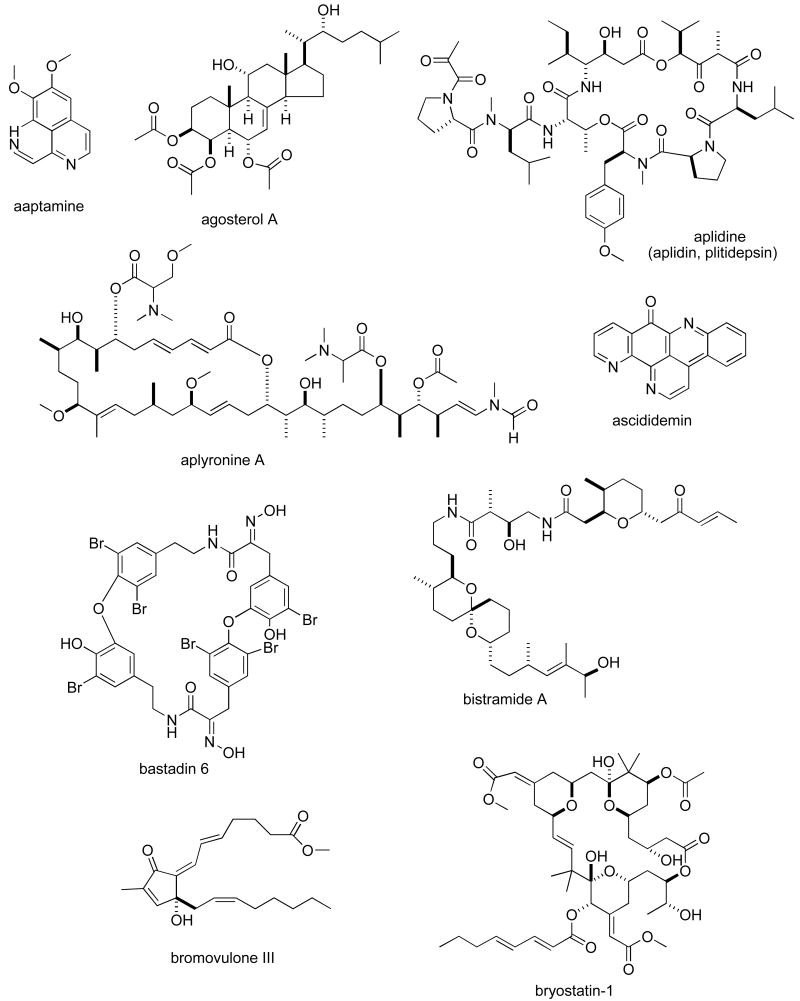
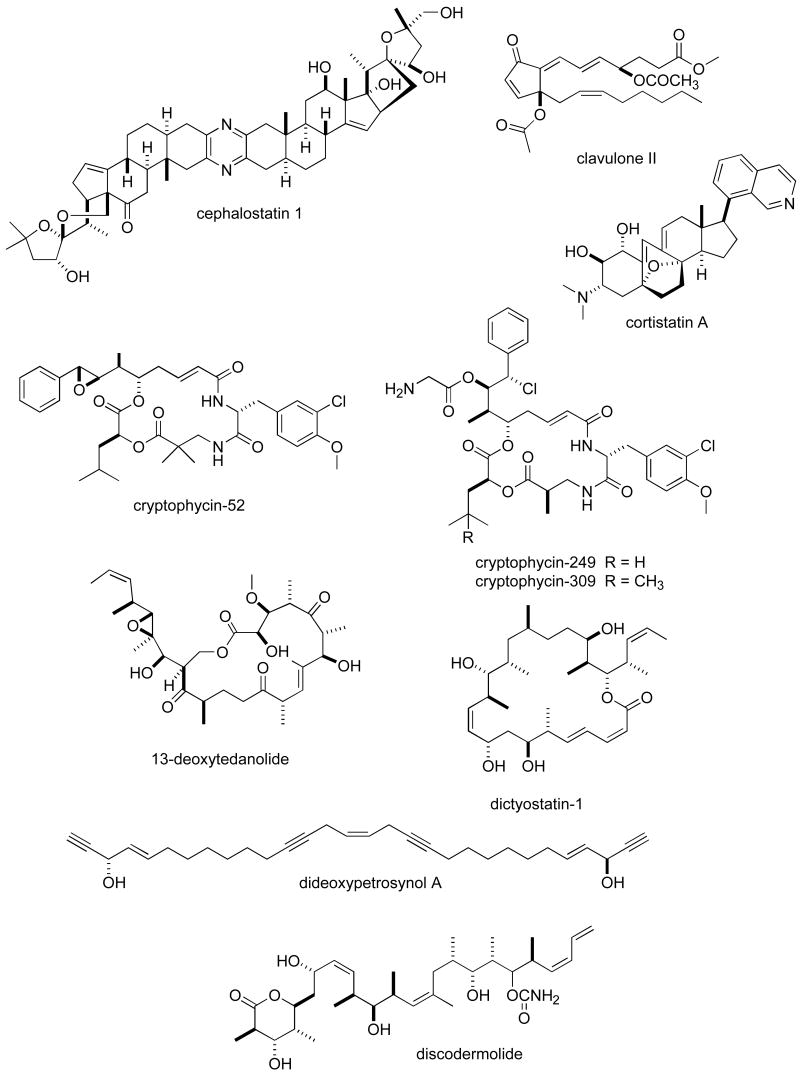
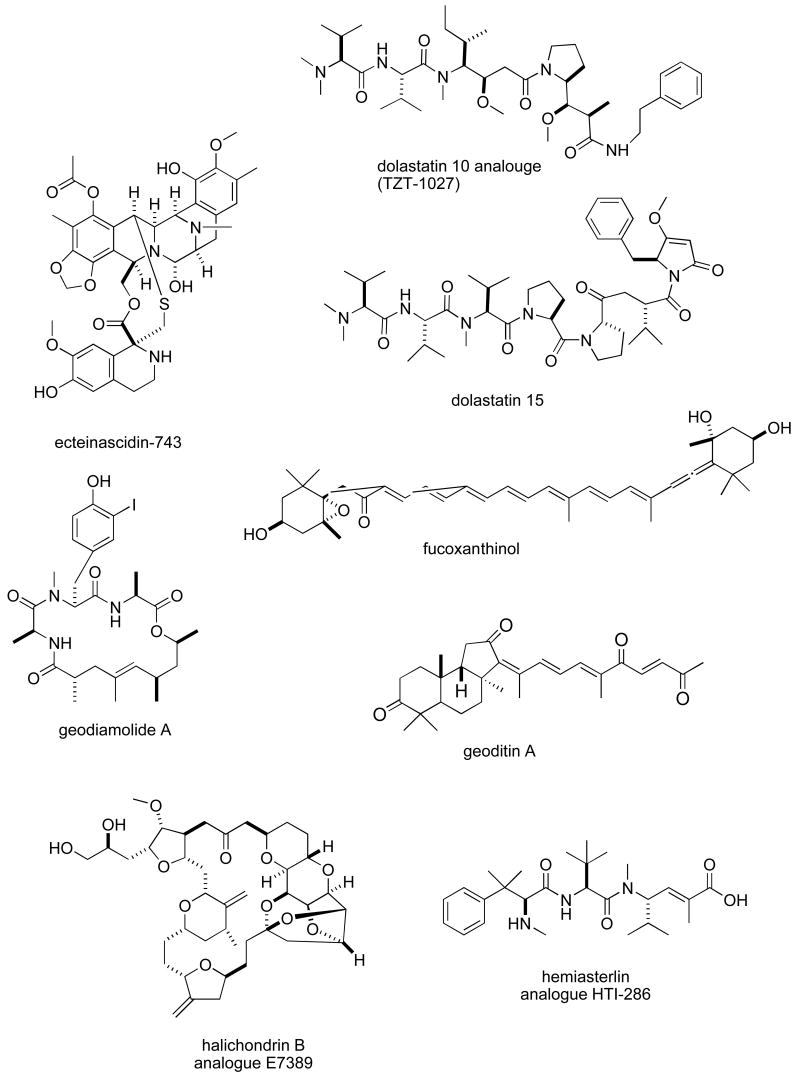
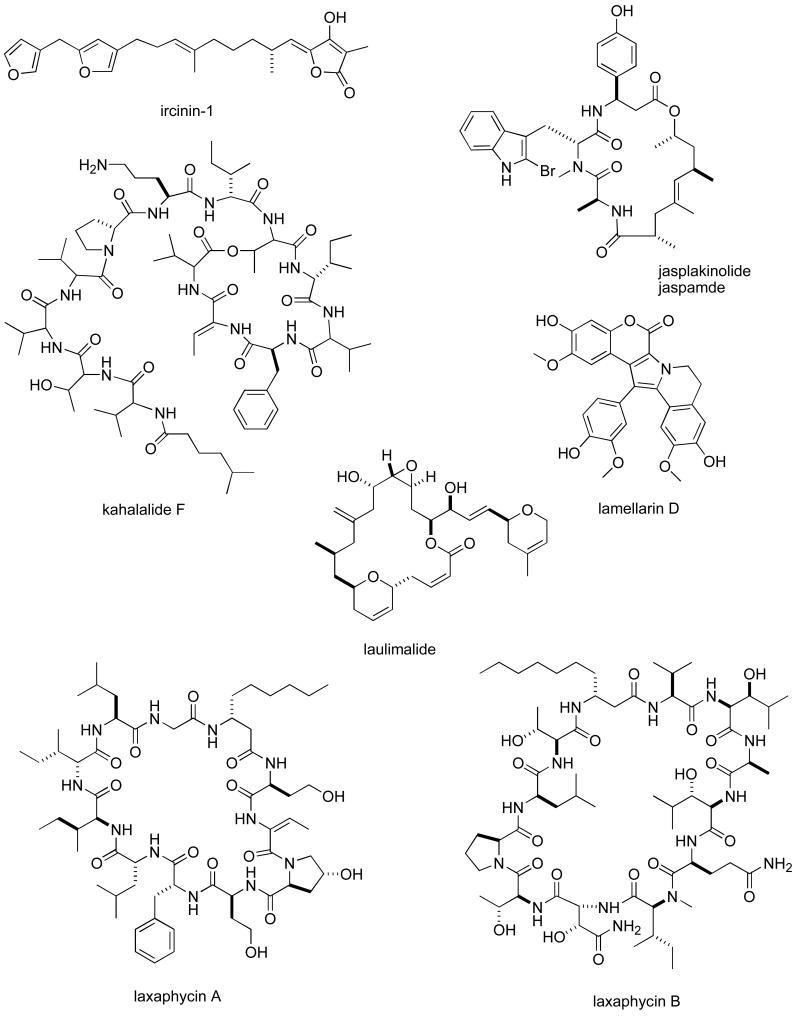
Abstract
During 2005 and 2006, marine pharmacology research directed towards the discovery and development of novel antitumour agents was reported in 171 peer-reviewed articles. The purpose of this article is to present a structured review of the antitumour and cytotoxic properties of 136 marine natural products, many of which are novel compounds that belong to diverse structural classes, including polyketides, terpenes, steroids, and peptides. The organisms yielding these bioactive marine compounds included invertebrate animals, algae, fungi and bacteria. Antitumour pharmacological studies were conducted with 42 structurally defined marine natural products in a number of experimental and clinical models which further defined their mechanisms of action. Particularly potent in vitro cytotoxicity data generated with murine and human tumour cell lines was reported for 94 novel marine chemicals with as yet undetermined mechanisms of action. Noteworthy is the fact that marine anticancer research was sustained by a global collaborative effort, involving researchers from Australia, Belgium, Benin, Brazil, Canada, China, Egypt, France, Germany, India, Indonesia, Italy, Japan, Mexico, the Netherlands, New Zealand, Panama, the Philippines, Slovenia, South Korea, Spain, Sweden, Taiwan, Thailand, United Kingdom, and the United States. Finally, this 2005-6 overview of the marine pharmacology literature highlights the fact that the discovery of novel marine antitumour agents continued at the same active pace as during 1998-2004.
Keywords: Marine, antitumor, drugs, agents, antineoplastic, cancer, chemotherapy, pharmacology, review, screening
1. Introduction
This article reviews the 2005-6 research literature in the field of marine antitumour pharmacology using a format similar to the one used in our previous five reports, which covered 1998-2004 (1-5). The pharmacology of marine compounds with antihelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; those affecting the cardiovascular and nervous systems, and other miscellaneous mechanisms of action have been reviewed elsewhere (6-10).
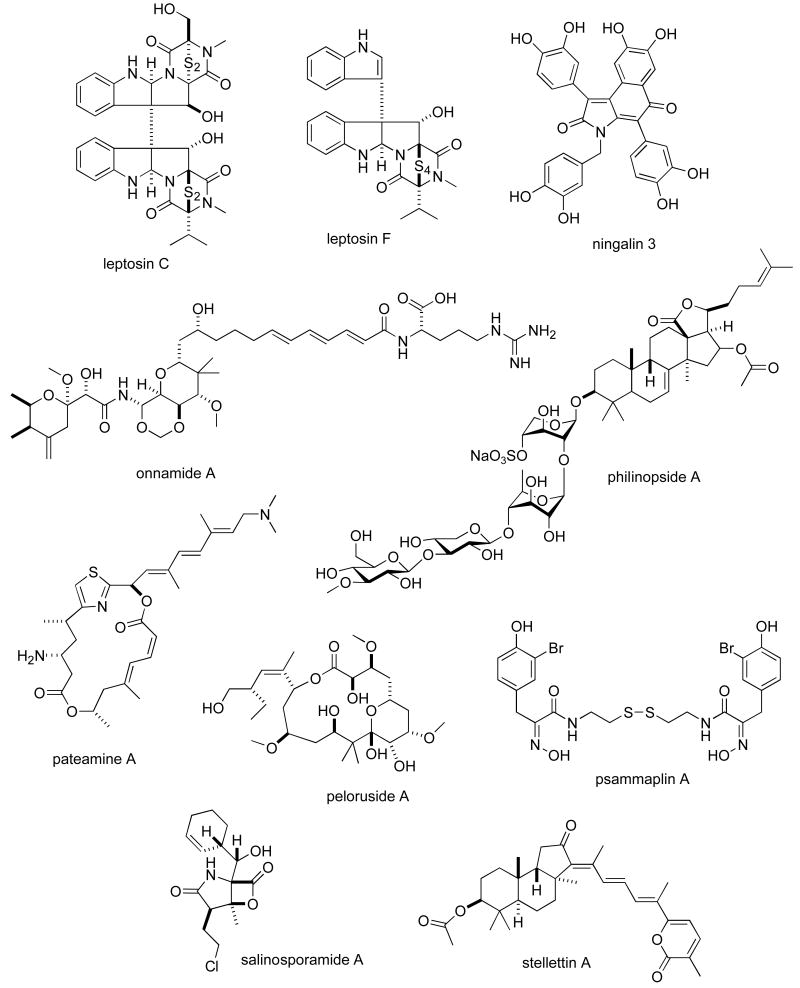
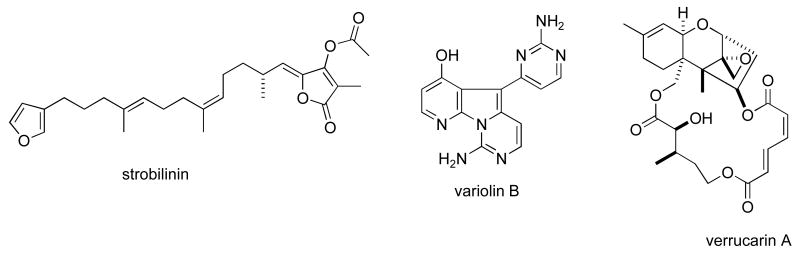
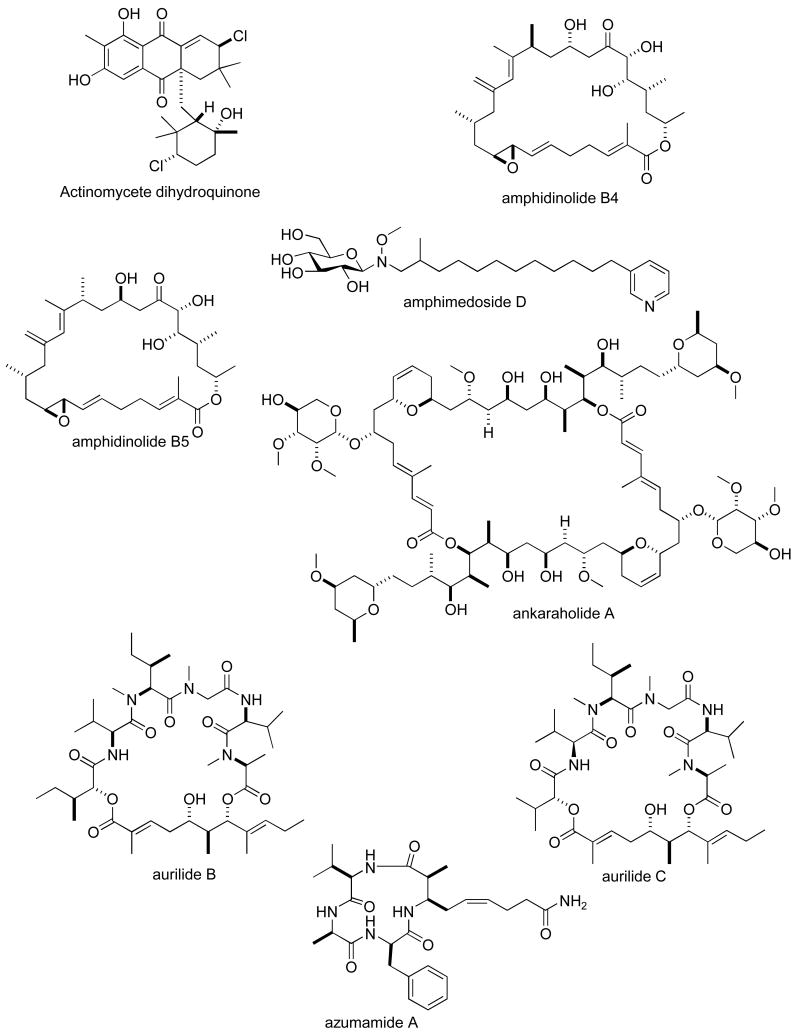
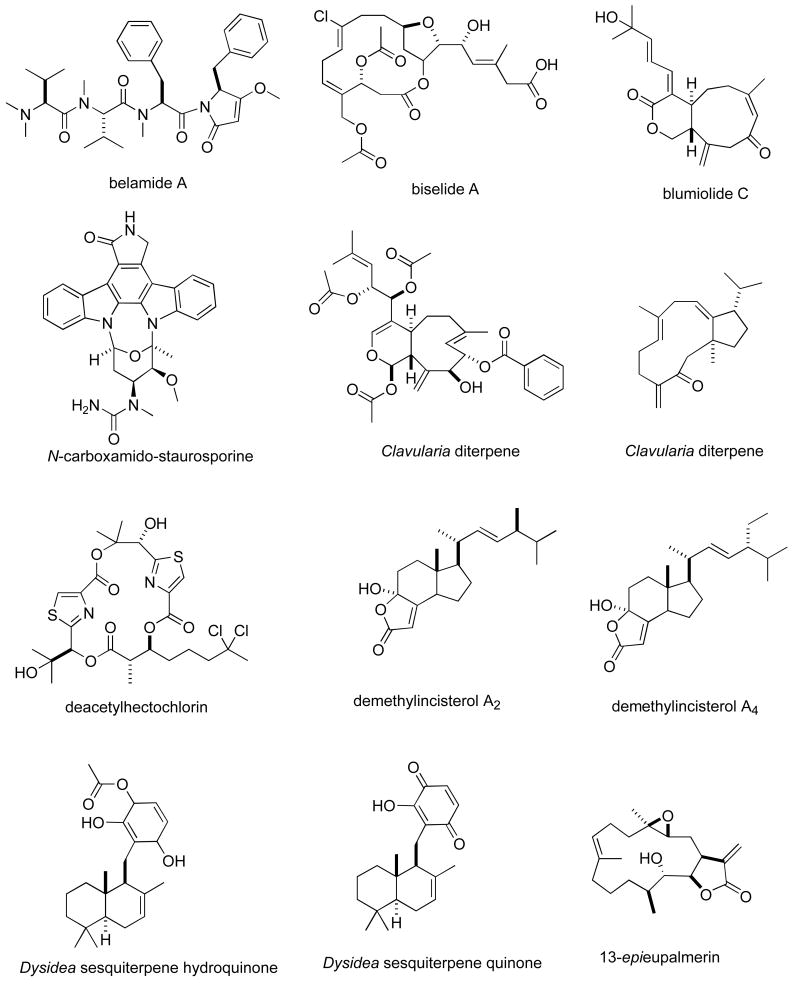
Consistent with our previous five reviews, only those articles reporting on antitumour pharmacology or cytotoxicity of marine compounds with well defined chemical structures (Figures 1 and 2) were included in the present review and are presented in alphabetical order in Table I or Table II. The literature reporting novel information on the preclinical and/or clinical pharmacology of marine chemicals with previously determined mechanisms of action has been summarized in Table I and is further discussed in the text of this review. On the other hand, reports on novel marine chemicals which demonstrated significant cytotoxicity but with as yet undetermined mechanisms of action are shown in Table II. With few exceptions, studies on the preclinical antitumour pharmacology of synthetic analogues of marine metabolites as well as reports on research with marine extracts or as yet structurally uncharacterized marine chemicals are not included in this review.
Figure 1.
Structures of marine natural products reported in 2005 and 2006 with established mechanisms of action
Figure 2.
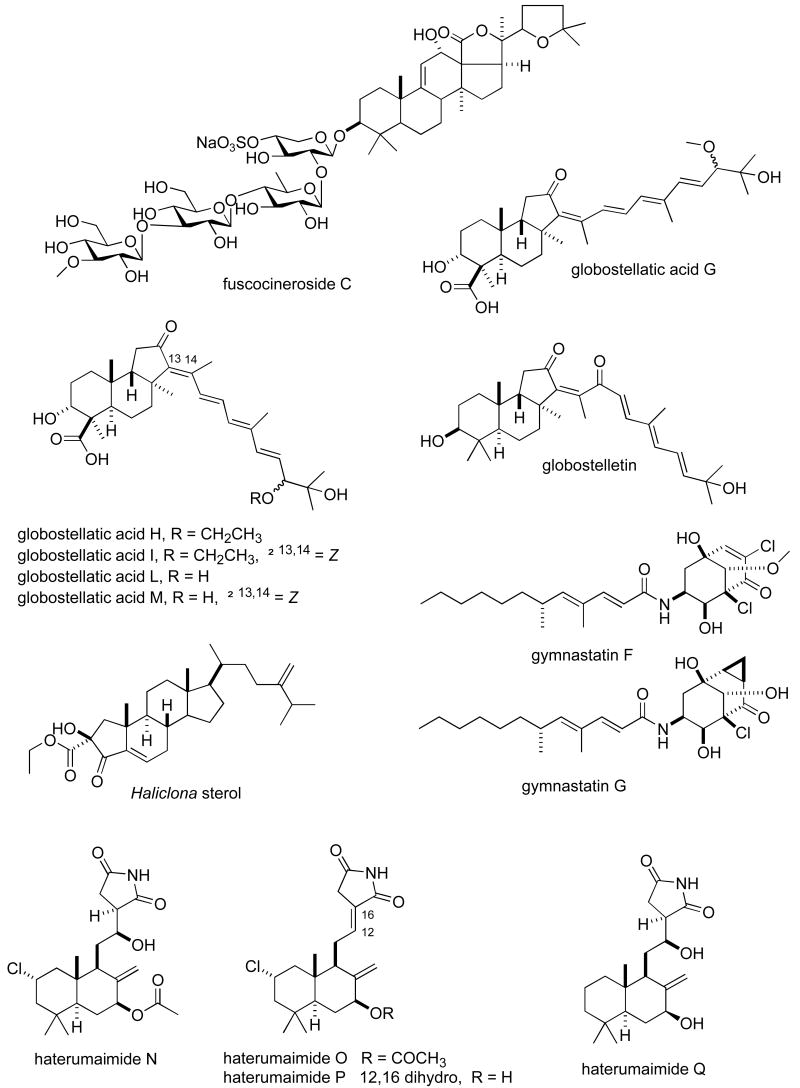
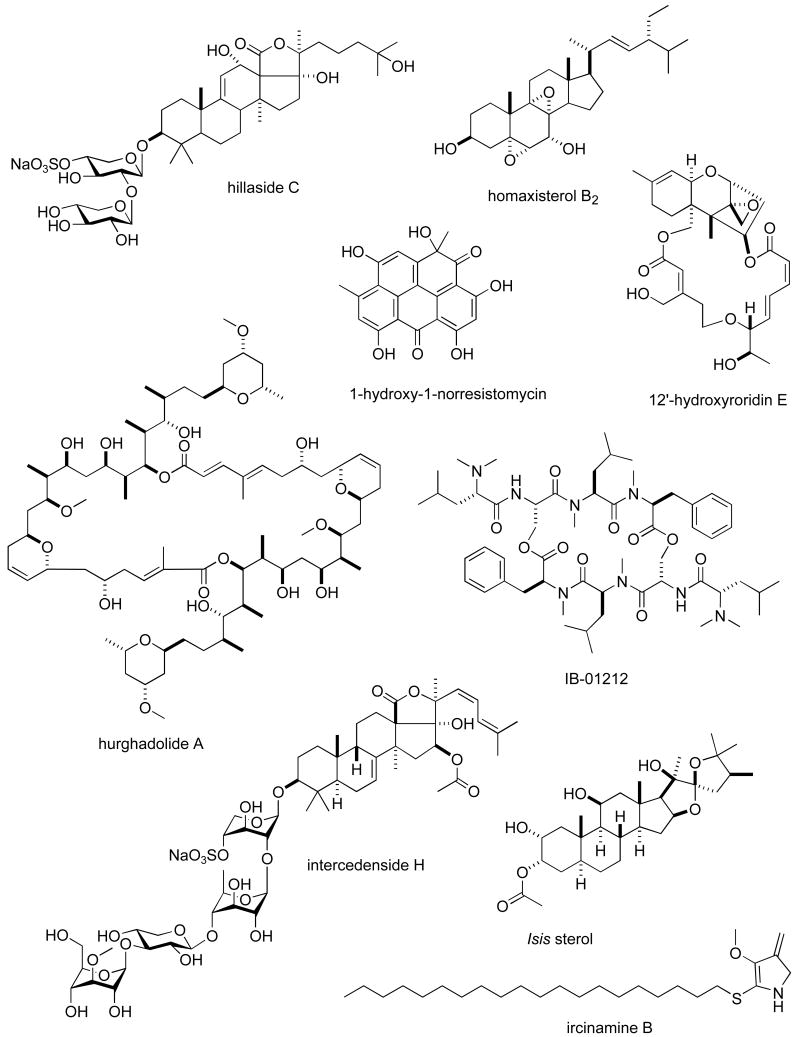
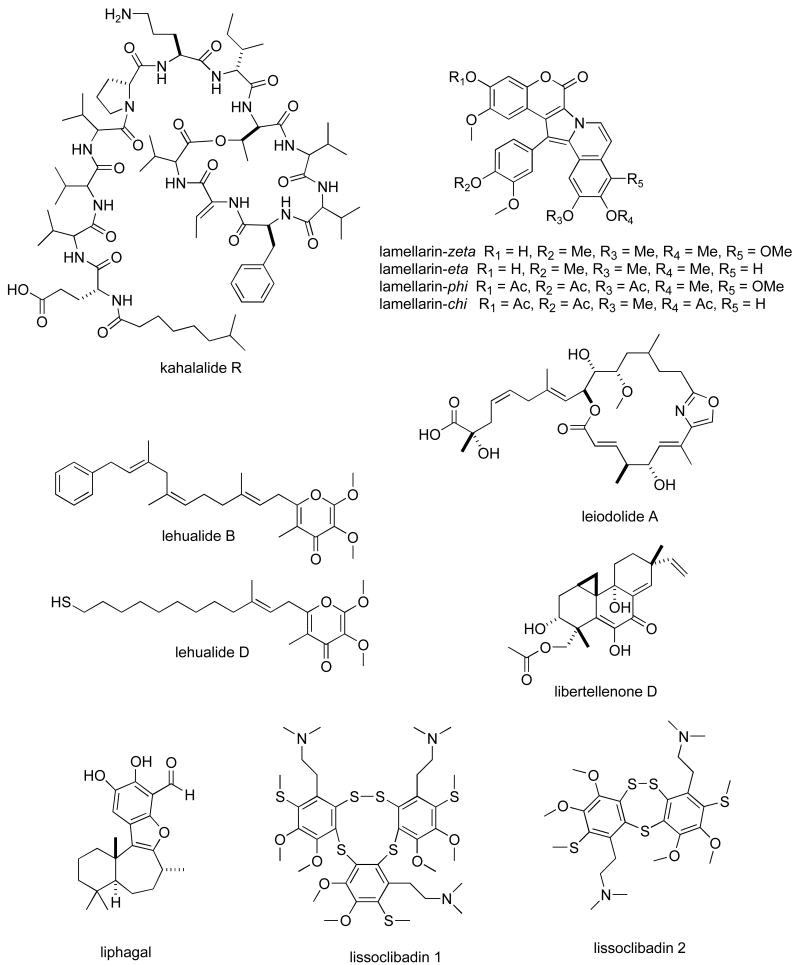
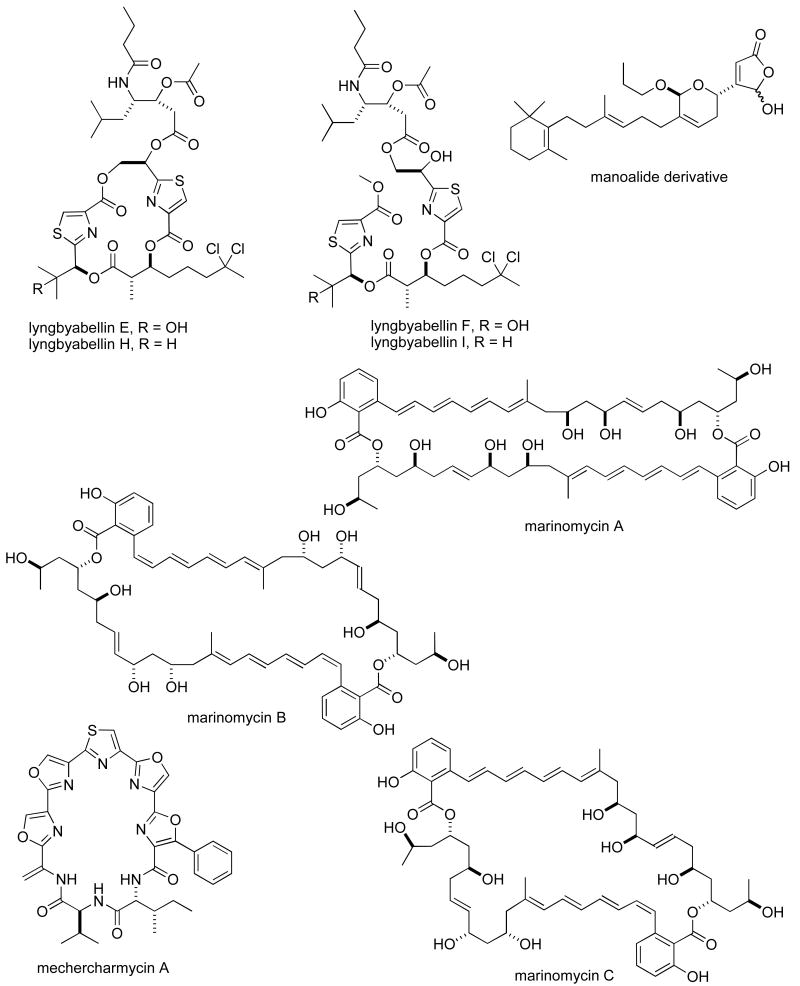
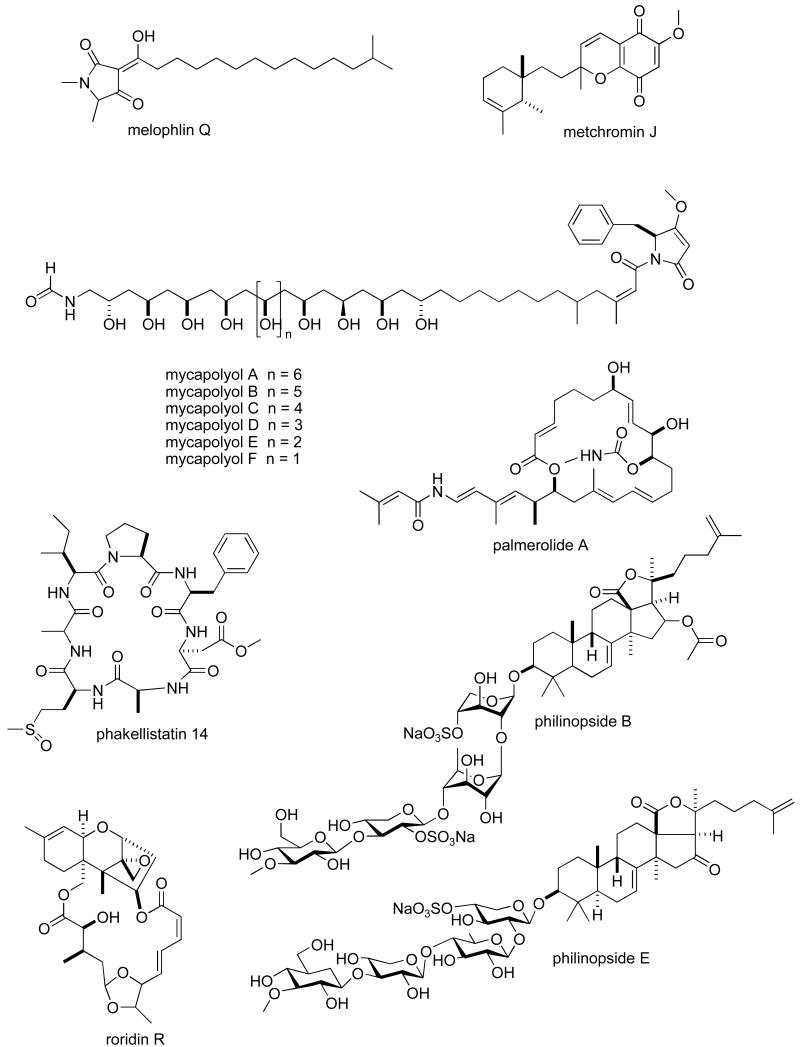
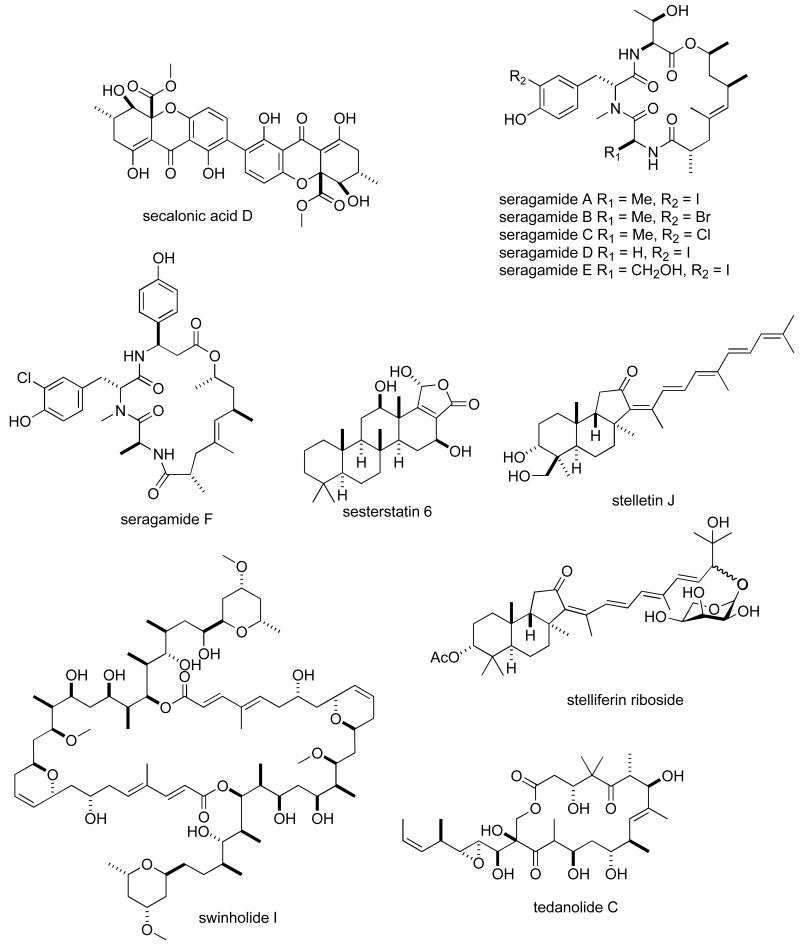
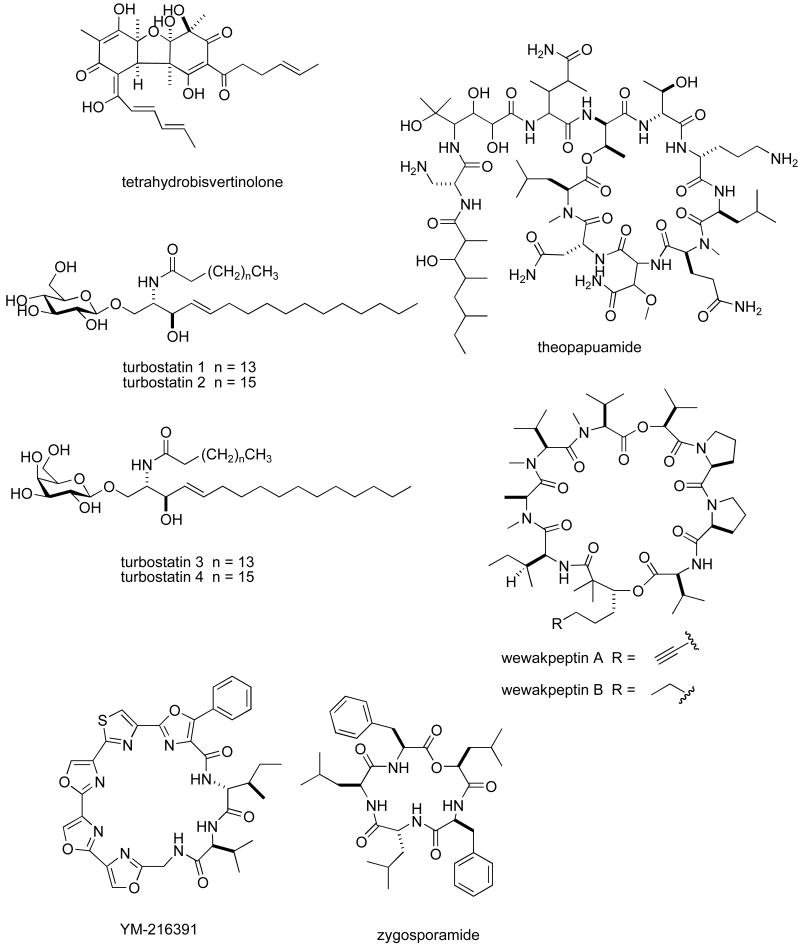
Structures of new marine natural products reported in 2005 and 2006 with undetermined mechanisms of action
Table I. 2005-6 Antitumour pharmacology of marine natural products with established mechanisms of action.
| Compound | Organism | Chemistry | Experimental or clinical model1 | Mechanism of action2 | Country3 | Ref. |
|---|---|---|---|---|---|---|
| aaptamine | Sponge | Alkaloid | HU osteosarcoma cell line | Induction of p21 gene and G2/M cell cycle arrest | INDO, JAPN | (90) |
| agosterol A | Sponge | Steroid | Insect cell expression of MRP1 wild type or mutants | [I125]-azido agosterol A binding to MRP1 abolished by ICL5 & ICL7 domain mutations | JAPN | (11) |
| alkylpyridinium salts | Sponge | Alkaloid | HU adenocarcinoma cell lines | Induction of apoptosis and reduced cell adhesion | ITA, SLO | (91) |
| aplidine | Ascidian | Depsipeptide | MU fibroblast cell line | Oxidation and inactivation of low molecular weight-protein tyrosine phosphatase activity | ITA, SPA | (12) |
| HU thyroid cancer cells | Cytostatic, blocks G1 phase of cell cycle, reduction of cyclin D1, cdk4 and p21 protein levels | SPA | (13) | |||
| Fas-positive and deficient HU leukemia cell line | Apoptosis promoted by concentration of death receptors, adaptors & signaling molecules in lipid rafts | SPA | (15) | |||
| HU-breast cancer cell line | JNK-dependent apoptosis affected by glutathione homeostasis, Rac1 GTPase activation & MKP-1 phosphatase | GER, SPA | (17) | |||
| aplyronine A | Sea hare | Macrolide | Synchrotron radiation method | Binding to hydrophobic cleft in actin molecule involving trimethylserine moiety | JAPN | (92) |
| ascididemin | Ascidian | Alkaloid | Assessment of DNA binding | Enhanced binding to telomeric DNA quadruplex | FRA, BEL | (21) |
| bastadin 6 | Sponge | Alkaloid | HU epidermoid carcinoma & leukemia cell lines | Inhibition of angiogenesis in vitro and in vivo involves apoptosis | JAPN | (93) |
| bistramide A | Ascidian | Polyketide | Depolymerization of F-actin, inhibition of G-actin polymerization, binding to actin subdomains 1 and 3 | USA | (27;28) | |
| bromovulone III | Soft coral | Prostanoid | HU hepatocarcinoma cell line | Apoptosis and endoplasmic reticulum stress, activation of caspase-12 | TAIW | (29) |
| Hormone-resistant prostate cancer cell line | Apoptosis via induction of Fas clustering and caspase-8/Bid/caspase-9 cascade | TAIW | (30) | |||
| bryostatin-1 | Bryozoan | Macrolide | HU prostate cancer cell lines | Apoptosis induction enhanced by PKCε overexpression | USA | (31) |
| HU cervical carcinoma cells | Enhanced cisplatin sensitivity associated to PKCδ regulation | USA | (32) | |||
| HU monocytic cell lines | Transcriptional and posttranscriptional regulation of IFNγ receptor 2 | USA | (35) | |||
| MU keratinocytes | Modulation of RasGRP1 receptor | USA | (34) | |||
| cephalostatin 1 | Tube worm | Steroid | HU Jurkat T cell line | Bcl-2 hyperphosphorylation independent of M-phase arrest and DNA damage | GER | (39) |
| HU Jurkat T cell line | Apoptosis induction via ER stress response signaling & caspase -4 & -9 activations | USA, GER | (40) | |||
| clavulone II | Soft coral | Prostanoid | HU leukemia cell line | G1 cell cycle arrest & apoptosis | TAIW | (94) |
| cortistatin A | Sponge | Alkaloid | HU normal and tumour cell lines | Selective inhibition of angiogenesis | JAPN, INDO | (95) |
| cryptophycins 52, 53, 249, & 309 | Bacterium | Depsipeptide | HU & MU glutathione metabolism | Cytosolic GSTs metabolize C52 & C53 | USA | (41) |
| 13-deoxytedanolide | Sponge | Macrolide | Ribosomal binding and polypeptide synthesis | Binding to 80S ribosome & 60S subunit; inhibition of polypeptide elongation | JAPN | (96) |
| dictyostatin-1 | Sponge | Macrolide | HU ovarian adenocarcinoma cell lines | Tubulin binding, taxoid site binding and antiproliferative effects comparable to discodermolide | USA | (44) |
| dideoxypetrosynol A | Sponge | Polyacetylene fatty acid | HU monocytic leukemia | Induction of Cdk inhibitor p16 expression & down-regulation of pRB phosphorylation | S. KOR | (46) |
| discodermolide | Polyketide | Direct photoaffinity labeling | Binding to amino acid residues 305-359 in the S9-S10 loop in β-tubulin | USA | (47) | |
| HU tumour cell lines | Inhibition of hypoxia-inducible factor 1α | SPA, USA | (48) | |||
| HU lung, colon, breast and cervical carcinoma | Induction of accelerated senescence | USA | (49) | |||
| dolastatin 10 & 15 | Mollusc | Peptide | HU tumour panel and MU cell line | Antitumour action & DNA fragmentation time-dependent & less affected by P-gp | JAPN | (52) |
| ecteinascidin-743 (ET-743) | Ascidian | Isoquinoline alkaloid | NIH 3T3 fibroblasts and HU hepatoma cell lines | Cell-cycle promoters are selectively affected | ITA | (62) |
| Lipo-, leio-, osteo- and chondrosarcoma cell lines | Up-regulation of 86 genes, down-regulation of 244 genes, cDNA microarray analysis with 6,700 cancer genes | SPA | (69) | |||
| S. pombo yeast model system | ET-743 binding to Arg314 in a 46-amino acid region of DNA-binding domain of human nuclease FEN-1 | SPA | (127) | |||
| fucoxanthinol | Ascidian | Carotenoid | HU leukemia, breast and colon tumour cell lines | Induction of apoptosis and decrease in Bcl-2 protein | JAPN | (97) |
| geodiamolides | Sponge | Peptide | HU breast cancer cell lines | Disorganization of actin filaments | BRA, JAPN | (98) |
| geoditin A | Sponge | Triterpene | HU leukemia cell line | Reactive oxygen species generation and caspase-3 mediated apoptosis | CHI | (99) |
| halichondrin B analogue E7389 | Sponge/Synthetic | Macrolide derivative | HU breast tumour cell line | Suppression of microtubule dynamics both in vitro and in vivo | USA | (80) |
| HU tumour cell lines, molecular modeling | Proposed binding site on tubulin results in unstable, aberrant tubulin polymers | USA, NZEL | (81) | |||
| hemiasterlin analogue HTI-286 | Sponge/synthetic | Tripeptide | Molecular docking experiments | Binding to specific residues in the β-tubulin | USA | (82) |
| ircinin-1 | Sponge | Sesterterpene | HU melanoma cell line | G1 phase inhibition and apoptosis induction | S. KOR | (100) |
| jasplakinolide | Sponge | Depsipeptide | HU breast and lung cancer cell lines | Enhanced motility of A549 lung cancer cells but not MCF7 breast tumour cells | BEL | (83) |
| kahalalide F | Mollusk | Depsipeptide | HU vulval, hepatic, colon and breast carcinoma cell lines | ErbB3 protein and PI3K-Akt pathway involved in necrosis induction | SPA, NETH | (84) |
| lamellarin D | Mollusk | Alkaloid | HU and MU tumour cell lines | Apoptosis induced by effect on mitochondria at μM concentrations | FRA, SPA | (86) |
| laxaphycins A & B | Bacterium | Cyclic peptides | HU lymphoblastic cell lines | Increased polyploidy by putative topoisomerase II alterations | FRA, BEN | (101) |
| leptosins C & F | Fungus | Alkaloid | HU lymphoblastoid cell line | DNA topoisomerase I & II inhibition, apoptosis induction & Akt/PKB inactivation | JAPN | (102) |
| ningalins | Ascidian/synthetic | Alkaloid | HU leukemia and breast cancer cell lines | Inhibition of PgP and MDR-reversing activity | USA | (103) |
| onnamide A | Sponge | Polyketide | HU leukemia cell line | Protein synthesis inhibition, activation of stress-activated protein kinases & apoptosis | JAPN | (104) |
| pateamine A | Sponge | Macrolide | HU T cell leukemia | Sequestration of RNA helicase elF4A inhibiting translation initiation | CAN, NZEL, USA | (87) |
| peloruside A | Sponge | Macrolide | HU breast cancer cell line | Synergistic effect with taxoid site drugs but not with laulimalide | NZEL, UK, USA | (88) |
| philinopside A | Sea cucumber | Saponin | HU adenocarcinoma cell lines | Inhibition of angiogenesis and receptor tyrosine kinases | CHI | (105) |
| psammaplin A | Sponge | Alkaloid | HU tumour cell lines | Activation of PPARγ and apoptosis induction | USA | (89) |
| salinosporamide A | Bacterium | Alkaloid | HU tumour cell lines | SAR studies revealed importance of chloroethyl group | USA | (106) |
| stellettin A | Sponge | Triterpene | HU leukemia cell line | Induction of oxidative stress & FasL-caspase 3 apoptotic pathway | CHI | (107) |
| strobilinin-felixinin | Sponge | Sesterterpene | HU tumour cell line | Cell cycle S phase arrest & inhibition of topoisomerase I and pol α-primase | S.KOR | (108) |
| variolin B | Sponge | Alkaloid | HU colon, leukemia & ovarian cell lines | Inhibition of cyclin-dependent kinases and apoptosis induction | FRA, ITA, SPA | (109) |
| verrucarin A | Fungus | Macrolide | HU leukemia cell lines | Inhibition of MAP kinase, enhanced p38 & JNK phosphorylation | JAPN | (110) |
Experimental or clinical model: HU: human; MU; murine;
Mechanism of action: SAR: Structure-activity relationship
Country: BEL: Belgium, BEN: Benin, BRA: Brazil, CAN: Canada, CHI: China, FRA: France, GER: Germany, INDO: Indonesia, ITA: Italy, JAPN: Japan, NETH: The Netherlands, NZEL: New Zealand, S.KOR: South Korea, SLO: Slovenia, SPA: Spain, TAIW: Taiwan, UK: United Kingdom.
Table II. 2005-6 Antitumour pharmacology of marine natural products with undetermined mechanism of action.
| Compound | Organism | Chemistry | Preclinical tumour cell line model1 | 50 % growth inhibition or cytotoxicity | Country2 | Ref. |
|---|---|---|---|---|---|---|
| Actinomycete dihydroquinone | Bacterium | Terpene | HU | 0.97 μg/mL | MEX, USA | (128) |
| amphidinolides B4 & B5 | Alga | Macrolide | HU & MU | 0.1-4 ng/mL | JAPN | (129) |
| amphimedoside D | Sponge | Alkaloid | MU | 0.45 μg/mL | JAPN, NETH | (130) |
| ankaraholides A & B | Bacterium | Macrolide | HU & MU | 8.9-262 nM | USA | (119) |
| aurilides B & C | Bacterium | Depsipeptide | HU & MU | 0.01-0.13 μM | USA | (131) |
| belamide A | Bacterium | Peptide | HU | 0.74-1.6 μM | PAN, USA | (132) |
| biselide A | Ascidian | Polyketide | HU | 0.513-9.35 μg/mL | JPAN | (133) |
| blumiolide C | Soft coral | Diterpene | HU & MU | 0.2-0.5 μg/mL | EGPT, TAIW | (134) |
| N-carboxamido-staurosporine | Bacterium | Alkaloid | 37-cell line panel | 0.016 μg/mL | GER, CHI | (135) |
| Clavularia inflata diterpenes | Soft Coral | Diterpene | MU | 0.5-0.6 μg/mL | TAIW | (136) |
| deacetylhectochlorin | Sea hare | Lipopeptide | HU | 0.31-1.03 μM | THAI, JAPN | (137) |
| Dysidea cf. cristagalli sesquiterpenes | Sponge | Sequiterpene | HU | 0.34-0.37 μM | N. ZEL | (138) |
| 13-epipalmerin | Soft coral | Diterpene | HU | 0.5-5 μg/mL | SPA, USA | (139) |
| fuscocineroside C | Sea cucumber | Triterpene glycoside | HU | 0.58-0.88 μM | CHI | (140) |
| gymnastatins F & G | Fungus | Polyketide | MU | 0.03-0.13 μg/mL | JAPN | (141) |
| globostelletin & globostellastic acid G, H, J, L & M | Sponge | Triterpene | MU | 0.31-0.92 nM | CHI, GER | (142) |
| Haliclona sterol | Sponge | Steroid | HU | 0.33-0.73 μg/mL | CHI, GER | (143) |
| haterumaimides N-Q | Ascidian | Alkaloid | MU | 3.4-50 ng/mL | JAPN | (144) |
| hillaside C | Sea cucumber | Triterpene | HU | 0.15-3.20 μg/mL | CHI | (145) |
| homaxisterol B2 | Sponge | Steroid | HU | 0.55-1.51 μg/mL | S. KOR | (146;147) |
| hurghadolide A | Sponge | Macrolide | HU | 0.36 μM | EGPT, USA | (111) |
| hydroxy-norresistomycin | Bacterium | Polyketide | HU | 9-14 ng/mL | IND | (148) |
| IB-01212 | Fungus | Depsipeptide | HU | 10 nM | SPA | (149) |
| intercedenside D-I | Sea cucumber | Triterpene glycoside | HU | 0.96-5 μg/mL | CHI, USA | (150) |
| ircininamine B | Sponge | Fatty acid | MU | 0.28 μg/mL | JAPN | (151) |
| Isis sterol | Soft coral | Steroid | HU | 0.21-1.07 μg/mL | TAIW | (152) |
| kahalalide R | Mollusk | Depsipeptide | HU & MU | 0.14-4.3 μM | CHI, GER | (153) |
| lamellarins | Ascidian | Alkaloid | HU | 0.2-178 nM | IND | (154) |
| lehualides B & D | Sponge | Polyketide | HU | 0.23-0.83 μM | NZEL, USA | (155) |
| leiodolides A & B | Sponge | Macrolide | HU 60-cell line panel | 0.25-0.26 μM | NZEL, USA | (156) |
| libertellone D | Fungus | Diterpene | HU | 0.76 μM | USA | (157) |
| liphagal | Sponge | Sesquiterpene | HU | 0.58-1.58 μM | CAN, NETH, USA | (116) |
| lissoclibadins 1 & 2 | Ascidian | Alkaloid | HU | 0.21-5.5 μM | JAPN | (158) |
| lyngbyabellins E-I | Bacterium | Lipopeptide | HU & MU | 0-2-4.8 μM | USA | (118) |
| manoalide derivative | Sponge | Sesterterpene | HU | 0.32 μg/mL | CHI, USA | (159) |
| marinomycins A-C | Bacterium | Macrolide | HU 60-cell line panel | 0.005-2.7 μM | USA | (160) |
| mechercharmycin A | Bacterium | Cyclic peptide | HU | 0.04 μM | JAPN | (161) |
| melophlin Q | Sponge | Fatty acid | MU | 0.85 μM | JAPN | (162) |
| metachromins J | Sponge | Sesquiterpene | HU & MU | 1-9.9 μg/mL | JAPN, AUS | (163) |
| mycapolyols A-F | Sponge | Polyketide | HU | 0.06-0.90 μg/mL | THAIL, JAPN | (164) |
| palmerolide A | Tunicate | Macrolide | HU | 18 nM | USA | (114) |
| phakellistatin 14 | Sponge | Peptide | HU & MU | 0. 75-5 μg/mL | USA | (165) |
| philinopsides A & B | Sea cucumber | Triterpene | HU | 0.75-3.5 μg/mL | CHI, USA | (112) |
| philinopside E | Sea cucumber | Triterpene | HU & MU | 0.62-5.53 μg/mL | CHI | (166) |
| roridin R & 12′-hydroxyroridin E | Fungus | Macrolide | MU | 0.19-0.45 μM | JAPN, INDO | (167) |
| roseotoxin B | Fungus | Depsipeptide | HU | 0.14-1.30 μg/mL | BRA, USA | (168) |
| secalonic acid D | Fungus | Polyketide | HU & MU | 0.03-5.76 μM | CHI | (120) |
| seragamides A-E | Sponge | Depsipeptide | MU | 0.064-0.58 μM | JAPN, USA | (117) |
| sesterstantin 6 | Sponge | Sesterterpene | HU & MU | 0.17-4.9 μg/mL | USA | (169) |
| stellettin J | Sponge | Triterpene | HU | 0.28-1.2 μM | USA | (115) |
| stelliferin riboside | Sponge | Triterpene | MU | 0.22 nM | CHI, GER | (142) |
| Streptomyces nobilis peptide | Bacterium | Cyclic peptide | HU | 14 nM | JAPN | (170) |
| swinholide I | Sponge | Macrolide | HU | 5.6 nM | EGPT, USA | (111) |
| tedanolide C | Sponge | Macrolide | HU | 0.057 μM | USA | (113) |
| tetrahydrobisvertinolone | Fungus | Polyketide | HU & MU | 0.52-1.7 μM | CHI | (171) |
| theopapuamide | Sponge | Depsipeptide | HU | 0.5-0.9 μM | USA | (172) |
| turbostatins | Mollusk | Fatty acid | HU & MU | 0.15-2.6 μg/mL | USA | (173) |
| wewakpeptins A & B | Bacterium | Depsipeptide | HU & MU | 0.2-0.65 μM | USA | (174) |
| zygosporamide | Fungus | Depsipeptide | NCI 60-cell line panel | 5.0-6.5 nM | USA | (175) |
HU: human, MU: murine;
Country: AUS: Australia, BRA: Brazil, CAN: Canada, CHI: China, EGPT: Egypt, FRA: France, GER: Germany, IND: India, INDO: Indonesia, JAPN: Japan, MEX: Mexico, NETH: Netherlands, NZEL: New Zealand, PAN: Panama, S. KOR: South Korea, SPA: Spain, SWE: Sweden, THAI: Thailand, TAIW: Taiwan.
2005-6 Antitumour pharmacology of marine natural products with established mechanisms of action
Table I summarizes novel mechanism of action research from preclinical studies of 42 marine compounds (selected structures are shown in Figure 1). Reports on clinical trials with some of these marine compounds are excluded from Table I, but discussed in this section of the article.
New information was published during 2005-6 on the preclinical and clinical pharmacology of 24 marine compounds which we have previously reviewed (1-5): agosterol A, aplidine, ascididemin, auristatin, bistramide A, bromovulone III, bryostatin-1, cephalostatin-1, cryptophycins, dictyostatin-1, didemnin B, dideoxypetrosynol A, discodermolide, dolastatins, ecteinascidin-743, fascaplysin, halichondrin B, hemiasterlin, jasplakinolide, kahalalide F, lamellarin D, pateamine A, peloruside A and psammaplin A.
One study was published on the preclinical pharmacology of agosterol A, a polyhydroxylated sterol acetate isolated from the marine sponge Spongia sp. Ren and colleagues (11) determined the functional role of intracellular loops (ICL) on the 190 kDa human membrane multidrug resistance protein 1 (MRP1), a transporter in non-P-glycoprotein-mediated multidrug resistance in tumour cells. Interestingly, mutations of the ICL5 or ICL7 domains directly affected ATP and azido agosterol A binding to MRP1, demonstrating the role of both ICL domains on the drug- binding properties of MRP1, and its concomitant drug transporter function.
Research on the cyclic depsipeptide aplidine, a second-generation didemnin analogue also known as aplidin or dehydrodidemnin B, and isolated from the Mediterranean marine tunicate Aplidium albicans continued at an active pace. Seven preclinical studies, which characterized the cellular and molecular pharmacology of aplidine, and two clinical articles were published during 2005-2006. Taddei and colleagues (12) demonstrated that aplidine's cytotoxic activity in NIH3T3 cells involved the production of mitochondrial reactive oxygen species that induced oxidation and inactivation of low molecular weight protein-tyrosine phosphatase activity, an enzyme that appears to play a role in both tumour onset and development. Bravo and colleagues (13) investigated the actions of aplidine in human thyroid cancer cells. Aplidine blocked in vitro cell progression into the G1 phase of the cell cycle, with markedly reduced levels of cyclin D1, cdk4 and p21 protein levels, at plasma concentrations similar to those observed in vivo in phase I/II clinical studies. Biscardi and colleagues (14) confirmed aplidine's cytotoxic and apoptotic activity in three human myeloid leukemia cell lines and in cells derived from patients with acute myeloid leukemia. At in vitro concentrations achievable in patients (100 nM) aplidine induced G1 cell cycle arrest and vascular endothelial growth factor inhibition. Gajate & Mollinedo (15) discovered that the mechanism of aplidine-induced apoptosis involved a novel and potent cell-killing mechanism that required Fas activation and clustering of additional death receptors, membrane-bound FasL and downstream signaling molecules into “aggregated lipid rafts” through a cytoskeleton-mediated process. The observation that aplidine was rapidly incorporated into lipid rafts highlighted the significance of these lipid aggregates in the regulation of apoptosis and cancer chemotherapy. Tognon and colleagues (16) found that aplidine-induced resistance in a human ovarian cancer cell line over a period of several months, was related to the expression of the multidrug transporter Pgp, “potentially useful pharmacological information” that may have clinical relevance. Gonzalez-Santiago and colleagues (17) examined the molecular mechanism of apoptosis induction by aplidine in human breast cancer cells. They demonstrated that aplidine disrupted glutathione homeostasis by increasing the ratio of oxidized to reduced forms, thus leading to increases in reactive oxygen species and oxidative stress. Furthermore, aplidine caused rapid activation of Rac1 small GTPase resulting in Jun N-terminal kinase (JNK) phosphorylation, which was considered critical for aplidine-induced apoptosis. There was a concomitant decrease of MKP-1 phosphatase, an enzyme overexpressed in human breast cancer and considered a “viable target for therapeutic intervention” by enabling expression of pro-apoptotic activity of JNK. Straight and colleagues (18) tested the hypothesis that aplidine would reduce the growth of anaplastic thyroid xenografts in mice. Interestingly, aplidine reduced tumour growth as well as the expression of 16 out of 20 angiogenic genes investigated, suggesting that aplidine might be an effective adjunctive therapy for anaplastic thyroid cancer, an aggressive and highly lethal cancer.
Two Phase I trials evaluated the clinical pharmacology of aplidine during 2005-6. Faivre and colleagues (19) completed a Phase I and pharmacokinetic study on sixty-seven patients who received aplidine as a 24-hour intravenous infusion for advanced malignancies. Although muscle toxicity was noted as dose limiting at doses ≥ 5 mg/m2, aplidine induced minor responses and tumour stabilizations in eight patients, and clinical benefit in six patients with endocrine tumours. Maroun and colleagues (20) conducted a Phase I study in thirty-seven patients with refractory solid tumours in which a 1-hour infusion of aplidine was given for 5 days every 3 weeks. Even though the regimen was well tolerated, only nine patients with progressive disease at study entry had stable disease, while two patients with non-small cell lung cancer and one with colorectal cancer evidenced minor responses.
A preclinical study by Guittat and colleagues (21) reported that ascididemin, a pyridoacridine alkaloid isolated from the marine sponge Amphimedon sp., inhibited telomerase function (IC50 = 87μM) by preferentially binding to G-quadruplex DNA structures, thus potentially affecting telomere structure and cancer cell replication.
Five studies in 2005-6 described the preclinical pharmacology of auristatin, a synthetic antitubulin agent related to the marine natural product dolastatin 10 (see below). Sutherland and colleagues (22) demonstrated that lysosomal trafficking and cysteine protease metabolism enabled the target-specific cellular cytotoxicity by valine-citrulline linked anti-CD 30-auristatin conjugates, which were previously shown by Sanderson and colleagues (23) to have the “longest reported drug-linker half-life to date” in plasma and cell culture. Taken together these findings provide the basis for pronounced specificity and antitumour activity of anti-CD30 monoclonal antibody-monomethylauristatin E conjugates towards CD30+ tumour cells. Using a similar antibody-drug conjugate approach, Ma and colleagues (24) evaluated the antitumour activity of auristatin-conjugated to a human monoclonal antibody to prostate-specific membrane antigen (PSMA), a membrane glycoprotein which is highly upregulated in prostate cancer. The novel conjugate eliminated PSMA-expressing cells with picomolar efficiency in vitro as well as showed therapeutic efficacy in a mouse xenograft model of androgen-independent human prostate cancer. These findings suggested this approach merits further development as a molecularly targeted therapy of hormone-refractory prostate cancer, the second leading cause of death in men in the United States. Smith and colleagues (25) as well as Tse and colleagues (26), reported encouraging in vitro and in vivo results with auristatin-containing antibody-drug conjugates to target melanoma cells expressing either melanotransferrin/p97 or glycoprotein NMB, respectively. Their studies indicated these agents may provide a new type of selective and efficient treatment for late stage malignant melanoma, for which current therapeutic options are limited.
Two studies extended the preclinical pharmacology of bistramide A, a polyketide derivative isolated from the marine ascidian Lissoclinum bistratum. In a detailed mechanistic study Statsuk and colleagues (27) identified actin as the cellular receptor for bistramide A and reported that this marine compound disrupted the actin cytoskeleton, depolymerized F-actin in vitro and bound directly to monomeric G-actin in a 1:1 ratio with a Kd = 7 nM. Furthermore, the discovery by Rizvi and colleagues (28) that bistramide A spans the entire deep binding cleft between actin's subdomains 1 and 3, while forming a network of extensive hydrogen-bonding contacts, has provided the required structural information for the rational development of novel bistramide analogues for studying the actin cytoskeleton and as potential therapeutic leads.
Two preclinical studies were described for the cyclopentenone prostanoid bromovulone III, which was isolated from the soft coral Clavularia viridis. In 2005 Chiang and colleagues (29) reported that bromovulone III induced apoptosis in hepatocellular carcinoma cells through a mechanism that involved endoplasmic reticulum stress as well as activation of the transcription factor CHOP/GADD153 and caspase-12. Then in 2006, this same research group (30) reported that bromovulone III induced apoptosis in human hormone-resistant prostate cancer cells by a mechanism that required the rapid redistribution and clustering of Fas, as well as activation of the caspase-9/Bid/caspase-9 signaling cascades.
Several preclinical and clinical studies published during 2005-6 extended the pharmacology of bryostatin-1, a macrocyclic lactone derived from the marine bryozoan Bugula neritina that continued to receive considerable attention in view of its demonstrated antineoplastic activity in vitro and in vivo. Five studies contributed new information on the molecular pharmacology of bryostatin-1 at both the cellular and molecular level. Powell & Yin (31) discovered that overexpression of PKCε sensitized human prostate cancer cells to the induction of apoptosis by bryostatin-1, an observation that may have implications for therapy because overexpression of PKCε has been reported in human prostate cancer. Mohanty and colleagues (32) investigated bryostatin-1's influence on protein kinase Cδ in human cervical carcinoma HeLa cells and its cisplatin-resistant variants. The observation that modulation of PKCδ by bryostatin-1 enhanced sensitivity to cisplatin in both HeLa cells and cisplatin-resistant HeLa cells “could be used to enhance cellular sensitivity to cisplatin” as well as increase the molecular understanding of bryostatin-1's effect on PKCδ regulation. Interestingly, Choi and colleagues (33) also noted that bryostatin-1 down-regulation of PKCδ was associated with enhanced proliferation of a non-small cell lung cancer cell line. Taken together these observations may help identify systems in which bryostatin-1 has a rational therapeutic application. Tuthill and colleagues (34) examined the effect of bryostatin-1 on the regulation and activation of mouse epidermal keratinocyte RasGRP1, an exchange factor for the Ras small GTPases which also activates three classic Ras proteins both in vitro and in vivo. These results support the hypothesis that non-PKC receptors are also involved in the molecular mechanism of action of bryostatin-1, and further the molecular understanding of the antitumour effects in the skin. Garcia and colleagues (35) demonstrated for the first time that bryostatin-1 enhanced expression of the IFN-γ receptor 2 in human monocytic cells and primary human monocytes, by a dual mechanism involving transcriptional and post-transcriptional events that did not require protein synthesis. The authors speculated that their observations might help “overcome some of the immune defects observed in cancer patients”, and thus proposed in vivo preclinical research of the combination of bryostatin-1 and IFN-γ in murine tumour models.
Two clinical trials with bryostatin-1 were reported during 2005-6. El-Rayes and colleagues (36) completed a Phase I study with bryostatin-1 and gemcitabine with 36 patients who had nonhematologic cancer that was refractory to conventional treatment. Based on the preclinical data and the tolerability of this combination, the authors suggested that a Phase II trial in breast and pancreatic cancer represented a rational approach for the development of this regimen. Ajani and colleagues (37) reported a multi-center Phase II study of sequential paclitaxel and bryostatin-1 in 35 patients with untreated, advanced gastric and gastroesophageal adenocarcinoma. Although the sequential use of paclitaxel plus bryostatin-1 resulted in a 29% response as compared to paclitaxel alone (17% response), further development of this synergic combination will require the amelioration or prevention of myalgia. Peterson and colleagues (38) reported a Phase II trial of interleukin-2 in combination with four different doses of bryostatin-1 in 33 patients with renal cell carcinoma, a type of cancer that in the US has an estimated incidence of 319,000 new cases per year. Although the addition of bryostatin-1 to IL-2 was well tolerated, and four patients demonstrated evidence of tumour shrinkage, the overall rate of response was low (3.2%), leading this group of researchers to state that they did not “recommend further studies investigating this combination”.
Two studies extended the preclinical pharmacology of cephalostatin 1, a bis-steroidal marine natural product isolated from the Indian Ocean hemichordate Cephalodiscus gilchristi. Müller and colleagues (39) discovered that cephalostatin 1 inactivated the antiapoptotic mitochondrial protein Bcl-2 by hyperphosphorylation which was independent of M-phase arrest and DNA damage, a finding that could potentially help treatment of drug-resistant cancers. Lopez-Antón and colleagues (40) reported that cephalostatin 1 activated an endoplasmic reticulum (ER) stress response which was accompanied by caspase-4 activation and apoptosis induction without requirement of the classical mitochondrial pathway. The fact that cephalostatin 1 appears to enable the ER stress signaling pathway may be advantageous for the treatment of chemoresistant tumours with potential defects in the mitochondrial pathway.
Two preclinical and one clinical study were reported during 2005-6 with the cryptophycins, macrocyclic depsipeptides isolated from the marine cyanobacterium Nostoc sp. Cannady and colleagues (41) examined the enzyme kinetics of glutathione conjugation of cryptophycin 52 and 53 by cytosolic glutathione S-transferases (GST) and epoxide hydrolases, and discovered that human, rat, and mouse cytosolic GSTs are responsible for metabolism of cryptophycin 52 to the GSH conjugate. Their results provide firm support for ongoing Phase II metabolism studies. Liang and colleagues (42) reported extensive preclinical evaluation of the synthetic and formulation-stable glycinate esters of cryptophycins-309, 249, as well as other analogues in mouse and human tumours. Based on the expectation that these second generation analogues will produce 100 to 1000 fold greater activity than the first clinical candidates (e.g. cryptophycin 52), and their formulation stability advantage compared to cryptophycin C-52, the authors noted both “C-309 and C-249 are being considered as second-generation clinical candidates”. D'Agostino and colleagues (43) described a multicenter Phase II study of a synthetic cryptophycin analogue LY355703 in 26 patients with platinum-resistant ovarian cancer, a leading cause of death from gynecological tumours in Western countries. Although only a modest level of activity was observed, the lack of severe side effects in this poor-prognosis population suggested that this compound might deserve further investigation.
One study during 2005-6 described the pharmacology of dictyostatin, a 22-membered macrolactone isolated from the sponge Spongia sp. collected in the Republic of Maldives. Madiraju and colleagues (44) observed that dictyostatin's induction of tubulin polymerization, taxoid site binding, and antiproliferative activity against human ovarian carcinoma cells was comparable to that of discodermolide, and thus concluded that the macrocyclic structure of dictyostatin might represent a template for the bioactive conformation of discodermolide.
Didemnin B, a cyclic depsipeptide produced by ascidians of the family Didemnidae was the focus of one pharmacologic investigation in 2005-6. Beasley and colleagues (45) completed a study of the excretion and tissue concentrations of [3H] didemnin B in mice after intraperitoneal administration. Interestingly, they found that the pancreas had the greatest concentration of radiolabel at both the high and low doses 7 days after administration, which suggested possible efficacy in animal models for the treatment of pancreatic cancer.
Park and colleagues (46) examined the pharmacology of dideoxypetrosynol A, a polyacetylene from the marine sponge Petrosia sp., by investigating the molecular mechanism involved in cell cycle arrest at the G1 to S phase transition in human monocytic leukaemia cells. A careful study of G1/S transition regulatory proteins revealed an enhanced expression of the Cdk inhibitor p16/INK4a with a concomitant decrease of retinoblastoma protein phosphorylation, thus suggesting that both p16 and pRB proteins play an important role in G1 cell cycle arrest induced by this compound in human leukemia cells.
There were four studies of discodermolide, a compound originally isolated from the sponge Discodermia dissoluta that suppresses microtubule dynamics. Xia and colleagues (47) using a photoaffinity-labeled analogue to investigate and define the drug binding pocket in tubulin, observed that while the analogue had no hypernucleation effect in an in vitro microtubule polymerization assay, it labeled amino acid residues 305-359 in the S9-S10 loop in β-tubulin. This sequence is very close to the Taxol binding site, thus enabling the construction of a computationally-derived binding model of both the discodermolide analogue and native discodermolide binding to β-tubulin. Escuin and colleagues (48) observed that discodermolide, as well as other microtubule-disrupting agents, down-regulated hypoxia-inducible factor-1α (HIF-1α) protein levels, but not mRNA, in a dose-dependent manner. This study directly linked β-tubulin drug binding with HIF-1α protein inhibition, a discovery of considerable clinical significance because HIF-1α over expression is present in over 70% of all human tumours and their metastasis, thus making HIF-1α “a prime target for anticancer therapies”. Klein and colleagues (49) evaluated discodermolide in several human cancer cell lines and determined that it induced accelerated senescence with a potency similar to doxorubicin with concomitant Erk1/2 activation and upregulation of two markers of senescence, namely the p66Shc and PAI-1 proteins. These findings provide the first demonstration of a microtubule stabilizing agent that inhibits tumour cell growth by the mechanism of accelerated senescence. Huang and colleagues (50) investigated the combination of discodermolide and taxol in human ovarian cancer cells and an in vivo model of ovarian carcinoma, and reported that both agents interacted synergistically at drug concentrations that resulted in aneuploidy rather than mitotic arrest. Although the mechanism for the synergistic efficacy of these two agents was not determined, the data supported concurrent use of low doses of these two compounds for treatment of epithelial ovarian carcinoma, a leading cause of death from gynecologic malignancies. In an effort to develop novel therapies for poorly vascularized and hypoxic tumours, a “longstanding problem in clinical oncology”, Smith and colleagues (51) tested discodermolide analogues as potent chemical components of combination bacteriolytic therapy. Interestingly, a single intravenous injection of (+)-2,3 anhydrodiscodermolide plus genetically modified Clostridium novyi-NT spores into mice bearing colorectal cancer xenografts caused rapid and complete obliteration of the tumours.
Nine studies were published during 2005-6 on the preclinical and clinical evaluation of the dolastatins, a family of modified peptides originally isolated from the marine mollusc Dolabella auricularia that induce actin assembly in vivo. Watanabe and colleagues (52) investigated the antitumour activity of TZT-1027 (Soblidotin), a newly synthesized dolastatin 10 derivative, using a panel of human tumours that included Pgp overexpressing sublines. They concluded that TZT-1027 antitumour activity was superior to that of paclitaxel, docetaxel and vincristine, and thus anticipated that TZT-1027 would provide benefit in the chemotherapy of tubulin inhibitor-unresponsive tumours.
Five Phase I trials were conducted with TZT-1027 and a third-generation dolastatin-15 analogue, tasidotin hydrochloride (ILX651). Jonge and colleagues (53) in the Netherlands, completed a Phase I and pharmacokinetic study with TZT-1027 in 17 patients with advanced solid tumours. In this trial one patient with a refractory metastatic liposarcoma demonstrated a response, and eight patients experienced stabilization of their tumour, and the study defined a dose of TZT-1027 that was “well tolerated”, with the main dose-limiting toxicities being reversible neutropenia and infusion arm pain. A three-institution Phase I study with TZT-1027 in 18 Japanese patients with advanced solid tumours was reported by Tamura and colleagues (54). The researchers noted that one patient with metastatic esophageal cancer achieved a partial response, and that TZT-1027 was “active at a tolerable dose” with neutropenia and infusion reaction (phlebitis) the most frequent toxicities that were observed. Greystoke and colleagues (55) published data of a Phase I study with TZT-1027 administered in combination with carboplatin in 14 patients with advanced solid tumours, which resulted in one patient with pancreatic adenocarcinoma achieving a partial response. Although peripheral reversible neuropathy was observed in 36% of the patients, the combination of TZT-1027 and carboplatin appeared to be “relatively well tolerated”. Cunningham and colleagues (56) conducted a Phase I and pharmacokinetic study with the pentapeptide tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, in 32 patients with advanced solid tumours refractory to standard treatment. While the best antitumour response consisted of stable disease in 10 patients, in contrast to TZT-1027, tasidotin's neurotoxicity and cardiovascular toxicity were diminished, with neutropenia observed as the principal dose-limiting toxicity. Mita and colleagues (57) reported a Phase I and pharmacokinetic study with tasidotin hydrochloride in thirty patients with advanced solid tumours. Although neutropenia was observed as the principal toxicity, the researchers concluded that the mild myelosuppression and manageable nonhematologic toxicities observed concomitant to antitumour activity in three patients with lung, hepatocellular and renal cell carcinoma “warranted further disease-directed evaluations” with this agent.
Three Phase II trials of dolastatin-10 and TZT-1027 were described during 2005-6. Perez and colleagues (58) reported a Phase II study in 22 patients with advanced breast cancer who were treated with dolastatin-10 as a single agent. While the observed hematological toxicity was moderate and one patient had a partial response, the study was terminated due to the lack of tumour response. The results of this Phase II study were judged to be “disappointing”, probably a result of dolastatin 10's “true lack of activity”. Kindler and colleagues (59) completed a Phase II trial of dolastatin-10 in 16 patients with advanced hepatobiliary cancer and 12 patients with metastatic pancreatic adenocarcinoma, malignancies that are known to be refractory to most chemotherapy and for which novel therapeutic agents are needed. Due to a lack of tumour response, and only a slight increase in patient survival, the authors concluded that unfortunately further evaluation of “dolastatin-10 in pancreaticobiliary malignancies is not warranted”. Patel and colleagues (60) reported the results of a Phase II study of intravenous TZT-1027 in 28 patients with advanced or metastatic soft-tissue sarcomas with prior exposure to anthracycline-based chemotherapy, usually doxorubicin. While no confirmed responses were observed in any of the patients enrolled in this study, TZT-1027 was found to be safe and well tolerated, with the most common hematologic toxicity being neutropenia.
Ten preclinical and seven clinical articles contributed to the preclinical and clinical pharmacology of the tetrahydroisoquinoline alkaloid ecteinascidin-743 (ET-743; Trabectedin, Yondelis®), an antitumour agent originally isolated from the Caribbean sea squirt Ecteinascidia turbinata (61).
New insights into the molecular pharmacology of ET-743 was provided by several studies during this period. Minuzzo and colleagues (62) tested the hypothesis that ET-743 specifically targeted cell-cycle genes involved in transcription. Their data demonstrated that ET-743 was not a general inhibitor of inducible genes, but its effects were downstream from transcription factor binding, and that histone acetylation was largely unaffected. David-Cordonnier and colleagues (63) carried out the first structure-activity study with ET-743 analogues, and demonstrated that the DNA-interacting activity of this molecule is dependent on the C21-hydroxyl group, and that changes in the C ring of ET-743 can affect the DNA binding affinity. The data also suggested the existence of an additional non-DNA target for ET-743, probably a protein, “located close to DNA”, that would cause formation of a ternary complex that would trigger apoptosis and cell cycle arrest. Marco & Gago (64) used unrestrained molecular dynamics simulations to investigated the interaction of two ET-743 molecules with a self complementary dodecanucleotide d(GTATGGCCATAC). These simulations revealed that it was possible for two ET-743 molecules to bind in a tail-tail arrangement to two adjacent TGG sites placed on opposite DNA strands. This interaction led to structural distortions in the DNA oligonucleotide that could affect the binding of transcription factors acting as activators or repressors of gene transcription. Dziegielewska and colleagues (65) determined the effects of ET-743 on DNA helicase/nuclease activity of RecBCD from Escherichia coli, an enzyme involved in unwinding the DNA helix, and used as a model to study DNA-damaging agents. They reported that ET-743 significantly inhibited unwinding, enhanced degradation of DNA, and completely disrupted the RecBCD's enzyme, thus resulting in inhibition of repair, replication and transcription processes. Herrero and colleagues (66) tested the hypothesis that ET-743 induced lethal DNA strand breaks by interacting with the nucleotide exchange excision repair proteins, which are involved in DNA damage repair caused by several anticancer drugs, eg. cisplatin. Specifically, the researchers discovered that ET-743-induced DNA cytotoxicity depended on the interaction of this agent with an arginine residue (Arg314) in the DNA-binding region in human nuclease FEN-1, and the putative formation of a DNA-ET-743 ternary complex which caused tumour cell death.
Preclinical cellular pharmacology of ET-743 was described in several studies during 2005-6. Brandon and colleagues (67) reported on the cytotoxicity of ET-743 in a human hepatic carcinoma cell line growing in vitro. The results demonstrated that because ET-743 is metabolized by cytochromes (CYP) P450 3A4, and 2C9, 2C19 and 2E1, and by Phase II enzymes, combination therapy with other CYP inhibitors (e.g. cisplatin, paclitaxel and doxorubicin) may result in hepatotoxicity due to drug-drug interactions. More recently, Brandon and colleagues (68) examined the human biotransformation and cytochrome P450 reaction phenotype of ET-743 and confirmed that CYP3A4 has a major role in the metabolism of ET-743 in vitro with lesser involvement of CYP2C9, 2C19, 2D6 and 2E1 isozymes. Clearly assessment of the biotransformation and CYP reaction phenotype is important for interpreting pharmacokinetic data from clinical trials and predicting potential ET-743-drug interactions, in particular hepatic toxicity. In an effort to correlate gene expression profiles with in vitro sensitivity to ET-743, Martinez and colleagues (69) investigated a panel of 11 chemonaïve, low-passage soft-tissue sarcoma cell lines established from patient biopsies using a cDNA microarray containing 6,700 cancer-related genes. Although the gene expression profile revealed that 244 genes were down-regulated and 86 genes up-regulated in 8 of the 11 sarcoma cell lines in this study, a noteworthy finding was the upregulation of genes related to cell cycle control, stress, DNA-damage response and apoptosis. Confirmation of these results in patient tumour specimens will be necessary to help identify subsets of soft-tissue sarcomas that may show increased sensitivity to ET-743. To investigate the mechanism of in vitro resistance, Marchini and colleagues (70) evaluated microarray-based gene expression profiles in ET-743-sensitive and - resistant ovarian and chondrosarcoma cell lines. Microarray studies on a panel of 2400 cDNAs revealed that a subset of 70 genes, 21 of which were upregulated and 49 downregulated, consistently showed differential expression in the ET-743-resistant cell lines, thus shedding new light on molecular pathways involved in chemoresistance to ET-743.
One study detailed the preclinical in vivo pharmacology of ET-743. Meco and colleagues (71) found that the combination of ET-743 and irinotecan, a water-soluble derivative of camptothecin that targets topoisomerase I, resulted in only weak cytotoxicity to human rhabdomyosarcoma in vitro, but this same drug combination produced a “strong and long-lasting” effect on the growth of rhabdomyosarcoma tumour xenografts in vivo. Although the mechanism of the in vivo synergism of ET-743 and irinotecan was not investigated, the authors hypothesized that the discrepancy between in vitro and in vivo effects might result from a combination of direct cytotoxic and indirect anti-inflammatory effects of ET-743.
One Phase I and pharmacokinetic study as well as 6 Phase II trials extended the clinical pharmacology of ET-743 during 2005-6. Lau and colleagues (72) conducted a Phase I and pharmacokinetic study with ET-743 given as a 3-hour i.v. infusion every 21 days in 12 children with refractory solid tumours. ET-743 was generally well tolerated with reversible hepatotoxicity the most common adverse effect (58% of patients). The observation that one patient with a recurrent Ewing sarcoma showed a complete response with resolution of pulmonary metastases has resulted in the development of a Phase II trial in children with refractory Ewing or soft tissue sarcomas. Garcia-Carbonero and colleagues (73) reported a Phase II and pharmacokinetic study with ET-743 in 36 previously untreated patients with advanced soft tissue sarcomas, mainly leiomyosarcoma and liposarcoma. ET-743 evidenced “manageable” toxicity in this study, with one complete and five partial responses to ET-743 being observed (17.1% response rate). This led the investigators to state that ET-743 “demonstrates for the first time the safety, tolerability, and antitumour activity” in chemotherapy-naïve patients with advanced soft tissue sarcomas when used as a single agent, and it justified “some prudent optimism” for patients who fail doxorubicin or ifosfamide therapy. Huygh and colleagues (74) reported a retrospective Phase II study of 89 patients with advanced, pretreated soft tissue and bone sarcoma, who were treated with ET-743 as a 24-hour continuous infusion. While the toxicities were mainly an asymptomatic elevation of transaminases and neutropenia, the treatment resulted in one complete remission, 5 partial remissions, one minimal response and 16 patients with disease stabilization of 6 months or more, thus strongly suggesting that “further evaluation of the activity of ET-743 in sarcomas is therefore warranted”. Le Cesne and colleagues (75) communicated a Phase II study with 104 patients from 8 European institutions with pretreated advanced soft tissue sarcomas, that were provided with a 24-hour continuous infusion every 3 weeks. The fact that 8 partial responses were observed in leiomyosarcomas, a tumour subtype usually considered to be resistant to doxorubicin and/or ifosfamide regimens, prompted the authors to “demand(s) further evaluations” of ET-743 as a second-line agent and in combination studies. Sessa and colleagues (76) assessed the efficacy and toxicity of ET-743 in 59 patients with advanced ovarian cancer who had experienced treatment failure after platinum or taxane therapy. Using a Phase II 3-hour infusion schedule every 3 weeks which allowed for outpatient administration, the study reported that ET-743 was tolerable with “promising activity” in relapsed ovarian cancer, showing a 43% response rate in patients with platinum-insensitive disease. Zelek and colleagues (77) from 3 French institutions determined the activity of ET-743 in a Phase II study that investigated the use of this compound as a 24-hour continuous intravenous infusion every 3 weeks in 27 patients with advanced breast cancer who were resistant or had relapsed after conventional chemotherapy. Although the partial response rate (14%) was judged to be modest, it was considered to be in the range of “what can be expected for an active drug in this setting”. Tewari and colleagues (78) reported a remarkable case of activity of ET-743 when used as a single agent in one patient with refractory metastatic uterine leiomyosarcoma in whom 4 prior regimens had failed. The patient had a durable partial response lasting at least 8 months, and the authors concluded that ET-743's activity in uterine leiomyosarcomas definitely warrants further investigation.
One report in 2005-6 examined the pharmacology of fascaplysin, an alkaloid obtained from the Papua New Guinea sponge Fascaplysinopsis reticulata. Subramanian and colleagues (79) showed therapeutic efficacy in a novel pharmacology paradigm designed to rapidly move prospective anticancer drugs from discovery phase through pharmacology testing and into therapeutic trial assessment, as well as complete proteomics analysis to reveal “pathways involved in the drug's cytotoxicity”.
Two reports extended the preclinical pharmacology of halichondrin B, a large polyether macrolide found in a variety of marine sponges. Jordan and colleagues (80) observed that ET389, a synthetic macrocyclic ketone analogue that is currently in Phase I and Phase II clinical trials, inhibited microtubule polymerization in an in vitro human breast cancer cell line. It significantly suppressed microtubule growth rate, length and duration, thus resulting in the suppression of the metaphase/anaphase transition. A putatively novel mechanism of action involving E7389's ability to “aggregate tubulin and selectively suppress microtubule growing events” was proposed by the authors. Dabydeen and colleagues (81), examined the biochemical mechanism of action of E7389 in direct comparison with halichondrin B. They found that ET389 was more potent than halichondrin B in all biochemical assays, and by extensive molecular modeling studies gained new insight into the interaction of both compounds with a cleft between αβ-heterodimers in tubulin. This interaction appeared to involve contacts with α-subunit residues Phe244, Ala247, Leu248, and Tyr257 and β-subunit residues Gli81, Pro82, Thr223 and Gly225.
One report extended the pharmacology of the peptidic antimitotic agent hemiasterlin, a compound isolated from numerous marine sponges. Ravi and colleagues (82) reported progress in the structure-based identification of the tubulin binding site for HTI-286, a synthetic analogue of hemiasterlin. They proposed a binding model of HTI-286 to key residues on β tubulin that appears to be supported by significant experimental data, including biophysical characterization, photolabeling and NMR studies, and by biological activity data.
One report extended the pharmacology of jasplakinolide (jaspamide), a cyclic depsipeptide originally isolated from Jaspis sponges, that induces actin polymerization. While investigating the anti-migratory potential of this agent, Hayot and colleagues (83) used an in vitro pharmacological strategy that included spectrofluorometry to monitor the kinetics of actin polymerization, and videomicroscopy to investigate cell motility. They observed that jasplakinolide enhanced the motility of A549 lung cancer cells but not that of MCF7 breast tumour cells, and this led the authors to conclude that the use of “multi-assays with different levels of sophistication” is required for the characterization of anti-migratory and potentially novel antimetastatic agents.
During 2005-2006 two reports described the preclinical and clinical pharmacology of kahalalide F, a complex depsipeptide isolated from the Hawaiian marine mollusk Elysia rufescens that is currently under clinical investigation. Janmmat and colleagues (84) determined that kahalalide F induced cytotoxicity in breast, vulval, non-small-cell lung, and hepatic carcinoma cell lines via a necrosis-like cell death process. This process was positively correlated to ErbB3 (HER3) receptor protein levels and downstream in the PI3K-Akt pathway in vitro, findings that the investigators proposed “may have important clinical relevance”. Lakhai and colleagues in the Netherlands (85) detailed a Phase I and pharmacokinetic study in 32 patients with androgen-refractory prostate cancer that received kalahalide F as a 1-hour i.v. infusion for five consecutive days every three weeks. While noting that kalahalide F dose-limiting toxicity was reversible, mainly an increase in transaminases, they observed that one patient had a partial response with a prostate-specific antigen declining by 50% for greater than four weeks, while five patients (16%) showed evidence of stable disease.
One report extended the pharmacology of the marine pyrrole alkaloid lamellarin D, a submicromolar inhibitor of topoisomerase I, that was isolated form the prosobranch mollusk Lamellaria sp. In an effort to find treatments for chemoresistant cancer, Kluza and colleagues (86) investigated the targets and pathways involved in apoptosis induced by lamellarin D. A detailed mechanistic study revealed that lamellarin D had an effect on the structural and functional integrity of cancer cell mitochondria at micromolar concentrations, which suggested lamellarin D should be considered a “bifunctional pharmacologic effector” agent.
During 2005-2006 one report described the preclinical pharmacology of pateamine A, a complex macrolide isolated from the marine sponge Micale sp. In an elegant molecular study, Bordeleau and colleagues (87) demonstrated that pateamine A is “the first example of a chemical inducer of dimerization that forces an engagement” between the RNA helicase eukaryotic initiation factor 4FA subunit (elF4A) and RNA. This interaction prevents elF4A from participating in the ribosome-recruitment of translation initiation, which is considered the rate-limiting step of protein synthesis in eukaryotes.
Preclinical research continued during 2005-6 with the macrolide peloruside A, a microtubule-stabilizing agent which is currently available both synthetically and from aquaculture grown samples of the New Zealand marine sponge Mycale hentscheli. Hamel and colleagues (88) confirmed that peluroside A bound to the laulimalide site on tubulin which is distinct from the taxoid site. Furthermore the researchers found that although peloruside A and laulimalide were unable to synergize with each other, both compounds could act synergistically on tubulin assembly with taxoid site-binding marine agents such as discodermolide, dictyostatin, and eleutherobin. This finding led the authors to conclude that other combinations of these agents may be worth investigating both preclinically and clinically.
In an effort to identify novel natural product-derived peroxisome proliferator-activated receptor γ (PPRγ) activators for breast cancer treatment, Mora and colleagues (89) investigated the effects of psammaplin A, a known histone deacetylase inhibitor originally isolated from the marine sponge Pseudoceratina rhax. Psammaplin A activated PPRγ in a cell-based reporter assay and induced apoptosis in human breast cancer cells in vitro. This suggests that PPARγ activators may provide new molecularly targeted lead compounds for the design of novel classes of antitumour agents.
Table I also includes a number of marine natural products which were not previously reviewed (2-4): aaptamine, polymeric alkylpyridinium salts, aplyronine A, bastadin 6, clavulone II, 13-deoxytedanolide, fucoxanthinol, geodiamolides, geoditin A, halocynthiaxanthin, ircinin-1, laxaphycins A & B, leptosins C & F, ningalins, onnamide A, philinopside A, salinosporamide A, stellettin A, strobilinin-felixinin, and variolin B.
As the result of a screening effort to discover agents that target the cyclin-dependent kinase inhibitor Cip/Kip p21 protein, Aoki and colleagues (90) reported the isolation of a benzonaphthyridene alkaloid aaptamine from the Indonesian marine sponge Aaptos suberitoides. The in vitro studies demonstrated that aaptamine induced expression of p21 protein in a p53-independent manner, arresting the cell cycle at the G2/M phase. Paleari and colleagues (91) showed that polymeric alkylpyridinium salts isolated from the marine sponge Reniera sarai induced apotosis and cell-cell adhesion in non-small cell lung cancer cells. The selectivity of these polymeric alkylpyridinium salts, which were recently described as irreversible acetylcholinesterase inhibitors, towards cholinergic receptor-expressing tumors led the investigators to propose them as “alternative inhibitors of lung cancer with low systemic toxicity”.
Hirata and colleagues (92) extended the molecular characterization of the sea hare metabolite aplyronine A, which had previously been shown to inhibit polymerization of globular actin to fibrous actin. Using synchrotron X-ray analysis, the crystal structure of the actin-aplyronine A complex was investigated. Aplyronine A was observed to bind to a hydrophobic cleft by intercalating its aliphatic tail into the actin molecule, an interaction shown to be essential to depolymerize actin and for cytotoxicity against human HeLa tumour cell lines.
Aoki and colleagues (93) described molecular pharmacology studies of bastadin 6, a macrocyclic and tetrameric bromotyrosine derivative isolated from the marine sponge Lanthella basta. Bastadin 6 was observed to inhibit angiogenesis and in vivo neovascularization, probably by an apoptotic mechanism. It therefore shows potential for further development as an inhibitor of tumour angiogenesis, although the actual molecular target has not yet been determined.
Huang and colleagues (94) investigated the molecular pharmacology of the marine prostaglandin analogue clavulone II, originally derived from the Japanese soft coral Clavularia viridis and previously shown to have antitumour and antiviral activity. Working with a human acute promyelocytic leukemia, clavulone II induced downregulation of cyclin D1 expression and G1 arrest of the cell cycle at lower concentrations (1.5 μM), while at higher concentrations (3 μM) clavulone II induced apoptosis with concomitant modulation of caspases and Bcl-2 family proteins.
Aoki and colleagues (95) reported the isolation of the steroidal alkaloids cortistatins A-D from the marine sponge Corticium simplex. Interestingly, cortistatin A exhibited highly selective anti-proliferative activity against human umbilical vein endothelial cells (IC50 = 0.0018-1.1 μM), in comparison with normal human dermal fibroblasts and several human tumor cell lines. Although the molecular mechanism of action of the cortistatins is currently under investigation, they clearly appear to be promising new inhibitors of angiogenesis and thus putative antitumour compounds.
Nishimura and colleagues (96) continued the characterization of the antitumour macrolide 13-deoxytedanolide, isolated from the marine sponge Mycale adhaerens. A high-affinity binding site on the Saccharomyces cerevisiae 60S large ribosomal unit was elegantly demonstrated to be the molecular target of 13-deoxytedanolide which caused potent inhibition of polypeptide synthesis (IC50 = 0.15 μM). The authors noted this compound was the first macrolide that bound the eukaryotic ribosome and therefore it represented “an important tool for elucidating structure and function of eukaryotic ribosomes”.
Konishi and colleagues (97) investigated the carotenoids fucoxanthinol and halocynthiaxantin isolated from the sea squirt Halocynthia roretzi. Both carotenoids inhibited the growth of human leukemia, breast and colon cancer cells in vitro in a dose- and time-dependent manner by a mechanism that required induction of apoptosis and the concomitant reduction of the apoptosis-suppressing protein Bcl-2.
Rangel and colleagues (98) reported new mechanistic information on the cyclic peptides geodiamolides A, B, H and I isolated from the marine sponge Geodia corticostylifera from Brazil. The researchers noted that peptides A & H had potent antiproliferative activity against two human breast cancer cell lines (IC50 = 18-90 nM) and disorganized F-actin filaments in a dose-dependent manner. Interestingly, normal cell lines did not show cytoskeleton alterations after treatment with the geodiamolides, thus suggesting a putative biomedical potential for these novel compounds.
As part of a research program to evaluate bioactive secondary metabolites of marine organisms, Liu and colleagues (99) compared the cytotoxicity of the isomalabaricane triterpenes geoditins A and B isolated from the marine sponge Geodia japonica. Geoditin A, which differs in an acetyl group at the C3 position with geoditin B, was found to be the most cytotoxic (IC50 = 5 μg/mL) to human HL60 promyelocytic leukemia cells, probably due to a dose-dependent increase of reactive oxygen species, a decrease in mitochondrial potential, and caspase-3 mediated apoptosis.
In a effort to find new agents for the treatment of melanoma, Choi and colleagues (100) completed a detailed mechanistic study of ircinin-1, isolated from the marine sponge Sarcotragus sp. Ircinin-1 inhibited the growth of a human melanoma cell line in vitro, by a dual mechanism that involved arrest of cell cycle progression at the G1 phase and induction of apoptosis via the Fas/Fas-L pathway.
Gbankoto and colleagues (101) studied the cytotoxic effects of laxaphycins A and B, cyclic depsipeptides isolated form the marine cyanobacterium Lyngbya majuscula. Working with three established human lymphoblastic cell lines and a protocol of triple labeling with vital dyes and multifluorescence image analysis, both peptides were observed to act synergistically to produce an increase in the polyploid cell population. It was hypothesized that this effect “could result from alteration of topoisomerase II activity”.
Yanagihara and colleagues (102) extended the molecular pharmacology of the sulphur-containing indole derivatives leptosins C and F, isolated from the marine fungus Leptoshaeria sp. Both compounds inhibited DNA topoisomerase II (IC50 = 3-10 μM), while only leptosin C inhibited topoisomerase I (IC50 = 10-30 μM) in vitro and in vivo. Furthermore, the compounds induced apoptosis in vivo, as measured by caspase-3 activation, while inactivating the survival Akt/protein kinase B pathway.
Chou and colleagues (103) evaluated the pharmacological properties of synthetic analogues of the ningalins, aromatic alkaloids originally isolated from the marine ascidian Didemnum sp. By a mechanism that involved a direct and dose-dependent interaction with the multidrug resistance drug transporter Pgp, the ningalins markedly enhanced the antitumour cytotoxicity of vinblastine, doxorubicin, and taxol both in vitro and/or in vivo. This observation supported the proposition that these agents “may allow a reduction in the dosage of anticancer drugs while enhancing or achieving a curative effect”.
While screening 20,000 samples for agents that might activate the tumour-suppressing transforming growth factor-β (TGF-β) signaling cascade, Lee and colleagues (104) discovered that onnamide A and theopederin B, isolated from the marine sponge Mycale sp., induced activation of the PAI-1 promoter gene, a well-characterized TGF-β-responsive gene. Since onnamide A and theopederin B both potently inhibited protein synthesis (IC50 = 30 nM and 1.9 nM, respectively) and activated p38 kinase and c-Jun N-terminal kinase, as well as inhibited proliferation of several human cancer cell lines in the nanomolar range, the researchers concluded that these marine agents might serve as lead candidates for anticancer drug development.
During a screening effort for potential angiogenesis inhibitors, Tong and colleagues (105) discovered a novel sulfated saponin philinopside A, isolated from the sea cucumber Pentacta quandrangulari, that possessed dual antiangiogenic and antitumour effects. Philinopside A inhibited angiogenesis (IC50 = 0.98-1.4 μM) in human microvascular endothelial cells as well as tumour growth both in vitro (IC50 = 1.5-2.4 μM) and in vivo by a synergistic mechanism that appeared to involve inhibition of 4 receptor tyrosine kinases (IC50 = 2.6-4.9 μM).
Macherla and colleagues (106) contributed structure-activity relationship (SAR) studies of salinosporamide A (NPI-0052), a novel marine bacterium-derived alkaloid shown to potently inhibit the proteasome, a multicatalytic proteolytic complex that is involved in the regulation of cellular protein degradation. With 16 analogues of salinosporamide A generated by either fermentation or derivatization, SAR studies were completed using a variety of well characterized cytotoxicity, proteasome inhibition and NF-κB activation assays. These studies demonstrated a marked reduction in potency resulted from replacement of the chloroethyl group in salinosporamide A with nonhalogenated substituents.
Liu and colleagues (107) reported novel preclinical pharmacology for the isomalabaricane triterpene stelletin A, isolated from the marine sponge Geodia japonica. These investigators observed differential cytotoxicity of stelletin A between human leukemia HL-60 cells (IC50 = 0.4 μg/mL) and human prostate cancer LNCaP cells (IC50 = 120 μg/ml) with the concomitant upregulation of the pro-apoptotic marker proteins, FasL, and caspase-3. Interestingly, in HL-60 cells stelletin A stimulated a dose-dependent increase of the NADPH oxidase components and generation of reactive oxygen radicals.
Jiang and colleagues (108) reported preclinical mechanism of action studies on the furanosesterterpene strobilinin-felixinin which was isolated from the sponge Psammocinia sp. and previously reported to display cytotoxicity towards several cancer cell lines. Cell cycle analysis revealed that the marine compounds arrested HeLa cells in the S phase, probably as a result of DNA synthesis inhibition, with topoisomerase I and polymerase α-primase the “two main target molecules”.
Simone and colleagues (109) extended the molecular pharmacology of variolin B, a guanidine alkaloid isolated from the Antarctican marine sponge Kirkpatrickia variolosa. Both variolin B and its analogue deoxy-variolin B, which has greater stability and solubility, were shown to activate apoptosis in a p53-independent fashion. They appeared to preferentially inhibit cyclin-dependent kinases (CDK) 1/cyclin B, CDK2/cyclin A, and CDK2/cyclin E. Thus variolin B may be effective against tumours with mutation or deletion of the p53 gene.
Oda and colleagues (110) extended the preclinical pharmacology of verrucarin A, isolated from a culture broth of the marine fungus Myrothecium roridum. Using human promyelocytic and erythroleukemia cell lines, the investigators determined that verrucarin A's strong cytotoxicity against these cell lines was concomitant to inhibition of the p38 and C-Jun mitogen-activated protein kinases in the nanomolar range.
2. 2005-6 Antitumour pharmacology of marine natural products with undetermined mechanisms of action
Table II, encompasses 94 novel marine natural products published during 2005-6 that demonstrated particularly potent activity in cytotoxicity assays (IC50 equal or less than 1.0 μg/mL) and whose structures are shown in Figure 2. The preclinical pharmacology completed with these marine compounds consisted mainly of in vitro and/or in vivo cytotoxicity testing with panels of either human or murine tumour cell lines. In a few reports cytotoxicity studies were more extensive and included the National Cancer Institute 60-tumour cell line screen. It is clear that additional pharmacological testing will be required to help determine if the potent cytotoxicity observed with these marine chemicals resulted from a specific pharmacologic effect rather than a general toxic effect on the tumour cells used in these investigations. Although contrasting with the extensive preclinical and clinical investigations completed with the marine compounds presented in Table I, mechanism of action research was reported for only a few of the compounds listed in Table 2: swinholide I and hurghadolide A caused disruption of the actin cytoskeleton at nM concentrations (111); philinopside A inhibited proliferation, migration and tube formation of human microvascular endothelial cells(112); exposure of human colorectal cancer cells to tedanolide C resulted in accumulation of cells in S-phase(113); palmerolide A inhibited V-ATPase (IC50 = 2 nM) (114); stelletin J promoted binding of DNA with DNA polymerase β (115); liphagal inhibited phosphatidylinositol-3-kinase α (IC50 = 100nM) (116); seragamide A facilitated G-actin polymerization (20-200 nM) (117); lyngbyabellin E (60 nM) and ankaraholide A caused disruption of cellular microfilament network and inhibition of cytokinesis (118;119) and secalonic acid D inhibited the cell cycle at the G0/G1 phase in a concentration-dependent manner (120).
Although less potent than the marine natural products included in Table 2, numerous additional reports were published during 2005-6 describing novel structurally characterized molecules with cytotoxic activity (IC50) mostly in the greater than 1 to 5.0 μg/mL range. Although only cytotoxicity against selected murine or human cancer cells was determined in vitro in the majority of these reports, mechanistic work was reported in a few of these studies, e.g. inhibition of Tie2 kinase, an enzyme that supports angiogenesis, by polybrominated diphenyl ethers (121); inhibition of human telomerase by axinelloside A (122); inhibition of FOX01a, a transcription factor, by psammaplysenes (123); potent histone deacetylase inhibition and anti-angiogenic effects by the cyclic peptides azumamides A-E (124) and significant antimetastatic activity by the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine (125).
3. Conclusion
Antitumour marine pharmacology research in 2005-6 consisted of a combination of preclinical research focused on the molecular and cellular pharmacology of marine cytotoxic agents, as well as clinical studies with a limited number of marine compounds, i.e., aplidine, bryostatin 1, cryptophycins, dolastatins, and ecteinascidin-743 (Trabectedin, Yondelis®). Although during 2005-6 no marine natural product was approved for cancer patient treatment by the U.S. Food and Drug Administration (FDA), ecteinascidin-743 (Trabectedin, Yondelis®) has recently been granted Orphan Drug designation from the European Commission, and the FDA for soft tissue sarcomas and ovarian cancer. Our 2005-6 overview of the antitumour and cytotoxic pharmacology of marine chemicals demonstrates that more than 54 years after the discovery by Bergman and colleagues (126) of spongothymidine and spongouridine, global research aimed at the discovery of novel and clinically useful antitumour agents derived from marine organisms continues at a remarkably active pace.
Acknowledgments
This publication was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Additional support to AMSM from Midwestern University's office of Research and Sponsored Programs is gratefully acknowledged. The excellent support for literature searches and article retrieval by library staff members as well as medical and pharmacy students of Midwestern University is most gratefully recognized. The authors wish to specially thank Mrs. Victoria Sears and Ms. Mary Hall for careful and dedicated assistance in the preparation of this manuscript.
Footnotes
Conflict of interest statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mayer AMS. Marine Pharmacology in 1998: antitumor and cytotoxic compounds. The Pharmacologist. 1999;41(4):159–64. [Google Scholar]
- 2.Mayer AMS, Lehmann VKB. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Research. 2001;21:2489–500. [PubMed] [Google Scholar]
- 3.Mayer AM, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer. 2003;105(3):291–9. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- 4.Mayer AM, Gustafson KR. Marine pharmacology in 2001-2: antitumour and cytotoxic compounds. Eur J Cancer. 2004;40(18):2676–704. doi: 10.1016/j.ejca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Mayer AM, Gustafson KR. Marine pharmacology in 2003-2004: anti-tumour and cytotoxic compounds. Eur J Cancer. 2006;42(14):2241–70. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities;with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. The Pharmacologist. 2000;42(2):62–9. [Google Scholar]
- 7.Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities;affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 2002;132:315–39. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 8.Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY) 2004;6(1):37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer AM, Hamann MT. Marine pharmacology in 2001--2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140(34):265–86. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer AM, Rodriguez AD, Berlinck RG, Hamann MT. Marine pharmacology in 2003-4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145(4):553–81. doi: 10.1016/j.cbpc.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren XQ, Furukawa T, Yamamoto M, Aoki S, Kobayashi M, Nakagawa M, et al. A functional role of intracellular loops of human multidrug resistance protein 1. J Biochem (Tokyo) 2006;140(3):313–8. doi: 10.1093/jb/mvj155. [DOI] [PubMed] [Google Scholar]
- 12.Taddei ML, Chiarugi P, Cuevas C, Ramponi G, Raugei G. Oxidation and inactivation of low molecular weight protein tyrosine phosphatase by the anticancer drug Aplidin. Int J Cancer. 2006;118(8):2082–8. doi: 10.1002/ijc.21602. [DOI] [PubMed] [Google Scholar]
- 13.Bravo SB, Garcia-Rendueles ME, Seoane R, Dosil V, Cameselle-Teijeiro J, Lopez-Lazaro L, et al. Plitidepsin has a cytostatic effect in human undifferentiated (anaplastic) thyroid carcinoma. Clin Cancer Res. 2005;11(21):7664–73. doi: 10.1158/1078-0432.CCR-05-0455. [DOI] [PubMed] [Google Scholar]
- 14.Biscardi M, Caporale R, Balestri F, Gavazzi S, Jimeno J, Grossi A. VEGF inhibition and cytotoxic effect of aplidin in leukemia cell lines and cells from acute myeloid leukemia. Ann Oncol. 2005;16(10):1667–74. doi: 10.1093/annonc/mdi311. [DOI] [PubMed] [Google Scholar]
- 15.Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem. 2005;280(12):11641–7. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- 16.Tognon G, Bernasconi S, Celli N, Faircloth GT, Cuevas C, Jimeno J, et al. Induction of resistance to Aplidin in a human ovarian cancer cell line related to MDR expression. Cancer Biol Ther. 2005;4(12):1325–30. doi: 10.4161/cbt.4.12.2157. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Santiago L, Suarez Y, Zarich N, Munoz-Alonso MJ, Cuadrado A, Martinez T, et al. Aplidin induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation. Cell Death Differ. 2006;13(11):1968–81. doi: 10.1038/sj.cdd.4401898. [DOI] [PubMed] [Google Scholar]
- 18.Straight AM, Oakley K, Moores R, Bauer AJ, Patel A, Tuttle RM, et al. Aplidin reduces growth of anaplastic thyroid cancer xenografts and the expression of several angiogenic genes. Cancer Chemother Pharmacol. 2006;57(1):7–14. doi: 10.1007/s00280-005-0014-7. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S, Chieze S, Delbaldo C, Ady-Vago N, Guzman C, Lopez-Lazaro L, et al. Phase I and pharmacokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J Clin Oncol. 2005;23(31):7871–80. doi: 10.1200/JCO.2005.09.357. [DOI] [PubMed] [Google Scholar]
- 20.Maroun JA, Belanger K, Seymour L, Matthews S, Roach J, Dionne J, et al. Phase I study of Aplidine in a dailyx5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada Clinical Trials Group study: NCIC CTG IND 115. Ann Oncol. 2006;17(9):1371–8. doi: 10.1093/annonc/mdl165. [DOI] [PubMed] [Google Scholar]
- 21.Guittat L, De CA, Rosu F, Gabelica V, De PE, Delfourne E, et al. Ascididemin and meridine stabilise G-quadruplexes and inhibit telomerase in vitro. Biochim Biophys Acta. 2005;1724(3):375–84. doi: 10.1016/j.bbagen.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11(2 Pt 1):843–52. [PubMed] [Google Scholar]
- 24.Ma D, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, et al. Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen. Clin Cancer Res. 2006;12(8):2591–6. doi: 10.1158/1078-0432.CCR-05-2107. [DOI] [PubMed] [Google Scholar]
- 25.Smith LM, Nesterova A, Alley SC, Torgov MY, Carter PJ. Potent cytotoxicity of an auristatin-containing antibody-drug conjugate targeting melanoma cells expressing melanotransferrin/p97. Mol Cancer Ther. 2006;5(6):1474–82. doi: 10.1158/1535-7163.MCT-06-0026. [DOI] [PubMed] [Google Scholar]
- 26.Tse KF, Jeffers M, Pollack VA, McCabe DA, Shadish ML, Khramtsov NV, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12(4):1373–82. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- 27.Statsuk AV, Bai R, Baryza JL, Verma VA, Hamel E, Wender PA, et al. Actin is the primary cellular receptor of bistramide A. Nat Chem Biol. 2005;1(7):383–8. doi: 10.1038/nchembio748. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi SA, Tereshko V, Kossiakoff AA, Kozmin SA. Structure of bistramide A-actin complex at a 1.35 angstroms resolution. J Am Chem Soc. 2006;128(12):3882–3. doi: 10.1021/ja058319c. [DOI] [PubMed] [Google Scholar]
- 29.Chiang PC, Chien CL, Pan SL, Chen WP, Teng CM, Shen YC, et al. Induction of endoplasmic reticulum stress and apoptosis by a marine prostanoid in human hepatocellular carcinoma. J Hepatol. 2005;43(4):679–86. doi: 10.1016/j.jhep.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Chiang PC, Kung FL, Huang DM, Li TK, Fan JR, Pan SL, et al. Induction of Fas clustering and apoptosis by coral prostanoid in human hormone-resistant prostate cancer cells. Eur J Pharmacol. 2006;542(13):22–30. doi: 10.1016/j.ejphar.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Powell CT, Yin L. Overexpression of PKCepsilon sensitizes LNCaP human prostate cancer cells to induction of apoptosis by bryostatin 1. Int J Cancer. 2006;118(6):1572–6. doi: 10.1002/ijc.21511. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty S, Huang J, Basu A. Enhancement of cisplatin sensitivity of cisplatin-resistant human cervical carcinoma cells by bryostatin 1. Clin Cancer Res. 2005;11(18):6730–7. doi: 10.1158/1078-0432.CCR-05-0450. [DOI] [PubMed] [Google Scholar]
- 33.Choi SH, Hyman T, Blumberg PM. Differential effect of bryostatin 1 and phorbol 12-myristate 13-acetate on HOP-92 cell proliferation is mediated by down-regulation of protein kinase Cdelta. Cancer Res. 2006;66(14):7261–9. doi: 10.1158/0008-5472.CAN-05-4177. [DOI] [PubMed] [Google Scholar]
- 34.Tuthill MC, Oki CE, Lorenzo PS. Differential effects of bryostatin 1 and 12-O-tetradecanoylphorbol-13-acetate on the regulation and activation of RasGRP1 in mouse epidermal keratinocytes. Mol Cancer Ther. 2006;5(3):602–10. doi: 10.1158/1535-7163.MCT-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia CS, Curiel RE, Mwatibo JM, Pestka S, Li H, Espinoza-Delgado I. The antineoplastic agent bryostatin-1 differentially regulates IFN-gamma receptor subunits in monocytic cells: transcriptional and posttranscriptional control of IFN-gamma R2. J Immunol. 2006;177(4):2707–16. doi: 10.4049/jimmunol.177.4.2707. [DOI] [PubMed] [Google Scholar]
- 36.El-Rayes BF, Gadgeel S, Shields AF, Manza S, Lorusso P, Philip PA. Phase I study of bryostatin 1 and gemcitabine. Clin Cancer Res. 2006;12(23):7059–62. doi: 10.1158/1078-0432.CCR-06-1419. [DOI] [PubMed] [Google Scholar]
- 37.Ajani JA, Jiang Y, Faust J, Chang BB, Ho L, Yao JC, et al. A multi-center phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Invest New Drugs. 2006;24(4):353–7. doi: 10.1007/s10637-006-6452-1. [DOI] [PubMed] [Google Scholar]
- 38.Peterson AC, Harlin H, Karrison T, Vogelzang NJ, Knost JA, Kugler JW, et al. A randomized phase II trial of interleukin-2 in combination with four different doses of bryostatin-1 in patients with renal cell carcinoma. Invest New Drugs. 2006;24(2):141–9. doi: 10.1007/s10637-006-5935-4. [DOI] [PubMed] [Google Scholar]
- 39.Muller IM, Dirsch VM, Rudy A, Lopez-Anton N, Pettit GR, Vollmar AM. Cephalostatin 1 inactivates Bcl-2 by hyperphosphorylation independent of M-phase arrest and DNA damage. Mol Pharmacol. 2005;67(5):1684–9. doi: 10.1124/mol.104.004234. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Anton N, Rudy A, Barth N, Schmitz ML, Pettit GR, Schulze-Osthoff K, et al. The marine product cephalostatin 1 activates an endoplasmic reticulum stress-specific and apoptosome-independent apoptotic signaling pathway. J Biol Chem. 2006;281(44):33078–86. doi: 10.1074/jbc.M607904200. [DOI] [PubMed] [Google Scholar]
- 41.Cannady EA, Chien C, Jones TM, Borel AG. In vitro metabolism of the epoxide substructure of cryptophycins by cytosolic glutathione S-transferase: species differences and stereoselectivity. Xenobiotica. 2006;36(8):659–70. doi: 10.1080/00498250600720593. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Moore RE, Moher ED, Munroe JE, Al-awar RS, Hay DA, et al. Cryptophycins-309, 249 and other cryptophycin analogs: preclinical efficacy studies with mouse and human tumors. Invest New Drugs. 2005;23(3):213–24. doi: 10.1007/s10637-005-6729-9. [DOI] [PubMed] [Google Scholar]
- 43.D'Agostino G, del CJ, Mellado B, Izquierdo MA, Minarik T, Cirri L, et al. A multicenter phase II study of the cryptophycin analog LY355703 in patients with platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2006;16(1):71–6. doi: 10.1111/j.1525-1438.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 44.Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, et al. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry. 2005;44(45):15053–63. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- 45.Beasley VR, Bruno SJ, Burner JS, Choi BW, Rinehart KL, Koritz GD, et al. Fate of tritiated didemnin B in mice: excretion and tissue concentrations after an intraperitoneal dose. Biopharm Drug Dispos. 2005;26(8):341–51. doi: 10.1002/bdd.466. [DOI] [PubMed] [Google Scholar]
- 46.Park C, Kim GY, Kim GD, Lee WH, Cheong JH, Kim ND, et al. Suppression of U937 human monocytic leukemia cell growth by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., via induction of Cdk inhibitor p16 and down-regulation of pRB phosphorylation. Oncol Rep. 2006;16(1):171–6. [PubMed] [Google Scholar]
- 47.Xia S, Kenesky CS, Rucker PV, Smith AB, III, Orr GA, Horwitz SB. A photoaffinity analogue of discodermolide specifically labels a peptide in beta-tubulin. Biochemistry. 2006;45(39):11762–75. doi: 10.1021/bi060497a. [DOI] [PubMed] [Google Scholar]
- 48.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65(19):9021–8. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein LE, Freeze BS, Smith AB, III, Horwitz SB. The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle. 2005;4(3):501–7. doi: 10.4161/cc.4.3.1550. [DOI] [PubMed] [Google Scholar]
- 50.Huang GS, Lopez-Barcons L, Freeze BS, Smith AB, III, Goldberg GL, Horwitz SB, et al. Potentiation of taxol efficacy and by discodermolide in ovarian carcinoma xenograft-bearing mice. Clin Cancer Res. 2006;12(1):298–304. doi: 10.1158/1078-0432.CCR-05-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith AB, III, Freeze BS, LaMarche MJ, Sager J, Kinzler KW, Vogelstein B. Discodermolide analogues as the chemical component of combination bacteriolytic therapy. Bioorg Med Chem Lett. 2005;15(15):3623–6. doi: 10.1016/j.bmcl.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe J, Minami M, Kobayashi M. Antitumor activity of TZT-1027 (Soblidotin) Anticancer Res. 2006;26(3A):1973–81. [PubMed] [Google Scholar]
- 53.de Jonge MJ, van der GA, Planting AS, van DL, Lems A, Boot I, et al. Phase I and pharmacokinetic study of the dolastatin 10 analogue TZT-1027, given on days 1 and 8 of a 3-week cycle in patients with advanced solid tumors. Clin Cancer Res. 2005;11(10):3806–13. doi: 10.1158/1078-0432.CCR-04-1937. [DOI] [PubMed] [Google Scholar]
- 54.Tamura K, Nakagawa K, Kurata T, Satoh T, Nogami T, Takeda K, et al. Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative and inhibitor of tubulin polymerization, which was administered to patients with advanced solid tumors on days 1 and 8 in 3-week courses. Cancer Chemother Pharmacol. 2007;60(2):285–93. doi: 10.1007/s00280-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 55.Greystoke A, Blagden S, Thomas AL, Scott E, Attard G, Molife R, et al. A phase I study of intravenous TZT-1027 administered on day 1 and day 8 of a three-weekly cycle in combination with carboplatin given on day 1 alone in patients with advanced solid tumours. Ann Oncol. 2006;17(8):1313–9. doi: 10.1093/annonc/mdl097. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham C, Appleman LJ, Kirvan-Visovatti M, Ryan DP, Regan E, Vukelja S, et al. Phase I and pharmacokinetic study of the dolastatin-15 analogue tasidotin (ILX651) administered intravenously on days 1, 3, and 5 every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2005;11(21):7825–33. doi: 10.1158/1078-0432.CCR-05-0058. [DOI] [PubMed] [Google Scholar]
- 57.Mita AC, Hammond LA, Bonate PL, Weiss G, McCreery H, Syed S, et al. Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin Cancer Res. 2006;12(17):5207–15. doi: 10.1158/1078-0432.CCR-06-0179. [DOI] [PubMed] [Google Scholar]
- 58.Perez EA, Hillman DW, Fishkin PA, Krook JE, Tan WW, Kuriakose PA, et al. Phase II trial of dolastatin-10 in patients with advanced breast cancer. Invest New Drugs. 2005;23(3):257–61. doi: 10.1007/s10637-005-6735-y. [DOI] [PubMed] [Google Scholar]
- 59.Kindler HL, Tothy PK, Wolff R, McCormack RA, Abbruzzese JL, Mani S, et al. Phase II trials of dolastatin-10 in advanced pancreaticobiliary cancers. Invest New Drugs. 2005;23(5):489–93. doi: 10.1007/s10637-005-2909-x. [DOI] [PubMed] [Google Scholar]
- 60.Patel S, Keohan ML, Saif MW, Rushing D, Baez L, Feit K, et al. Phase II study of intravenous TZT-1027 in patients with advanced or metastatic soft-tissue sarcomas with prior exposure to anthracycline-based chemotherapy. Cancer. 2006;107(12):2881–7. doi: 10.1002/cncr.22334. [DOI] [PubMed] [Google Scholar]
- 61.Fayette J, Coquard IR, Alberti L, Ranchere D, Boyle H, Blay JY. ET-743: a novel agent with activity in soft tissue sarcomas. Oncologist. 2005;10(10):827–32. doi: 10.1634/theoncologist.10-10-827. [DOI] [PubMed] [Google Scholar]
- 62.Minuzzo M, Ceribelli M, Pitarque-Marti M, Borrelli S, Erba E, DiSilvio A, et al. Selective effects of the anticancer drug Yondelis (ET-743) on cell-cycle promoters. Mol Pharmacol. 2005;68(5):1496–503. doi: 10.1124/mol.105.013615. [DOI] [PubMed] [Google Scholar]
- 63.vid-Cordonnier MH, Gajate C, Olmea O, Laine W, de l I, Perez C, et al. DNA and non-DNA targets in the mechanism of action of the antitumor drug trabectedin. Chem Biol. 2005;12(11):1201–10. doi: 10.1016/j.chembiol.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Marco E, Gago F. DNA structural similarity in the 2:1 complexes of the antitumor drugs trabectedin (Yondelis) and chromomycin A3 with an oligonucleotide sequence containing two adjacent TGG binding sites on opposing strands. Mol Pharmacol. 2005;68(6):1559–67. doi: 10.1124/mol.105.015685. [DOI] [PubMed] [Google Scholar]
- 65.Dziegielewska B, Beerman TA, Bianco PR. Inhibition of RecBCD enzyme by antineoplastic DNA alkylating agents. J Mol Biol. 2006;361(5):898–919. doi: 10.1016/j.jmb.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 66.Herrero AB, Martin-Castellanos C, Marco E, Gago F, Moreno S. Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin. Cancer Res. 2006;66(16):8155–62. doi: 10.1158/0008-5472.CAN-06-0179. [DOI] [PubMed] [Google Scholar]
- 67.Brandon EF, Meijerman I, Klijn JS, den Arend D, Sparidans RW, Lazaro LL, et al. In-vitro cytotoxicity of ET-743 (Trabectedin, Yondelis), a marine anti-cancer drug, in the Hep G2 cell line: influence of cytochrome P450 and phase II inhibition, and cytochrome P450 induction. Anticancer Drugs. 2005;16(9):935–43. doi: 10.1097/01.cad.0000180121.16407.38. [DOI] [PubMed] [Google Scholar]
- 68.Brandon EF, Sparidans RW, Guijt KJ, Lowenthal S, Meijerman I, Beijnen JH, et al. In vitro characterization of the human biotransformation and CYP reaction phenotype of ET-743 (Yondelis, Trabectidin), a novel marine anti-cancer drug. Invest New Drugs. 2006;24(1):3–14. doi: 10.1007/s10637-005-4538-9. [DOI] [PubMed] [Google Scholar]
- 69.Martinez N, Sanchez-Beato M, Carnero A, Moneo V, Tercero JC, Fernandez I, et al. Transcriptional signature of Ecteinascidin 743 (Yondelis, Trabectedin) in human sarcoma cells explanted from chemo-naive patients. Mol Cancer Ther. 2005;4(5):814–23. doi: 10.1158/1535-7163.MCT-04-0316. [DOI] [PubMed] [Google Scholar]
- 70.Marchini S, Marrazzo E, Bonomi R, Chiorino G, Zaffaroni M, Weissbach L, et al. Molecular characterisation of two human cancer cell lines selected in vitro for their chemotherapeutic drug resistance to ET-743. Eur J Cancer. 2005;41(2):323–33. doi: 10.1016/j.ejca.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 71.Riccardi A, Meco D, Ubezio P, Mazzarella G, Marabese M, Faircloth GT, et al. Combination of trabectedin and irinotecan is highly effective in a human rhabdomyosarcoma xenograft. Anticancer Drugs. 2005;16(8):811–5. doi: 10.1097/01.cad.0000172837.67766.6a. [DOI] [PubMed] [Google Scholar]
- 72.Lau L, Supko JG, Blaney S, Hershon L, Seibel N, Krailo M, et al. A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children' s Oncology Group study. Clin Cancer Res. 2005;11(2 Pt 1):672–7. [PubMed] [Google Scholar]
- 73.Garcia-Carbonero R, Supko JG, Maki RG, Manola J, Ryan DP, Harmon D, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol. 2005;23(24):5484–92. doi: 10.1200/JCO.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 74.Huygh G, Clement PM, Dumez H, Schoffski P, Wildiers H, Selleslach J, et al. Ecteinascidin-743: evidence of activity in advanced, pretreated soft tissue and bone sarcoma patients. Sarcoma. 2006;2006:1–11. doi: 10.1155/SRCM/2006/56282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le CA, Blay JY, Judson I, Van OA, Verweij J, Radford J, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23(3):576–84. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 76.Sessa C, De BF, Perotti A, Bauer J, Curigliano G, Noberasco C, et al. Trabectedin for women with ovarian carcinoma after treatment with platinum and taxanes fails. J Clin Oncol. 2005;23(9):1867–74. doi: 10.1200/JCO.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 77.Zelek L, Yovine A, Brain E, Turpin F, Taamma A, Riofrio M, et al. A phase II study of Yondelis (trabectedin, ET-743) as a 24-h continuous intravenous infusion in pretreated advanced breast cancer. Br J Cancer. 2006;94(11):1610–4. doi: 10.1038/sj.bjc.6603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tewari D, Saffari B, Cowan C, Wallick AC, Koontz MZ, Monk BJ. Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma. Gynecol Oncol. 2006;102(3):421–4. doi: 10.1016/j.ygyno.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Subramanian B, Nakeff A, Tenney K, Crews P, Gunatilaka L, Valeriote F. A new paradigm for the development of anticancer agents from natural products. J Exp Ther Oncol. 2006;5(3):195–204. [PMC free article] [PubMed] [Google Scholar]
- 80.Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086–95. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 81.Dabydeen DA, Burnett JC, Bai R, Verdier-Pinard P, Hickford SJ, Pettit GR, et al. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol. 2006;70(6):1866–75. doi: 10.1124/mol.106.026641. [DOI] [PubMed] [Google Scholar]
- 82.Ravi M, Zask A, Rush TS., III Structure-based identification of the binding site for the hemiasterlin analogue HTI-286 on tubulin. Biochemistry. 2005;44(48):15871–9. doi: 10.1021/bi051268b. [DOI] [PubMed] [Google Scholar]
- 83.Hayot C, Debeir O, Van HP, Van DM, Kiss R, Decaestecker C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol Appl Pharmacol. 2006;211(1):30–40. doi: 10.1016/j.taap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Janmaat ML, Rodriguez JA, Jimeno J, Kruyt FA, Giaccone G. Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol Pharmacol. 2005;68(2):502–10. doi: 10.1124/mol.105.011361. [DOI] [PubMed] [Google Scholar]
- 85.Rademaker-Lakhai JM, Horenblas S, Meinhardt W, Stokvis E, de Reijke TM, Jimeno JM, et al. Phase I clinical and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. Clin Cancer Res. 2005;11(5):1854–62. doi: 10.1158/1078-0432.CCR-04-1534. [DOI] [PubMed] [Google Scholar]
- 86.Kluza J, Gallego MA, Loyens A, Beauvillain JC, Sousa-Faro JM, Cuevas C, et al. Cancer cell mitochondria are direct proapoptotic targets for the marine antitumor drug lamellarin D. Cancer Res. 2006;66(6):3177–87. doi: 10.1158/0008-5472.CAN-05-1929. [DOI] [PubMed] [Google Scholar]
- 87.Bordeleau ME, Cencic R, Lindqvist L, Oberer M, Northcote P, Wagner G, et al. RNA-mediated sequestration of the RNA helicase eIF4A by Pateamine A inhibits translation initiation. Chem Biol. 2006;13(12):1287–95. doi: 10.1016/j.chembiol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, et al. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol Pharmacol. 2006;70(5):1555–64. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 89.Mora FD, Jones DK, Desai PV, Patny A, Avery MA, Feller DR, et al. Bioassay for the identification of natural product-based activators of peroxisome proliferator-activated receptor-gamma (PPARgamma): the marine sponge metabolite psammaplin A activates PPARgamma and induces apoptosis in human breast tumor cells. J Nat Prod. 2006;69(4):547–52. doi: 10.1021/np050397q. [DOI] [PubMed] [Google Scholar]
- 90.Aoki S, Kong D, Suna H, Sowa Y, Sakai T, Setiawan A, et al. Aaptamine, a spongean alkaloid, activates p21 promoter in a p53-independent manner. Biochem Biophys Res Commun. 2006;342(1):101–6. doi: 10.1016/j.bbrc.2006.01.119. [DOI] [PubMed] [Google Scholar]
- 91.Paleari L, Trombino S, Falugi C, Gallus L, Carlone S, Angelini C, et al. Marine sponge-derived polymeric alkylpyridinium salts as a novel tumor chemotherapeutic targeting the cholinergic system in lung tumors. Int J Oncol. 2006;29(6):1381–8. [PubMed] [Google Scholar]
- 92.Hirata K, Muraoka S, Suenaga K, Kuroda T, Kato K, Tanaka H, et al. Structure basis for antitumor effect of aplyronine a. J Mol Biol. 2006;356(4):945–54. doi: 10.1016/j.jmb.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 93.Aoki S, Cho SH, Ono M, Kuwano T, Nakao S, Kuwano M, et al. Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anticancer Drugs. 2006;17(3):269–78. doi: 10.1097/00001813-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Huang YC, Guh JH, Shen YC, Teng CM. Investigation of anticancer mechanism of clavulone II, a coral cyclopentenone prostaglandin analog, in human acute promyelocytic leukemia. J Biomed Sci. 2005;12(2):335–45. doi: 10.1007/s11373-005-3009-9. [DOI] [PubMed] [Google Scholar]
- 95.Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J Am Chem Soc. 2006;128(10):3148–9. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]
- 96.Nishimura S, Matsunaga S, Yoshida M, Hirota H, Yokoyama S, Fusetani N. 13-Deoxytedanolide, a marine sponge-derived antitumor macrolide, binds to the 60S large ribosomal subunit. Bioorg Med Chem. 2005;13(2):449–54. doi: 10.1016/j.bmc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 97.Konishi I, Hosokawa M, Sashima T, Kobayashi H, Miyashita K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142(12):53–9. doi: 10.1016/j.cbpc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Rangel M, Prado MP, Konno K, Naoki H, Freitas JC, hado-Santelli GM. Cytoskeleton alterations induced by Geodia corticostylifera depsipeptides in breast cancer cells. Peptides. 2006;27(9):2047–57. doi: 10.1016/j.peptides.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 99.Liu WK, Ho JC, Che CT. Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia HL60 cells. Cancer Lett. 2005;230(1):102–10. doi: 10.1016/j.canlet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 100.Choi HJ, Choi YH, Yee SB, Im E, Jung JH, Kim ND. Ircinin-1 induces cell cycle arrest and apoptosis in SK-MEL-2 human melanoma cells. Mol Carcinog. 2005;44(3):162–73. doi: 10.1002/mc.20084. [DOI] [PubMed] [Google Scholar]
- 101.Gbankoto A, Vigo J, Dramane K, Banaigs B, Aina E, Salmon JM. Cytotoxic effect of Laxaphycins A and B on human lymphoblastic cells (CCRF-CEM) using digitised videomicrofluorometry. In Vivo. 2005;19(3):577–82. [PubMed] [Google Scholar]
- 102.Yanagihara M, Sasaki-Takahashi N, Sugahara T, Yamamoto S, Shinomi M, Yamashita I, et al. Leptosins isolated from marine fungus Leptoshaeria species inhibit DNA topoisomerases I and/or II and induce apoptosis by inactivation of Akt/protein kinase B. Cancer Sci. 2005;96(11):816–24. doi: 10.1111/j.1349-7006.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chou TC, Guan Y, Soenen DR, Danishefsky SJ, Boger DL. Potent reversal of multidrug resistance by ningalins and its use in drug combinations against human colon carcinoma xenograft in nude mice. Cancer Chemother Pharmacol. 2005;56(4):379–90. doi: 10.1007/s00280-005-1019-y. [DOI] [PubMed] [Google Scholar]
- 104.Lee KH, Nishimura S, Matsunaga S, Fusetani N, Horinouchi S, Yoshida M. Inhibition of protein synthesis and activation of stress-activated protein kinases by onnamide A and theopederin B, antitumor marine natural products. Cancer Sci. 2005;96(6):357–64. doi: 10.1111/j.1349-7006.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tong Y, Zhang X, Tian F, Yi Y, Xu Q, Li L, et al. Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. Int J Cancer. 2005;114(6):843–53. doi: 10.1002/ijc.20804. [DOI] [PubMed] [Google Scholar]
- 106.Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao TH, Nicholson B, et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48(11):3684–7. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- 107.Liu WK, Cheung FW, Che CT. Stellettin A induces oxidative stress and apoptosis in HL-60 human leukemia and LNCaP prostate cancer cell lines. J Nat Prod. 2006;69(6):934–7. doi: 10.1021/np058122y. [DOI] [PubMed] [Google Scholar]
- 108.Jiang Y, Ahn EY, Ryu SH, Kim DK, Park JS, Kang SW, et al. Mechanism of cell cycle arrest by (8E,13Z,20Z)-strobilinin/(7E,13Z,20Z)-felixinin from a marine sponge Psammocinia sp. Oncol Rep. 2005;14(4):957–62. [PubMed] [Google Scholar]
- 109.Simone M, Erba E, Damia G, Vikhanskaya F, Di Francesco AM, Riccardi R, et al. Variolin B and its derivate deoxy-variolin B: new marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur J Cancer. 2005;41(15):2366–77. doi: 10.1016/j.ejca.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Oda T, Namikoshi M, Akano K, Kobayashi H, Honma Y, Kasahara T. Verrucarin a inhibition of map kinase activation in a pma-stimulated promyelocytic leukemia cell line. Marine Drugs. 2005;3:64–73. [Google Scholar]
- 111.Youssef DT, Mooberry SL. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J Nat Prod. 2006;69(1):154–7. doi: 10.1021/np050404a. [DOI] [PubMed] [Google Scholar]
- 112.Yi YH, Xu QZ, Li L, Zhang SL, Wu HM, Ding J, et al. Philinopsides A and B, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Helvetica Chimica Acta. 2006;89(1):54–63. [Google Scholar]
- 113.Chevallier C, Bugni TS, Feng X, Harper MK, Orendt AM, Ireland CM. Tedanolide C: a potent new 18-membered-ring cytotoxic macrolide isolated from the Papua New Guinea marine sponge Ircinia sp. J Org Chem. 2006;71(6):2510–3. doi: 10.1021/jo052285+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diyabalanage T, Amsler CD, McClintock JB, Baker BJ. Palmerolide A, a cytotoxic macrolide from the antarctic tunicate Synoicum adareanum. J Am Chem Soc. 2006;128(17):5630–1. doi: 10.1021/ja0588508. [DOI] [PubMed] [Google Scholar]
- 115.Clement JA, Li M, Hecht SM, Kingston DG. Bioactive isomalabaricane triterpenoids from Rhabdastrella globostellata that stabilize the binding of DNA polymerase beta to DNA. J Nat Prod. 2006;69(3):373–6. doi: 10.1021/np0504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marion F, Williams DE, Patrick BO, Hollander I, Mallon R, Kim SC, et al. Liphagal, a Selective inhibitor of PI3 kinase alpha isolated from the sponge Akacoralliphaga: structure elucidation and biomimetic synthesis. Org Lett. 2006;8(2):321–4. doi: 10.1021/ol052744t. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka C, Tanaka J, Bolland RF, Marriott G, Higa T. Seragamides A-F, new actin-targeting depsipeptides from the sponge Suberites japonicus Thiele. Tetrahedron. 2006;62(15):3536–42. [Google Scholar]
- 118.Han BN, McPhail KL, Gross H, Goeger DE, Mooberry SL, Gerwick WH. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron. 2005;61(49):11723–9. [Google Scholar]
- 119.Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, et al. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org Lett. 2005;7(7):1375–8. doi: 10.1021/ol050188x. [DOI] [PubMed] [Google Scholar]
- 120.Ren H, Tian L, Gu Q, Zhu W. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch Pharm Res. 2006;29(1):59–63. doi: 10.1007/BF02977469. [DOI] [PubMed] [Google Scholar]
- 121.Xu YM, Johnson RK, Hecht SM. Polybrominated diphenyl ethers from a sponge of the Dysidea genus that inhibit Tie2 kinase. Bioorganic & Medicinal Chemistry. 2005;13(3):657–9. doi: 10.1016/j.bmc.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 122.Warabi K, Hamada T, Nakao Y, Matsunaga S, Hirota H, van Soest RW, et al. Axinelloside A, an unprecedented highly sulfated lipopolysaccharide inhibiting telomerase, from the marine sponge, Axinella infundibula. J Am Chem Soc. 2005;127(38):13262–70. doi: 10.1021/ja052688r. [DOI] [PubMed] [Google Scholar]
- 123.Schroeder FC, Kau TR, Silver PA, Clardy J. The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68(4):574–6. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- 124.Nakao Y, Yoshida S, Matsunaga S, Shindoh N, Terada Y, Nagai K, et al. Azumamides A-E: histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew Chem Int Ed Engl. 2006;45(45):7553–7. doi: 10.1002/anie.200602047. [DOI] [PubMed] [Google Scholar]
- 125.Sawant SS, Youssef DT, Reiland J, Ferniz M, Marchetti D, El Sayed KA. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J Nat Prod. 2006;69(7):1010–3. doi: 10.1021/np050527v. [DOI] [PubMed] [Google Scholar]
- 126.Bergmann W, Burke DC. Contributions to the study of marine products. XXXIX. The nucleosides of sponges. III. Spongothymidine and spongouridine. J Org Chem. 1955;20:1501–7. [Google Scholar]
- 127.Herrero AB, Martin-Castellanos C, Marco E, Gago F, Moreno S. Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin. Cancer Res. 2006;66(16):8155–62. doi: 10.1158/0008-5472.CAN-06-0179. [DOI] [PubMed] [Google Scholar]
- 128.Soria-Mercado IE, Prieto-Davo A, Jensen PR, Fenical W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J Nat Prod. 2005;68(6):904–10. doi: 10.1021/np058011z. [DOI] [PubMed] [Google Scholar]
- 129.Tsuda M, Kariya Y, Iwamoto R, Fukushi E, Kawabata J, Kobayashi J. Amphidinolides B4 and B5, potent cytotoxic 26-membered macrolides from dinoflagellate Amphidinium species. Marine Drugs. 2005;3:1–8. [Google Scholar]
- 130.Takekawa Y, Matsunaga S, van Soest RW, Fusetani N. Amphimedosides, 3-alkylpyridine glycosides from a marine sponge Amphimedon sp. J Nat Prod. 2006;69(10):1503–5. doi: 10.1021/np060122q. [DOI] [PubMed] [Google Scholar]
- 131.Han B, Gross H, Goeger DE, Mooberry SL, Gerwick WH. Aurilides B and C, cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2006;69(4):572–5. doi: 10.1021/np0503911. [DOI] [PubMed] [Google Scholar]
- 132.Simmons TL, McPhail KL, Ortega-Barria E, Mooberry SL, Gerwick WH. Belamide A, a new antimitotic tetrapeptide from a Panamanian marine cyanobacterium. Tetrahedron Lett. 2006;47(20):3387–90. [Google Scholar]
- 133.Teruya T, Suenaga K, Maruyama S, Kurotaki M, Kigoshi H. Biselides A-E: novel polyketides from the Okinawan ascidian Didemnidae sp. Tetrahedron. 2005;67(27):6561–7. [Google Scholar]
- 134.El Gamal AA, Chiang CY, Huang SH, Wang SK, Duh CY. Xenia diterpenoids from the formosan soft coral Xenia blumi. J Nat Prod. 2005;68(9):1336–40. doi: 10.1021/np058047r. [DOI] [PubMed] [Google Scholar]
- 135.Wu SJ, Fotso S, Li F, Qin S, Kelter G, Fiebig HH, et al. N-carboxamido-staurosporine and selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J Antibiot (Tokyo) 2006;59(6):331–7. doi: 10.1038/ja.2006.46. [DOI] [PubMed] [Google Scholar]
- 136.Wang SK, Huang MJ, Duh CY. Cytotoxic constituents from the formosan soft coral Clavularia inflata var. luzoniana. J Nat Prod. 2006;69(10):1411–6. doi: 10.1021/np0601253. [DOI] [PubMed] [Google Scholar]
- 137.Suntornchashwej S, Chaichit N, Isobe M, Suwanborirux K. Hectochlorin and morpholine derivatives from the Thai sea hare, Bursatella leachii. J Nat Prod. 2005;68(6):951–5. doi: 10.1021/np0500124. [DOI] [PubMed] [Google Scholar]
- 138.McNamara CE, Larsen L, Perry NB, Harper JL, Berridge MV, Chia EW, et al. Anti-inflammatory sesquiterpene-quinones from the New Zealand sponge Dysidea cf. cristagalli. J Nat Prod. 2005;68(9):1431–3. doi: 10.1021/np050171n. [DOI] [PubMed] [Google Scholar]
- 139.Nieto MI, Gonzalez N, Rodriguez J, Kerr RG, Jimenez C. New cytotoxic cembranolides: isolation, biogenetic studies, and synthesis of analogues. Tetrahedron. 2006;62(50):11747–54. [Google Scholar]
- 140.Zhang SY, Yi YH, Tang HF. Bioactive triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J Nat Prod. 2006;69(10):1492–5. doi: 10.1021/np060106t. [DOI] [PubMed] [Google Scholar]
- 141.Amagata T, Minoura K, Numata A. Gymnastatins F-H, cytostatic metabolites from the sponge-derived fungus Gymnascella dankaliensis. J Nat Prod. 2006;69(10):1384–8. doi: 10.1021/np0600189. [DOI] [PubMed] [Google Scholar]
- 142.Fouad M, Edrada RA, Ebel R, Wray V, Muller WE, Lin WH, et al. Cytotoxic isomalabaricane triterpenes from the marine sponge Rhabdastrella globostellata. J Nat Prod. 2006;69(2):211–8. doi: 10.1021/np050346t. [DOI] [PubMed] [Google Scholar]
- 143.Yu S, Deng Z, Proksch P, Lin W. Oculatol, oculatolide, and A-nor sterols from the sponge Haliclona oculata. J Nat Prod. 2006;69(9):1330–4. doi: 10.1021/np0600494. [DOI] [PubMed] [Google Scholar]
- 144.Uddin J, Ueda K, Siwu ER, Kita M, Uemura D. Cytotoxic labdane alkaloids from an ascidian Lissoclinum sp.: isolation, structure elucidation, and structure-activity relationship. Bioorg Med Chem. 2006;14(20):6954–61. doi: 10.1016/j.bmc.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 145.Wu J, Yi YH, Tang HF, Zou ZR, Wu HM. Structure and cytotoxicity of a new lanostane-type triterpene glycoside from the sea cucumber Holothuria hilla. Chem Biodivers. 2006;3(11):1249–54. doi: 10.1002/cbdv.200690126. [DOI] [PubMed] [Google Scholar]
- 146.Mansoor TA, Hong J, Lee CO, Bae SJ, Im KS, Jung JH. Cytotoxic sterol derivatives from a marine sponge Homaxinella sp. J Nat Prod. 2005;68(3):331–6. doi: 10.1021/np0496690. [DOI] [PubMed] [Google Scholar]
- 147.Mansoor TA, Lee YM, Hong J, Lee CO, Im KS, Jung JH. 5,6:8,9-diepoxy and other cytotoxic sterols from the marine sponge Homaxinella sp. J Nat Prod. 2006;69(1):131–4. doi: 10.1021/np0502950. [DOI] [PubMed] [Google Scholar]
- 148.Gorajana A, Kurada BV, Peela S, Jangam P, Vinjamuri S, Poluri E, et al. 1-Hydroxy-1-norresistomycin, a new cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. J Antibiot (Tokyo) 2005;58(8):526–9. doi: 10.1038/ja.2005.72. [DOI] [PubMed] [Google Scholar]
- 149.Cruz LJ, Insua MM, Baz JP, Trujillo M, Rodriguez-Mias RA, Oliveira E, et al. IB-01212, a new cytotoxic cyclodepsipeptide isolated from the marine fungus Clonostachys sp. ESNA-A009. J Org Chem. 2006;71(9):3335–8. doi: 10.1021/jo051600p. [DOI] [PubMed] [Google Scholar]
- 150.Zou Z, Yi Y, Wu H, Yao X, Du L, Jiuhong W, et al. Intercedensides D-I, cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J Nat Prod. 2005;68(4):540–6. doi: 10.1021/np040205b. [DOI] [PubMed] [Google Scholar]
- 151.Sato S, Kuramoto M, Ono N. Ircinamine B, bioactive alkaloid from marine sponge Dactylia sp. Tetrahedron Lett. 2006;47(45):7871–3. [Google Scholar]
- 152.Chao CH, Huang LF, Yang YL, Su JH, Wang GH, Chiang MY, et al. Polyoxygenated steroids from the gorgonian Isis hippuris. J Nat Prod. 2005;68(6):880–5. doi: 10.1021/np050033y. [DOI] [PubMed] [Google Scholar]
- 153.Ashour M, Edrada R, Ebel R, Wray V, Watjen W, Padmakumar K, et al. Kahalalide derivatives from the Indian sacoglossan mollusk Elysia grandifolia. J Nat Prod. 2006;69(11):1547–53. doi: 10.1021/np060172v. [DOI] [PubMed] [Google Scholar]
- 154.Reddy SM, Srinivasulu M, Satyanarayana N, Kondapi AK, Venkateswarlu Y. New potent cytotoxic lamellarin alkaloids from Indian ascidian Didemnum obscurum. Tetrahedron. 2005;61(39):9242–7. [Google Scholar]
- 155.Sata N, Abinsay H, Yoshida WY, Horgen FD, Sitachitta N, Kelly M, et al. Lehualides A-D, metabolites from a Hawaiian sponge of the genus Plakortis. J Nat Prod. 2005;68(9):1400–3. doi: 10.1021/np0500528. [DOI] [PubMed] [Google Scholar]
- 156.Sandler JS, Colin PL, Kelly M, Fenical W. Cytotoxic macrolides from a new species of the deep-water marine sponge Leiodermatium. J Org Chem. 2006;71(19):7245–51. doi: 10.1021/jo060958y. [DOI] [PubMed] [Google Scholar]
- 157.Oh DC, Jensen PR, Kauffman CA, Fenical W. Libertellenones A-D: induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg Med Chem. 2005;13(17):5267–73. doi: 10.1016/j.bmc.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 158.Liu HW, Fujiwara T, Nishikawa T, Mishima Y, Nagai H, Shida T, et al. Lissoclibadins 1-3, three new polysulfur alkaloids, from the ascidian Lissoclinum cf. badium. Tetrahedron. 2005;61(36):8611–5. [Google Scholar]
- 159.Zhou GX, Molinski TF. Manoalide derivatives from a sponge, Luffariella sp. J Asian Nat Prod Res. 2006;8(12):15–20. doi: 10.1080/10286020500246022. [DOI] [PubMed] [Google Scholar]
- 160.Kwon HC, Kauffman CA, Jensen PR, Fenical W. Marinomycins a-d, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “marinispora”. J Am Chem Soc. 2006;128(50):1622–32. doi: 10.1021/ja0558948. [DOI] [PubMed] [Google Scholar]
- 161.Kanoh K, Matsuo Y, Adachi K, Imagawa H, Nishizawa M, Shizuri Y. Mechercharmycins A and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J Antibiot (Tokyo) 2005;58(4):289–92. doi: 10.1038/ja.2005.36. [DOI] [PubMed] [Google Scholar]
- 162.Xu J, Hasegawa M, Harada K, Kobayashi H, Nagai H, Namikoshi M. Melophlins p, q, R, and s: four new tetramic Acid derivatives, from two palauan marine sponges of the genus melophlus. Chem Pharm Bull (Tokyo) 2006;54(6):852–4. doi: 10.1248/cpb.54.852. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi Y, Tsuda M, Fromont J, Kobayashi J. Metachromins J and K, new sesquiterpenoids from marine sponge Spongia species. Heterocycles. 2006;67(2):791–6. [Google Scholar]
- 164.Phuwapraisirisan P, Matsunaga S, Fusetani N. Mycapolyols A-F, new cytotoxic metabolites of mixed biogenesis from the marine sponge Mycale izuensis. Org Lett. 2005;7(11):2233–6. doi: 10.1021/ol050648m. [DOI] [PubMed] [Google Scholar]
- 165.Pettit GR, Tan R. Isolation and structure of phakellistatin 14 from the Western Pacific marine sponge Phakellia sp. J Nat Prod. 2005;68(1):60–3. doi: 10.1021/np040092w. [DOI] [PubMed] [Google Scholar]
- 166.Zhang SL, Li L, Yi YH, Sun P. Philinopsides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat Prod Res. 2006;20(4):399–407. doi: 10.1080/14786410500185584. [DOI] [PubMed] [Google Scholar]
- 167.Xu J, Takasaki A, Kobayashi H, Oda T, Yamada J, Mangindaan RE, et al. Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J Antibiot (Tokyo) 2006;59(8):451–5. doi: 10.1038/ja.2006.63. [DOI] [PubMed] [Google Scholar]
- 168.Lira SP, Vita-Marques AM, Seleghim MH, Bugni TS, LaBarbera DV, Sette LD, et al. New destruxins from the marine-derived fungus Beauveria felina. J Antibiot (Tokyo) 2006;59(9):553–63. doi: 10.1038/ja.2006.76. [DOI] [PubMed] [Google Scholar]
- 169.Pettit GR, Tan R, Cichacz ZA. Antineoplastic agents. 542. Isolation and structure of sesterstatin 6 from the Indian Ocean sponge Hyrtios erecta. J Nat Prod. 2005;68(8):1253–5. doi: 10.1021/np0402221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sohda KY, Nagai K, Yamori T, Suzuki K, Tanaka A. YM-216391, a novel cytotoxic cyclic peptide from Streptomyces nobilis. I. fermentation, isolation and biological activities. J Antibiot (Tokyo) 2005;58(1):27–31. doi: 10.1038/ja.2005.2. [DOI] [PubMed] [Google Scholar]
- 171.Liu W, Gu Q, Zhu W, Cui C, Fan G. Two new benzoquinone derivatives and two new bisorbicillinoids were isolated from a marine-derived fungus Penicillium terrestre. J Antibiot (Tokyo) 2005;58(7):441–6. doi: 10.1038/ja.2005.57. [DOI] [PubMed] [Google Scholar]
- 172.Ratnayake AS, Bugni TS, Feng X, Harper MK, Skalicky JJ, Mohammed KA, et al. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J Nat Prod. 2006;69(11):1582–6. doi: 10.1021/np060229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pettit GR, Tang Y, Knight JC. Antineoplastic agents. 545. Isolation and structure of turbostatins 1-4 from the Asian marine mollusk Turbo stenogyrus. J Nat Prod. 2005;68(7):974–8. doi: 10.1021/np040107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han B, Goeger D, Maier CS, Gerwick WH. The wewakpeptins, cyclic depsipeptides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J Org Chem. 2005;70(8):3133–9. doi: 10.1021/jo0478858. [DOI] [PubMed] [Google Scholar]
- 175.Oh DC, Jensen PR, Fenical W. Zygosporamide, a cytotoxic cyclic depsipeptide from the marine-derived fungus Zygosporium masonii. Tetrahedron Lett. 2006;47(48):8625–8. [Google Scholar]