-03 | ||
| SAR1439 | dfrB | dihydrofolate reductase type I | 2.13 | 6.34E-03 | |
| SAR1440 | thyA | thymidylate synthase | 5.32 | 1.58E-03 | |
| SAR1585 | malR | maltose operon transcriptional repressor | 2.21 | 2.20E-02 | |
| SAR1655 | putative methyltransferase | 2.25 | 6.08E-03 | ||
| SAR2210 | aldehyde dehydrogenase family protein | 5.48 | 1.51E-03 | ||
| SAR2352 | moaA | putative molybdenum cofactor biosynthesis protein A | 2.07 | 1.86E-03 | |
| SAR2385 | putative Na+/H+ antiporter | 2.35 | 1.34E-02 | ||
| SAR2395 | inositol monophosphatase family protein | 2.90 | 7.59E-04 | ||
| SAR2413 | putative short chain dehydrogenase | 4.63 | 3.66E-03 | ||
| SAR2460 | putative acetyltransferase (GNAT) family protein | 2.26 | 6.97E-04 | ||
| SAR2485 | narH | nitrate reductase beta chain | 2.16 | 1.02E-02 | |
| SAR2541 | putative carboxylesterase | 2.45 | 1.79E-02 | ||
| SAR2544 | ABC transporter ATP-binding protein | 6.01 | 1.68E-03 | ||
| SAR2559 | putative short chain dehydrogenase | 6.85 | 6.16E-04 | ||
| SAR2659 | putative short chain dehydrogenase | 2.65 | 1.76E-03 | ||
| SAR2661 | putative hydrolase | 8.11 | 8.27E-04 | ||
| SAR2754 | hisIE | putative histidine biosynthesis bifunctional protein | 2.09 | 2.19E-02 | |
| SAR2778 | putative nickel transport protein | 2.51 | 3.59E-03 | ||
| Hypothetical Genes | SAR0111 | putative myosin-crossreactive antigen | 5.96 | 2.43E-03 | |
| SAR0112 | putative membrane protein | 3.57 | 4.80E-04 | ||
| SAR0171 | hypothetical protein | 2.64 | 3.01E-02 | ||
| SAR0269 | LacI family regulatory protein | 2.57 | 3.59E-03 | ||
| SAR0299 | hypothetical protein | 2.05 | 4.99E-02 | ||
| SAR0305 | putative membrane protein | 3.89 | 6.02E-03 | ||
| SAR0390 | putative lipoprotein | 3.97 | 1.68E-03 | ||
| SAR0392 | putative membrane protein | 2.54 | 1.20E-02 | ||
| SAR0405 | hypothetical protein | 2.76 | 1.07E-02 | ||
| SAR0444 | putative lipoprotein | 2.31 | 2.16E-03 | ||
| SAR0498 | yabJ | putative regulatory protein | 3.65 | 1.07E-03 | |
| SAR0499 | spoVG | stage V sporulation protein G | 2.83 | 1.11E-02 | |
| SAR0601 | putative DNA-binding protein | 2.15 | 4.48E-04 | ||
| SAR0670 | putative sensor histidine kinase protein | 2.01 | 4.80E-04 | ||
| SAR0721 | multicopper oxidase protein | 2.29 | 4.39E-03 | ||
| SAR0733 | conserved hypothetical protein | 3.04 | 1.99E-03 | ||
| SAR0734 | conserved hypothetical protein | 2.23 | 1.59E-03 | ||
| SAR0821 | conserved hypothetical protein | 3.19 | 6.54E-03 | ||
| SAR0825 | conserved hypothetical protein | 5.06 | 1.86E-03 | ||
| SAR0840 | putative membrane protein | 5.25 | 2.63E-03 | ||
| SAR0849 | hypothetical protein | 2.81 | 6.05E-03 | ||
| SAR0850 | hypothetical protein | 2.94 | 6.81E-04 | ||
| SAR0854 | hypothetical protein | 4.07 | 1.56E-02 | ||
| SAR0855 | hypothetical protein | 2.53 | 1.94E-03 | ||
| SAR0867 | hypothetical protein | 2.54 | 4.55E-03 | ||
| SAR0877 | conserved hypothetical protein | 2.34 | 3.01E-02 | ||
| SAR0879 | NifU-like protein | 2.06 | 3.39E-02 | ||
| SAR0880 | conserved hypothetical protein | 2.14 | 3.47E-03 | ||
| SAR0882 | putative membrane protein | 4.05 | 4.12E-03 | ||
| SAR0931 | putative membrane protein | 7.87 | 4.28E-04 | ||
| SAR1055 | hypothetical protein | 4.50 | 3.08E-03 | ||
| SAR1077 | putative membrane protein | 2.54 | 5.69E-03 | ||
| SAR1227 | conserved hypothetical protein | 2.11 | 1.71E-02 | ||
| SAR1258 | putative DNA-binding protein | 2.12 | 3.07E-04 | ||
| SAR1289 | putative exported protein | 3.49 | 1.65E-03 | ||
| SAR1306 | hypothetical protein | 2.20 | 5.66E-03 | ||
| SAR1429 | putative membrane protein | 5.74 | 1.84E-03 | ||
| SAR1528 | hypothetical phage protein | 6.04 | 4.99E-02 | ||
| SAR1623 | conserved hypothetical protein | 2.29 | 1.39E-02 | ||
| SAR1669 | conserved hypothetical protein | 2.01 | 1.03E-02 | ||
| SAR1670 | conserved hypothetical protein | 2.53 | 5.36E-03 | ||
| SAR1671 | probable nicotinate-nucleotide adenylyltransferase | 2.03 | 8.07E-03 | ||
| SAR1816 | putative membrane protein | 2.82 | 6.16E-04 | ||
| SAR1854 | hypothetical protein | 4.98 | 1.27E-03 | ||
| SAR1965 | ThiJ/PfpI family protein | 2.25 | 4.83E-02 | ||
| SAR1970 | conserved hypothetical protein | 2.17 | 4.68E-02 | ||
| SAR1972 | putative exported protein | 5.71 | 6.59E-03 | ||
| SAR2010 | hypothetical protein | 3.49 | 6.58E-03 | ||
| SAR2047 | hypothetical phage protein | 2.12 | 1.16E-02 | ||
| SAR2085 | hypothetical phage RecT family protein | 2.18 | 9.84E-04 | ||
| SAR2088 | hypothetical phage protein | 2.62 | 2.28E-02 | ||
| SAR2094 | hypothetical phage protein | 2.69 | 3.06E-03 | ||
| SAR2095 | hypothetical phage protein | 4.03 | 5.44E-03 | ||
| SAR2098 | hypothetical phage protein | 2.02 | 2.87E-03 | ||
| SAR2189 | putative membrane protein | 2.94 | 6.07E-03 | ||
| SAR2232 | conserved hypothetical protein | 8.26 | 2.06E-03 | ||
| SAR2245 | putative transcriptional antiterminator | 6.03 | 1.76E-03 | ||
| SAR2270 | hypothetical IucA/IucC family protein | 3.36 | 3.80E-03 | ||
| SAR2274 | putative membrane protein | 4.59 | 2.04E-03 | ||
| SAR2275 | putative membrane protein | 3.98 | 6.16E-04 | ||
| SAR2347 | putative membrane protein | 2.21 | 6.54E-03 | ||
| SAR2392 | conserved hypothetical protein | 3.03 | 5.20E-03 | ||
| SAR2393 | putative molydopterin dinucleotide binding domain protein | 3.26 | 2.50E-03 | ||
| SAR2444 | putative membrane protein | 4.38 | 2.77E-04 | ||
| SAR2469 | putative pyridoxamine 5′-phosphate oxidase | 4.72 | 1.11E-03 | ||
| SAR2496 | putative solute binding lipoprotein | 2.60 | 3.66E-03 | ||
| SAR2525 | hypothetical protein | 5.28 | 2.33E-05 | ||
| SAR2532 | CapD domain protein | 2.48 | 4.16E-03 | ||
| SAR2542 | putative transport protein | 2.01 | 5.64E-03 | ||
| SAR2543 | putative membrane protein | 6.37 | 6.16E-04 | ||
| SAR2568 | hypothetical protein | 4.66 | 1.65E-03 | ||
| SAR2656 | conserved hypothetical protein | 3.35 | 6.16E-04 | ||
| SAR2657 | hypothetical protein | 2.40 | 8.80E-04 | ||
| SAR2658 | TetR family regulatory protein | 2.22 | 4.39E-04 | ||
| SAR2660 | conserved hypothetical protein | 7.26 | 6.16E-04 | ||
| SAR2665 | conserved hypothetical protein | 2.19 | 4.06E-03 | ||
| SAR2666 | hypothetical protein | 2.75 | 1.87E-03 | ||
| SAR2667 | hypothetical protein | 2.19 | 1.37E-02 | ||
| SAR2688 | hypothetical protein | 7.55 | 3.70E-04 | ||
| SAR2689 | hypothetical protein | 2.53 | 1.84E-02 | ||
| SAR2726 | conserved hypothetical protein | 5.07 | 1.80E-03 | ||
| SAR2727 | glycosyl transferase, group 1 family protein | 4.11 | 6.70E-03 | ||
| SAR2739 | conserved hypothetical protein | 4.21 | 2.06E-03 | ||
| SAR2740 | conserved hypothetical protein | 2.05 | 3.09E-02 | ||
| SAR2777 | putative DNA-binding protein | 2.40 | 1.90E-03 | ||
| SAR2780 | putative membrane protein | 7.38 | 4.80E-04 |
Table 2. MRSA252 genes down-regulated following the addition of linoleic acid (0.1 mM) to exponentially growing cells (linoleic acid challenge).
| Group Functions | MRSA 252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Down Regulated | P-value |
| Virulence Factors and Regulators | SAR0105 | plc | 1-phosphatidylinositol phosphodiesterase | 3.85 | 4.39E-04 |
| SAR1574 | fur | iron uptake regulatory protein | 2.08 | 2.46E-02 | |
| SAR1984 | ferritin | 2.56 | 4.39E-03 | ||
| SAR2001 | staphopain protease | 2.44 | 3.00E-04 | ||
| SAR2474 | sarZ | MarR family virulence regulator | 2.22 | 2.18E-02 | |
| SAR2155 | rsbU | putative sigma factor sigB regulation protein | 2.56 | 1.58E-03 | |
| SAR2715 | argR | arginine repressor family protein | 2.27 | 4.27E-03 | |
| SAR2716 | aur | zinc metalloproteinase aureolysin precursor | 2.17 | 1.76E-03 | |
| Energy Metabolism | SAR0234 | ldh1 | L-lactate dehydrogenase 1 | 2.94 | 2.99E-05 |
| SAR0235 | putative PTS system, IIBC component | 2.22 | 1.30E-03 | ||
| SAR0242 | putative galactitol PTS component | 2.13 | 1.76E-02 | ||
| SAR0263 | putative PTS system protein | 2.50 | 3.44E-02 | ||
| SAR0355 | Cys/Met metabolism PLP-dependent enzyme | 2.04 | 2.74E-02 | ||
| SAR0522 | putative pyridoxal 5-phosphate biosynthesis protein | 3.03 | 3.00E-04 | ||
| SAR0523 | SNO glutamine amidotransferase family protein | 2.70 | 3.70E-04 | ||
| SAR0752 | putative phosphofructokinase | 2.38 | 3.27E-02 | ||
| SAR0753 | fruA | fructose-specific PTS system component | 2.50 | 3.78E-02 | |
| SAR0766 | glutamine amidotransferase class-I protein | 2.04 | 6.16E-04 | ||
| SAR1088 | putative pyruvate carboxylase | 2.5 | 8.78E-04 | ||
| SAR1450 | tdcB | putative threonine dehydratase | 2.22 | 5.47E-03 | |
| SAR1451 | ald2 | alanine dehydrogenase 2 | 3.03 | 1.15E-03 | |
| SAR1777 | pfkA | 6-phosphofructokinase | 2.86 | 2.87E-03 | |
| SAR1789 | ackA | acetate kinase | 2.33 | 9.23E-03 | |
| SAR2143 | ilvC | ketol-acid reductoisomerase | 2.22 | 2.80E-02 | |
| SAR2213 | fba | putative tagatose-bisphosphate aldolase | 3.13 | 6.59E-03 | |
| SAR2262 | putative uridylyltransferase | 2.27 | 8.35E-03 | ||
| SAR2579 | gtaB | putative uridylyltransferase | 2.63 | 6.84E-03 | |
| SAR2720 | putative PTS system component | 4.17 | 3.54E-03 | ||
| SAR2721 | pmi | mannose-6-phosphate isomerase | 3.57 | 2.76E-03 | |
| Cell Wall Synthesis | SAR0228 | putative glutamine amidotransferase class-I | 2.13 | 2.08E-03 | |
| SAR0257 | lytS | autolysin sensor kinase protein | 3.33 | 3.00E-03 | |
| SAR0258 | lytR | autolysin response regulator protein | 3.57 | 9.15E-03 | |
| SAR0259 | lrgA | holin-like protein | 2.22 | 1.20E-03 | |
| SAR0646 | tagA | teichoic acid biosynthesis protein | 2.78 | 6.80E-03 | |
| SAR0648 | tagG | teichoic acid ABC transporter permease protein | 2.38 | 1.32E-02 | |
| SAR0649 | tagB | teichoic acid biosynthesis protein | 2.50 | 4.41E-04 | |
| SAR1143 | putative carbamate kinase | 2.27 | 6.79E-03 | ||
| SAR1752 | hemA | glutamyl-tRNA reductase | 2.27 | 3.93E-02 | |
| SAR1807 | putative transglycosylase | 2.04 | 8.78E-04 | ||
| SAR2472 | gltT | putative proton/sodium-glutamate symport protein | 2.04 | 2.97E-02 | |
| SAR2621 | cidA | holin-like protein | 2.27 | 1.18E-02 | |
| SAR2669 | putative dihydroorotate dehydrogenase | 2.86 | 3.75E-03 | ||
| Fatty Acid Metabolism | SAR0225 | fadD | putative acyl-CoA dehydrogenase | 2.17 | 3.27E-02 |
| SAR0227 | fadX | putative acetyl-CoA transferase | 2.13 | 4.95E-02 | |
| SAR0803 | conserved hypothetical protein | 3.23 | 1.02E-02 | ||
| Carotenoid Biosynthesis | SAR1278 | miaA | putative isopentenylpyrophosphate transferase | 2.00 | 9.23E-03 |
| SAR1479 | putative heptaprenyl diphosphate synthase | 2.78 | 1.62E-02 | ||
| SAR1480 | menH | heptaprenylnaphthoquinone methyltransferase | 2.27 | 3.54E-02 | |
| SAR1481 | putative hexaprenyl diphosphate synthase | 3.13 | 1.79E-02 | ||
| DNA Repair and Replication | SAR0001 | dnaA | chromosomal replication initiator protein DnaA | 2.04 | 2.17E-03 |
| SAR0004 | recF | DNA replication and repair protein RecF | 2.08 | 8.51E-03 | |
| SAR0028 | repB | replication protein (pseudogene) | 4.35 | 1.48E-02 | |
| SAR0485 | holB | putative DNA polymerase III, delta' subunit | 3.03 | 1.26E-02 | |
| SAR0711 | putative replication initiation protein | 2.50 | 3.43E-02 | ||
| SAR2429 | putative 3-methylpurine glycosylase | 2.22 | 1.87E-03 | ||
| Metabolism | SAR0246 | ispD | conserved hypothetical protein | 2.00 | 2.27E-03 |
| SAR0261 | putative nitric oxide reductase | 2.22 | 6.16E-04 | ||
| SAR0302 | putative formate/nitrite transporter | 2.38 | 8.03E-03 | ||
| SAR0524 | nupC | nucleoside permease | 2.94 | 3.96E-03 | |
| SAR0562 | putative deoxyadenosine kinase protein | 2.17 | 2.64E-02 | ||
| SAR0563 | putative deaminase | 2.50 | 3.75E-03 | ||
| SAR0569 | putative glycosyl transferase | 2.13 | 4.40E-03 | ||
| SAR0642 | ABC transporter permease protein | 2.56 | 9.65E-03 | ||
| SAR0643 | ABC transporter ATP-binding protein | 3.70 | 9.25E-03 | ||
| SAR0655 | putative Na+ dependent nucleoside transporter | 2.17 | 2.25E-03 | ||
| SAR0743 | putative sodium:sulfate symporter protein | 2.22 | 4.39E-04 | ||
| SAR0847 | nuc | thermonuclease precursor | 3.33 | 3.70E-04 | |
| SAR0916 | putative peptidyl-prolyl cis-trans isomerase | 2.13 | 6.54E-03 | ||
| SAR1008 | putative glycosyl transferases | 4.00 | 1.65E-03 | ||
| SAR1014 | acetyltransferase (GNAT) family protein | 2.27 | 6.54E-03 | ||
| SAR1090 | ctaB | putative protoheme IX farnesyltransferase | 2.04 | 2.66E-02 | |
| SAR1185 | putative guanylate kinase | 2.78 | 6.16E-03 | ||
| SAR1449 | amino acid permease | 2.50 | 2.65E-03 | ||
| SAR1478 | ndk | putative nucleoside diphosphate kinase | 2.38 | 3.03E-02 | |
| SAR1598 | arginine repressor | 2.50 | 3.23E-03 | ||
| SAR1627 | 5-formyltetrahydrofolate cyclo-ligase family protein | 2.78 | 2.27E-03 | ||
| SAR1707 | putative ATPase | 2.13 | 1.36E-02 | ||
| SAR1714 | relA | GTP pyrophosphokinase | 2.27 | 3.96E-03 | |
| SAR1717 | secF | putative protein-export membrane protein | 2.27 | 6.05E-03 | |
| SAR1804 | putative acyltransferase | 2.44 | 2.99E-02 | ||
| SAR2129 | scrR | sucrose operon repressor | 2.56 | 1.38E-02 | |
| SAR2130 | ammonium transporter family protein | 2.04 | 1.65E-03 | ||
| SAR2340 | acetyltransferase (GNAT) family protein | 3.03 | 8.77E-04 | ||
| SAR2363 | modA | putative molybdate-binding lipoprotein precursor | 2.08 | 2.97E-02 | |
| SAR2432 | CorA-like Mg2+ transporter protein | 2.44 | 5.61E-03 | ||
| SAR2493 | putative formate/nitrite transporter | 2.22 | 8.71E-03 | ||
| SAR2594 | ABC transporter ATP-binding protein | 2.38 | 1.65E-03 | ||
| SAR2789 | putative subtilase family protease | 2.04 | 2.27E-03 | ||
| Hypothetical Genes | SAR0013 | putative membrane protein | 2.17 | 1.81E-02 | |
| SAR0024 | conserved hypothetical protein | 3.03 | 2.27E-03 | ||
| SAR0030 | hypothetical protein | 2.38 | 6.16E-03 | ||
| SAR0048 | putative membrane protein | 2.08 | 1.08E-02 | ||
| SAR0061 | putative membrane protein | 2.08 | 4.25E-02 | ||
| SAR0063 | hypothetical protein | 2.56 | 1.02E-02 | ||
| SAR0075 | hypothetical protein | 2.04 | 6.16E-04 | ||
| SAR0078 | hypothetical protein | 2.08 | 9.65E-03 | ||
| SAR0097 | putative DNA-binding protein | 2.17 | 2.99E-03 | ||
| SAR0145 | putative lipoprotein | 2.13 | 1.56E-02 | ||
| SAR0197 | hypothetical protein | 286 | 2.14E-02 | ||
| SAR0216 | putative lipoprotein | 2.04 | 6.16E-04 | ||
| SAR0338 | putative membrane protein | 2.86 | 2.40E-03 | ||
| SAR0383 | abortive infection bacteriophage resistance related | 4.76 | 1.99E-02 | ||
| SAR0618 | putative iron compound-binding protein | 2.27 | 4.08E-02 | ||
| SAR0673 | conserved hypothetical protein | 2.70 | 4.55E-03 | ||
| SAR0694 | putative bacteriocin | 2.38 | 3.75E-03 | ||
| SAR0695 | putative bacteriocin-immunity membrane protein | 2.22 | 2.08E-03 | ||
| SAR0718 | putative membrane protein | 3.33 | 9.93E-04 | ||
| SAR0761 | putative lipoprotein | 2.86 | 3.00E-04 | ||
| SAR0793 | hypothetical protein | 2.56 | 1.58E-02 | ||
| SAR0846 | secreted von Willebrand factor-binding homolog | 2.17 | 1.94E-02 | ||
| SAR0890 | conserved hypothetical protein | 2.56 | 8.06E-04 | ||
| SAR0893 | putative membrane protein | 2.13 | 4.74E-02 | ||
| SAR0898 | conserved hypothetical protein | 2.70 | 1.59E-02 | ||
| SAR0899 | conserved hypothetical protein | 2.33 | 4.94E-03 | ||
| SAR0915 | kinase-associated protein B | 2.44 | 8.06E-04 | ||
| SAR0970 | protozoan/cyanobacterial globin family protein | 2.38 | 1.11E-02 | ||
| SAR0971 | conserved hypothetical protein | 2.78 | 1.87E-03 | ||
| SAR0979 | putative membrane protein | 2.50 | 2.25E-03 | ||
| SAR0981 | putative esterase | 2.44 | 1.55E-03 | ||
| SAR0982 | putative restriction-modification system protein | 2.44 | 1.37E-03 | ||
| SAR0983 | putative restriction-modification system protein | 2.56 | 2.42E-03 | ||
| SAR0985 | putative 2′,5′ RNA ligase family | 2.13 | 2.32E-02 | ||
| SAR0987 | putative monogalactosyldiacylglycerol synthase | 2.56 | 6.54E-03 | ||
| SAR1066 | putative lipoprotein | 2.50 | 4.74E-02 | ||
| SAR1085 | conserved hypothetical protein | 2.33 | 3.44E-02 | ||
| SAR1086 | conserved hypothetical protein | 3.45 | 6.54E-03 | ||
| SAR1095 | conserved hypothetical protein | 2.86 | 2.16E-02 | ||
| SAR1114 | putative cell division protein ZapA | 2.38 | 3.96E-03 | ||
| SAR1148 | putative DNA-binding protein | 2.38 | 2.66E-02 | ||
| SAR1154 | MraZ protein | 2.50 | 3.00E-03 | ||
| SAR1312 | hypothetical protein | 3.85 | 3.27E-02 | ||
| SAR1315 | hypothetical protein | 2.38 | 2.99E-03 | ||
| SAR1316 | hypothetical protein | 2.27 | 1.79E-02 | ||
| SAR1320 | hypothetical protein | 4.00 | 1.46E-02 | ||
| SAR1335 | putative exported protein | 2.27 | 7.38E-03 | ||
| SAR1389 | conserved hypothetical protein (pseudogene) | 2.33 | 5.63E-03 | ||
| SAR1448 | major facilitator superfamily transporter protein | 2.04 | 4.57E-03 | ||
| SAR1556 | putative phage regulatory protein | 2.08 | 5.64E-03 | ||
| SAR1558 | putative phage lipoprotein | 2.44 | 1.24E-03 | ||
| SAR1559 | hypothetical phage protein | 2.33 | 4.80E-04 | ||
| SAR1560 | hypothetical phage protein | 2.04 | 1.72E-02 | ||
| SAR1561 | putative phage membrane protein | 2.13 | 1.10E-03 | ||
| SAR1581 | conserved hypothetical protein | 2.86 | 1.81E-02 | ||
| SAR1592 | conserved hypothetical protein | 2.27 | 1.16E-02 | ||
| SAR1699 | conserved hypothetical protein | 2.00 | 3.92E-03 | ||
| SAR1706 | putative transcriptional regulator | 3.45 | 2.16E-02 | ||
| SAR1708 | conserved hypothetical protein | 2.04 | 3.14E-03 | ||
| SAR1770 | putative membrane protein | 2.13 | 2.99E-03 | ||
| SAR1834 | putative leucyl-tRNA synthetase | 2.17 | 3.65E-02 | ||
| SAR1885 | hypothetical protein | 2.63 | 6.97E-04 | ||
| SAR1897 | hypothetical protein | 3.03 | 2.86E-02 | ||
| SAR1935 | probable phosphoesterase | 2.78 | 2.37E-03 | ||
| SAR1938 | putative DNA-binding protein | 2.38 | 2.80E-02 | ||
| SAR2020 | putative membrane protein | 2.44 | 6.39E-03 | ||
| SAR2035 | putative exported protein | 2.86 | 1.61E-02 | ||
| SAR2113 | hypothetical protein | 2.86 | 4.64E-02 | ||
| SAR2114 | hypothetical protein | 2.56 | 3.43E-02 | ||
| SAR2115 | hypothetical protein | 2.86 | 4.22E-02 | ||
| SAR2118 | putative membrane protein | 2.00 | 3.28E-03 | ||
| SAR2119 | membrane anchored protein | 2.44 | 1.08E-03 | ||
| SAR2156 | pemK-like protein | 3.03 | 5.61E-03 | ||
| SAR2219 | hypothetical protein | 2.78 | 6.21E-03 | ||
| SAR2261 | putative membrane protein | 2.08 | 6.02E-04 | ||
| SAR2263 | putative membrane protein | 2.17 | 2.89E-03 | ||
| SAR2299 | hypothetical protein | 2.04 | 3.47E-03 | ||
| SAR2369 | putative acyl-CoA dehydrogenase | 2.86 | 1.94E-03 | ||
| SAR2425 | putative membrane protein | 2.13 | 5.63E-03 | ||
| SAR2428 | putative membrane protein | 2.00 | 3.23E-03 | ||
| SAR2435 | putative acyl hydrolase | 2.50 | 3.02E-02 | ||
| SAR2439 | tetR family regulatory protein | 2.22 | 3.23E-03 | ||
| SAR2473 | putative exported protein | 3.85 | 3.97E-03 | ||
| SAR2500 | putative lipoprotein | 2.86 | 8.48E-04 | ||
| SAR2546 | putative lipoprotein | 3.13 | 2.76E-02 | ||
| SAR2595 | putative membrane protein | 2.78 | 1.65E-03 | ||
| SAR2718 | putative exported protein | 2.04 | 4.80E-04 | ||
| SAR2719 | transcriptional regulator | 3.13 | 3.07E-04 | ||
| SAR2792 | putative membrane protein | 3.85 | 2.78E-03 | ||
| SAR2793 | putative membrane protein | 3.70 | 8.17E-04 |
Table 3. MRSA252 genes up-regulated during growth in the presence of linoleic acid (0.01mM) (linoleic acid growth exposure).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Up Regulated | P-value |
| Virulence Factors and Regulators | SAR0279 | esxA | virulence factor esxA | 5.93 | 7.03E-08 |
| SAR0280 | esaA | putative membrane protein | 4.32 | 4.53E-06 | |
| SAR0281 | essA | putative membrane protein | 2.70 | 1.66E-03 | |
| SAR0282 | esaB | conserved hypothetical protein | 2.65 | 3.35E-03 | |
| SAR0284 | essC | putative membrane protein | 2.56 | 1.25E-03 | |
| SAR0284v | essC | putative membrane protein | 2.49 | 4.23E-03 | |
| SAR2123 | agrB | putative autoinducer processing protein | 9.36 | 1.74E-05 | |
| SAR2125 | agrC | autoinducer sensor protein | 5.39 | 4.33E-05 | |
| SAR2126 | agrA | autoinducer sensor protein response regulator protein | 2.25 | 1.41E-03 | |
| agrIII | agrIII | Class III accessory gene regulator (agr) locus | 8.71 | 4.16E-06 | |
| RNAIII | RNAIII | RNAIII accessory gene regulator (agr) locus | 10.20 | 1.21E-05 | |
| Metabolism | SAR0150 | adhE | putative aldehyde-alcohol dehydrogenase | 2.25 | 1.67E-02 |
| SAR0190 | glcA | glucose-specific PTS transporter protein, IIABC component | 2.05 | 3.76E-02 | |
| SAR0829 | pgk | phosphoglycerate kinase | 2.76 | 2.16E-03 | |
| SAR0830 | tpiA | triosephosphate isomerase | 2.75 | 1.69E-03 | |
| SAR0831 | pgm | putative phosphoglycerate mutase | 2.83 | 2.22E-03 | |
| SAR0832 | eno | putative enolase | 2.15 | 5.88E-03 | |
| SAR2296 | alsD | putative acetolactate decarboxylase | 2.43 | 3.32E-03 | |
| SAR2297 | alsS | putative acetolactate synthase | 2.17 | 1.41E-03 | |
| SAR2618 | glcB | PTS system, glucose-specific IIABC component | 2.78 | 1.41E-02 | |
| SAR2711 | arcC | carbamate kinase | 2.40 | 3.41E-02 | |
| SAR2712 | arcD | arginine/ornithine antiporter | 2.21 | 1.88E-02 | |
| SAR2713 | arcB | putative ornithine carbamoyltransferase | 2.31 | 1.88E-02 | |
| SAR2714 | arcA | arginine deiminase | 2.89 | 1.41E-02 | |
| Hypothetical Genes | SAR0111 | putative myosin-crossreactive antigen | 2.44 | 6.52E-05 | |
| SAR0277 | putative exported protein | 3.76 | 4.22E-05 | ||
| SAR0278 | putative CHAP domain protein | 2.89 | 1.22E-04 | ||
| SAR0299 | possible pseudogene | 2.95 | 3.30E-03 | ||
| SAR0301 | putative membrane protein | 3.44 | 1.93E-03 | ||
| SAR0385 | similar to putative pathogenicity island gene orf3 | 4.09 | 1.93E-03 | ||
| SAR0839 | putative lipoprotein | 3.36 | 5.41E-05 | ||
| SAR1564 | hypothetical protein | 2.09 | 5.32E-04 | ||
| SAR1565 | putative lipoprotein | 2.38 | 3.02E-03 | ||
| SAR2426 | putative membrane protein | 2.09 | 2.11E-03 | ||
| SAR2427 | ABC transporter ATP-binding protein | 2.14 | 4.82E-03 | ||
| SAR2428 | putative membrane protein | 3.73 | 1.21E-05 | ||
| SAR2569 | hypothetical protein | 6.01 | 4.75E-02 |
Table 4. MRSA252 genes down-regulated during growth in the presence of linoleic acid (0.01mM ) (linoleic acid growth exposure).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Down Regulated | P-value |
| Stess Response | SAR0525 | ctsR | stress regulatory protein | 3.57 | 6.52E-05 |
| SAR0526 | uvrB/uvrC domain protein | 4.35 | 4.16E-06 | ||
| SAR0528 | clpC | putative stress response-related Clp ATPase | 4.17 | 5.47E-05 | |
| SAR0823 | clpP | putative ATP-dependent Clp protease proteolytic subunit | 2.04 | 3.82E-04 | |
| SAR0938 | clpB | putative ATPase subunit of an ATP-dependent protease | 9.09 | 5.72E-06 | |
| SAR1657 | dnaK | chaperone protein | 2.94 | 5.41E-05 | |
| SAR1658 | grpE | GrpE protein | 3.57 | 7.03E-08 | |
| SAR2116 | groEL | 60 kDa chaperonin | 2.44 | 1.05E-03 | |
| SAR2117 | groES | 10 kDa chaperonin | 2.78 | 1.92E-04 | |
| Metabolism | SAR0189 | putative thiamine pyrophosphate enzyme | 2.94 | 1.51E-04 | |
| SAR0208 | putative sugar transport system permease | 2.94 | 2.68E-02 | ||
| SAR0209 | putative oxidoreductase | 4.75 | 1.21E-02 | ||
| SAR0210 | putative oxidoreductase | 9.09 | 3.75E-03 | ||
| SAR0527 | putative phosphotransferase | 4.55 | 7.03E-08 | ||
| SAR0752 | putative phosphofructokinase | 2.44 | 3.20E-02 | ||
| SAR0753 | fruA | fructose-specific PTS system component | 3.45 | 1.21E-02 | |
| SAR1274 | glpF | putative glycerol uptake facilitator protein | 3.70 | 3.36E-03 | |
| SAR1275 | glpK | glycerol kinase | 4.17 | 6.42E-04 | |
| SAR1276 | glpD | glycerol-3-phosphate dehydrogenase | 7.69 | 4.83E-06 | |
| SAR2244 | mtlA | mannitol-specific PTS system component | 2.08 | 4.75E-02 | |
| SAR2445 | hrtA | Heme-regulated transporter ATPase | 2.94 | 3.11E-04 | |
| SAR2594 | ABC transporter ATP-binding protein | 2.33 | 3.32E-03 | ||
| Hypothetical Genes | SAR0100 | putative membrane protein | 2.56 | 2.28E-02 | |
| SAR0211 | conserved hypothetical protein | 11.11 | 3.02E-03 | ||
| SAR0584 | vraX | predicted role in ipenimen resistance | 2.27 | 3.15E-02 | |
| SAR0750 | conserved hypothetical protein | 2.22 | 1.32E-02 | ||
| SAR0939 | LysR family regulatory protein | 2.94 | 5.81E-05 | ||
| SAR2595 | putative membrane protein | 2.04 | 7.18E-03 |
Table 5. MRSA252 genes up-regulated during growth in the presence of oleic acid (0.01 mM) (oleic acid growth exposure).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Up Regulated | P-value |
| Virulence Factors and Regulators | SAR0279 | esxA | virulence factor EsxA | 3.20 | 1.51E-05 |
| SAR0280 | esaA | putative membrane protein | 2.67 | 1.07E-04 | |
| SAR2122 | hld | delta-hemolysin precursor | 6.02 | 5.53E-04 | |
| SAR2123 | agrB | putative autoinducer processing protein | 6.54 | 3.55E-07 | |
| SAR2125 | agrC | autoinducer sensor protein | 3.77 | 9.89E-05 | |
| SAR2126 | agrA | autoinducer sensor protein response regulator protein | 2.01 | 2.21E-04 | |
| agrIII | agrIII | Class III accessory gene regulator (agr) locus | 6.30 | 1.12E-07 | |
| RNAIII | RNAIII | RNAIII accessory gene regulator (agr) locus | 7.02 | 3.21E-05 | |
| Metabolism | SAR0753 | fruA | fructose-specific PTS system component | 2.07 | 1.34E-02 |
| SAR2296 | alsD | conserved hypothetical protein | 2.05 | 4.08E-03 | |
| SAR2297 | alsS | putative acetolactate synthase | 2.49 | 1.19E-03 | |
| SAR2711 | arcC | carbamate kinase | 4.09 | 6.41E-03 | |
| SAR2712 | arcD | arginine/ornithine antiporter | 3.55 | 7.34E-04 | |
| SAR2713 | arcB | putative ornithine carbamoyltransferase | 3.41 | 1.97E-03 | |
| SAR2714 | arcA | arginine deiminase | 4.03 | 3.28E-03 | |
| Hypothetical Genes | SAR0277 | putative exported protein | 2.00 | 7.31E-03 | |
| SAR0301 | putative membrane protein | 2.15 | 2.54E-02 | ||
| SAR0385 | putative membrane protein | 2.88 | 8.02E-03 | ||
| SAR0839 | putative lipoprotein | 2.07 | 1.97E-03 | ||
| SAR1448 | major facilitator superfamily | 2.03 | 1.32E-02 | ||
| SAR2710 | putative regulatory protein | 2.62 | 6.93E-05 |
Table 6. MRSA252 genes down-regulated during growth in the presence of oleic acid (0.01 mM) (oleic acid growth exposure).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Down Regulated | P-value |
| Stress Response | SAR0525 | ctsR | stress regulatory protein | 3.23 | 3.26E-05 |
| SAR0526 | uvrB/uvrC domain protein | 4.17 | 3.26E-05 | ||
| SAR0527 | putative phosphotransferase | 3.85 | 2.20E-07 | ||
| SAR0528 | clpC | putative stress response-related Clp ATPase | 3.23 | 1.01E-04 | |
| SAR0938 | clpB | putative ATPase subunit of an ATP-dependent protease | 8.33 | 1.30E-06 | |
| SAR1119 | uvrC | putative excinuclease ABC subunit C | 3.70 | 7.00E-03 | |
| SAR1657 | dnaK | chaperone protein | 2.63 | 1.01E-04 | |
| SAR1658 | grpE | GrpE protein | 2.86 | 4.78E-06 | |
| SAR2116 | groEL | 60 kDa chaperonin | 2.38 | 1.82E-03 | |
| SAR2117 | groES | 10 kDa chaperonin | 2.44 | 2.21E-04 | |
| Metabolism | SAR0120 | putative ornithine cyclodeaminase | 2.38 | 4.46E-02 | |
| SAR0354 | putative homocysteine S-methyltransferase | 2.13 | 1.60E-02 | ||
| SAR0452 | putative NADH-Ubiquinone protein | 2.00 | 1.32E-02 | ||
| SAR1274 | glpF | putative glycerol uptake facilitator protein | 4.35 | 9.16E-03 | |
| SAR1275 | glpK | glycerol kinase | 3.57 | 1.59E-02 | |
| SAR1276 | glpD | aerobic glycerol-3-phosphate dehydrogenase | 4.76 | 3.26E-05 | |
| SAR1849 | proline dehydrogenase | 3.23 | 8.02E-03 | ||
| SAR2445 | hrtA | Heme-regulated transporter ATPase | 2.94 | 1.01E-04 | |
| SAR2446 | hrtB | Heme-regulated transporter permease | 2.22 | 3.98E-02 | |
| SAR2582 | gntP | putative gluconate permease | 5.88 | 4.53E-03 | |
| SAR2583 | gntK | putative gluconokinase | 4.55 | 3.60E-02 | |
| Hypothetical Genes | SAR0939 | LysR family regulatory protein | 2.86 | 2.76E-04 | |
| SAR2581 | hypothetical protein | 4.55 | 3.99E-02 |
The sudden imposition of linoleic acid during exponential growth at OD600 = 3 (linoleic acid challenge) resulted in large-scale transcriptional reprogramming of genes in four major discernible categories, including: virulence, energy metabolism, stress resistance and cell wall synthesis. In contrast, the presence of linoleic at 0.01 mM, a non-growth limiting concentration (linoleic acid growth exposure), resulted in changes in transcription of fewer genes in the same categories, with the exception of cell wall synthesis.
Effect of linoleic acid on S. aureus MRSA252 transcription
A distinctive feature of linoleic acid addition to cells of MRSA252 under both challenge and growth exposure conditions was observed to be the 10- and 2-fold up-regulation of the virulence regulator RNAIII, respectively (Table 1, 3). Previous studies have not reported changes in regulation of this locus after exposure to FFAs in S. aureus [34], [35]. Moreover, after linoleic acid challenge the virulence regulator sarA was up-regulated as was clfA, encoding clumping factor A and genes required for capsule formation (capF, capM, capN), while the genes encoding the proteases staphopain and aureolysin were down-regulated (Table 1, 2). Further virulence-associated loci up-regulated in the presence of linoleic acid during growth included the esxA locus encoding ESAT-6-like proteins and the genes coding for their synthesis/secretion [41] and tcaR that encodes a MarR-like regulator of SarS and SasF expression [42] (Table 3).
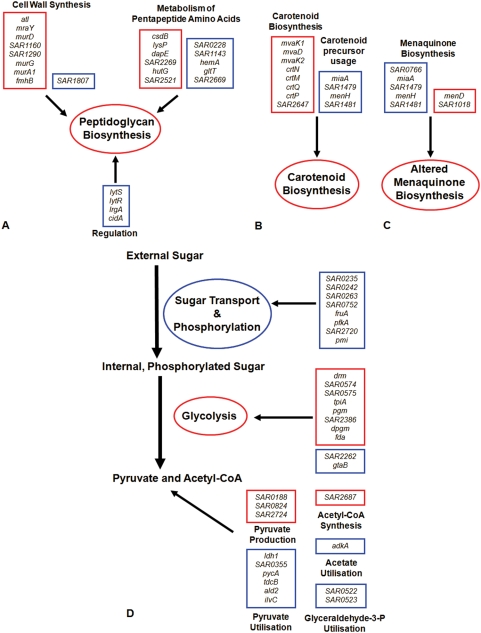
Many genes involved in sugar metabolism showed altered levels of regulation. In particular, several genes in the fructose and mannose metabolism pathways were down-regulated. These include SAR0753 (fruA) and SAR0752, involved in the importation and phosphorylation of fructose, respectively. Genes with similar functions involving the importation and phosphorylation of glucose, mannose, maltose and galactitol, namely SAR0235, SAR1777 (pfkA), SAR2720, SAR2721 (pmi), SAR0242 and SAR0263 were also down-regulated. This could indicate an alteration of central metabolism via the action of the linoleic acid. Here, in concert with these changes, many genes in glycolysis were up-regulated, including SAR2684 (fda), SAR0830 (tpiA), SAR0831 (pgm) and SAR2506 (dpgm). In addition, the SAR0141 (drm), SAR0574 and SAR0575 genes involved in pentose and glucuronate interconversions were up-regulated, which would increase the availability of substrates for glycolysis or pentose phosphate pathways. The down-regulation of the putative UTP-glucose-1-phosphate uridylyltransferases SAR2262 and SAR2579 (gtaB), which are predicted to catalyse the conversion of glucose-1-phosphate to UDP-glucose, would maintain the pool of phosphorylated glucose available for glycolysis.
In addition to increased transcription of genes encoding glycolytic enzymes, the cells exposed to a linoleic acid challenge alter metabolism to maintain levels of pyruvate. The up-regulation of SAR0824 which encodes malate dehydrogenase (converting malate to pyruvate) is predicted to increase pyruvate levels. Concomitantly, there was down-regulation of genes involved in pyruvate utilisation, including ldh1, SAR1088 (pycA), ald2 and SAR0355 converting pyruvate to lactate, oxaloacetate, alanine and cysteine, respectively. Reduced transcription of SAR2143 (ilvC) could further lower the expenditure of cellular pyruvate via amino acid synthesis, and down-regulation of SAR0522 and SAR0523 encoding predicted enzymes utilising glyceraldehyde-3-phosphate would prevent diversion of this intermediate from glycolysis. The reduced importation of substrates for glycolysis would explain increased levels of glycolytic enzymes and modulation of other pathways to increase pyruvate production. Under such potentially energy starved conditions, the pool of pyruvate would be pushed toward energy creation at the expense of less critical pathways.
There was up-regulation of many genes involved in cellular stress responses, including the CtsR regulon genes clpB, dnaJ and dnaK suggesting that linoleic acid addition is perceived by S. aureus as a stressor. Moreover, the transcripts of several σB-regulated genes were up-regulated, including katA, asp23 and clpL, and the crtM, crtN, crtO, crtP, crtQ genes involved in staphyloxanthin biosynthesis. The mevalonate pathway generates the isopentenyl-diphosphate precursor for biosynthesis of this carotenoid, and the pathway genes mvaK1, mvaD and mvaK2 were up-regulated accordingly (Table 1). Linoleic acid has been proposed to interfere with membrane function by increasing fluidity, which has the potential to perturb the electron transport chain. The production of carotenoids, which insert into the membrane has been reported to decrease fluidity and counteract the effect of LC-uFFAs [26]. In response to linoleic acid challenge the menaquinone biosynthesis pathway genes SAR1017 (menD) and SAR1018 involved in the conversion of chorismate to menaquinone (MK), and present in an operon with menB, were up-regulated indicating an increase in MK biosynthesis. This up-regulated MK synthesis could be a response to perturbation of the electron transport chain. The SAR1479, SAR1480 (menH) and SAR1481 genes synthesise heptaprenyl diphosphate for the isoprenoid moiety of MK-7, while SAR1278 (miaA) is a predicted isopentenyl-pyrophosphate transferase. These genes were down-regulated, which is consistent with a reduction of the MK-7 isoprenolog. S. aureus synthesises various MK isoprenologs, up to MK-9, and alters their ratio in response to changes in temperature and oxygen levels [43].
Genes concerned with cell wall biosynthesis were observed to be modulated in linoleic acid challenge conditions but not in the growth exposure conditions. The genes mraY, murD, murG and murA1 involved in the synthesis of the pentaglycine precursor in PG synthesis were up-regulated, as was atl, encoding the major cellular autolysin (Table 1) [44]. There was down-regulation of the two-component regulatory system lytRS, the holin-like lrgA and cidA and the putative transglycosylase SAR1807, which have cell wall modulatory roles (Table 2) [45], [46]. In addition to these changes, an assortment of transcriptionally modulated genes was observed, which would function to maintain the level of constituents for the PG-pentapeptide precursor. SAR2109 (dapE), which catalyses the formation of a substrate for lysine biosynthesis and the lysine-specific permease SAR1761 (lysP) were up-regulated, and this would increase the pool of L-lysine in the cell. Up-regulation of SAR2420 (hutG), and down-regulation of SAR2669 encoding a putative dihydroorotate dehydrogenase, SAR0228 encoding a putative glutamine amidotransferase and SAR1752 (hemA), in concert, would maintain glutamate levels within the cell. SAR2269, a putative alanine racemase, was up-regulated thereby increasing synthesis of D-alanine by isomerising L-alanine. The microarray data also revealed increased transcription of the tagA, tagG and tagB genes concerned with teichoic acid biosynthesis.
The fatty acid biosynthesis enzyme FabI was previously reported to be inhibited by linoleic acid and was therefore proposed to be a key target for its antibacterial activity [23]. Here, within fatty acid metabolism, only fabZ was up-regulated in linoleic acid challenge conditions, whereas fadD, fadX and plc were down-regulated. fabZ is directly downstream of murA1 within a predicted operon which may explain why fabZ alone is up-regulated amongst the fatty acid biosynthesis genes.
Quantitative Real-Time PCR
Confirmation of the microarray data was performed using qRT-PCR to test selected transcriptional changes of known genes from different functional subsets. To this end, the expression level of genes involved in staphyloxanthin synthesis (crtM), PG biosynthesis (murG, cidA and lytR), stress responses (katA and clpB), virulence (RNAIII, sarA, arcA, hla and spa) and fatty acid metabolism (fabZ, fabI, fadD and fadA) were analysed. In addition, the sasF gene was analysed to confirm the particularly high levels of transcript that were observed under the challenge experimental conditions. Most genes tested showed the same pattern of up- or down-regulation (Table 7) that was identified by microarray analysis under any given set of conditions. The only exceptions were the fatty acid degradation pathway genes fadD and fadA. While fadD was 2.15 fold down-regulated after linoleic acid challenge when analysed by microarray, this was identified as a 3.16 fold up-regulation when tested by qRT-PCR. The fadA gene lies within a predicted operon with fadD and would thus be co-regulated. A 3.1 fold up-regulation of fadA was similarly measured by qRT-PCR when the cells were challenged with linoleic acid, which supports the reproducibility of the qRT-PCR analysis of fadD and its likely operon arrangement with fadA. Therefore, with the exception of the fad operon, the microarray data was shown to be consistent when tested by qRT-PCR.
Table 7. qRT-PCR analysis of gene expression in MRSA252.
| ORF | Gene | Linoleic Challenge | Linoleic Growth | Oleic Growth | Linoleic Growth | Oleic Growth |
| OD600 = 3 | OD600 = 3 | OD600 = 8 | OD600 = 8 | |||
| SAR0114 | spa | 1.46 (0.37) | 1.01 (0.07) | −1.08 (0.11) | −3.03 (0.14) | −2.92 (0.24) |
| SAR0223 | fadA | 3.10 (0.08) | nd | nd | nd | nd |
| SAR0225 | fadD | 3.16 (0.61) | −1.34 (0.03) | −1.88 (0.02) | −1.06 (0.03) | −1.01 (0.02) |
| SAR0258 | lytR | −5.03 (0.01) | nd | nd | nd | nd |
| SAR0625 | sarA | 3.84 (0.81) | 1.10 (0.11) | −1.12 (0.07) | 1.52 (0.55) | 2.05 (1.12) |
| SAR2621 | cidA | −1.93 (0.02) | 1.52 (0.03) | 1.41 (0.03) | −2.75 (0.01) | −1.39 (0.02) |
| SAR0938 | clpB | 3.90 (0.08) | −8.55 (0.01) | −10.31 (0.01) | −3.16 (0.01) | −3.00 (0.01) |
| SAR0978 | fabI | 1.25 (0.02) | nd | nd | nd | nd |
| SAR1136 | hla | −1.60 (0.20) | 1.19 (0.19) | −1.71 (0.09) | 6.38 (2.91) | 7.88 (5.16) |
| SAR1344 | katA | 7.27 (0.18) | nd | nd | nd | nd |
| SAR1430 | murG | 7.59 (0.22) | nd | nd | nd | nd |
| SAR2187 | fabZ | 3.37 (0.64) | −1.10 (0.02) | 1.40 (0.02) | 1.10 (0.04) | 1.20 (0.06) |
| SAR2643 | crtM | 3.72 (0.08) | nd | nd | nd | nd |
| SAR2714 | arcA | 1.61 (0.03) | 1.86 (0.04) | 2.17 (0.04) | −2.19 (0.02) | −1.50 (0.01) |
| SAR2725 | sasF | 31.86 (0.69) | nd | nd | nd | nd |
| RNAIII | 7.86 (0.15) | 56.14 (1.40) | 34.28 (0.74) | 156.12 (6.95) | 153.30 (2.81) |
The values correspond to the fold change for each gene tested under the relevant fatty acid treatment conditions when compared to the untreated control. The standard deviation for each measurement is in parentheses. nd, not detemined. ORF indicates the gene locus in MRSA252 (http://www.genedb.org/genedb/saureusMRSA/).
The transcription of a subset of genes was examined by qRT-PCR during mid-exponential growth phase and late exponential-phase (OD600 = 8) (Table 7), to examine the potential effect of the increased levels of the density-signalling effector RNAIII on transcription of regulated genes (e.g. spa, hla and sarA). qRT-PCR analysis was performed on MRSA252 genes under linoleic and oleic acid growth exposure conditions. The RNAIII and clpB transcripts were consistently up- or down-regulated, respectively, at all of the points tested during growth; at OD600 = 8 RNAIII was massively up-regulated (>150-fold) in the presence of either linoleic or oleic acid. The transcription of sarA was up-regulated 1.5- to 2-fold in post-exponential phase in these conditions. Post-exponential transcription of hla was >6-fold higher after growth with either linoleic or oleic acid in comparison with the untreated control. Interestingly, this increase was moderate compared to that observed for RNAIII of the agr locus, which is known to up-regulate expression of hla. This reflects the complex regulation of hla and may be due to the increase in sarA levels.
Several genes showed fluctuations in relative transcript levels during the growth cycle. For example, arcA transcription varied over the different sample points, with gene up-regulation at OD600 = 3.0 for the linoleic growth experiment as per the microarray results. However, arcA was down-regulated in post-exponential phasef growth phase in the presence of linoleic acid.
The observation of increased expression of RNAIII, hla and spa in MRSA252 in response to LC-uFFAs is significantly different to previously published experiments for these transcripts in alternative strains [34], [35]. The expression of a large subset of genes, confirmed by qRT-PCR to be altered following exposure of MRSA252 to linoleic acid (Table 7), were subsequently examined in SH1000 to determine whether they were similarly regulated (Table 8). This revealed that in SH1000 the up- or down-regulation of several genes was in direct contrast to the pattern observed in MRSA252. For example, where both microarray data and qRT-PCR data showed that there was a large up-regulation of RNAIII after challenge or growth exposure in MRSA252, pronounced down-regulation was observed in SH1000 by qRT-PCR. Contrasts in regulation between MRSA252 and SH1000 were also observed for sarA, spa and sasF. However, several genes not predicted to be RNAIII-regulated, including lytR, clpB, fabI, murG, and arcA exhibited similar patterns of regulation in both strains under the conditions tested.
Table 8. qRT-PCR analysis of gene expression in SH1000.
| ORF | Gene | Linoleic Challenge | Linoleic Growth | Oleic Growth |
| OD600 = 3 | OD600 = 3 | |||
| SAR0114 | spa | 2.19 (0.09) | −1.94 (0.02) | 1.66 (0.04) |
| SAR0258 | lytR | −2.31 (0.02) | nd | nd |
| SAR0625 | sarA | 1.26 (0.05) | −3.79 (0.02) | −3.45 (0.03) |
| SAR0938 | clpB | 1.95 (0.08) | −2.11 (0.02) | −2.58 (0.03) |
| SAR0978 | fabI | −1.20 (0.04) | nd | nd |
| SAR1136 | hla | −3.60 (0.01) | −2.11 (0.02) | −2.58 (0.03) |
| SAR1430 | murG | 1.84 (0.11) | nd | nd |
| SAR2643 | crtM | 1.32 (0.05) | nd | nd |
| SAR2714 | arcA | nd | 2.19 (0.10) | 4.39 (0.18) |
| SAR2725 | sasF | 1.49 (0.06) | nd | nd |
| RNAIII | −1.79 (0.03) | −3.29 (0.01) | −1.95 (0.01) |
The values correspond to the fold change for each gene tested under the relevant fatty acid treatment conditions when compared to the untreated control. The standard deviation for each measurement is in parentheses. nd, not detemined. ORF indicates the gene locus in MRSA252 (http://www.genedb.org/genedb/saureusMRSA/) that was tested in SH1000.
Proteomic analysis
The proteome of MRSA252 was analysed by 2D-PAGE to identify protein expression changes in exponentially growing cells that were exposed to linoleic acid under the challenge conditions used for the microarray experiments. This analysis was performed to determine whether the large-scale transcriptional modulation described above was translated into a correspondingly large-scale proteomic shift. Under these conditions, 58 proteins were significantly (P≤0.05) up-regulated ≥2-fold and 15 proteins were significantly (P≤0.05) down-regulated ≥2-fold. MALDI-MS was used to identify the most intense protein spots on the gel corresponding to proteins that were modulated by linoleic acid, and the identities of 38 up-regulated and 5 down-regulated proteins were unambiguously determined (Table 9 and 10). There was strong agreement between the observed changes in protein expression due to linoleic acid challenge exposure and the encoded functions of the genes modulated in the microarray experiments. In terms of the assigned metabolic pathways, the interpreted effects of the fatty acid upon the cell were therefore corroborated. Proteins associated with stress responses and PG and MK biosynthesis were modulated in response to linoleic acid. Similarly, the CapA protein involved in capsule biosynthesis was up-regulated over 3-fold. From the proteomic data, challenge with linoleic acid resulted in up-regulation of glycolysis pathway proteins and those linked to pyruvate metabolism. Moreover, the proteomic data were often complementary to those from the microarrays. Several proteins within the glycolysis and pyruvate metabolism pathways were up-regulated (e.g Gap1, Pgi), whereas all glycolytic genes except eno were up-regulated in the microarray experiment. A few contradictions were observed between the microarray and proteomics data. The ald2, ackA, ispD, SAR0985 and SAR2369 proteins were determined by proteomics to be up-regulated but were down-regulated according to microarray analysis.
Table 9. MRSA252 proteins up-regulated following the addition of linoleic acid (0.1 mM) to exponentially growing cells (linoleic acid challenge).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Up Regulated | P-value |
| Virulence Factors and Regulators | SAR2745 | capA | Capsular polysaccharide biosynthesis protein | 3.36 | 2.27E-04 |
| Energy Metabolism | SAR0140 | deoC1 | deoxyribose-phosphate aldolase | 4.34 | 2.49E-03 |
| SAR0217 | formate acetyltransferase | 2.30 | 1.50E-03 | ||
| SAR0394 | phosphoglycerate mutase family protein | 2.94 | 1.67E-02 | ||
| SAR0828 | gap1 | glyceraldehyde 3-phosphate dehydrogenase 1 | 2.07 | 3.53E-03 | |
| SAR0924 | pgi | glucose-6-phosphate isomerase | 4.18 | 7.43E-03 | |
| SAR1451 | ald2 | alanine dehydrogenase 2 | 2.13 | 1.32E-03 | |
| SAR1789 | ackA | acetate kinase | 2.81 | 6.97E-04 | |
| SAR2506 | dpgm | putative phosphoglycerate mutase | 2.15 | 3.52E-02 | |
| SAR2685 | mqo2 | malate:quinone oxidoreductase | 3.39 | 8.08E-03 | |
| DNA Repair and Replication | SAR1639 | dnaG | DNA primase | 2.66 | 2.61E-03 |
| SAR1996 | lig | DNA Ligase | 2.09 | 6.11E-04 | |
| Protein Synthesis | SAR0552 | fus | elongation factor G | 4.47 | 2.51E-04 |
| SAR0552 | fus | elongation factor G | 2.10 | 2.09E-02 | |
| SAR1216 | trmD | putative tRNA (guanine-7-)-methyltransferase | 2.33 | 4.84E-04 | |
| SAR1720 | queA | S-adenosylmethionine:tRNA ribosyltransferase-isomerase | 2.11 | 1.68E-02 | |
| SAR2309 | rpoA | RNA polymerase alpha subunit | 2.61 | 2.40E-03 | |
| Peptidoglycan Synthesis | SAR0470 | lysR | family regulatory protein | 2.20 | 1.07E-02 |
| SAR1762 | thrS | threonyl-tRNA synthetase | 2.77 | 9.07E-04 | |
| SAR1991 | gatB | aspartyl/glutamyl-tRNA amidotransferase subunit B | 2.25 | 3.52E-02 | |
| SAR2201 | glyA | serine hydroxymethyltransferase | 3.55 | 8.43E-03 | |
| SAR2201 | glyA | serine hydroxymethyltransferase | 2.15 | 3.74E-03 | |
| Carotenoid Biosynthesis | SAR1378 | prephenate dehydrogenase | 2.37 | 8.08E-03 | |
| Miscellaneous | SAR0218 | putative pyruvate formate-lyase activating enzyme | 2.60 | 3.46E-02 | |
| SAR0403 | putative DNA binding protein | 2.72 | 3.48E-02 | ||
| SAR2007 | putative oxygenase/mitric oxide synthase | 2.04 | 7.13E-03 | ||
| SAR2007 | putative nitric oxide synthase | 2.66 | 2.38E-02 | ||
| Metabolism | SAR0150 | adhE | putative aldehyde-alcohol dehydrogenase | 3.48 | 5.07E-03 |
| SAR0246 | ispD | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | 2.43 | 4.84E-04 | |
| SAR0246 | ispD | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | 2.21 | 8.75E-03 | |
| SAR0564 | putative haloacid dehalogenase-like hydrolase | 2.22 | 1.01E-02 | ||
| SAR1070 | pdhD | dihydrolipoamide dehydrogenase | 2.22 | 2.62E-02 | |
| SAR2353 | mobA | molybdopterin-guanine dinucleotide biosynthesis protein | 3.12 | 8.32E-04 | |
| SAR2513 | 6-carboxyhexanoate–CoA ligase | 4.84 | 4.13E-05 | ||
| SAR2641 | putative aminotransferase | 2.07 | 1.54E-02 | ||
| Hypothetical Proteins | SAR0985 | putative RNA ligase protein | 2.31 | 2.12E-02 | |
| SAR2064 | hypothetical phage protein | 2.06 | 1.41E-03 | ||
| SAR2369 | Acyl-CoA dehydrogenase-related protein | 3.36 | 4.69E-03 |
Table 10. MRSA252 proteins down-regulated following the addition of linoleic acid (0.1 mM) to exponentially growing cells (linoleic acid challenge).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Down Regulated | P-value |
| Protein Synthesis | SAR0927 | spsB | signal peptidase Ib | 5.88 | 9.88E-04 |
| SAR1755 | tig | trigger factor | 2.56 | 1.93E-02 | |
| SAR2179 | putative membrane protein | 2.17 | 2.05E-02 | ||
| Peptidoglycan Synthesis | SAR1284 | glnA | glutamine synthetase | 2.33 | 5.00E-02 |
| Metabolism | SAR0814 | hprK | kinase/phosphorylase | 3.03 | 6.66E-03 |
In addition to linoleic acid, the effect of the skin-associated LC-uFFA hexadecenoic acid [C16:1 (n-6)] on the cellular proteome was studied to determine whether there was a common response to LC-uFFAs on S. aureus MRSA252. Analysis of 2D-SDS-PAGE gels revealed strong spot conservation for proteins exhibiting modulated expression in response to hexadecenoic acid compared to linoleic acid. Under challenge conditions with 0.1 mM hexadecenoic acid, 95 proteins were significantly (P≤0.05) up-regulated ≥2-fold and 7 proteins were significantly (P≤0.05) down-regulated ≥2-fold. MALDI-MS was used to identify 63 of the most intense protein spots on the gel corresponding to proteins that were modulated by linoleic acid and the identities of 56 up-regulated and 5 down-regulated proteins were unambiguously determined (Table 11 and 12). Many of the same proteins, or different proteins within the same metabolic pathways e.g. glycolysis and pyruvate metabolism, were identified after exposure to hexadecenoic acid and linoleic acid. This indicates that there is commonality in the metabolic response to the cellular perturbations caused by exposure to these LC-uFFAs, which differ in chain length, and the number and position of double bonds.
Table 11. MRSA252 proteins up-regulated following the addition of hexadecenoic acid (0.1 mM) to exponentially growing cells (hexadecenoic acid challenge).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Up regulated | P-value |
| Stress Response | SAR2116 | groEL | chaperonin | 2.03 | 3.49E-02 |
| SAR2273 | asp23 | alkaline shock protein 23 | 2.89 | 8.90E-03 | |
| SAR2461 | pyridine nucleotide-disulphide oxidoreductase family protein | 2.05 | 3.99E-02 | ||
| SAR2461 | pyridine nucleotide-disulphide oxidoreductase family protein | 2.00 | 2.13E-03 | ||
| SAR2461 | pyridine nucleotide-disulphide oxidoreductase family protein | 2.59 | 2.49E-02 | ||
| Energy Metabolism | SAR0140 | deoC1 | deoxyribose-phosphate aldolase | 4.21 | 4.86E-03 |
| SAR0394 | phosphoglycerate mutase family protein | 4.79 | 1.06E-03 | ||
| SAR0828 | gap1 | glyceraldehyde 3-phosphate dehydrogenase 1 | 2.86 | 2.51E-03 | |
| SAR0830 | tpiA | triosephosphate isomerase | 3.40 | 4.42E-03 | |
| SAR0832 | eno | enolase | 3.20 | 1.83E-02 | |
| SAR0832 | eno | enolase | 3.09 | 4.60E-03 | |
| SAR0832 | eno | enolase | 2.07 | 5.15E-03 | |
| SAR0924 | pgi | glucose-6-phosphate isomerase | 2.31 | 2.73E-02 | |
| SAR1068 | pdhB | putative pyruvate dehydrogenase E1 component | 2.72 | 2.29E-04 | |
| SAR1068 | pdhB | putative pyruvate dehydrogenase E1 component | 2.72 | 2.29E-04 | |
| SAR1121 | sdhA | putative succinate dehydrogenase flavoprotein | 2.24 | 1.36E-02 | |
| SAR1451 | ald2 | alanine dehydrogenase 2 | 2.21 | 2.30E-03 | |
| SAR2605 | ddh | D-lactate dehydrogenase | 3.11 | 8.87E-03 | |
| SAR2685 | mqo2 | malate:quinone oxidoreductase | 4.79 | 1.06E-03 | |
| SAR2685 | mqo2 | malate:quinone oxidoreductase | 3.13 | 2.18E-03 | |
| DNA Repair and Replication | SAR1997 | pcrA | ATP-dependent DNA helicase | 2.23 | 3.49E-02 |
| Protein Synthesis | SAR0552 | fus | translation elongation factor G | 3.56 | 3.84E-02 |
| SAR0553 | tuf | translation elongation factor Tu | 2.65 | 3.41E-02 | |
| SAR0553 | tuf | elongation factor Tu | 4.13 | 7.56E-03 | |
| SAR1216 | trmD | putative tRNA (guanine-7-)-methyltransferase | 2.80 | 1.69E-03 | |
| SAR1485 | rpsA | putative 30S ribosomal protein S1 | 2.91 | 4.91E-02 | |
| SAR1719 | tgt | queuine tRNA-ribosyltransferase | 2.01 | 4.96E-02 | |
| SAR1720 | queA | S-adenosylmethionine:tRNA ribosyltransferase-isomerase | 2.49 | 1.44E-02 | |
| SAR2309 | rpoA | DNA-directed RNA polymerase subunit alpha | 2.44 | 9.73E-03 | |
| Peptidoglycan Synthesis | SAR1048 | purD | putative phosphoribosylamine–glycine ligase | 3.98 | 2.08E-02 |
| SAR1762 | thrS | threonyl-tRNA synthetase | 2.51 | 1.51E-02 | |
| SAR2212 | murA2 | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 2.13 | 1.36E-02 | |
| Cell Division | SAR1795 | ezrA | putative septation ring formation regulator | 3.11 | 3.71E-03 |
| SAR1795 | ezrA | putative septation ring formation regulator | 2.56 | 5.16E-03 | |
| Miscellaneous | SAR0218 | putative pyruvate formate-lyase activating enzyme | 2.39 | 4.03E-03 | |
| SAR0403 | putative DNA-binding protein | 2.84 | 8.32E-03 | ||
| SAR2007 | putative oxygenase | 2.85 | 2.92E-02 | ||
| Metabolism | SAR0351 | thl | acetyl-CoA acetyltransferase | 2.85 | 2.59E-04 |
| SAR0351 | thl | acetyl-CoA acetyltransferase | 2.77 | 9.07E-04 | |
| SAR0514 | putative O-acetylserine (thiol)-lyase | 2.41 | 4.43E-03 | ||
| SAR1142 | otc | ornithine carbamoyltransferase | 2.03 | 5.43E-03 | |
| SAR2352 | moaA | putative molybdenum cofactor biosynthesis protein A | 3.73 | 6.34E-03 | |
| SAR2352 | moaA | putative molybdenum cofactor biosynthesis protein A | 2.25 | 9.64E-03 | |
| SAR2460 | putative acetyltransferase (GNAT) family protein | 5.37 | 6.22E-05 | ||
| SAR2460 | putative acetyltransferase (GNAT) family protein | 5.20 | 2.03E-02 | ||
| SAR2641 | putative aminotransferase | 2.12 | 8.47E-03 | ||
| SAR2694 | nrdG | putative anaerobic ribonucleotide reductase activating protein | 3.38 | 4.66E-04 | |
| SAR2694 | nrdG | putative anaerobic ribonucleotide reductase activating protein | 2.48 | 3.43E-02 | |
| Hypothetical Proteins | SAR0246 | ispD | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | 3.37 | 2.20E-04 |
| SAR0246 | ispD | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | 2.90 | 1.69E-03 | |
| SAR0985 | putative RNA ligase protein | 2.11 | 1.68E-02 | ||
| SAR1105 | isdD | hypothetical protein | 2.25 | 3.60E-03 | |
| SAR2063 | hypothetical phage protein | 2.06 | 1.41E-03 | ||
| SAR2369 | putative acyl-CoA dehydrogenase | 2.61 | 2.43E-04 | ||
| SAR2545 | M42 glutamyl aminopeptidase | 2.08 | 1.72E-02 | ||
| SAR2674 | hypothetical protein | 2.45 | 5.46E-04 |
Table 12. MRSA252 proteins down-regulated following the addition of hexadecenoic acid (0.1 mM) to exponentially growing cells (hexadecenoic acid challenge).
| Group Functions | MRSA252 ORF | MRSA252 Gene | MRSA252 Gene Product | Fold Change Down Regulated | P-value |
| Protein Synthesis | SAR0927 | spsB | signal peptidase Ib | 4.55 | 8.44E-03 |
| Peptidoglycan Synthesis | SAR0920 | NAD-specific glutamate dehydrogenase | 2.17 | 8.21E-03 | |
| Miscellaneous | SAR2622 | lysR | family regulatory protein | 2.13 | 1.21E-02 |
| Metabolism | SAR0483 | tmk | putative thymidylate kinase | 2.27 | 1.04E-03 |
| SAR1399 | pstB | ABC transporter ATP-binding protein | 3.23 | 1.80E-03 |
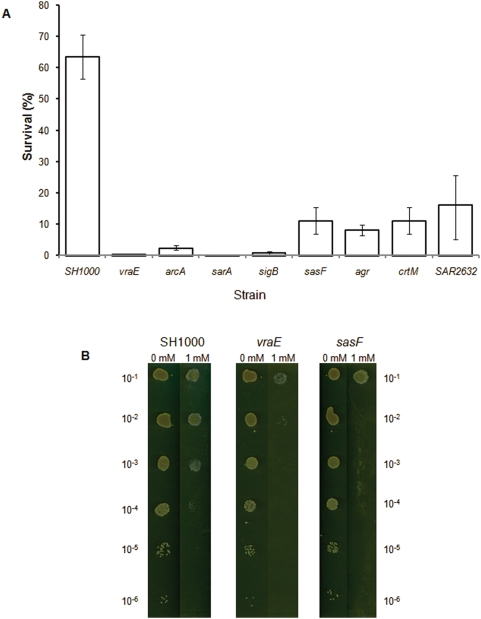
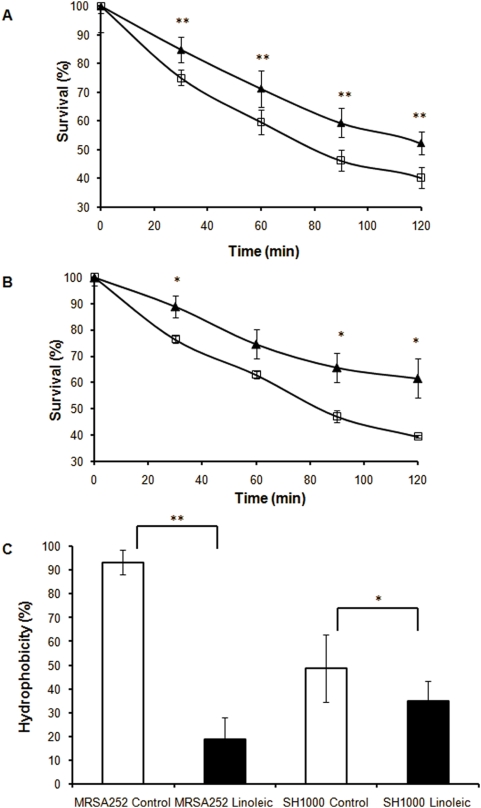
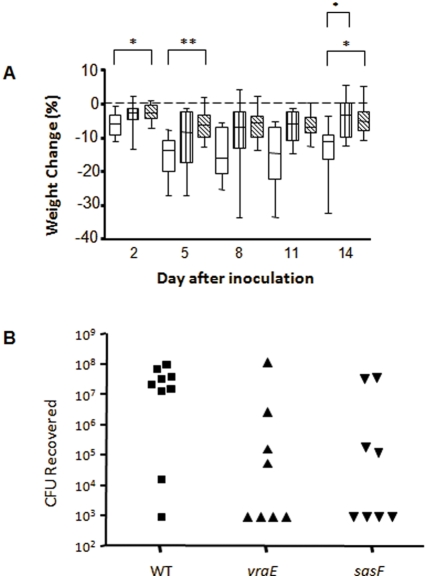
Identification of survival mutants
Allelic replacement mutants were constructed in the genes sasF and arcA, which displayed altered transcription in response to linoleic acid and a further mutant was constructed in vraS, encoding a cell wall synthesis regulator. The contribution of these genes to survival in the presence of LC-uFFAs was tested on agar plates containing linoleic acid. Additionally, existing mutants of genes identified by microarray analysis to display altered transcription in response to linoleic acid, or regulators of these genes, were tested. Furthermore, a 5,000 clone Tn917 mutant library was also screened to identify survival mutants. Analysis of the mutant clones was performed in SH1000 since MRSA252 is resistant to most antibiotics used for gene inactivation studies. Importantly, many of the mutant strains tested exhibited increased sensitivity to LC-uFFAs when compared to the wild-type, including those harbouring mutations in the genes: sasF (Liv694), crtM (Liv681), arcA (Liv692), sigB (Liv130), agr (Liv038) and sarA (Liv039) (Fig. 2A, 2B). In contrast clfA (Liv442), vraS (Liv718), katA (Liv750), lytSR (Liv101) and clpC (Liv671) did not contribute to survival under the conditions tested in a SH1000 background. Screening of the Tn917 transposon library identified two further clones with defective survival. Sequencing upstream and downstream of the transposon in these mutants revealed insertion of Tn917 in the SAR2632 (Liv766) and vraE (Liv753) genes. Complementation of the fatty acid sensitivity of the sasF, arcA, vraE and SAR2632 mutants was achieved by individually cloning each gene into the low copy number shuttle vector pSK5630 [75] and transforming each mutant with the relevant plasmid. Complementation restored survival of each mutant in LC-uFFA resistance assays (data not shown).
Figure 2. Plate based survival assay.
A Graph showing the percentage survival of WT and mutant variants of SH1000 when serial dilutions of the strains were plated on BHI agar containing 1 mM linoleic acid. Survival is expressed as a percentage of viable cell counts obtained for control plates lacking linoleic acid. Values are the mean of multiple independent experiments. Error bars indicate standard errors of the mean. p<0.005 for each mutant by Student's t-test. B Plates showing the relative survival of SH1000 and the sasF (Liv694) and vraE (Liv753) mutants on BHI agar containing 0 or 1 mM linoleic acid. The 10−1 to 10−6 dilution series of cultures are as indicated.
Autolysis assays
Cells grown in the presence of linoleic acid under constant growth conditions displayed reduced expression of the CtsR regulon, which is known to impact on cell autolysis [47]. Consequently we addressed the impact of the presence of LC-uFFAs upon autolysis of treated and control cells of MRSA252 and SH1000. A significantly increased rate of autolysis was observed in linoleic acid treated cells of each strain (Fig. 3A, 3B). This increase is in accordance with the reduced expression of the CtsR regulon in treated cells.
Figure 3. Physiological effects of linoleic acid on S. aureus.
The result of growth of MRSA252 and SH1000 in the absence (closed triangle) or presence (open box) of 0.01mM linoleic acid on autolysis is shown in A and B, respectively. Survival is expressed as a percentage of OD600 at T = 0. Values are from three independent experiments. Error bars indicate standard errors of the mean. **p<0.01, *p<0.05 by Student's t test. C Relative hydrophobicity of the MRSA252 and SH1000 strains following overnight growth in BHI +/− 0.1 mM linoleic acid. Values are from three independent experiments. Error bars indicate standard errors of the mean. **p<0.01, *p<0.05 by Student's t test.
Cell hydrophobicity
IsdA reduces cell surface hydrophobicity and acts to increase staphylococcal resistance to LC-uFFAs [34] while a GML resistant mutant of Enterococcus faecalis was found to be less hydrophobic than the wild type parent strain [48]. Partitioning of cells in the non-polar solvent hexadecane was measured to determine whether modulating cell hydrophobicity was a S. aureus response to growth in the presence of fatty acids. Growth in the presence of 0.1 mM linoleic acid resulted in both strains exhibiting decreased partitioning indicating a decrease in cell surface hydrophobicity (Fig. 3C). The change in cell hydrophobicity was particularly dramatic for MRSA252 with partitioning reduced from over 90% to less than 20% of cells upon growth in the presence of linoleic acid. The adaptive decrease in cell hydrophobicity makes conditions less favourable for interactions between the cell and the amphipathic fatty acid. Alterations to cell surface charge via the dlt and mprF loci have also been linked to S. aureus evasion of a number of innate immune system components including cationic antimicrobial peptides [49]–[51]. The SH1000 mutants vraE, sasF, or SAR2632, identified in this study as having decreased survival upon exposure to linoleic acid, did not exhibit altered hydrophobicity in this partitioning assay (data not shown). This indicates that the products of these three genes interact with LC-uFFAs in a manner that does not involve alterations to cell surface hydrophobicity.
Murine arthritis virulence assay
A murine arthritis model of infection was used to determine a role for the LC-uFFA survival genes sasF and vraE in pathogenesis. This model of infection also reports on systemic inflammation and abscess formation in kidneys and was therefore relevant for in vivo investigation of fatty acid survival mutants. Neither the sasF nor vraE mutations showed a significant reduction in arthritis development of SH1000 in this model (data not shown). However, a significantly reduced weight loss (P<0.05) was observed for both sasF and the vraE mutants for 3 out of 5 weight measurements over the 14 day experiment, when compared to the SH1000 parent strain (Fig. 4A). In contrast, while a reduced bacterial load of both mutant strains was observed in the kidney compared to the wild type this was not found to be significant (p = 0.075) (Fig. 4B). Collectively, these data suggest that SasF and VraE might make contributions to the pathogenesis of systemic inflamation, but not to the development of arthritis.
Figure 4. Contribution of vraE and sasF to virulence.
A Effect of WT SH1000 (open box) and mutations of vraE (vertical hatched box) and sasF (diagonal hatched box) on percentage change in weight of infected mice. *p<0.05, **p<0.01 by Dunn's test. B Effect of mutations of vraE (closed triangle) and sasF (closed inverted triangle) on cfu of S. aureus SH1000 (closed box) in kidneys of infected mice.
Discussion
Analysing the response of MRSA252, an EMRSA-16 clone, to the LC-uFFAs linoleic [C18:2 (n-6,9)] and oleic [C18:1 (n-6)] acid revealed modulated expression of many genes, including those encoding virulence determinants. After exposure of exponentially growing cells to linoleic acid there was a very large increase in RNAIII compared to control cells, and this was also observed at all stages of growth when either linoleic or oleic acid were present from the time of inoculation. This observed up-regulation of RNAIII synthesis was unexpected given previous reports on the effects of GML, a lauric acid monoester, and the LC-uFFA hexadecenoic acid [C16:1 (n-6)] on S. aureus gene expression [34], [35]. In those studies, there was no change in agr (RNAIII) expression, but down-regulation of agr-regulated virulence determinants, including alpha toxin (hla). MRSA252 has a nonsense mutation in hla, which does not affect hla mRNA measurements by qRT-PCR but ablates activity of the encoded protein preventing activity measurements [52]. In this study, transcription of hla in MRSA252 was only up-regulated in the presence of linoleic or oleic acid in the post-exponential growth phase demonstrating maintenance of its temporal expression, despite up-regulation of RNAIII at earlier phases of growth. Analysis by qRT-PCR revealed contrasting regulation of RNAIII synthesis in SH1000 and MRSA252 in response to treatment with LC-uFFAs. RNAIII levels were reduced after growth exposure to linoleic or oleic acid during growth of SH1000. The data reported here therefore highlights important differences between the effects of these LC-FFAs between strains. Previous studies identified a fatty acid modifying enzyme (FAME) in strains of S. aureus, which esterifies LC-FFAs with cholesterol, thereby reducing toxicity [32]. However, this activity was demonstrated to be agr-regulated [29], [53], producing the anomaly that in strains with SH1000-like regulation, expression of the detoxifying enzyme would be down-regulated upon exposure to its substrate. MRSA252 is a successful epidemic strain of S. aureus and the ability to persist in an environment containing LC-uFFAs such as on the skin surface (hexadecenoic acid) or in skin infections (linoleic and oleic acid) would aid the transmission of the organism. In this scenario, the specific up-regulation of agr in response to LC-uFFAs observed in MRSA252 (EMRSA-16) may contribute towards its success as an epidemic strain. Superior skin colonisation was previously suggested as a reason for the epidemic nature of the EMRSA-15 and -16 strains, which together are responsible for over 95% of MRSA from cases of nosocomial bacteraemia in the UK [54], [55].
Microarray analysis revealed further virulence factors exhibiting increased transcription, including the esx locus, which encodes a specific secretion system and the ESAT-6-like proteins that have been confirmed as having a role in the pathogenesis of murine abscesses [41]. Increased transcription of the esx locus was only observed after growth exposure to linoleic or oleic acid and not in response to linoleic acid challenge conditions. Increased transcription of the arcABDC operon, encoding the arginine deiminase (ADI) pathway enzymes, was observed under the same conditions where the esx locus is up-regulated. The ADI pathway enables the utilisation of arginine as an energy source under anaerobic conditions of growth. Concomitant with the expression of the ADI pathway, there was an up-regulation of many glycolytic enzymes, suggesting that a net effect of growth exposure to linoleic acid was metabolic alterations leading toward anaerobic growth. To test the importance of the ADI pathway under these conditions, an arcA allelic replacement mutant of SH1000 was generated (arcA was transcriptionally up-regulated in both SH1000 and MRSA252). The arcA strain was found to display a reduction in growth on agar plates containing 1 mM linoleic acid, with a 25-fold lower survival than the parental strain. The alteration in metabolism via up-regulation of the ADI pathway is therefore important for survival under these conditions. The ADI pathway may also contribute to virulence since some ST8-SCCmecIVa (USA300) MRSA clones carry the arginine catabolism mobile element (ACME), which contains an extra copy of the arc operon [56]. This leads to the hypothesis that the arcABDC operon facilitates pathogenicity by increasing survival of S. aureus in the presence of LC-uFFAs.
The sasF gene showed the largest change in expression of any gene in response to linoleic acid challenge (>16-fold and >30-fold up-regulation in MRSA252 by microarray and qRT-PCR, respectively). Expression of SasF, an LPXAG motif cell wall-anchored surface protein, is repressed by TcaR, the teicoplanin-associated locus regulator [42], [57]. The tcaR gene was found by microarray analysis to be up-regulated (>3-fold) in MRSA252 under the linoleic challenge conditions (Table 1). However, the SH1000 strain harbours a truncated copy of tcaR [42], [58] and synthesises a non-functional protein. This could explain why the sasF gene was only slightly up-regulated in SH1000 since its transcription may already be very high as its expression is reduced as part of the TcaR regulon. Many of the differences observed in the transcriptional responses of SH1000 and MRSA252 to the presence of fatty acids (Table 7, 8) are thus likely to be due to differential responses modulating RNAIII production, altered sarA transcription and differences between the strains in respect of the functionality of TcaR. The importance of sasF transcription for adaptation and survival of S. aureus to linoleic acid was tested by constructing an allelic replacement mutant in SH1000. The sasF mutant showed much reduced survival on agar plates containing 1 mM linoleic acid, exhibiting a 6-fold lower level of survival than the parental strain (Fig. 2A). The expression of this cell wall-anchored protein is therefore important for survival under these conditions. SasF may also contribute to virulence since in a murine arthritis model of infection a sasF allelic replacement mutant of SH1000 caused significantly less weight loss of the animals compared to control cells (Fig. 4A). Reduced numbers of bacteria were harvested from the kidney in mice infected with the sasF mutant strain compared to the control but the difference was not significant (Fig. 4B). SasF did not significantly affect development or severity of arthritis.
A screen for additional mutants of SH1000 that were defective for survival in the presence of linoleic acid identified several Tn917 transposants from a 5,000 clone library of mutants. Sequencing located the transposons within genes encoding VraE (ABC transporter permease) and SAR2632 (MMPL domain, putative efflux pump). The mutants Liv753 (vraE) and Liv766 (SAR2632) had reduced survival using this agar plate-based assay exhibiting 130-fold and 4-fold reductions in viability, respectively, at 1 mM linoleic acid (Fig. 2A). Each of these genes encodes transporter proteins of unknown function. The gene vraE is located downstream of vraD in a bicistronic operon and is a member of the GraSR regulon proposed to regulate traffic of cell wall substrates [59], [60]. Two studies have shown that S. aureus vraE mutants display decreased resistance to meticillin and increased susceptibility to human β-defensin 3. [61], [62]. VraE may also contribute to virulence, since in a murine arthritis model of infection a vraE allelic replacement mutant of SH1000 resulted in significantly less weight loss of the animals compared to control cells (Fig. 4A). Reduced numbers of bacteria were harvested from the kidney in mice infected with the vraE mutant strain compared to control but the difference was not significant (Fig. 4B). VraE did not significantly affect the development or severity of arthritis (data not shown). SAR2632 is a predicted transporter protein of the MMPL domain family proposed to be involved in lipid transport [63].
The identification of cell envelope mutants correlated with the gene expression and proteomic data, in which altered levels of cell wall synthesis and regulation components was observed (Fig. 5A). An increase in autolysis was observed under growth exposure conditions, although whether this is due to changes in expression of PG synthesis genes or down-regulation of the CtsR regulon remains unelucidated. The overall up-regulation of many cell wall synthesis genes could have two possible explanations. Firstly, the increased synthesis may be required to maintain the integrity of the cell wall, damaged due to loss of material through the precipitation of PG by LC-uFFAs as described by Campbell et al. [25]. The binding of LC-uFFAs to PG would not be unexpected given that chitosan, which has a very similar structure to PG, has been shown to bind lipids [64]. Secondly, an increase in cell wall and capsule synthesis could act as a defense mechanism since an increase in ionically charged material surrounding the cell would mitigate against access of the non-polar carbon chain of LC-uFFAs to the cell membrane. Reduced cell surface hydrophobicity and increased thickness of the cell wall have been suggested as Gram-positive defense mechanisms to limit interactions with lipids [34], [48]. Therefore, the reduced cell surface hydrophobicity of both the MRSA252 and SH1000 strains, observed here following overnight growth in the presence of linoleic acid, represents a pathogen countermeasure to this component of the innate immune system.
Figure 5. Schematic representation of cellular pathways displaying changes in gene transcription in response to linoleic acid challenge conditions.
Sections A, B, C and D highlight the various genes involved in peptidoglycan, carotenoid, menaquinone and energy metabolism respectively. Genes in red and blue boxes are up- and down-regulated, respectively. See text for details.
Previous studies have used gene expression profiling to determine the cellular pathway targeted by antimicrobial agents [65], [66]. In this study, there was no change in expression of fatty acid biosynthesis genes, other than fabZ, which is located on the same operon as the PG synthesis gene murA1. This suggests that the anti-staphylococcal toxicities of the LC-uFFAs used in this study are not a consequence of inhibiting fatty acid biosynthesis. Prior studies on the action of LC-uFFAs upon cells of S. aureus demonstrated membrane perturbations [12], [18], [22], [26]. This supports the finding of Chamberlain et al. that increased fluidity of S. aureus membranes resulted from exposure to LC-uFFAs [26]. Furthermore, these authors demonstrated that carotenoid-dependent pigmentation in non-isogenic clinical isolates positively correlated with increased survival from LC-uFFAs and showed that the carotenoid staphyloxanthin acted to decrease membrane fluidity and reduce its damaging effects. Interestingly, genes involved in carotenoid biosynthesis were up-regulated in response to LC-uFFAs (Fig. 5B), and Liv681 (crtM), which cannot produce staphyloxanthin, was shown here to have a >5-fold reduced survival to 1 mM linoleic acid. Staphyloxanthin production is regulated via σB, which also regulates many stress response components observed to be up-regulated in the array data. Consequently, the general stress response, including the production of staphyloxanthin serves as an important component of defence against LC-uFFAs, given the discovery that Liv130 (sigB) exhibited a >75-fold reduction in survival to 1 mM linoleic acid. sigB has previously been shown to contribute to S. aureus resistance to antimicrobials [67]. The CtsR regulon was strongly up-regulated after linoleic acid challenge, but was down-regulated after growth exposure and may therefore also participate in the adaptation to this environment.
A consequence of LC-uFFAs inserting in the cell membrane could be the disruption of the electron transport chain. This would explain the numerous changes in expression of genes associated with energy creation within the cell (Fig. 5C, 5D) and appears to constitute the main cellular response to LC-uFFAs. The overall trend is one of increasing levels of pyruvate and alterations in menaquinone synthesis. Moreover, the ADI pathway for anaerobic utilisation of arginine was up-regulated under growth exposure conditions. The genes affected by LC-uFFAs include those involved in the glycolytic and fermentative pathways, cell wall synthesis, cell division, and capsule synthesis. These pathways have also been shown to be modulated in a S. aureus small colony variant, which has a mutation in the hemB gene of the electron transport chain [68]. This supports a mode of action for LC-uFFAs of disturbing cell energetics via membrane disruption. Finally, the overall similarities in responses to the LC-uFFAs employed in this study appear to indicate a common mode of action amongst the linoleic, oleic and hexadecenoic acids. This corroborated dataset on the transcriptional and translational responses of S. aureus should provide a useful resource for further studies on this pathogens response to the host environment.
Materials and Methods
Bacterial strains, plasmid and growth conditions
Strains and plasmids used in this study are listed in Table 13. Unless indicated otherwise, bacteria were grown in brain heart infusion broth (BHI)(Merck) at 37°C with shaking at 125 rpm. When included, antibiotics were added at the following concentrations: erythromycin, 5 µg ml−1; lincomycin, 25 µg ml−1; tetracycline, 5 µg ml−1, chloramphenicol 10 µg ml−1.
Table 13. Strains and plasmids used in this study.
| Strain or Plasmid | Comment | Reference or Source |
| Strains: | ||
| E. coli: | ||
| DH5α | ø80 (lacZ)M15 (argF-lac)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | [86] |
| S. aureus: | ||
| SH1000 | Functional rsbU derivative of 8325-4 rsbU + | [87] |
| MRSA252 | Wild-type clinical isolate | [52] |
| RN4220 | Restriction-deficient transformation recipient mutant of 8325-4 | [88] |
| N315 | Wild-type pharyngeal smear clinical isolate | [89] |
| MSSA476 | Wild-type clinical isolate | [52] |
| Liv033 (katA) | 8325-4 katA::Tn917 | [87] |
| Liv038 (agr) | SH1000 agr::tet | [87] |
| Liv039 (sarA) | SH1000 sarA::kan | [87] |
| Liv101 (lytS) | SH1000 lytS::pER1 | [45] |
| Liv130 (sigB) | SH1000 sigB::tet | [87] |
| Liv142 (atl) | SH1000 atl::lacZ pAZ106 | [44] |
| Liv405 (clfA) | 8325-4 clfA::lacZ pAZ106 | T. Foster |
| Liv442 (clfA) | SH1000 clfA trandsduced from Liv405 | This Study |
| Liv671 (clpC) | SH1000 clpC::erm | [90] |
| Liv673 (crtM) | Newman crtM::cat | [91] |
| Liv681 (crtM) | SH1000 crtM::cat transduced from Liv673 | This study |
| Liv684 (sasF) | RN4220 sasF::tet | This Study |
| Liv686 (arcA) | RN4220 arcA::tet | This Study |
| Liv692 (arcA) | SH1000 arcA::tet transduced from Liv686 | This Study |
| Liv694 (sasF) | SH1000 sasF::tet transduced from Liv684 | This Study |
| Liv718 (vraS) | SH1000 vraS::tet transduced from Liv723 | This Study |
| Liv723 (vraS) | RN4220 vraS::tet | This Study |
| Liv750 (katA) | SH1000 katA::Tn917 transduced from Liv033 | This study |
| Liv753 (vraE) | SH1000 SAR2782::Tn917 | This Study |
| Liv766 (SAR2632) | SH1000 SAR2632::Tn917 | This Study |
| Liv994 | RN4220 pSK5630+sasF | This Study |
| Liv995 | RN4220 pSK5630+arcA | This Study |
| Liv996 | RN4220 pSK5630+vraE | This Study |
| Liv997 | RN4220 pSK5630+SAR2632 | This Study |
| Liv1000 (sasF) | Liv694 complemented with pSK5630+sasF | This Study |
| Liv1001 (arcA) | Liv692 complemented with pSK5630+arcA | This Study |
| Liv1002 (vraE) | Liv753 complemented with pSK5630+vraE | This Study |
| Liv1003 (SAR2632) | Liv766 complemented with pSK5630+SAR2632 | This Study |
| Plasmids: | ||
| pLTV1 | Temperature-sensitive plasmid harbouring Tn917 | [69] |
| pAZ106 | Promoterless lacZ erm insertion vector | [92] |
| pDG1513 | pMTL22 derivative [tetr] | [73] |
| pSK5630 | Low copy number E. coli-S. aureus shuttle vector [cmr] | [75] |
Transposon mutagenesis and screening for sensitivity to LC-uFFAs
Transposon mutagenesis was performed on the SH1000 strain of S. aureus using the Tn917 containing plasmid pLTV1, as described previously [69]. Single colonies from a transposon library grown on BHI agar containing erythromycin and lincomycin were innoculated into 96-well plates containing 200 µl of BHI. From this library 5,000 clones were cultured and stored at −80°C in 10% glycerol. After repeat growth of clones overnight at 37°C, without shaking, cultures were diluted 100-fold before 5 µl was spotted onto BHI agar with or without 0.5 mM linoleic acid. After overnight incubation, strains with decreased resistance to linoleic acid, when compared to wild type (WT) SH1000, were selected. The transposon-mediated mutations in these strains were transduced into the WT SH1000 using ø11 as described previously [70]. The linoleic acid sensitivity of these mutants was reconfirmed, proving the phenotype was transposon-linked, by repeat assay using serial dilutions of the mutant strains onto BHI agar containing millimolar concentrations of linoleic acid. The locations of the Tn917 insertions within the genome of mutants were determined using arbitrary primed nested PCR and DNA sequencing of regions upstream and downstream of the transposon [71].
Construction of sasF, arcA and vraS insertional mutants and complementation plasmids
Construction of sasF, arcA and vraS mutants was performed as described by Horsburgh et al. [72] using the oligonucleotides described in Table 14. Briefly this was as follows: the sasF, arcA or vraS genes were amplified as upstream and downstream fragments using primer pairs sasF_BamHI/sasF_NotI and sasF_KpnI/sasF_EcoRI, or arcA_BamHI/arcA_NotI and arcA_KpnI/arcA_EcoRI or vraS_BamHI/vraS_NotI and vraS_KpnI/vraS_EcoRI, respectively. The tetracycline resistance gene from pDG1513 [73] was amplified by using the primer pair Tet_NotI/Tet_KpnI. The upstream, downstream and tet gene fragments were digested with BamHI and NotI, or KpnI and EcoRI, or NotI and KpnI, respectively, and simultaneously ligated into pAZ106, which had been previously digested with BamHI and EcoRI. The resulting constructs were confirmed by restriction digest and then used to transform electrocompetent S. aureus RN4220 by the method of Schenk and Ladagga [74]. Strains of RN4220 containing the Campbell integration of the plasmid were resolved in SH1000 by transductional outcross using ø11. Clones of SH1000, which had now lost the plasmid and contained an allelic replacement with the tetracycline resistance gene, were confirmed as mutants by PCR amplification. Correct allelic replacement was confirmed in each case.
Table 14. Oligonucleotides used for the construction of mutants.
| Oligonucleotides | Sequence (5′ to 3′) |
| sasF_BamHI | CCACGGATCCGGTAGTGATGTTTTGG |
| sasF_NotI | ATAACTGCGGCCGCTTGAAACGGTTTCCCTCG |
| sasF_KpnI | CCGGTACCGTTATCACGACGCAATAAG |
| sasF_EcoRI | ACATGAATTCAAACAAGGAGTTCGGAC |
| arcA_BamHI | CCACGGATCCACAAGTAGTAGATATGTG |
| arcA_NotI | ATAACTGCGGCCGCTTAATTGGACCATCTGTC |
| arcA_KpnI | CCGGTACCGACACTTTCTAATCAAG |
| arcA_EcoRI | ACATGAATTCTGCTTTGGTAAATCAC |
| vraS_BamHI | CCACGGATCCGCATGCTAGCTGCATTTC |
| vraS_NotI | ATAACTGCGGCCGCCATTTCATGATCATCCAC |
| vraS_KpnI | CCGGTACCCAAGCTGTCATCTATGCATTC |
| vraS_EcoRI | ACATGAATTCGCTGAAACATCTACTC |
| Tet_NotI | ATAACTGCGGCCGCGGCGGATTTTATGACCGATGAAG |
| Tet_KpnI | CCGGTACCTTAGAAATCCCTTTGAGAATGTTT |
| Complementation | |
| Sar2725_SasF_For | ACGCGTCGACTAATATGATGTTAGCGACATGG |
| Sar2725_SasF_Rev | ACGCGGATCCAATGATGGACAATCTATTCATTGC |
| arcA_For | ACGCGTCGACGTGAATATAATCACATGTAAGCG |
| arcA_Rev | ACGCGGATCCTCTGTCATTATTTTCACCCTCG |
| Sar2632_For | ACGCGTCGACTTTATAACTCGTAAATCAGTCTC |
| Sar2632_Rev | ACGCGGATCCCATGTAAAATTTGCGACATTGC |
| Sar2782_vraE_For | ACGCGTCGACTGTCATCATGCTAAAAGATGGC |
| Sar2782_vraE_Rev | ACGCGGATCCAGTTAATAGTTATACTGCATTGC |
Complementation of the sasF, arcA, vraE and SAR2632 mutants was performed by amplifying each gene with sufficient upstream and downstream DNA using the primer pairs listed in Table 14. The fragments were ligated into pSK5630 [75] following digestion with BamHI/SalI, and the resulting constructs and the control plasmid were transformed into E. coli DH5α, with selection on agar plates containing ampicillin. The resulting constructs were confirmed by restriction digest and then used to transform electrocompetent S. aureus RN4220. The plasmids were then purified from RN4220 and transformed into the corresponding mutant strains.
Microarray analysis
To ascertain the transcriptional responses of MRSA252 to fatty acids, overnight cultures (18 h) of MRSA252 were used to inoculate 100 ml of BHI (Merck) with or without 10 µM of oleic or linoleic acid in 250 ml conical flasks. These 100 ml cultures were placed in a shaking water bath at 37°C at 250 rpm and 10 ml samples were taken from the flask when the cultures reached late exponential phase (OD600 = 3). Identical inoculations were performed to 100 ml of BHI lacking additional fatty acids. 100 µM of linoleic acid in ethanol or an equal volume of the ethanol used to dilute the fatty acid was added to these cultures at an OD600 = 3.0 and the RNA extracted from treated and untreated cells 20 min post-treatment. Each treatment and control culture was performed in biological triplicate. The concentrations of fatty acids used in these experiments did not alter the pH of the media. RNA was extracted from 10 ml samples of culture taken at the indicated time intervals and stabilised by the addition of 20 ml of RNA Bacteria Protect (Qiagen). The cells were subsequently harvested by centrifugation at 5000 rpm for 10 min and cell pellets resuspended in lysis buffer (10 mM Tris, pH8.0) containing 200 U ml−1 of lysostaphin and 400 U ml−1 of mutanolysin, and incubated for 90 min at 37°C with gentle shaking every 10–15 min. The RNA was subsequently extracted using the RNeasy Midi kit (Qiagen) and DNase treated whilst on the purification column using the RNase-Free DNase Set according to manufacturers instructions (Qiagen). The quantity and quality of the RNA was assessed on an Agilent 2100 bioanalyzer by using the RNA 6000 Nano LabChip Kit. The RNA was converted to cDNA and labelled by incorporation of Cy5 dCTP during reverse transcription of RNA using the enzyme Superscript II (Amersham). DNA used in the microarray hybridisations was extracted from 5 ml of an overnight culture (18 h) of MRSA252 using the Edge Biosystems Bacterial Genomic DNA purification kit according to manufacturer's instructions. The DNA was labeled by the incorporation of Cy3 dCTP using Klenow (Invitrogen). cDNA derived from RNA and genomic DNA were pooled and hybridized on whole-genome microarrays supplied by the Bacterial Microarray Group at St. George's Hospital (BμG@S [http://bugs.sgul.ac.uk]) before washing and scanning [76]. Microarrays were scanned using an Affymetrix 428 scanner and image data extracted using ImaGene 5.2 (BioDiscovery). Fully annotated microarray data have been deposited in BμG@Sbase (accession number E-BUGS-68; http://bugs.sgul.ac.uk/E-BUGS-68) and also ArrayExpress (accession number E-BUGS-68). Two independent labelling reactions and hybridisations were carried out for each RNA sample. Image data was analysed using the GeneSpring 7.3.1 software (Silicon Genetics). Briefly, data were normalized relative to the corresponding untreated controls. Signals below 0.01 were taken as 0.01. Genes were then filtered on expression level to remove non-changing genes, with only those genes that changed by at least two-fold considered biologically significant. Changing genes were then filtered on confidence applying the Benjamini and Hochberg false discovery rate algorithm with a maximum significance cut-off at 0.05 to eliminate the chance of false-positives [77].
Quantitative Real-Time PCR
To confirm the validity of microarray data gene specific mRNAs were quantified from treated and untreated cultures by quantitative real-time PCR (qRT-PCR). Cells were grown in biological triplicate exactly as described above for the microarray experiments and bacterial RNA was isolated using the Pro-Blue Fast RNA kit (MP Biomedicals). DNA was removed from the samples by DNase I treatment (Ambion) according to manufacturer's instructions. The purified RNA was quantified using the nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific) and the integrity assessed by electrophoresis. 0.5 µg of RNA was reverse transcribed with 100 U of Bioscript Reverse Transcriptase (Bioline) using 0.2 µg of random hexamer primers (Promega) according to manufacturer's instructions. qRT-PCR was performed using the 7500 Fast System (Applied Biosystems) and the QuantiFast SYBR Green PCR kit (Qiagen) according to manufacturer's instructions. The relative levels of gene expression in fatty acid treated cells and the non-treated controls were calculated by relative quantification using gyrB as the endogenous reference gene. The choice of gyrB as a single reference gene was based on its consistent levels in microarray in all conditions and at all timpoints that were analysed. The oligonucleotides used for qRT-PCR are listed in Table 15. All samples were amplified in triplicate and the data analysis was carried out using the 7500 Fast System Software (Applied Biosystems).
Table 15. Oligonucleotides used for qRT-PCR analysis.
| Oligonucleotide | SAR Number | Sequence (5′ to 3′) |
| gyrB_For | SAR0005 | ATCGACTTCAGAGAGAGGTTTG |
| gyrB_Rev | SAR0005 | CCGTTATCCGTTACTTTAATCCA |
| spa_For | SAR0114 | GAAGCAACCAGCAAACCATGC |
| spa_Rev | SAR0114 | ACGTCCAGCTAATAACGCTGC |
| fadA_For | SAR0223 | GAAGATGTCATTGTTGGTACGG |
| fadA_Rev | SAR0223 | TGTAATCCTGATGAGCAGTAGC |
| fadD_For | SAR0225 | TTCATTGCTAGAAAGTAAGTACCG |
| fadD_Rev | SAR0225 | TGGCGTTTGGACGATCCTTGT |
| lytR_For | SAR0258 | TTTTTGCAACTGCACATGACCAA |
| lytR_Rev | SAR0258 | TTATCATCTTTGGCTTTAGTCGC |
| sarA_For | SAR0625 | TAAACTACAAACAACCACAAGTTG |
| sarA_Rev | SAR0625 | TTCGATTTTTTTACGTTGTTGTGC |
| clpB_For | SAR0938 | GAACGAGCAAATATTGAGGTAGA |
| clpB_Rev | SAR0938 | GCCTTAGTTATCAATTGGTTTGC |
| fabI_For | SAR0978 | GTGATGGGTGTTGCTAAAGCG |
| fabI_Rev | SAR0978 | AACCACCCACACCTTTTGCAC |
| hla_For | SAR1136 | GTTGCAACTACCTGATAATGAAG |
| hla_Rev | SAR1136 | CCAATTTTTCCAGAATCATCACC |
| katA_For | SAR1344 | AATAGTATGACAGCAGGGCCTA |
| katA_Rev | SAR1344 | AATGTCCCAAATGCACCAGAAC |
| murG_For | SAR1430 | ATCCCGAGGCGACCAAATTGA |
| murG_Rev | SAR1430 | AATTCGAGTTCTTTCCTGTTCCA |
| fabZ_For | SAR2186 | AATATGAAGAAGGTCAACGTTGC |
| fabZ_Rev | SAR2186 | ACCGCACCTGTTTGAGCTAACG |
| cidA_For | SAR2621 | GCCGGCAGTATTGTTGGTCTA |
| cidA_Rev | SAR2621 | TAATACCTACAACTGACGGTATG |
| crtM_For | SAR2643 | TGATGACAGTATAGATGTTTATGG |
| crtM_Rev | SAR2643 | ACATGCTGAAGGGCCATCATG |
| arcA_For | SAR2714 | GTCAGGAGTACGTAAGGAAGA |
| arcA_Rev | SAR2714 | GTGTCCTATTGAGGCTTGTGG |
| sasF-For | SAR2725 | CACAAATCGGAAGATTCAGC |
| sasF_Rev | SAR2725 | TGAGTCGATTACTATGGCTTTGA |
| RNAIII_For | RNAIII | ACATGGTTATTAAGTTGGGATGG |
| RNAIII_Rev | RNAIII | TAAAATGGATTATCGACACAGTGA |
Sample preparation for 2D-PAGE
Cultures of MRSA252 (100 ml) were grown to late exponential phase (OD600 = 3.0) and exposed to 0.1 mM linoleic acid or 0.1 mM hexadecenoic acid as described above. Cells were harvested by centrifugation at 5000 g for 10 min at 4°C. After two washes in PBS the cells were resuspended in 2 ml of lysis buffer (PBS, 1 mg/ml DNase I, 100 µM benzamidine, 100 µM PMSF, 1 mg/ml RNase, 2 mg/ml lysostaphin) and incubated at 37°C for 20 min before chilling on ice. Cell debris and insoluble material was pelleted by centrifugation at 4°C for 20,000 g for 20 min. The supernatant was stored at −20°C. Protein samples were quantified using the BioRad Protein assay. The protein samples were desalted using Slide-A-Lyzer Mini Dialysis Units with a 3.5 kDa MWCO (Thermo Scientific).
2D-PAGE
Soluble protein (120 µg ) was brought up to 320 µl with rehydration buffer (8 M urea, 2M thiourea, 4% (w/v) CHAPS, 20 mM DTT, 1% (v/v) ASB 14 detergent and 0.5% (v/v) carrier ampholytes (Bio-lyte 3/10, Bio-Rad)). Samples were incubated for an hour at room temperature with gentle shaking, before centrifugation at 8,000 g for 5 min. Samples were in-gel rehydrated and focused on 17 cm, pH 4–7 IPG strips (Bio-Rad) for a total of 40000 V h (150V for 1h, 300V for 1h, 600V for 1h, 1200V for 1h, 1200–8000V over 1h (linear gradient), 8000 V to 40000 v (steady state)), using a Protean IEF Cell (Bio-Rad). After focusing, strips were equilibrated in 50 mM Tris (pH 6.8), 6 M urea, 2% (w/v) SDS, 30% (w/v) glycerol, and bromophenol blue, containing 20 mM DTT in the reduction step (15 min) and 25 mM iodoacetamide in the alkylation step (15 min). IPG strips were run in the second dimension on 20×20cm 12.5% SDS-PAGE gels using a Protean II xi 2D Cell (Bio-Rad). Gels were run in triplicate, silver-stained [78] and scanned (GS-710 Densitometer, Bio-Rad) as gray-scale tiff files at 16 bit and 300 dpi and uploaded into the Progenesis ‘SameSpots’ (Non Linear Dynamics) gel image analysis Software. Quantitative analysis was based on average gels created from three gel replicates. Spots in the treated samples with a p≤0.05 and ≥ two-fold difference from the control sample were considered statistically significant. For protein identification by mass spectrometry 2 gels containing 800 µg each of soluble protein (a pool from each growth condition) were prepared as above and stained with Colloidal Coomassie Brilliant Blue [79]. The scanned images were uploaded into Progenesis ‘SameSpots’ and matched to the analytical gels.
Trypsin digestion and mass spectrometric identification of proteins
Spots for identification were excised and digested in-gel with trypsin. Gel Plugs were destained in 50% (v/v) acetonitrile:50% (v/v) 50 mM ammonium bicarbonate (37°C), dehydrated in 100% acetonitrile (37°C), and rehydrated overnight (37°C) in 10 µl of 50mM ammonium bicarbonate containing trypsin (1 µl of 100 ng trypsin stock reconstituted in 50 mM acetic acid (Promega)). Supernatants containing the extracted peptides were removed and analyzed by MALDI-TOF.
Peptide Mass Fingerprinting (PMF) was conducted on a reflectron MALDI-TOF instrument (MLDIWatersMicromass,UK ). Samples were mixed in a 1∶1 ratio with a saturated solution of α cyano-4-hydroxycinnamic acid ACN: water:TFA (50∶49∶1 (v/v/v)). The acquired spectra were analysed using MassLynx v 4.0 (Waters-Micromass,UK) and were all externally calibrated with a mixture of peptides. For each sample, all acquired spectra were combined and processed as follows using MassLynx v 4.0: smoothing, 2× smooth using a Savitzky Golay method set at +/− 3 channels and background subtraction using a polynomial of order 1 and 40% below the curve in order to reduce background noise. To get accurate mono isotopic peak data all processed spectra were centred using the top 80% of each peak. Peak lists were generated using ProteinLynx, part of MassLynx v 4.0. Monoisotopic peptide masses in the mass range of 800–4000 Da were used in the database search. The resulting peptide mass maps were used to interrogate S. aureus MRSA252 sequences to generate statistically significant candidate identifications using the MASCOT search engine (Matrix Science). Searches were performed allowing for complete carbamidomethylation (alkylation) of cysteine residues, partial oxidation of methionine residues, one missed cleavage and a mass error of 250 ppm. Molecular Weight Search (MOWSE) scores [80], number of matched ions, percent protein sequence coverage, and correlation of gel region with predicted mass and pI were collectively considered for each protein identification.
Cell surface hydrophobicity assays
Cell surface hydrophobicity of S. aureus strains was measured as described previously [81]. Briefly, stationary-phase cells (18-h cultures) grown in the presence or absence of 0.1 mM linoleic acid were harvested, washed three times and resuspended to an OD440 of 0.5 in PBS. 3 ml aliquots of each of these bacterial suspensions were vortexed for 1 min with 200 µl n-hexadecane (Sigma). After 15 min incubation to enable partitioning, 1 ml was removed from the aqueous layer and the OD440 recorded. Cell surface hydrophobicity was calculated as the percentage decrease in OD as a result of cells partitioning into the hexadecane.
Cell Autolysis Assay
Cell autolysis rates were determined on cells exposed to linoleic acid using an assay modified from that described by Blackman et al. [82]. Briefly, cells were grown in 100 ml volumes of BHI to an OD600 of 0.8–1.0 in the presence or absence of 0.01 mM linoleic acid. Following harvesting of the cells by centrifugation, the cells were washed in PBS and resuspended to an OD600 = 0.6 in 0.5% (v/v) Triton X-100. The cells were incubated with shaking at 37°C and the OD600 was monitored over time.
Experimental septic arthritis
A well described mouse model of septic arthritis was used to test the in vivo role of genes implicated in resistance to fatty acids in the strains [83]–[85]. Seven week old female NMRI mice were obtained from Charles River Laboratories (Sulzfeld, Germany) and maintained in the animal facility of the Department of Rheumatology and Inflammation Research, University of Göteborg, Sweden. All mice were maintained according to the local ethic board animal husbandry standards. The mice were housed 10 to a cage under standard conditions of temperature and light and were fed standard laboratory chow and water ad libitum. Bacteria were grown on blood agar plates for 24 h, harvested and stored frozen at −20°C in PBS containing 5% bovine serum albumin and 10% dimethyl sulfoxide. Before injection into animals, the bacterial suspensions were thawed, washed in PBS, and adjusted to appropriate cell concentrations. Mice were inoculated in the tail vein with 0.2 ml of bacterial suspension. The number of viable bacteria was measured in conjunction with each challenge by counting colonies following culture at 37°C for 24 hours on blood agar plates. Ten mice were infected with each strain of S. aureus by i.v. injection in the tails of 3.2–3.5×106 CFU of bacteria for induction of septic arthritis. The mice were weighed regularly and examined for arthritis until death by cervical dislocation 14 days after challenge. The kidneys were aseptically dissected and kept on ice, homogenized, diluted in PBS and inoculated on blood agar plates. Data were presented as CFU per kidney pair.
Acknowledgments
We would like to thank Alan McCarthy and Heather Allison for critical reading of the manuscript and Timothy Foster, Dorte Frees, Friedrich Götz and Simon Foster for kindly supplying mutant strains.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was enabled by the BBSRC grant BB/D003563/1 awarded to MJH. EJ, IMJ and AT were supported by LUA/ALF, Göteborg Medical Society, Göteborg Rheumatism Association, King Gustaf V's 80 Years Foundation, Swedish Research Council, Swedish Rheumatism Association, The Sigurd and Elsa Golje Memorial Foundation and the Family Thöléns and Kristlers Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus aureus. Am J Clin Dermatol. 2004;5:217–223. doi: 10.2165/00128071-200405040-00002. [DOI] [PubMed] [Google Scholar]
- 2.Schäfer L, Kragballe K. Abnormalities in epidermal lipid metabolism in patients with atopic dermatitis. J Invest Dermatol. 1991;96:10–15. doi: 10.1111/1523-1747.ep12514648. [DOI] [PubMed] [Google Scholar]
- 3.Takigawa H, Nakagawa H, Kuzukawa M, Mori H, Imokawa G. Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology. 2005;211:240–248. doi: 10.1159/000087018. [DOI] [PubMed] [Google Scholar]
- 4.Ansari MN, Nicolaides N, Fu HC. Fatty acid composition of the living layer and stratum corneum lipids of human sole skin epidermis. Lipids. 1970;5:838–845. doi: 10.1007/BF02531977. [DOI] [PubMed] [Google Scholar]
- 5.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Pappas A, Anthonavage M, Gordon JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002;118:164–171. doi: 10.1046/j.0022-202x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- 7.Do TQ, Moshkani S, Castillo P, Anunta S, Pogosyan A, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol. 2008;181:4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye ES, Kapral FA. Characterization of a bactericidal lipid developing within staphylococcal abscesses. Infect Immun. 1981;32:98–104. doi: 10.1128/iai.32.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engler HD, Kapral FA. The production of a bactericidal monoglyceride in staphylococcal abscesses. J Med Microbiol. 1992;37:238–244. doi: 10.1099/00222615-37-4-238. [DOI] [PubMed] [Google Scholar]
- 10.Shryock TR, Kapral FA. The production of bactericidal fatty acids from glycerides in staphylococcal abscesses. J Med Microbiol. 1992;36:288–92. doi: 10.1099/00222615-36-4-288. [DOI] [PubMed] [Google Scholar]
- 11.Bergsson G, Arnfinnsson J, Steingrímsson O, Thormar H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS. 2001;109:670–678. doi: 10.1034/j.1600-0463.2001.d01-131.x. [DOI] [PubMed] [Google Scholar]
- 12.Galbraith H, Miller TB. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J Appl Bacteriol. 1973;36:659–675. doi: 10.1111/j.1365-2672.1973.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 13.Heczko PB, Lütticken R, Hryniewicz W, Neugebauer M, Pulverer G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J Clin Microbiol. 1979;9:333–335. doi: 10.1128/jcm.9.3.333-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelsey JA, Bayles KW, Shafii B, McGuire MA. Fatty acids and monoacylglycerols inhibit growth of Staphylococcus aureus. Lipids. 2006;41:951–961. doi: 10.1007/s11745-006-5048-z. [DOI] [PubMed] [Google Scholar]
- 16.Knapp HR, Melly MA. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986;154:84–94. doi: 10.1093/infdis/154.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Nieman C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954;18:147–161. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speert DP, Wannamaker LW, Gray ED, Clawson CC. Bactericidal effect of oleic acid on group A streptococci: mechanism of action. Infect Immun. 1979;26:1202–1210. doi: 10.1128/iai.26.3.1202-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilmarsson H, Traustason BS, Kristmundsdóttir T, Thormar H. Virucidal activities of medium- and long-chain fatty alcohols and lipids against respiratory syncytial virus and parainfluenza virus type 2: comparison at different pH levels. Arch Virol. 2007;152:2225–2236. doi: 10.1007/s00705-007-1063-5. [DOI] [PubMed] [Google Scholar]
- 20.Hilmarsson H, Larusson LV, Thormar H. Virucidal effect of lipids on visna virus, a lentivirus related to HIV. Arch Virol. 2006;151:1217–1224. doi: 10.1007/s00705-005-0699-2. [DOI] [PubMed] [Google Scholar]
- 21.Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenway DL, Dyke KG. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979;115:233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- 23.Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, et al. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Won SR, Hong MJ, Kim YM, Li CY, Kim JW, et al. Oleic acid: an efficient inhibitor of glucosyltransferase. FEBS Lett. 2007;581:4999–5002. doi: 10.1016/j.febslet.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Campbell IM, Crozier DN, Pawagi AB, Buivids IA. In vitro response of Staphylococcus aureus from cystic fibrosis patients to combinations of linoleic and oleic acids added to nutrient medium. J Clin Microbiol. 1983;18:408–415. doi: 10.1128/jcm.18.2.408-415.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain NR, Mehrtens BG, Xiong Z, Kapral FA, Boardman JL, et al. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect Immun. 1991;59:4332–4337. doi: 10.1128/iai.59.12.4332-4337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grinsted J, Lacey RW. Ecological and genetic implications of pigmentation in Staphylococcus aureus. J Gen Microbiol. 1973;75:259–267. doi: 10.1099/00221287-75-2-259. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Z, Kapral FA. Carotenoid pigment levels in Staphylococcus aureus and sensitivity to oleic acid. J Med Microbiol. 1992;37:192–194. doi: 10.1099/00222615-37-3-192. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain NR, Imanoel B. Genetic regulation of fatty acid modifying enzyme from Staphylococcus aureus. J Med Microbiol. 1996;44:125–129. doi: 10.1099/00222615-44-2-125. [DOI] [PubMed] [Google Scholar]
- 30.Kapral FA, Smith S, Lal D. The esterification of fatty acids by Staphylococcus aureus fatty acid modifying enzyme (FAME) and its inhibition by glycerides. J Med Microbiol. 1992;37:235–237. doi: 10.1099/00222615-37-4-235. [DOI] [PubMed] [Google Scholar]
- 31.Long JP, Hart J, Albers W, Kapral FA. The production of fatty acid modifying enzyme (FAME) and lipase by various staphylococcal species. J Med Microbiol. 1992;37:232–234. doi: 10.1099/00222615-37-4-232. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen JE, Shryock TR, Kapral FA. Modification of bactericidal fatty acids by an enzyme of Staphylococcus aureus. J Med Microbiol. 1992;36:293–298. doi: 10.1099/00222615-36-4-293. [DOI] [PubMed] [Google Scholar]
- 33.Lee EH, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol. 1999;33:839–845. doi: 10.1046/j.1365-2958.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 34.Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, et al. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Projan SJ, Brown-Skrobot S, Schlievert PM, Vandenesch F, Novick RP. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J Bacteriol. 1994;176:4204–4209. doi: 10.1128/jb.176.14.4204-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruzin A, Novick RP. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J Bacteriol. 2000;182:2668–2671. doi: 10.1128/jb.182.9.2668-2671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlievert PM, Deringer JR, Kim MH, Projan SJ, Novick RP. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother. 1992;36:626–631. doi: 10.1128/aac.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson ML, Schlievert PM. Glycerol monolaurate inhibits the effects of Gram-positive select agents on eukaryotic cells. Biochemistry. 2006;45:2387–2397. doi: 10.1021/bi051992u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruzin A, Novick RP. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J Bacteriol. 1998;180:182–185. doi: 10.1128/jb.180.1.182-185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vetter SM, Schlievert PM. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis. Antimicrob Agents Chemother. 2005;49:1302–1305. doi: 10.1128/AAC.49.4.1302-1305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burts ML, Williams WA, DeBord K, Missiakas DM. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci USA. 2005;102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCallum N, Bischoff M, Maki H, Wada A, Berger-Bächi B. TcaR, a putative MarR-like regulator of sarS expression. J Bacteriol. 2004;186:2966–2972. doi: 10.1128/JB.186.10.2966-2972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster SJ. Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J Bacteriol. 1995;177:5723–5725. doi: 10.1128/jb.177.19.5723-5725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunskill EW, Bayles KW. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol. 1996;178:611–618. doi: 10.1128/jb.178.3.611-618.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qoronfleh MW, Gustafson JE, Wilkinson BJ. Conditions that induce Staphylococcus aureus heat shock proteins also inhibit autolysis. FEMS Microbiol Lett. 1998;166:103–107. doi: 10.1111/j.1574-6968.1998.tb13189.x. [DOI] [PubMed] [Google Scholar]
- 48.Dufour M, Manson JM, Bremer PJ, Dufour JP, Cook GM, et al. Characterization of monolaurin resistance in Enterococcus faecalis. Appl Environ Microbiol. 2007;73:5507–5515. doi: 10.1128/AEM.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins LV, Kristian SA, Weidenmaier C, Faigle M, Van Kessel KP, et al. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis. 2002;186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 50.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weidenmaier C, Peschel A, Kempf VA, Lucindo N, Yeaman MR, et al. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect Immun. 2005;73:8033–8038. doi: 10.1128/IAI.73.12.8033-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlain NR, Brueggemann SA. Characterisation and expression of fatty acid modifying enzyme produced by Staphylococcus epidermidis. J Med Microbiol. 1997;46:693–697. doi: 10.1099/00222615-46-8-693. [DOI] [PubMed] [Google Scholar]
- 54.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother. 2005;56:455–462. doi: 10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]
- 55.Johnson AP, Aucken HM, Cavendish S, Ganner M, Wale MC, et al. Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J Antimicrob Chemother. 2001;48:143–144. doi: 10.1093/jac/48.1.143. [DOI] [PubMed] [Google Scholar]
- 56.Goering RV, McDougal LK, Fosheim GE, Bonnstetter KK, Wolter DJ, et al. Epidemiologic distribution of the arginine catabolic mobile element among selected methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:1981–1984. doi: 10.1128/JCM.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roche FM, Massey R, Peacock SJ, Day NP, Visai L, et al. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology. 2003;149:643–654. doi: 10.1099/mic.0.25996-0. [DOI] [PubMed] [Google Scholar]
- 58.Oscarsson J, Kanth A, Tegmark-Wisell K, Arvidson S. SarA is a repressor of hla (alpha-hemolysin) transcription in Staphylococcus aureus: its apparent role as an activator of hla in the prototype strain NCTC8325 depends on reduced expression of sarS. J Bacteriol. 2006;188:8526–8533. doi: 10.1128/JB.00866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, et al. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49:807–821. doi: 10.1046/j.1365-2958.2003.03599.x. [DOI] [PubMed] [Google Scholar]
- 60.Neoh HM, Cui L, Yuzawa H, Takeuchi F, Matsuo M, et al. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin-intermediate resistance to vancomycin-intermediate resistance. Antimicrob Agents Chemother. 2008;52:45–53. doi: 10.1128/AAC.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Lencastre H, Wu SW, Pinho MG, Ludovice AM, Filipe S, et al. Antibiotic resistance as a stress response: complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb Drug Resist. 1999;5:163–175. doi: 10.1089/mdr.1999.5.163. [DOI] [PubMed] [Google Scholar]
- 62.Sass V, Pag U, Tossi A, Bierbaum G, Sahl HG. Mode of action of human beta-defensin 3 against Staphylococcus aureus and transcriptional analysis of responses to defensin challenge. Int J Med Microbiol. 2008;298:619–633. doi: 10.1016/j.ijmm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Tekaia F, Gordon SV, Garnier T, Brosch R, Barrell BG, et al. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 64.Wydro P, Krajewska B, Hac-Wydro K. Chitosan as a lipid binder: a langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules. 2007;8:2611–2617. doi: 10.1021/bm700453x. [DOI] [PubMed] [Google Scholar]
- 65.Freiberg C, Fischer HP, Brunner NA. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob Agents Chemother. 2005;49:749–759. doi: 10.1128/AAC.49.2.749-759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutter B, Schaab C, Albrecht S, Borgmann M, Brunner NA, et al. Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob Agents Chemother. 2004;48:2838–2844. doi: 10.1128/AAC.48.8.2838-2844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riordan JT, O'Leary JO, Gustafson JE. Contributions of sigB and sarA to distinct multiple antimicrobial resistance mechanisms of Staphylococcus aureus. Int J Antimicrob Agents. 2006;28:54–61. doi: 10.1016/j.ijantimicag.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seggewiss J, Becker K, Kotte O, Eisenacher M, Yazdi MR, et al. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J Bacteriol. 2006;188:7765–7777. doi: 10.1128/JB.00774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camilli A, Portnoy A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 71.Begun J, Sifri CD, Goldman S, Calderwood SB, Ausubel FM. Staphylococcus aureus virulence factors identified by using a high-throughput Caenorhabditis elegans-killing model. Infect Immun. 2005;73:872–877. doi: 10.1128/IAI.73.2.872-877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horsburgh MJ, Wiltshire MD, Crossley H, Ingham E, Foster SJ. PheP, a putative amino acid permease of Staphylococcus aureus, contributes to survival in vivo and during starvation. Infect Immun. 2004;72:3073–3076. doi: 10.1128/IAI.72.5.3073-3076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerot-Fleury AM, Shazand K, Frandsen N, Stragier P. Antibiotic resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 74.Schenk S, Ladagga RA. Improved methods for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992;94:133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- 75.Grkovic S, Brown MH, Hardie KM, Firth N, Skurray RA. Stable low-copy-number Staphylococcus aureus shuttle vectors. Microbiol. 2003;149:785–794. doi: 10.1099/mic.0.25951-0. [DOI] [PubMed] [Google Scholar]
- 76.Witney AA, Marsden GL, Holden MTG, Stabler RA, Husain SE, et al. Design, validation and application of a seven strain Staphylococcus aureus microarray for comparative genomics. Appl Environ Microbiol. 2005;71:7504–7514. doi: 10.1128/AEM.71.11.7504-7514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 78.Yan J, Wait R, Berkelman T, Harry R, Westbrook J, et al. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 79.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 80.Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 81.Banner MA, Cunniffe JG, Macintosh RL, Foster TJ, Rohde H, et al. Localised tufts of fibrils on Staphylococcus epidermidis NCTC 11047 are comprised of the accumulation-associated protein. J Bacteriol. 2007;189:2793–2804. doi: 10.1128/JB.00952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blackman SA, Smith TJ, Foster SJ. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology. 1998;144:73–82. doi: 10.1099/00221287-144-1-73. [DOI] [PubMed] [Google Scholar]
- 83.Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Josefsson E, Kubica M, Mydel P, Potempa J, Tarkowski A. In vivo sortase A and clumping factor A mRNA expression during Staphylococcus aureus infection. Microb Pathog. 2008;44:103–110. doi: 10.1016/j.micpath.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arth Rheum. 1996;39:1596–1605. doi: 10.1002/art.1780390921. [DOI] [PubMed] [Google Scholar]
- 86.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, N.Y.: Cold Spring Laboratory Press; 1989. [Google Scholar]
- 87.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, et al. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Novick RP. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 89.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, et al. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 90.Frees D, Chastanet A, Qazi S, Sørensen K, Hill P, et al. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol Microbiol. 2004;54:1445–1462. doi: 10.1111/j.1365-2958.2004.04368.x. [DOI] [PubMed] [Google Scholar]
- 91.Clauditz A, Resch A, Wieland KP, Peschel A, Götz F Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan PF, Foster SJ, Ingham E, Clements MO. The Staphylococcus aureus alternative sigma factor óB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]