Abstract
Background & Aims
The Paneth cell α-defensins HD5 and HD6 contribute to the antimicrobial barrier against intestinal infection. We have previously demonstrated that both HD5 and HD6 mRNA were reduced in adults living in urban Zambia compared to adults living in London. The aim of this study was to determine if α-defensin expression in Zambian adults is related to susceptibility to diarrhea during 3 years of follow-up.
Methods
We analyzed intestinal biopsies from a longitudinal cohort study in 83 Zambian adults using quantitative reverse transcription polymerase chain reaction (RT-PCR), Western blotting, immunohistochemistry and in situ hybridization, and we measured diarrhea incidence.
Results
HD5 and HD6 mRNA in Paneth cells varied between individuals, over time and seasonally, and was strongly correlated with mucosal architecture. Gene expression was restricted to Paneth cells. The median (interquartile range, IQR) HD5 mRNA was 6.0 (5.6-6.7) log10 transcripts/μg total RNA in 18 participants who experienced diarrhea in two months after the biopsy was taken, compared to 6.8 (6.2-7.3) in 94 who did not (P=0.006), and a similar observation was made for HD6.
Conclusions
These data indicate that intestinal α-defensin expression is dynamic and seasonal, and suggest that susceptibility to intestinal infection is related to defensin expression.
Keywords: defensin, diarrhea, Africa, innate immunity, Paneth cell, incidence, enteropathy, HIV
Introduction
Intestinal infectious disease is a major contributor to morbidity and mortality in tropical countries, estimated to have caused 1.5 million deaths in children in developing countries in the year 20021. Although the death rate from acute diarrheal disease in children has fallen due to the widespread use of oral rehydration therapy, the more refractory problem of the persistent diarrhea-malnutrition syndrome remains a public health problem2. A similar syndrome has emerged in adults with HIV-related immunosuppression, and both adults and children with these disorders have high mortality rates3,4. In AIDS, in primary malnutrition, and during immunosuppressive therapy, there is a failure of cell mediated immunity, so there is an urgent need to elucidate pathways of intestinal host defense. An improved understanding of innate immunity may lead to new therapeutic avenues for persistent intestinal infections.
Antimicrobial peptides5,6 secreted into the intestine may constitute an important barrier to colonization of enterocytes (i.e. infection) and to the translocation of bacteria from the lumen into blood and lymph. Paneth cells are specialized intestinal epithelial cells which contain a rich armamentarium of antimicrobial peptides and proteins. In addition, cells of other lineages also secrete molecules with antimicrobial properties. The intestinal α-defensins, human defensin (HD)5 and HD6, are small cationic peptides synthesized by Paneth cells7,8 which probably make a major contribution to intestinal defence9,10,11. Mice with a deletion of the matrilysin gene cannot process α-defensins to their active form and are more susceptible to colonization with Salmonella spp.12. Also, transgenic mice which express HD5 are protected from lethal challenge with Salmonella typhimurium13 indicating that HD5 can function as an antimicrobial molecule in vivo.
We have previously observed that adults living in a crowded township in Lusaka, Zambia, had lower levels of mRNA of both HD5 and HD6 than adults living in London14, yielding approximately 10-fold less specific mRNA per μg total RNA. This is surprising as it might be expected that increased expression of antimicrobial peptides might confer a survival advantage in tropical populations where exposure to intestinal pathogens is frequent and intense. It has been known for many years that healthy members of tropical populations have a background environmental enteropathy. This ‘tropical enteropathy’15 appears to be a consequence of high exposure to enteropathogens as it is largely reversible in visitors to the tropics16, it is more closely related to economic circumstances than to climate17, and it shows seasonal variation18. Tropical enteropathy is characterized by reduced villous height, increased crypt depth, and T cell activation when compared to populations living in temperate climates, both in adults19 and children20. This pattern of crypt hyperplastic enteropathy also characterizes many infective states21,22, but within our Zambian population villous height and crypt depth were positively correlated18 as occurs during starvation23. We studied the pattern of Paneth cell defensin expression in adults in this tropical population and in relation to the individual risk of diarrhea during two years of a longitudinal cohort study.
Methods
Study groups and tissue collection
We studied 83 Zambian adults, drawn at random from a cohort study, fully representative of the population of a crowded shanty compound in Lusaka in which we have previously analyzed small intestinal architecture and function18,24. HIV seroprevalence in Lusaka is relatively stable at 25-30%25 so HIV status was established in those participants willing to undergo testing. Jejunal biopsies were obtained by enteroscopy approximately annually over this time period, and used for quantification of HD5 and HD6 mRNA14 and for localization of mRNA and peptide. Morphometry was carried out as previously described, including villous height, crypt depth, villous width, epithelial surface area, and villous compartment volume, but only the first two measurements were made on biopsies taken after baseline18. Paneth cells were counted as previously described26. Biopsies for morphometric analysis, immunohistochemistry and in situ hybridization were collected into formal-saline; biopsies for RT-PCR and peptide analysis were immediately snap-frozen in liquid nitrogen, stored at −80°C, and analyzed within 6 months. Approval for these studies was obtained from the research ethics committees of both the University of Zambia and the London School of Hygiene and Tropical Medicine.
Competitive RT-PCR for HD5 and HD6
We have previously described a quantitative assay for HD5 and HD6 mRNA14. Briefly, biopsies were treated with Trizol (Invitrogen, Paisley, UK) for RNA extraction, treated with DNase (Promega, UK), and co-reverse transcribed with known quantities of a standard synthetic RNA prior to PCR amplification. The threshold of detection was determined to be 104 transcripts per μg total RNA.
Immunohistochemistry and in situ hybridization
Immunohistochemistry was used to define expression of HD5 as previously described26. No antibody to HD6 was available. HD5 and HD6 mRNA distribution was defined using in situ hybridisation26.
Peptide isoforms
To determine if there was variation in the stored forms of HD5, tissue extracts of cationic peptides were analyzed by acid-urea gel electrophoresis, followed by Western blotting as previously described27. Recombinant HD5 and pro-HD5 peptides were used as markers of migration, and a control antibody from animals injected with vehicle only was used as a negative control.
Studies of diarrhea incidence
Participants in the Lusaka cohort study were interviewed every 2 weeks to ascertain whether they had experienced diarrhea in the previous 2 weeks. Participants who had experienced diarrhea were not invited for endoscopy until one month had elapsed, but otherwise dates of appointment were allocated randomly at any time of the year (except for July or August) in order to detect seasonal variation.
Data analysis
HD5 and HD6 mRNA content was expressed as log10 transcripts per μg total RNA. The levels were not normally distributed so results are presented as median and interquartile range, and in statistical comparisons non-parametric statistical tests, the Kruskal-Wallis test, Wilcoxon's matched-pair rank sum test, and Spearman's rank correlation coefficient, were used. Where the result of the RT-PCR was below threshold (i.e. below 104 transcripts/μg) the result was designated 4.0 for the purposes of analysis, which would not affect non-parametric test results. Seasonality was assessed by presenting villous height and mRNA in two-month periods from all years of data collection grouped together. Data on diarrhea incidence was used to estimate incidence as a dichotomous (yes/no) variable for the two months before or after the date of the biopsy. Analysis was performed using STATA version 8.0 (Stata Corp, College Station, Texas).
Results
Dynamics of α-defensin expression in Zambian adults
We analyzed biopsies taken from 83 participants in the first year of the study then analyzed biopsies taken from those individuals who remained under follow up; in the second year 46 of these were still under follow up and in the final year 40 were still included, so 169 biopsies altogether were available for analysis. Characteristics of the study participants at baseline are shown in Table 1 together with HIV status (where known), CD4 counts, morphometry of the biopsies, and defensin mRNA.
Table 1.
Demographic and clinical characteristics of Lusaka study participants at baseline
| All | HIV seronegative | HIV seropositive | P | |
|---|---|---|---|---|
| n | 83 | 47 | 31 | |
| Sex (M:F) | 30:53 | 19:28 | 10:21 | 0.48 |
| Age (years) | 32 (27-40) | 39 (29-45) | 29 (27-35) | 0.006 |
| VH (μm) | 256 (222-299) | 261 (217-310) | 253 (225-279) | 0.39 |
| CD (μm) | 153 (135-173) | 143 (131-161) | 169 (152-186) | 0.001 |
| CD4 (cells/μL) | 611 (389-800) | 722 (620-855) | 333 (194-471) | 0.0001 |
| CD8 (cells/μL) | 625 (391-774) | 506 (383-643) | 792 (589-1337) | 0.0005 |
| HD5 (log10 transcripts/μg) | 5.6 (4.6-6.7) | 5.9 (4.9-6.9) | 5.2 (4.0-6.2) | 0.31 |
| HD6 (log10 transcripts/μg) | 5.8 (5.0-6.5) | 6.0 (5.2-6.6) | 5.7 (4.6-6.2) | 0.22 |
83 participants were included in the study but HIV tests were performed only on 78. Values shown are given as median (interquartile range), and the P value given was derived from a Kruskal-Wallis test of the difference between HIV seropositives and HIV seronegatives. (VH, villous height; CD, crypt depth).
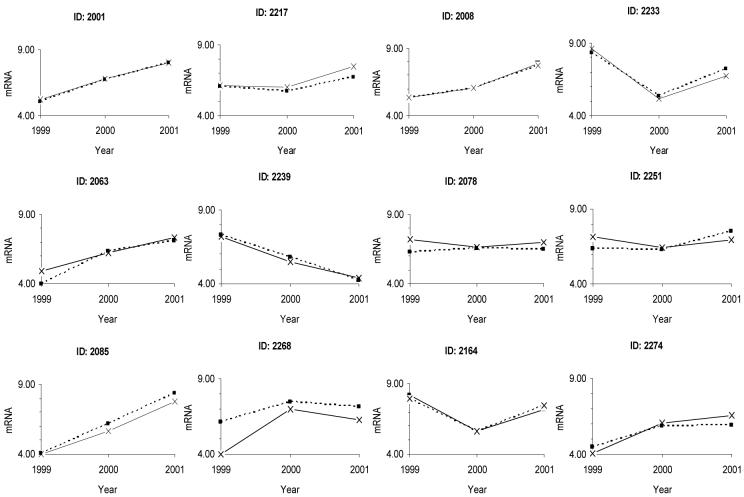
The number of transcripts in initial biopsies from 83 different individuals varied from 4 (i.e. threshold) to 8 log10 transcripts /μg total RNA, and a similar range was seen in every year of the study. This range of variation was not explained by inter-experimental variation, and exceeded the variation seen in triplicate biopsies taken from the same individual14. The correlation between HD5 and HD6 mRNA was strong (ρ=0.88; p<0.001). No overall differences were seen in HD5 and HD6 mRNA between HIV infected and uninfected adults (Table 1). No correlation was observed between mRNA content and CD4 count in HIV-infected participants. There was also substantial longitudinal variation between the values obtained from each individual over the two years of the study (Figure 1). In the group as a whole the longitudinal variation displayed a seasonal effect (Table 2).
Figure 1.
Longitudinal changes in HD5 (solid line) and HD6 (dashed line) mRNA at 3 time points over 2 years. Data from twelve participants from the Lusaka population have been selected to demonstrate the range of variation seen. mRNA is shown as log10 transcripts/μg total RNA.
Table 2.
Seasonal variation in defensin expression
| Months | n | HD5* | HD6* | VH | CD |
|---|---|---|---|---|---|
| Jan, Feb | 7 | 8.1 (8.0-8.2) | 8.4 (7.9-8.4) | 202 (176-215) | 157 (130-191) |
| Mar, Apr | 35 | 6.2 (5.3-6.9) | 6.3 (5.6-6.7) | 258 (234-304) | 164 (140-193) |
| May, Jun | 70 | 5.9 (5.0-7.2) | 6.2 (5.2-7.4) | 254 (231-302) | 156 (132-172) |
| Sep, Oct | 45 | 6.5 (5.6-7.0) | 6.4 (5.6-7.0) | 230 (218-258) | 152 (138-168) |
| Nov, Dec | 12 | 6.2 (4.0-6.9) | 6.2 (5.7-6.7) | 217 (194-262) | 149 (134-173) |
| P | 0.001 | 0.002 | 0.0001 | 0.59 | |
Values given are median (interquartile range) of the mRNA (log10 transcripts/μg total RNA) of all the biopsies taken during those months during the entire 2 year study period. Villous height (VH) and crypt depth (CD) are expressed in μm. P values refer to results of the Kruskal-Wallis test across all groups. No sampling was performed in July or August. Rainfall occurs during November – March with peak rainfall in January when mRNA was at its highest.
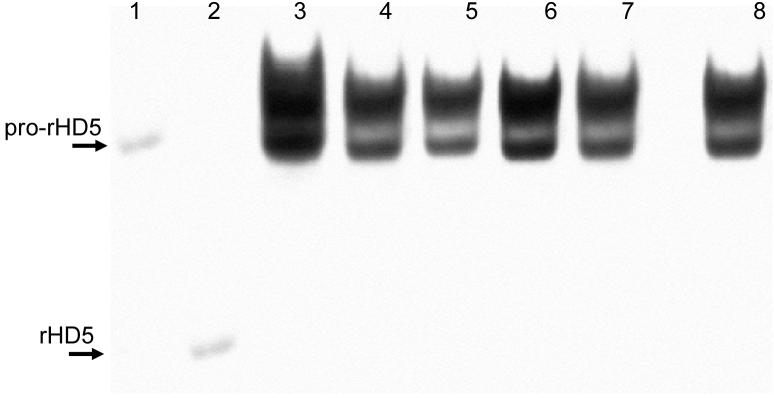
This variation in mRNA levels was not due to changes in the cell types expressing HD5 and HD6 as assessed by in situ hybridization and immunohistochemistry. HD5 and HD6 were almost exclusively expressed by Paneth cells (Figure 2) with only one or two cells higher up the crypt staining by either technique in all the 60 sections examined, and Paneth cell numbers varied little or not at all. As HD5 is stored as propeptide27 we looked for evidence for variation in quantity of stored propeptide or in post-translational processing accompanying the changes in mRNA quantity. Western blots of cationic peptide extracts of these biopsies showed the profile of peptide isoforms previously observed in human intestinal tissue27, including propeptide and O-glycan linked propeptide (Figure 3). Very little variation was observed in either the quantity or the migration pattern of HD5 propeptide isoforms stored in Paneth cell granules (Figure 3). As no antibody for HD6 was available, peptide analyses were only performed on HD5.
Figure 2.
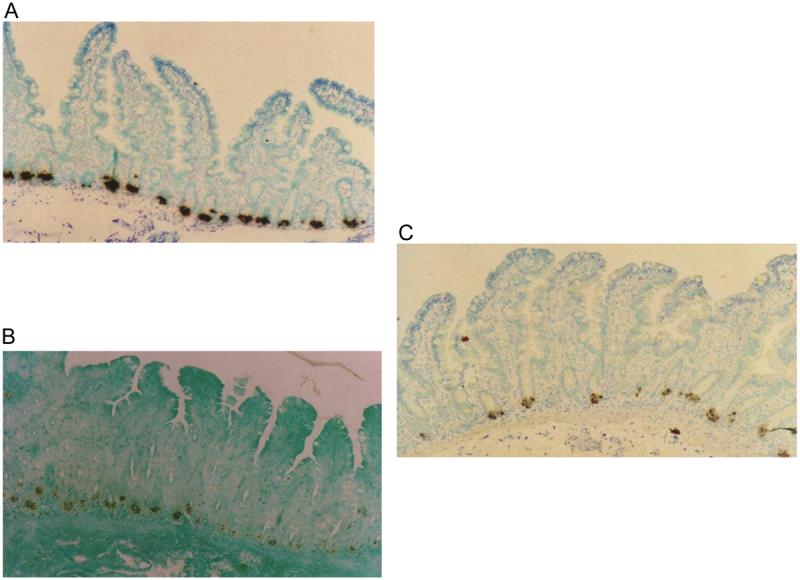
A In situ hybridization using antisense riboprobe to HD5. B Immunostaining using monoclonal anti-HD5 antibody. C In situ hybridization using antisense riboprobe to HD6. Labelling of mRNA and peptide was almost exclusively localized to the Paneth cell compartment.
Figure 3.
Western blot of tissue extracts of jejunal biopsies probed with anti-HD5 antibody. Gel electrophoresis was performed by acid-urea polyacrylamide gel electrophoresis in which peptides are separated by both size and charge. Recombinant peptides are therefore used as markers of migration. Lane 1, recombinant pro-HD5 (20-94); lane 2, recombinant HD5; lanes 3-7, individual biopsies; lane 8, control ileal tissue from healthy patient. The quantity and isoform distribution of propeptide shows little variation. The diffuse, slow migrating band above the pro-HD5 control corresponds to an O-linked glycan modification of the HD5 (20-94) propeptide (C. L. Bevins, unpublished data).
HD5 and HD6 mRNA varied with changes in mucosal architecture
In HIV seronegative adults, HD5 and HD6 mRNA expression at baseline were both inversely correlated with villous height, epithelial surface area and villous compartment volume. No correlation was seen with crypt depth (Table 3). Over 2 years of follow up, the changes in defensin mRNA expression showed strong inverse correlations with the changes in villous height and significant correlations were also seen with the observed changes in crypt depth (Table 3). The seasonal variation in defensin mRNA was inversely correlated with the seasonal variation in villous height (Table 2). In HIV seropositive individuals no correlations between defensin mRNA and determinants of mucosal architecture were observed.
Table 3.
Correlation between mucosal architecture and defensin mRNA in HIV seronegative participants, at baseline and over 2 years of follow up
| Architectural variable | ρ | P | ρ | P |
|---|---|---|---|---|
| Baseline parameters | HD5 | HD6 | ||
| Villous height | −0.39 | 0.01 | −0.36 | 0.01 |
| Crypt depth | −0.21 | ns | −0.15 | ns |
| Epithelial surface area | −0.42 | 0.006 | −0.38 | 0.01 |
| Villous compartment volume | −0.39 | 0.01 | −0.43 | 0.004 |
| Villous width | 0.32 | 0.04 | 0.21 | ns |
| Change over 2 years | ||||
| Villous height | −0.71 | 0.005 | −0.64 | 0.01 |
| Crypt depth | −0.54 | 0.04 | −0.53 | 0.04 |
Coefficients shown are Spearman's rank correlation coefficients (ρ) for biopsies from 42 participants at baseline and for 15 participants followed up for 2 years. The correlations in HIV seropositive participants were weak and statistically insignificant. (ns, not statistically significant).
Inverse correlation between defensin mRNA and incidence of diarrhea
Defensin mRNA was compared in biopsies from participants who went on to experience diarrhea in the two months after the biopsies were taken and those who did not. Median (IQR) HD5 mRNA was 6.0 (5.6-6.7) in 18 participants who experienced diarrhea compared to 6.8 (6.2-7.3) in 94 who did not (P=0.006). Similarly, HD6 mRNA was 6.0 (5.6-7.3) in 20 who experienced diarrhea compared to 6.6 (6.2-7.5) in 99 who did not (P=0.04).
Discussion
Innate immunity in the small intestine is likely to make a major contribution to host defense, being an initial barrier to the colonization or translocation of potential pathogens. Experiments in animals suggest that defensins have a significant role in vivo in intestinal defense, although recent in vitro data suggest that HD6 is not antimicrobial against a limited number of bacterial species of the intestinal microbiota28 and the authors speculate that HD6 may have activity against viral or other pathogens. There are few data from studies in humans, and confirmation of such a role would be of great interest. We found that small intestinal α-defensin expression is constitutive, in as much as it is always detectable. However, mRNA varied substantially between individuals, over time, and in a seasonal pattern. mRNA varied reciprocally over time with both villous height and crypt depth, which were positively correlated with each other18.
We also found that reduced gene expression was associated with an increased risk of diarrhea. There are two possible explanations for this. The first is that defensins make such an important contribution to intestinal defense that a 10-fold reduction in mRNA could significantly increase susceptibility to infection and therefore diarrhea. This is plausible as mRNA predicted peptide turnover in experiments in an ex vivo model (W. Dhaliwal, unpublished observations). The second is that increased exposure to tropical microbiota could simultaneously down-regulate defensin mRNA and increase diarrhea incidence. This hypothesis is supported by experiments which suggest that pathogens or their components can suppress expression of antimicrobial molecules29,30. However, our study design required that participants should have been free of diarrhea for the month prior to the date of biopsy, so we favor the first explanation, although the latter cannot be completely ruled out.
The strong correlations over time between defensin mRNA and mucosal architecture deserve further exploration. The very strong correlation between HD5 and HD6 made it important to establish whether the changes in defensin transcripts might be explained by changes in Paneth cell number or distribution, or changes in the lineage of cells expressing α-defensin genes, but no such changes were found. It is well known that T cell activation induces mucosal remodeling, with reduced villous height and increased crypt depth. This may explain the differences between temperate and tropical populations, in which T cell activation has been suggested to play a part19. However, it cannot explain the mucosal remodeling within this tropical population in which villous height and crypt depth were positively correlated18. We suspect that nutritional factors may play a part as starvation is the only context in which such a positive correlation has so far been observed. Although to our surprise there was no difference in mRNA quantity between HIV-infected and HIV-uninfected people, the correlation between mRNA and mucosal architecture was lost in HIV infected adults. This suggests that in HIV infection there is some dysregulation of α-defensin transcriptional control.
Diarrhea incidence was greater in participants who had reduced defensin mRNA. We can only find significance in this observation if we are reasonably confident that exposure to potential pathogens would be similar from one individual to another. This is highly probable in this cohort study, because all participants were drawn from one small subsection of one ‘compound’ in peri-urban Lusaka. This environment is characterized by overcrowding and poor sanitary facilities. Water and food outlets are few in number and invariably hygiene is poor. A majority of adults living in this area at baseline participated in our study, and we believe that exposure, although not uniform, was likely to be quite consistent. We can be confident that the reduced defensin mRNA in patients who went on to experience diarrhea was not confounded by HIV status, which although predictive of diarrhea incidence (data not shown), was not related to defensin mRNA as noted above. Future studies will be needed in other populations to confirm the association between reduced defensin expression and increased risk of diarrhea. It will also be necessary to establish whether this effect is through increased frequency of infection (by which we mean colonization), increased intensity of infection, or a permissive effect on virulence factor expression by pathogens.
Acknowledgements
We are grateful to Emmanuel Kunda, Rosemary Banda, Vera Yambayamba, Coillard Kaunga, John Samson Mbewe, Stayner Mwanamakondo, Rose Soko and Miriam Banda in the Misisi clinical team and the endoscopy unit of the University Teaching Hospital, and to our study participants. Bo Shen and Dipankar Ghosh of The Cleveland Clinic Foundation contributed to the immunostaining and Western blotting, respectively.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest
Financial support was obtained from The Wellcome Trust
This work was presented at the Gordon conference on antimicrobial peptides, Ventura, CA, March 6-11, 2005 in poster form
Corresponding author: Dr Paul Kelly, Tropical Gastroenterology & Nutrition group, Department of Medicine, University of Zambia School of Medicine, University Teaching Hospital, PO Box 531X, Ridgeway, Lusaka, Zambia, Tel and Fax: 00260 1 252269, email: guts@coppernet.zm
REFERENCES
- 1.World Health Organisation . The World Health Report 2003: Global Health – today's challenges. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Gracey M. Diarrheal disease in perspective. In: Gracey M, Walker-Smith JA, editors. Diarrheal Disease. Vol. 38. Philadelphia: Vevey/Lippincott-Raven; 1997. pp. 1–12. (Nestle Nutrition Workshop Series). [Google Scholar]
- 3.Zulu I, Veitch A, Sianongo S, et al. Albendazole chemotherapy for AIDS-related diarrhea in Zambia - clinical, parasitological and mucosal responses. Aliment Pharmacol Ther. 2002;16:595–601. doi: 10.1046/j.1365-2036.2002.01182.x. [DOI] [PubMed] [Google Scholar]
- 4.Amadi B, Kelly P, Mwiya M, et al. Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J Pediatr Gastroenterol Nutr. 2001;32:550–554. doi: 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 6.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 7.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 8.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 9.Bevins CL, Martin-Porter E, Ganz T. Defensins and innate host defense of the gastrointestinal tract. Gut. 1999;45:911–915. doi: 10.1136/gut.45.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz T. Defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 11.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 13.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal W, Bajaj-Elliott M, Kelly P. Intestinal defensin gene expression in human populations. Mol Immunol. 2003;40:469–475. doi: 10.1016/s0161-5890(03)00156-1. [DOI] [PubMed] [Google Scholar]
- 15.Baker SJ, Mathan VI. Tropical enteropathy and tropical sprue. Am J Clin Nutr. 1972;25:1047–1055. doi: 10.1093/ajcn/25.10.1047. [DOI] [PubMed] [Google Scholar]
- 16.Lindenbaum J, Harmon JW, Gerson CD. Subclinical malabsorption in developing countries. Am J Clin Nutr. 1972;25:1056–1061. doi: 10.1093/ajcn/25.10.1056. [DOI] [PubMed] [Google Scholar]
- 17.Menzies IS, Zuckerman MJ, Nukajam WS, et al. Geography of intestinal permeability and absorption. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–419. [PubMed] [Google Scholar]
- 19.Veitch AM, Kelly P, Zulu IS, Segal I, Farthing MJ. Tropical enteropathy: a T-cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol. 2001;13:1175–1181. doi: 10.1097/00042737-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–311. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 21.Savidge TC, Shmakov AN, Walker-Smith JA, Phillips AD. Epithelial cell proliferation in childhood enteropathies. Gut. 1996;39:185–193. doi: 10.1136/gut.39.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farthing MGJ. Acute diarrhea: pathophysiology. In: Gracey M, Walker-Smith JA, editors. Diarrheal Disease. Vol. 38. Philadelphia: Vevey/Lippincott-Raven; 1997. pp. 55–74. (Nestle Nutrition Workshop Series). [Google Scholar]
- 23.Guiraldes E, Hamilton JR. Effect of chronic malnutrition on intestinal structure, epithelial renewal, and enzymes in suckling rats. Pediatr Res. 1981;15:930–934. doi: 10.1203/00006450-198106000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kelly P, Zulu I, Amadi B, et al. Morbidity and nutritional impairment in relation to CD4 count in a Zambian population with high HIV prevalence. Acta Trop. 2002;83:151–158. doi: 10.1016/s0001-706x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 25.Central Statistical Office and ORC Macro . Zambia Demographic and Health Survey 2001 Final Report. Washington: ORC Macro; 2003. [Google Scholar]
- 26.Kelly P, Feakins R, Domizio P, et al. Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol. 2004;135:303–309. doi: 10.1111/j.1365-2249.2004.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh D, Porter E, Shen B, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 28.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human α-defensins. Antimicrob Ag Chemotherap. 2005;49:269–275. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam D, Bandholtz L, Nilsson J, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 30.Salzman NH, Chou MM, de Jong H, Liu L, Porter EM, Paterson Y. Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun. 2003;71:1109–1115. doi: 10.1128/IAI.71.3.1109-1115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]