Abstract
A number of bis(diazeniumdiolates) that we designed to release up to four moles of nitric oxide (NO) and which are structural analogues of the NO prodrug and anti-cancer lead compound, O2-{2,4-dinitro-5-[4-(N-methylamino)benzoyloxy]phenyl} 1-(N,N-dimethylamino)diazen-1-ium-1,2- diolate (PABA/NO), were synthesized and studied. A majority of these compounds yielded higher levels of NO, were better inhibitors of proliferation of a number of cancer cell lines, and more rapidly induced substantially increased levels of S-glutathionylation of cellular proteins in comparison with PABA/NO. In most cases, the anti-proliferative activity and extents of S-glutathionylation correlated well with levels of intracellular NO release. We report bis(diazeniumdiolates) to be a class of S-glutathionylating agents with potent anti-proliferative and S-glutathionylating activity.
Introduction
Nitric oxide (NOa) is intimately involved in mediating a plethora of biological processes.1–6 Numerous studies indicate that nitric oxide is also a promising therapeutic agent for the treatment of several physiological conditions and disease states, including cancer, culminating in the pursuit of nitric oxide prodrugs as potential drug candidates.7–11 The wide-ranging physiological involvement and applicability of NO offer several opportunities for drug discovery but also present enormous challenges, most notably among these the delivery of nitric oxide selectively to the desired physiological site in order to minimize any potential side effects.
Diazeniumdiolate anions are prodrugs of nitric oxide that are widely used as reliable sources of NO in a number of chemical and biological studies.12–16 Our laboratory has developed a number of promising diazeniumdiolate-based cancer drug candidates17–22 such as O2-{2,4-dinitro-5-[4-(N-methylamino)benzoyloxy]phenyl} 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate, PABA/NO, which has shown high potency as an anti-proliferative agent.23–25 PABA/NO was shown to have anti-cancer activity comparable to that of cisplatin in human ovarian cancer xenograft studies in a rodent model.24
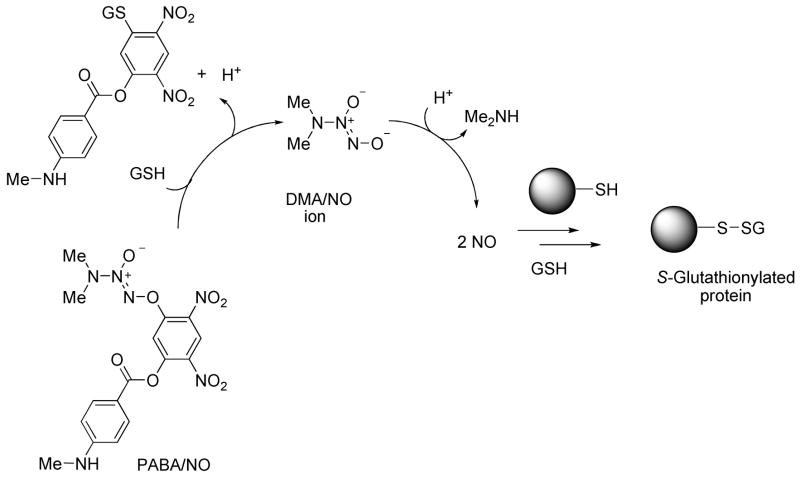
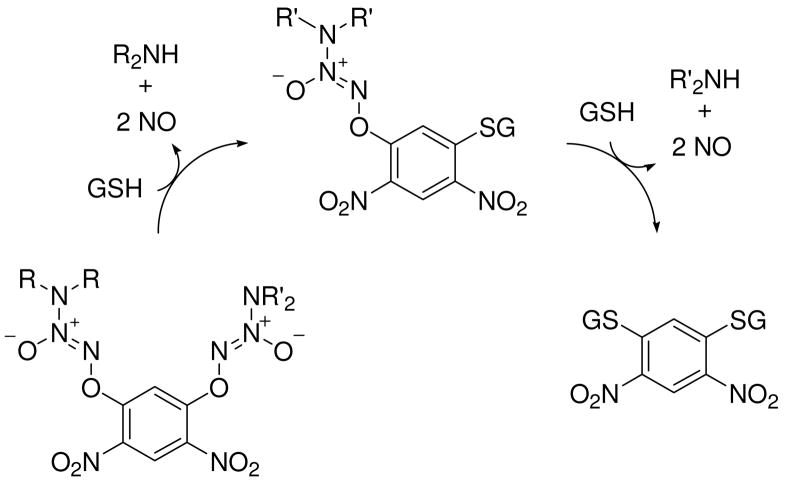
PABA/NO and analogous members of the arylated diazeniumdiolate class of prodrugs were conceived as substrates for glutathione S-transferase (GST),26–28 a phase II detoxification enzyme that is frequently over-expressed by cancer cells. Attack by glutathione (GSH) on the 2,4-dinitroaryl ring results in the release of a diazeniumdiolate anion, which dissociates to form NO (Scheme 1).23 This process was shown to be catalyzed by GST and thus presents one plausible mechanism for selective generation of potentially cytotoxic nitric oxide in tumors.
Scheme 1.
Mechanism of nitric oxide release from PABA/NO, an O2-arylated diazeniumdiolate that is designed to be activated by reaction with glutathione.23 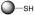 is a representative protein that can be S-glutathionylated and
is a representative protein that can be S-glutathionylated and 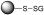 is a representative S-glutathionylated protein.
is a representative S-glutathionylated protein.
Addition of glutathione to the cysteine residues in proteins (S-glutathionylation) is a post-translational modification that alters protein structure and function.29–34 A variety of chemical pathways involving thiol oxidation or nitrosation have been proposed to account mechanistically for protein S-glutathionylation.35 Glutathionylation can serve as a protective mechanism against cysteine oxidation in proteins and is recognized as an important component of cellular redox signaling. Altered S-glutathionylation levels are observed in several pathological conditions such as melanoma, diabetes mellitus, HIV infection, and ischemia.30 S-Glutathionylation of several proteins has been reported; notable among these are PTP1B, caspase-3, HIV protease, p38, ERK, and JNK. In addition to activation of stress kinases such as p38 and JNK, PABA/NO (10 μM) also induced rapid (< 5 min) S-glutathionylation of ATP synthase, β-lactate dehydrogenase, protein disulfide isomerase, actin, and glucosidase-II. 24, 25
Recently, we reported the synthesis and study of a number of PABA/NO structural analogues.36 Abundant NO was released from most of these prodrugs when treated with GSH. Furthermore, it was observed that analogues that released higher amounts of NO had superior anti-proliferative activity, while analogues that formed negligible amounts of NO under these conditions were inactive against human leukemia HL-60 cells, suggesting that nitric oxide is a component of the cytotoxicity of such prodrugs. Hence, it was envisaged that any further increase of the levels of nitric oxide may result in prodrugs that have superior cytotoxic effects in comparison with PABA/NO. Furthermore, nitrosative stress caused by NO and its redox forms is also proposed to mediate protein S-glutathionylation by PABA/NO (Scheme 1), suggesting that increased nitric oxide levels in comparison with PABA/NO should also lead to higher extents of S-glutathionylation.24, 25
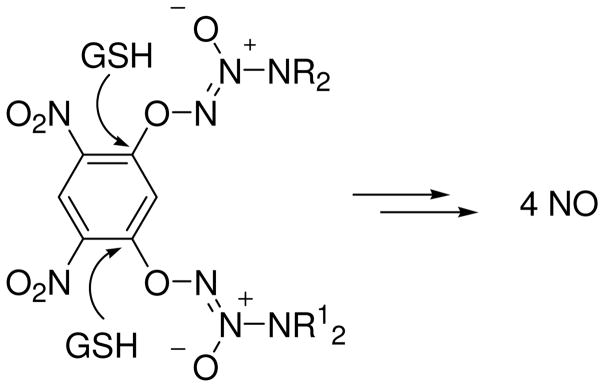
One strategy to increase the amount of NO released is to attach an additional diazeniumdiolate group to the aromatic ring; attack by two GSHs would result in the formation of up to 4 moles of NO per mole of prodrug (Scheme 2). Based on this design, we report the synthesis, glutathione-mediated nitric oxide release, S-glutathionylating ability, and in vitro anti-proliferative activity of a library of nitric oxide prodrugs possessing two diazeniumdiolate groups.
Scheme 2.
Design of bis(diazeniumdiolate) prodrugs that release elevated amounts of NO.
Results
Synthesis
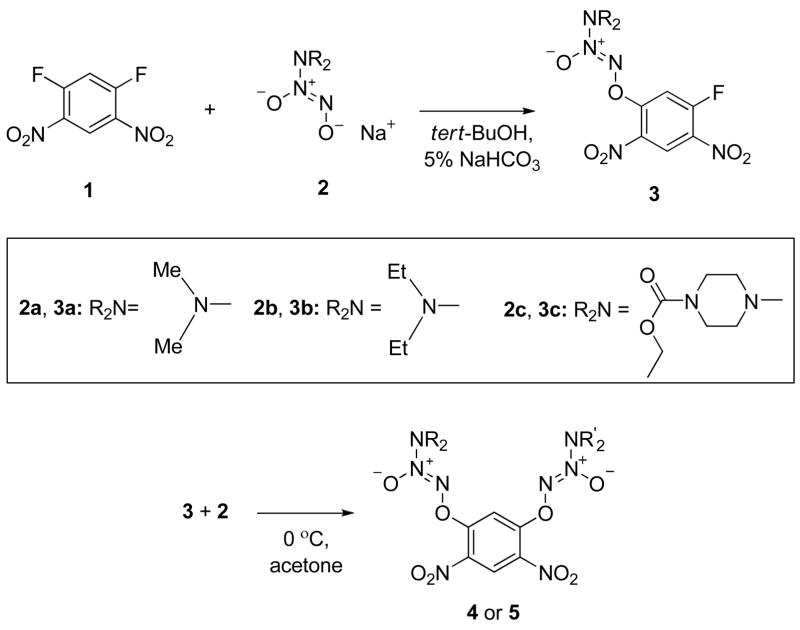
Earlier, we reported the synthesis of PABA/NO analogues from the sequential addition of a diazeniumdiolate ion then 4-aminobenzoic acid derivatives to 1,5-difluoro-2,4-dinitrobenzene.23 Using a similar strategy, we prepared the fluorides 3a, 3b, and 3c (Scheme 3). Treatment of 3a with a number of diazeniumdiolate anions (2b-2k, Table 1) afforded 4a-4j in excellent yields (Table 2). Using a similar procedure, compounds 5a, 5c, and 5d were prepared from 3b, again in excellent yields (Table 2); compound 5b was synthesized from 3c in high yield (Table 2).
Scheme 3.
Synthesis of bis(diazeniumdiolate) prodrugs.
Table 1.
Diazeniumdiolate Salts (R2N-N2O2−Na+) Used in the Synthesis of Aryl-bis(Diazeniumdiolates).
| R2N | compd | R2N | compd | R2N | compd |
|---|---|---|---|---|---|
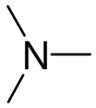
|
2a |
|
2e |
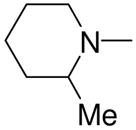
|
2i |
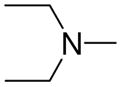
|
2b |
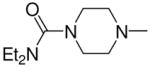
|
2f |
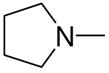
|
2j |
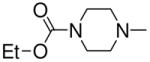
|
2c |
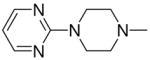
|
2g |
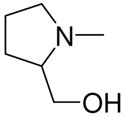
|
2k |
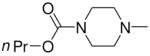
|
2d |
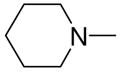
|
2h |
Table 2.
bis(Diazeniumdioalate) Prodrugs 4a-5d
| compd | R2N substituent | R2N substituent | precursors | yield (%) |
|---|---|---|---|---|
| 4a | Me2N– |
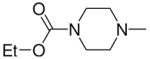
|
3a, 2c | 84 |
| 4b | Me2N– |
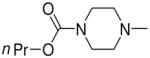
|
3a, 2d | 95 |
| 4c | Me2N– |
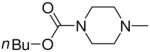
|
3a, 2e | 93 |
| 4d | Me2N– |
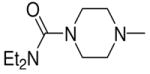
|
3a, 2f | 94 |
| 4e | Me2N– |
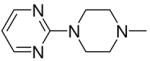
|
3a, 2g | 85 |
| 4f | Me2N– |
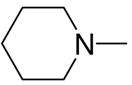
|
3a, 2h | 90 |
| 4g | Me2N– |
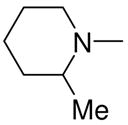
|
3a, 2i | 81 |
| 4h | Me2N– |
|
3a, 2j | 79 |
| 4i | Me2N– |
|
3a, 2k | 72 |
| 4j | Et2N– | Me2N– | 3a, 2b | 91 |
| 5a | Et2N– |
|
3b, 2c | 77 |
| 5b |
|
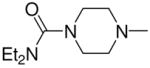
|
3c, 2f | 81 |
| 5c | Et2N– |
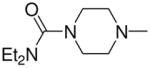
|
3b, 2f | 90 |
| 5d | Et2N– |
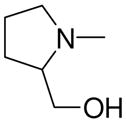
|
3b, 2k | 87 |
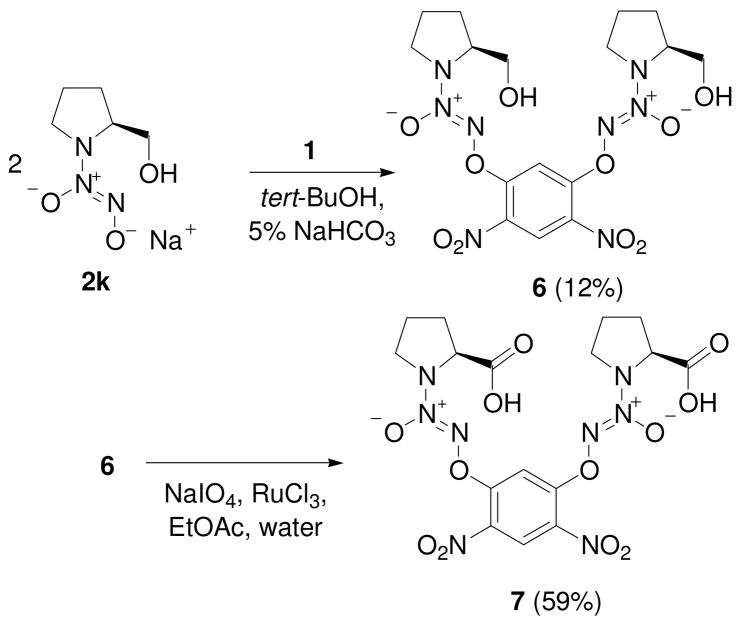
Finally, the diazeniumdiolate salt 2k was used to prepare compound 6 and a prodrug form of 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), 7 (Scheme 4).
Scheme 4.
Synthesis of bis(diazeniumdiolate) prodrug forms 6 and 7.
We assume that compound 4g is racemic, inasmuch as the supplier did not comment on its stereochemistry. Compounds 6, 5d, 4i, and 7 are assumed to be optically active and to have the configuration of the amino acid proline from which they were all ultimately prepared. The NMR spectra of 6 and 7 were those of single compounds, with no evidence of a second diastereomer that would be anticipated if significant racemization had occurred.
Glutathione-Activated Nitric Oxide Release
The amount of nitric oxide released from these compounds upon treatment with glutathione was determined (Table 3). PABA/NO released 1.4 moles of nitric oxide per mole under these conditions. A majority of these bis(diazeniumdiolate) prodrugs released at least twice as much NO as PABA/NO. Excellent yields (≥ 90 %) of nitric oxide from several compounds such as 4b, 4f, 4h, and 4j were observed.
Table 3.
Glutathione-Activated Nitric Oxide Release from bis(Diazeniumdiolate) Prodrugs and PABA/NO and Their in Vitro Anti-Proliferative Activity in the HL-60 Human Leukemia Cell Line.
| compound | moles of NO released | IC50 (μM)a | TGI (μM)b |
|---|---|---|---|
| PABA/NO | 1.4 | 3.9 | >10 |
| 4a | 2.7 | 1.2 | 2.0 |
| 4b | 3.7 | 1.6 | 3.9 |
| 4c | 3.1 | 0.9 | 2.7 |
| 4d | 3.5 | 1.9 | 4.9 |
| 4e | 3.5 | 1.2 | 5.0 |
| 4f | 3.7 | 1.7 | 4.9 |
| 4g | 3.0 | 1.5 | 4.9 |
| 4h | 3.6 | 2.3 | 4.9 |
| 4i | 3.0 | 1.0 | 2.0 |
| 4j | 3.8 | 1.0 | 2.3 |
| 5a | 3.1 | 2.1 | 5.0 |
| 5b | 2.9 | 0.9 | 3.0 |
| 5c | 3.0 | 2.9 | >10 |
| 5d | 3.0 | 1.8 | 4.0 |
| 6 | 2.5 | 4.0 | >10 |
| 7 | 3.5 | 9.6 | >10 |
IC50 = Concentration required to inhibit cell proliferation by 50 %.
TGI = Concentration providing total growth inhibition.
Anti-Proliferative Activity
Since PABA/NO has been shown to exhibit cytostatic activity against the leukemia HL-60 cell line,23 this line was used as an initial screen for the anti-proliferative activity of bis(diazeniumdiolates). All the PABA/NO analogues prepared in this work, excepting 7, were found to possess excellent anti-proliferative activity, with IC50 values ranging from 0.9 – 4.0 μM (mean 1.7 μM) and in most cases better than that of PABA/NO (Table 3).
Three analogues, 4a, 4i, and 5b, were then further tested for their anti-proliferative activity against a number of other human cancer cell lines. Leukemia U-937, non-small cell lung cancer H441 and A549, colon cancer HCT-116, HCT-15, and HT-29, ovarian cancer OVCAR-3 and OVCAR-5, and prostate cancer PC-3 cell lines were used for this study. Again, the compounds 4a, 4i and 5b were found to be superior inhibitors of cell proliferation and generally showed greater anti-proliferative activity in comparison with that of PABA/NO (Table 4), with the leukemia cell line appearing to be most sensitive.
Table 4.
Anti-Proliferative Activity of 4a, 4i, 5b and PABA/NO against Selected Cancer Cell Lines.
| 4a | 4i | 5b | PABA/NO | |||||
|---|---|---|---|---|---|---|---|---|
| cell line | IC50 (μM) | TGI (μM) | IC50 (μM) | TGI (μM) | IC50 (μM) | TGI (μM) | IC50 (μM) | TGI (μM) |
| U-937 | 1.3 | 3.0 | 0.8 | 1.2 | 0.9 | 3.0 | 2.7 | 5.5 |
| H441 | 5.0 | 7.0 | 2.1 | 4.5 | 1.7 | 4.0 | 4.9 | >10 |
| A549 | 9.1 | >30 | 5.1 | 6.0 | 3.9 | 10 | >10 | >10 |
| HCT-116 | 2.4 | 4.0 | 1.6 | 3.6 | 1.5 | 4.0 | 5.5 | >10 |
| HCT-15 | 2.5 | 4.0 | 1.7 | 3.3 | 1.6 | 4.5 | 5.7 | >10 |
| HT-29 | 4.5 | 10 | 2.4 | 4.5 | 2.5 | 6.5 | 7.0 | >10 |
| OVCAR-3 | 1.3 | 2.3 | 1.7 | 3.8 | 1.4 | 4.0 | 5.0 | 9.5 |
| OVCAR-5 | 3.3 | 9.0 | 2.5 | 4.0 | 1.9 | 5.5 | 7.8 | >10 |
| PC-3 | 5.0 | 7.0 | 3.3 | 7.0 | 3.3 | 8.0 | >10 | >10 |
Protein S-Glutathionylation
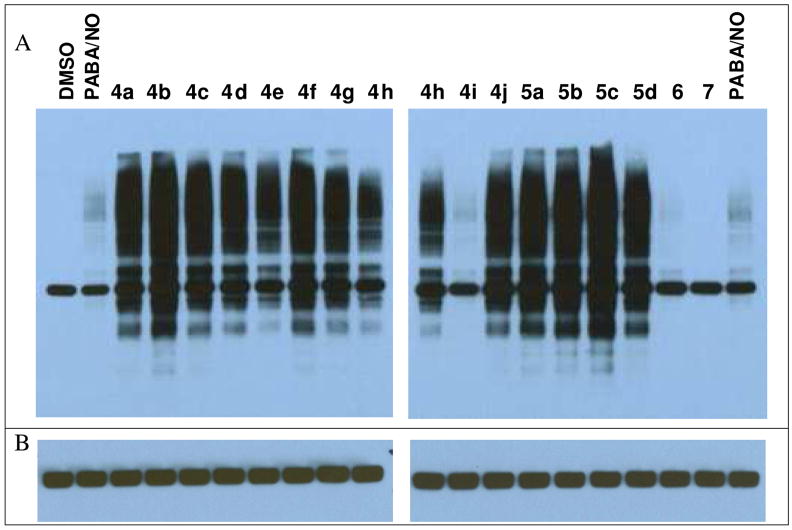
All but one of the bis(diazeniumdiolate) prodrugs induced rapid protein S-glutathionylation in human leukemia HL-60 cells (Figure 1). Treatment with compound 7 did not result in protein S-glutathionylation. The levels of S-glutathionylation for two compounds (4i and 6) were comparable to those of PABA/NO. The thirteen remaining compounds caused massive glutathionylation of intracellular proteins, much more intense than the levels resulting from PABA/NO.
Figure 1.
(A) Equal numbers of HL-60 cells were treated with 5 μM PABA/NO, with the bis(diazeniumdiolate) prodrugs, or with the vehicle DMSO. Ten micrograms of cellular protein lysates was separated by SDS-PAGE under nonreducing conditions and immunoblotted using anti-glutathione antibodies. (B) The immunoblots shown in (A) were stripped and re-probed with β-actin antibodies as a loading control. The band visible in the DMSO control in (A) is β-actin, which has been shown to be spontaneously glutathionylated at physiological conditions.37, 38
Intracellular Nitric Oxide Release
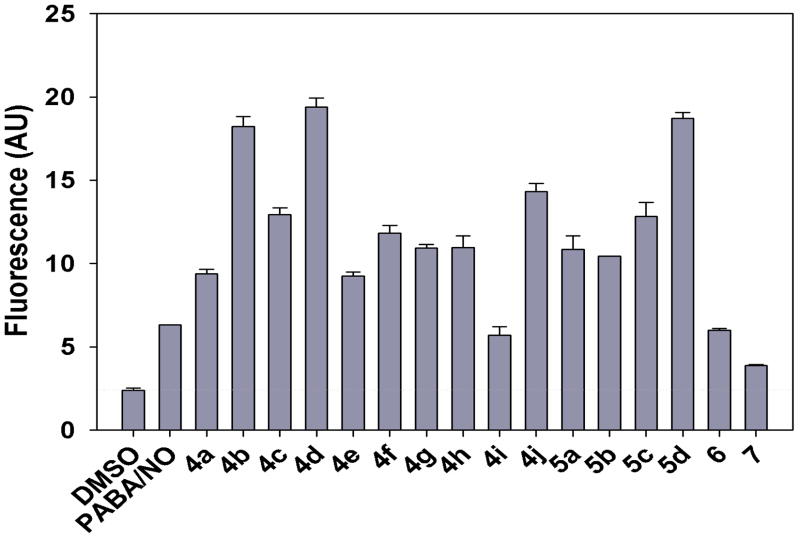
The intracellular level of nitric oxide after bis(diazeniumdiolate) treatment was quantified by measuring the extent of reaction with the nitric oxide-sensitive fluorophore, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate). HL-60 cells were pre-loaded with DAF-FM diacetate, followed by treatment with the bis(diazeniumdiolate) prodrugs, or the solvent (DMSO) control, or PABA/NO. Most bis(diazeniumdiolate) analogues of PABA/NO were found to produce significantly higher levels of fluorescence, indicating greater amounts of intracellular nitric oxide than that produced by PABA/NO (Figure 2). However, analogues 4i, 6, and 7 formed intracellular levels of NO comparable to or lower than those of PABA/NO. The amounts of NO released intracellularly did not correlate significantly with those obtained upon reaction with glutathione in simple phosphate buffer (P = 0.08). Lack of consistency between the buffered GSH solution and intracellular NO release measurements may reflect differences in cell permeability of individual compounds.
Figure 2.
Intracellular levels of NO measured as DAF-NO derivative fluorescence intensities in arbitrary units (AU). Error bars represent standard deviations of three independent experiments carried out with final concentration of the compound being 5 μM.
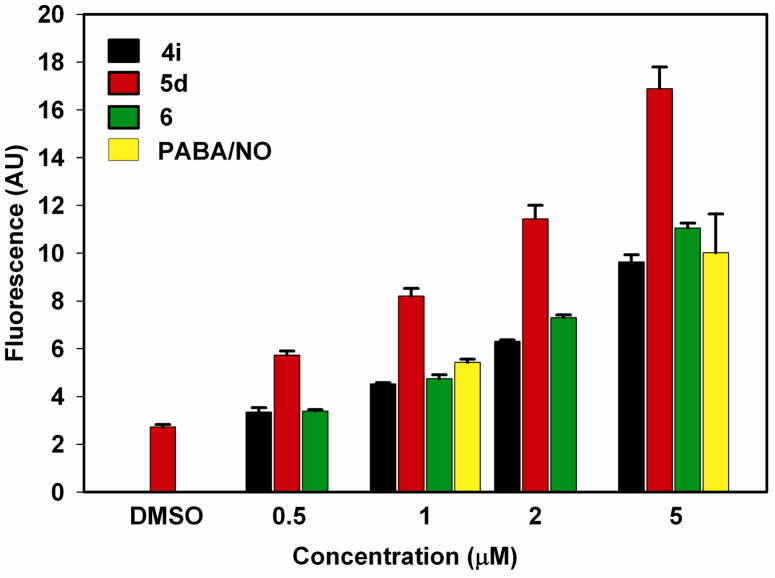
We were curious about the structure-property relationships among 4i, 5d, and 6. All three bear a prolinol diazeniumdiolate substitutent at one position of the dinitrophenyl ring, the difference being that the other substituent is the diazeniumdiolate of either dimethylamine (4i), diethylamine (5d), or a second prolinol (6). All three had low IC50 values in the HL-60 cell screen (1.0, 1.8, and 4.0 μM, respectively, Table 3), but the diethylamine derivative was far more active than the other two as an inducer of S-glutathionylation (Figure 1) and intracellular release of active NO (Figure 2). While we were confident that these were reproducible observations, a referee’s question led us to run an additional experiment In this case, the three compounds were compared with PABA/NO at four different concentrations ranging from 0.5–5 μM. The results, summarized in Figure 3, once again showed that 5d was substantially more active than the other three. The mechanistic origins of this difference remain to be determined.
Figure 3.
Intracellular levels of NO measured as DAF-NO derivative fluorescence intensities in arbitrary units (AU). Error bars represent standard deviations of three independent experiments.
Discussion
The results show that adding a second diazeniumdiolate group to the aromatic ring of previously reported arylated diazeniumdiolate prodrugs generally increased the amount of NO released (Scheme 5) on reaction with glutathione in neutral buffer solution relative to the mono(diazeniumdiolated) analogue PABA/NO, as expected, providing significantly higher anti-proliferative activity than PABA/NO across a diverse panel of tumor cell lines.
Scheme 5.
Proposed mechanism of nitric oxide release from bis(diazeniumdiolate) prodrugs.
Within the group of bis(diazeniumdiolates) reported here, however, no significant correlation between IC50 values and amounts of nitric oxide released upon treatment with GSH was derived. For example, the IC50 value of 4j, which released 3.8 moles of NO, was 1.0 μM while 4i showed a similar inhibitory effect but released only 3.0 moles of NO under similar conditions. While the analogue 6 showed good anti-proliferative activity against human leukemia HL-60 cells, the inhibitory activity of the PROLI/NO analogue 7 was diminished in comparison with all other prodrugs, although it formed 3.5 moles of NO when treated with GSH. This observation is consistent with our earlier finding that the 2,4-dinitrophenyl prodrug form of PROLI/NO did not significantly inhibit proliferation of HL-60 cells even at 50 μM.39
The total growth inhibition (TGI) index for several of these analogues was found to be less than 5 μM. Also, when one of the diazeniumdiolate groups was diazeniumdiolated dimethylamine 2a, the size and nature of the substituent at the 5-position of the aromatic ring did not appear to play any significant role in the anti-proliferative activity (IC50s of 4a-4j and 5a ranged from 0.9–2.3 μM; TGIs were 2–5 μM).
Intracellular NO release correlated well with the anti-proliferative activities and S-glutathionylating abilities, however. For example, from the intracellular NO release study with DAF-FM diacetate, compound 7, which had the lowest levels of fluorescence among the analogues, showed both diminished anti-leukemic activity and lowered S-glutathionylation levels. All compounds with superior TGIs (≤ 5 μM), with the exception of 4i, also formed greater intracellular levels of NO in comparison with PABA/NO (TGI > 10 μM). The intracellular NO release from 4i was comparable with that of the prolinol analogue 6 but both these analogues had diminished intracellular NO-releasing capacities in comparison with 5d. This observation was consistent over the concentration range of 0.5 – 5 μM (Figure 3). At this time, we are unable to provide a rationale to account for these observations. However, the levels of intracellular nitric oxide formed from the prolinol analogues 4i, 5d, and 6 correlated well with their S-glutathionylating abilities, suggesting that cell permeability is critical to the observed biological effects of this series of compounds.
Proliferation of the lung cancer cell line A549, which was resistant to PABA/NO, was significantly inhibited by 4i and 5b, whose IC50 values were 5.1 and 3.9 μM, respectively, and moderately inhibited by 4a (IC50 = 9.1 μM). Compound 4i was the most consistent inhibitor of proliferation in the range of cancer cell lines tested and in all cases, the TGI was less than 10 μM. Intracellular NO release and S-glutathionylation by 4i were at levels comparable to that of PABA/NO, showing that factors other than nitric oxide generation contribute to anti-proliferative activity.
Conclusions
A number of bis(diazeniumdiolated) dinitrobenzene derivatives that generate up to 4 moles of NO per mole of prodrug were synthesized and found to have excellent protein S-glutathionylating ability in human leukemia HL-60 cells. The anti-proliferative activities of a majority of these prodrugs were superior to those of PABA/NO, suggesting them as an improved class of anti-tumor agents with potent ability to induce S-glutathionylation of proteins in cells treated with them.
Experimental Section
NO was purchased from Matheson Gas Products (Montgomeryville, PA). Other starting materials were purchased from Aldrich Chemical Co. (Milwaukee, WI) unless otherwise indicated. NMR spectra were recorded on a Varian UNITY INOVA spectrometer; chemical shifts (d) are reported in parts per million (ppm) downfield from tetramethylsilane. Unless otherwise specified, the solvent used in all NMR experiments was CDCl3. Quantification of NO after treatment of compounds with glutathione (GSH) was carried out by chemiluminescence with a Thermal Energy Analyzer Model 502A instrument (Thermedics, Inc., Woburn, MA) or a Sievers 280i Nitric Oxide Analyzer (Boulder, CO). Ultraviolet (UV) spectra were recorded on an Agilent Model 8453 or a Hewlett-Packard model 8451A diode array spectrophotometer. Elemental analyses were performed by Midwest Microlab (Indianapolis, IN). Compounds 2a,40 2b,41 2c,42 2d-2e,20 2g,42 2h,41 2i,43 2j,44 2k,45 3a20 and 3c20 were prepared using reported methods.
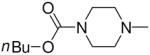
Sodium 1-(4-N,N-Diethylcarbamoyl-piperazin-1-yl)diazen-1-ium-1,2-diolate (2f)
Compound 2f was prepared according to reference 18, but the procedure reported for it there was incorrectly labeled as that of “ JS-K” in the Materials and Methods section of that paper. The correct procedure for 2f is as follows (summarized from reference 18). A solution of 5.3 g (0.029 mol) of N,N-diethylcarbamoylpiperazine in 15 mL of methanol was placed in a Parr bottle and treated with 6.7 mL (0.031 mol) of methanolic sodium methoxide. The solution was purged with 40 psi of NO and stirred at 25 °C for 72 h. The solid precipitate was filtered, washed with ether, and dried to give 4.54 g (60%) of diazeniumdiolate salt: UV (0.1 M NaOH) λmax (ε) 250 nm (9.8 mM−1 cm−1); 1H NMR (0.1 M NaOD in D2O) δ1.13 (t, J = 7.1 Hz, 6H), 3.13–3.16 (m, 4H), 3.27 (q, J = 7.1 Hz, 4H), 3.43–3.47 (m, 4H). 13C NMR (0.1 M NaOD in D2O) δ 29.7, 33.2, 36.5, 39.0 152.7.
O2-(2,4-Dinitro-5-fluorophenyl) 1-(N,N-Diethylamino)diazen-1-ium-1,2-diolate (3b)
A partial solution of 2 g (9.8 mmol) of 1,5-difluoro-2,4-dinitrobenzene in 25 mL of tert-butyl alcohol was placed in a round-bottom flask and stirred at room temperature. A solution of 0.70 g (4.4 mmol) of 2b in 15 mL of 5% sodium bicarbonate was added dropwise at a rapid rate. The reaction mixture was stirred for 30 min and a thick yellow solid was formed. Water was added, and the product was collected by filtration and recrystallized from methanol to give 3b (2.2 g, 70%): mp 59–60 °C; UV (ethanol) λmax (ε) 254 nm (12 mM−1cm−1); 1H NMR δ 1.26 (t, J = 7.1 Hz, 6H), 3.65 (q, J = 7.1 Hz, 4H), 7.44 (d, J = 11.7 Hz, 1H), 8.90 (d, J = 7.5 Hz, 1H); 13C NMR δ 11.40, 46.52, 106.31 (d, 2JC-F = 27.6 Hz), 130.71, 132.56, 155.19, 157.29, 160.01. Anal. (C10H12FN5O6) C, H, F, N.
General Procedure for the Synthesis of bis(Diazeniumdiolates)
A slurry of diazeniumdiolate salt (1.3 mmol) in 10 mL of acetone is added slowly to a solution of 1-diazeniumdiolated 5-fluoro-2,4-dinitrobenzene (1 mmol) in 10 mL of acetone that was previously cooled to 0 °C under nitrogen. The reaction mixture is allowed to warm gradually to room temperature. The progress of reaction is monitored by TLC. The product is extracted with dichloromethane and washed with 5% sodium bicarbonate solution. The organic layer is dried over anhydrous sodium sulfate, filtered and evaporated under vacuum to give the crude product. Purification is carried out by recrystallization or flash chromatography.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (4a)
A solution of 2c (282 mg, 1.3 mmol) in 10 mL of acetone was added to a solution of 3a (289 mg, 1 mmol) in 10 mL of acetone. Purification of the crude material was carried out on silica gel with 7:3 ethyl acetate:hexane as the eluant. The product 4a was obtained as an oil (410 mg, 84%): UV (ethanol) λmax (ε) 290 nm (22 mM−1m−1); 1H NMR δ 1.29 (t, J = 7.1 Hz, 3H), 3.28 (s, 6H), 3.61–3.64 (m, 4H), 3.71–3.74 (m, 4H), 4.18 (q, J = 7.1 Hz, 2H), 7.55 (s, 1H), 8.83 (s, 1H); 13C NMR δ 14.6, 41.8, 50.5, 62.1, 105.3, 125.5, 131.8, 131.9, 153.8, 154.2, 155.0. Anal. (C15H21N9O10) C, H, N.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(4-n-propoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (4b)
The product 4b (478 mg, 95 %) was obtained from 2d (254 mg, 1.00 mmol) and 3a (250 mg, 0.86 mmol). Purification of the crude material was carried out on silica gel with 10:1 dichloromethane:acetone as the eluant: mp 111–112 °C; UV (ethanol) λmax (ε) 290 nm (24 mM−1 cm−1); 1H NMR δ 0.97 (t, J = 7.2 Hz, 3H), 1.64–1.73 (m, 2H), 3.28 (s, 6H), 3.62–3.65 (m, 4H), 3.72–3.75 (m, 4H), 4.08 (t, J = 6.8 Hz, 2H), 7.54 (s, 1H), 8.82 (s, 1H); 13C NMR δ 10.2, 22.1, 41.6, 42.0, 50.3, 67.5, 104.7, 125.4, 131.2, 131.4, 153.8, 154.2, 155.0. Anal. (C16H23N9O10) C, H. N: calcd 25.14; found 24.62.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(4-n-butoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (4c)
A solution of 2e (270 mg, 1.00 mmol) in 10 mL of acetone was added to a solution of 3a (250 mg, 0.86 mmol) in 10 mL of acetone. Purification of the crude material was carried out by recrystallization from ethanol to give 4c (480 mg, 93%): mp 113–114 °C; UV (ethanol) λmax (ε) 290 nm (24 mM−1 cm−1); 1H NMR δ 0.95 (t, J = 7.3 Hz, 3H), 1.35–1.44 (m, 2H), 1.61–1.68 (m, 2H), 3.28 (s, 6H), 3.61–3.63 (m, 4H), 3.71–3.73 (m, 4H), 4.12 (t, J = 6.7 Hz, 2H), 7.55 (s, 1H), 8.83 (s, 1H); 13C NMR δ 13.7, 19.1, 30.9, 41.6, 42.1, 50.3, 65.9, 104.9, 125.4, 131.4, 131.6, 153.8, 154.2, 155.0. Anal. (C17H25N9O10) C, H. N: calcd 24.46; found 24.01.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[4-(N,N-diethylaminocarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (4d)
A solution of 2f (255 mg, 1.00 mmol) in 10 mL of acetone was added to a solution of 3a (250 mg, 0.86 mmol) in 10 mL of acetone. Purification of the crude material was carried out on silica gel with 7:3 ethyl acetate:hexane as the eluant. The product 4d was obtained as a pale yellow solid (487 mg, 94%): mp 133–134 °C; UV (ethanol) λmax (ε) 289 nm (25 mM−1cm−1); 1H NMR δ 1.15 (t, J = 7.1 Hz, 6H), 3.23–3.30 (m, 10H), 3.42 (t, J = 5.1 Hz, 4H), 3.63–3.68 (m, 4H), 7.57 (s, 1H), 8.85 (s, 1H); 13C NMR δ 13.2, 41.7, 41.8, 45.6, 50.2, 105.1, 125.5, 131.5, 131.7, 153.8, 154.0, 154.3, 163.6. Anal. (C17H26N10O9) C, H, N.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[4-(pyrimidin-2-yl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (4e)
A solution of 2g (200 mg, 0.81 mmol) in 8 mL of acetone was added to a solution of 3a (184 mg, 0.63 mmol) in 8 mL of acetone. Purification of the crude material was carried out by recrystallization from ethanol to give 4e (420 mg, 85%): mp 139–140 °C; UV (ethanol) λmax (ε) 290 nm (23 mM−1 cm−1); 1H NMR δ 3.28 (s, 6H), 3.69–3.72 (m, 4H), 4.07–4.09 (m, 4H), 6.61 (t, J = 4.8 Hz, 1H), 7.59 (s, 1H), 8.35 (d, J = 4.8 Hz, 2H), 8.86 (s, 1H); 13C NMR δ 41.8, 42.3, 50.5, 105.0, 111.1, 125.6, 131.6, 131.7, 154.0, 154.3, 157.8, 160.8. Anal. (C16H19N11O8) C, H, N.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-[(piperidin-1-yl)diazen-1-ium-1,2-diol-2-ato]benzene (4f)
The product 4f (375 mg, 90%) was obtained from 2h (250 mg, 0.86 mmol) and 3a (190 mg, 1.13 mmol). The crude compound 4f was purified by recrystallization from ethanol: mp 134–135 °C; UV (ethanol) λmax(ε) 292 nm (26 mM−1 cm−1); 1H NMR δ 1.57–1.63 (m, 2H), 1.80 (m, 4H), 3.28 (s, 6H), 3.61–6.64 (m, 4H), 7.59 (s, 1H), 8.86 (s, 1H); 13C NMR δ 23.2, 24.3, 29.7, 41.7, 51.6, 110.0, 125. 5, 131.5, 154.3. Anal. (C13H18N8O8) C, H, N.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-[(2-methylpiperidin-1-yl)diazen-1-ium-1,2-diol-2-ato]benzene (4g)
This reaction was carried out as described in the general procedure using 2i (260 mg, 1.45 mmol) and 3a (300 mg, 1 mmol). Purification of the crude material was carried out on silica gel with 7:3 ethyl acetate:hexane as the eluant to give 4g (350 mg, 81%): mp 114–115 °C; UV (ethanol) λmax (ε) 286 nm (22 mM−1 cm−1); 1H NMR δ 1.13 (d, J = 6.0 Hz, 3H), 1.44–1.62 (m, 3H), 1.66–1.74 (m, 1H), 1.80 (quintet, J = 6.0 Hz, 2H), 1.87–1.93 (m, 1H), 3.28 (s, 6H), 3.51–3.56 (m, 1H), 3.77–3.85 (m, 1H), 7.68 (s, 1H), 8.85 (s, 1H); 13C NMR δ 16.2, 21.1, 24.9, 31.7, 41.8, 51.2, 55.7, 105.6, 125.4, 131.8, 153.9, 154.3. Anal. (C14H20N8O8): C, H, N.
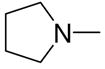
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-[(pyrrolidin-1-yl)diazen-1-ium-1,2-diol-2-ato]benzene (4h)
A solution of 2j (198 mg, 1.3 mmol) in 15 mL of acetone was added to a solution of 3a (289 mg, 1 mmol) in 10 mL of acetone. Purification of the crude material was carried out on silica gel with 10:1 dichloromethane:acetone as the eluant. The product 4h was obtained as a yellow powder (318 mg, 79%): mp 139–140 °C; UV (ethanol) λmax (ε) 293 nm (22 mM−1 cm−1); 1H NMR δ 2.05–2.09 (m, 4H), 3.27 (s, 6H), 3.74–3.78 (m, 4H), 7.58 (s, 1H), 8.86 (s, 1H); 13C NMR δ 23.4, 41.7, 50.5, 104.4, 125.5, 131.1, 131.2, 154.3, 154.7. Anal. (C12H16N8O8) C, H, N.
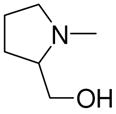
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-[(2-hydroxymethylpyrrolidin-1-yl)diazen-1-ium-1,2-diol-2-ato]benzene (4i)
A solution of 2k (475 mg, 2.6 mmol) in 15 mL of acetone was mixed with a solution of 3a (578 mg, 2 mmol) in 10 mL of acetone. Purification of the crude material was carried out on silica gel with 7:3 ethyl acetate:hexane as the eluant. The product 4i was obtained as an oil (290 mg, 72%): UV (ethanol) λmax (ε) 295 nm (21 mM−1cm−1); 1H NMR δ 2.00–2.20 (m, 5H), 3.28 (s, 6H), 3.71–3.77 (m, 2H), 3.83–3.92 (m, 2H), 4.33–4.37 (m, 1H), 7.58 (s, 1H), 8.86 (s, 1H); 13C NMR δ 23.2, 27.0, 41.7, 52.4, 64.2, 64.5, 104.3, 125.6, 131.0, 131.1, 154.3, 154.4. Anal. (C13H18N8O9) H, N. C: calcd 36.28; found 35.87.
1-[1-(N,N-Dimethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-[1-(N,N-diethylamino)diazen-1-ium-1,2-diol-2-ato]benzene (4j)
The product 4j (368 mg, 91%) was obtained from 2b (220 mg, 1.4 mmol) and 3a (300 mg, 1 mmol). Purification of the crude material was carried out by recrystallization from ethyl acetate:hexane (7:3): mp 130–131 °C ; UV (ethanol) λmax (ε) 292 nm (25 mM−1 cm−1); 1H NMR δ 1.26 (t, J = 7.0 Hz, 6H), 3.27 (s, 6H), 3.58 (q, J = 7.0 Hz, 4H), 7.59 (s, 1H), 8.88 (s, 1H); 13C NMR δ 11.6, 41.7, 46.9, 104.5, 125.6, 131.2, 131.1, 154.4, 154.5. Anal. C12H18N8O8 C, H, N.
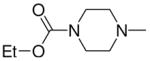
1-[1-(N,N-Diethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (5a)
A solution of 2c (390 mg, 1.79 mmol) in 10 mL of acetone was added to a solution of 3b (480 mg, 1.51 mmol) in 10 mL of acetone. Purification of the crude material was carried out on silica gel with 7:3 ethyl acetate:hexane as the eluant. The product 5a was obtained as an oil (400 mg, 77%): UV (ethanol) λmax (ε) 291 nm (24 mM−1 cm−1); 1H NMR δ 1.27 (t, J = 7.1 Hz, 6H), 1.29 (t, J = 7.2 Hz, 3H), 3.58–3.66 (m, 8H), 3.71–3.75 (m, 4H), 4.18 (q, J = 7.1 Hz, 2H), 7.57 (s, 1H), 8.86 (s, 1H); 13C NMR δ11.5, 14.5, 42.1, 46.8, 50.4, 62.0, 104.7, 125.5, 131.2, 131.5, 153.9, 154.4, 154.9. Anal. (C17H25N9O10) C, H, N.
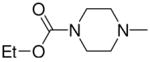
1-[4-(Ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[4-(N,N-diethylaminocarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (5b)
A solution of 3c (172 mg, 0.42 mmol) in 10 mL of acetone was added to a solution of 2f (154 mg, 0.60 mmol) in 10 mL of acetone. Purification of the crude material was carried out by recrystallization from ethanol to give 5b (215 mg, 81%): mp 94–95 °C; UV (ethanol) λmax (ε) 288 nm (26 mM−1 cm−1); 1H NMR δ1.16 (t, J = 6.8 Hz, 6H), 1.29 (t, J = 7.0 Hz, 3H), 3.26 (q, J = 7.0 Hz, 4H), 3.42–3.45 (m, 4H), 3.60–3.68 (m, 8H), 3.72–3.74 (m, 4H), 4.19 (q, J = 6.8 Hz, 2H), 7.55 (s, 1H), 8.81 (s, 1H); 13C NMR δ 13.4, 14.5, 41.7, 45.6, 50.2, 50.5, 62.0, 105.9, 125.4, 132.1, 153.7, 153.8, 154.9, 163.5. Anal. (C22H33N11O11) C, H, N.
1-[1-(N,N-Diethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(4-(N,N-diethyl-aminocarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (5c)
Product 5c (490 mg, 90%) was obtained from 2f (229 mg, 0.9 mmol) and 3b (240 mg, 0.75 mmol). Purification of the crude material was carried out on silica gel with 10:1 dichloromethane:acetone as the eluant to give 5c as a solid: mp 98–99 °C; UV (ethanol) λmax (ε) 291 nm (25 mM−1 cm−1); 1H NMR δ 1.15 (t, J = 6.8 Hz, 6H), 1.27 (t, J = 7.1 Hz, 6H), 3.26 (q, J = 7.1 Hz, 4H), 3.43 (m, 4H), 3.60 (q, J = 6.8 Hz, 4H), 3.63–3.67 (m, 4H), 7.58 (s, 1H), 8.87 (s, 1H); 13C NMR δ 11.5, 13.1, 41.7, 45.6, 46.7, 50.1, 104.5, 125.5, 131.0, 131.3, 154.0, 154.5, 163.5. Anal. (C19H30N10O9) C, H, N.
1-[1-(N,N-Diethylamino)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitro-5-{[(2-hydroxymethyl)-pyrrolidin-1-yl]diazen-1-ium-1,2-diol-2-ato}benzene (5d)
Product 5d (400 mg, 87%) was obtained from 2k (183 mg, 1.00 mmol) and 3b (412 mg, 1.3 mmol). Purification of the crude material was carried out on silica gel with 10:1 dichloromethane:acetone as the eluant: mp 116–117 °C ; UV (ethanol) λmax (ε) 297 nm (21 mM−1 cm−1); 1H NMR δ 1.25 (t, J = 7.1 Hz, 6H), 2.01–2.19 (m, 4H), 2.28 (t, J = 5.5 Hz, 1H), 3.58 (q, J = 7.1 Hz, 4H), 3.71–3.79 (m, 2H), 3.83–3.92 (m, 2H), 4.33–4.39 (m, 1H), 7.60 (s, 1H), 8.87 (s, 1H); 13C NMR δ 11.6, 23.2, 27.0, 46.9, 52.4, 64.0, 64.4, 104.2, 125.6, 131.0, 131.1, 154.4. Anal. (C15H22N8O9) C, H, N.
1,5-bis[(2-hydroxymethylpyrrolidin-1-yl)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitrobenzene (6)
Sodium 2-(hydroxymethylpyrrolidin-1-yl)diazen-1-ium-1,2-diolate, 2k (745 mg; 4.07 mmol), was dissolved in 5 mL of cold (4 °C) water and placed in a round bottom flask that had been immersed in an ice-water bath. A solution of 830 mg (4.07 mmol) of 1,5-difluoro-2,4-dinitrobenzene in tetrahydrofuran was prepared and to this was added 5 mL of tert-butyl alcohol. The resulting solution was added to the cold reagent and stirred at 0 °C for 15 min. The ice-bath was removed and the resulting yellow slurry was stirred overnight. Sodium bicarbonate solution (5%) was added and the resulting precipitate was collected by filtration to give 645 mg of a yellow solid. Flash chromatography on silica gel using 5:1 dichloromethane:ethyl acetate followed by 10:1 dichloromethane:methanol gave 243 mg (12%) of product: mp 131–132 °C; UV (ethanol) λmax (ε) 300 nm (22.5 mM−1 cm−1); 1H NMR δ1.99–2.21 (m, 10H), 3.76–3.81 (m, 4H), 3.83–3.91 (m, 4H), 4.34–4.39 (m, 2H), 7.54 (s, 1H), 8.88 (s, 1H); 13C NMR δ 23.1, 27.2, 52.4, 64.3, 64.4, 103.8, 125.7, 130.9, 154.6. Anal. (C16H22N8O10) C, H, N.
1,5-bis[(2-Carboxylatopyrrolidin-1-yl)diazen-1-ium-1,2-diol-2-ato]-2,4-dinitrobenzene (7)
To a solution of 6 (160 mg, 0.33 mmol) in 2 mL of acetonitrile and 2 mL of ethyl acetate were added 564 mg (2.64 mmol) of sodium periodate and 4 mL of water, followed by 4.6 mg (0.02 mmol) of ruthenium trichloride. The mixture was stirred at room temperature for 2 h. The reaction mixture was filtered, diluted with ethyl acetate and washed with water. The bis-carboxylic acid was extracted from the organic layer with 5% sodium bicarbonate. The aqueous layer was acidified with dilute HCl and extracted with ethyl acetate, dried over sodium sulfate, filtered and evaporated to give a solid. The solid was taken up in dichloromethane giving a cloudy solution that was filtered through magnesium sulfate and concentrated under vacuum to give 100 mg (59%) of product: mp 135–136 °C; UV (EtOH) λmax (ε) 295 nm (23 mM−1cm−1); 1H NMR δ 2.02–2.05 (m, 6H), 2.33–2.39 (m, 2H), 3.90 (broad, 4H), 4.71–4.73 (m, 2H), 7.55 (s, 1H), 8.88 (s, 1H), 13.17 (b, 1H); 13C NMR δ22.0, 27.9, 50.5, 61.8, 102.6, 125.7, 130.3, 154.1, 171.8. Anal. (C16H18N8O12·0.2 EtOAc) C, H, N.
Nitric Oxide Release
Chemiluminescence detection and quantification of NO evolving from the reactions of PABA/NO and its analogues were conducted by preparing 10 mM stock solutions of these compounds in pH 7.4 phosphate buffer. A pH 7.4 0.1 M phosphate buffer (3 mL) containing 50 μM diethyltriaminepentaacetic acid and GSH (4 mM) was sparged into the chemiluminescence detector with inert gas until a steady detector response was established. The stock solution of the diazeniumdiolate prodrug (20–100 μL, 0.31–1.87 μM) was injected into this GSH solution and the NO release profile was followed at 37 °C. Nitric oxide release was monitored until baseline signal was achieved (25 min – 5 h). The resulting curve was integrated to quantify the amount of NO released/mol of compound.
Intracellular NO Release
The intracellular level of nitric oxide after bis-(diazeniumdiolate) prodrug treatment was quantified using the NO-sensitive fluorophore 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate) (Invitrogen, Carlsbad, CA). HL-60 cells were loaded with 2.5 μM DAF-FM diacetate in Hanks’ balanced salt solution (HBSS) at 37 °C and 5% CO2. After 30 min of incubation the cells were collected by centrifugation, rinsed with HBSS to remove excess of probe, and resuspended in fresh HBSS. The compounds were added to the cells at 5 μM final concentration. After 40-min incubation the fluorescence of the benzotriazole derivative formed on DAF-FM’s reaction with aerobic NO was analyzed using a Perkin Elmer LS50B luminescence spectrometer with the excitation source at 495 nm and emission at 515 nm.
Cell Culture and Cytotoxicity Assays
Cell lines HL-60, U-937, H441 and A549 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and HCT-116, HCT-15, HT29, OVCAR-3, OVCAR-5 and PC-3 were from the NCI-Frederick DCTD Tumor/Cell Line Repository (Frederick, MD). Cells were maintained in RPMI 1640 medium (Gibco, Invitrogen, Carlsbad, CA), supplemented with 10% fetal calf serum (Gemini Bio-Products, Sacramento, CA), 100 U/mL penicillin/streptomycin and 2 mM glutamine at 37 °C and 5% CO2.
The CellTiter 96 non-radioactive cell proliferation assay (MTT assay; Promega, Madison, WI), performed according to the manufacturer’s protocol, was used to measure cell proliferation. Cells were seeded in 96-well plates at a density 104 per well and allowed to grow for 24 h before addition of the drugs. bis(Diazeniumdiolates) were prepared as 10 mM stock solution in dimethyl sulfoxide (Sigma, St. Louis, MO). Increasing drug concentrations in 10 μL of phosphate-buffered saline (PBS) were added to 100 μL of the culture medium and incubated at 37 °C for 72 h. Each compound concentration was represented in six repeats, and the screening was performed as at least two independent experiments.
S-Glutathionylating Activities of bis(Diazeniumdiolates)
HL-60 cells in complete growth medium (RPMI 1640 supplemented with 10% fetal bovine serum, glutamine and penicillin/streptomycin) were treated with 1 μM compound, or an equal volume of dimethyl sulfoxide as a control, for 15 min. Cells were collected by centrifugation, washed with PBS and prepared for immunoblotting by lysing in lysis buffer (25 mM Hepes buffer, pH 7.2, containing 150 mM NaCl, 10 mM MgCl2, 1% Nonidet P40, 0.25% sodium deoxycholate, 10% glycerol, 2.5 mM EDTA, and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Proteins in cell lysates were separated under nonreducing conditions on 4–12% NuPage Bis-Tris gels (Invitrogen Life Technologies, Carlsbad, CA) and transferred to PVDF membranes (Invitrogen) at 30 V for 1.5 h. After overnight blocking in 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T), the membranes were probed with anti-glutathione monoclonal antibodies (1:1000, Virogen, Watertown, MA) in 5% milk in TBS-T for 3 h at room temperature. After repeated washings in TBS-T, the blots were incubated for 60 min at room temperature with anti-mouse secondary antibody, 1:1000, conjugated with horseradish peroxidase (GE Healthcare, Piscataway, NJ), in 5% milk in TBS-T, then, after washing, developed using the chemiluminescence ECL kit (GE Healthcare, Piscataway, NJ). To show equal loading the blots were stripped with stripping buffer (Thermo Scientific, Rockford, IL) and re-probed with anti-beta-actin rabbit polyclonal antibodies (Abcam, Inc., Cambridge, MA).
Supplementary Material
Results from elemental analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as by National Cancer Institute contract N01-CO-12400 to SAIC-Frederick.
Footnotes
Abbreviations: NO, nitric oxide; PABA/NO, O2-{2,4-dinitro-5-[4-(N-methylamino)benzoyloxy]phenyl} 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate; GST, glutathione S-transferase; GSH, glutathione; DFM-FM diacetate, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate; DMSO, dimethyl sulfoxide; DEA/NO, sodium 1-(N,N-diethylamino)diazen-1-ium-1,2-diolate; DMA/NO, sodium 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate; PROLI/NO, disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate; HBSS, Hanks’s balanced salt solution; TBS-T, Tris-buffered saline-Tween 20; PBS, phosphate buffered saline; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
- 1.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in vascular system: An overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ. Nitric Oxide: A unique endogenous signaling molecule in vascular biology (Nobel Lecture) Angew Chem Int Ed Engl. 1999;38:1882–1892. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1882::AID-ANIE1882>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Herman AG, Vanhoutte P. Endothelium-derived relaxing factor is identified as nitric oxide. Trends Pharmacol Sci. 1987;8:365–368. [Google Scholar]
- 4.Moncada S, Radomski MW, Palmer RMJ. Endothelium-derived relaxing factor: Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 6.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling (Nobel Lecture) Angew Chem Int Ed Engl. 1999;38:1856–1868. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1856::AID-ANIE1856>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resistance Updates. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–1884. [PubMed] [Google Scholar]
- 9.Shami PJ, Moore JO, Gockerman JP, Hathorn JW, Misukonis MA, Weinberg JB. Nitric oxide modulation of the growth and differentiation of freshly isolated acute non- lymphocytic leukemia cells. Leukemia Res. 1995;19:527–533. doi: 10.1016/0145-2126(95)00013-e. [DOI] [PubMed] [Google Scholar]
- 10.Shami PJ, Sauls DL, Weinberg JB. Schedule and concentration-dependent induction of apoptosis in leukemia cells by nitric oxide. Leukemia. 1998;12:1461–1466. doi: 10.1038/sj.leu.2401131. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, Haney AF, Granger DL. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 12.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1- ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 13.Keefer LK. Nitric oxide (NO)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): Emerging commercial opportunities. Curr Top Med Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 14.Scatena R, Bottoni P, Martorana GE, Giardina B. Nitric oxide donor drugs: An update on pathophysiology and therapeutic potential. Expert Opin Investig Drugs. 2005;14:835–846. doi: 10.1517/13543784.14.7.835. [DOI] [PubMed] [Google Scholar]
- 15.King SB. Mechanisms and novel directions in the biological applications of nitric oxide donors. Free Radic Biol Med. 2004;37:735–736. doi: 10.1016/j.freeradbiomed.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Thatcher GRJ. An introduction to NO-related therapeutic agents. Curr Top Med Chem. 2005;5:597–601. doi: 10.2174/1568026054679281. [DOI] [PubMed] [Google Scholar]
- 17.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li CQ, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS, Saavedra JE, Keefer LK, Waalkes MP. Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol Cancer Ther. 2004;3:709–714. [PubMed] [Google Scholar]
- 19.Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI. JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways. J Cell Physiol. 2003;197:426–434. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- 20.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4- ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 21.Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK. JS- K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Ther. 2003;2:409–417. [PubMed] [Google Scholar]
- 22.Udupi V, Yu M, Malaviya S, Saavedra JE, Shami PJ. JS-K, a nitric oxide prodrug, induces cytochrome c release and caspase activation in HL-60 myeloid leukemia cells. Leukemia Res. 2006;30:1279–1283. doi: 10.1016/j.leukres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, Wilde TC, Citro ML, Cuellar M, Deschamps JR, Parrish D, Shami PJ, Findlay VJ, Townsend DM, Tew KD, Singh S, Jia L, Ji X, Keefer LK. PABA/NO as an anticancer lead: Analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem. 2006;49:1157–1164. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, Tew KD. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide- releasing prodrug. Mol Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, Ji X, Keefer LK, Tew KD. A glutathione S-transferase π–activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong RN. Glutathione S-transferases: Reaction mechanism, structure, and function. Chem. Res. Toxicol. 1991;4:131–140. doi: 10.1021/tx00020a001. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong RN. Glutathione S-transferases: Structure and mechanism of an archetypical detoxication enzyme. Adv Enzymol Relat Areas Mol Biol. 1994;69:1–44. doi: 10.1002/9780470123157.ch1. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 29.Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-Glutathionylation: From redox regulation to human diseases. J Cell Mol Med. 2008;8:201–212. doi: 10.1111/j.1582-4934.2004.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghezzi P, Di Simplicio P. Glutathionylation pathways in drug response. Curr Opin Pharmacol. 2007;7:398–403. doi: 10.1016/j.coph.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein gluathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Tew KD. Redox in redux: Emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem Pharmacol. 2007;73:1257–1269. doi: 10.1016/j.bcp.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Ruiz A, Lamas S. Signalling by NO-induced protein S-nitrosylation and S- glutathionylation: Convergences and divergences. Cardiovasc Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxidants Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 36.Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Synthesis, nitric oxide release, and anti-leukemic activity of glutathione-activated nitric oxide prodrugs: Structural analogues of PABA/NO, an anti-cancer lead compound. Bioorg Med Chem. 2008;16:2657–2664. doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: Investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci U S A. 2003;100:5103–5106. doi: 10.1073/pnas.0931345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 39.Chakrapani H, Goodblatt MM, Udupi V, Malaviya S, Shami PJ, Keefer LK, Saavedra JE. Synthesis and in vitro anti-leukemic activity of structural analogues of JS-K, an anti- cancer lead compound. Bioorg Med Chem Lett. 2008;18:950–953. doi: 10.1016/j.bmcl.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flippen-Anderson JL, Rice WG, Turpin JA, Davies KM, Keefer LK. The secondary amine/nitric oxide complex ion R2N[N(O)NO]– as nucleophile and leaving group in SNAr reactions. J Org Chem. 2001;66:3090–3098. doi: 10.1021/jo0016529. [DOI] [PubMed] [Google Scholar]
- 41.Drago RS, Karstetter BR. The reaction of nitrogen(II) oxide with various primary and secondary amines. J Am Chem Soc. 1961;83:1819–1822. [Google Scholar]
- 42.Saavedra JE, Booth MN, Hrabie JA, Davies KM, Keefer LK. Piperazine as a linkerfor incorporating the nitric oxide-releasing diazeniumdiolate group into other biomedically relevant functional molecules. J Org Chem. 1999;64:5124–5131. doi: 10.1021/jo9901539. [DOI] [PubMed] [Google Scholar]
- 43.Chakrapani H, Showalter BM, Citro ML, Keefer LK, Saavedra JE. Nitric oxide prodrugs: Diazeniumdiolate anions of hindered secondary amines. Org Lett. 2007;9:4551–4554. doi: 10.1021/ol7019636. [DOI] [PubMed] [Google Scholar]
- 44.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-a-induced apoptosis and toxicity in the liver. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 45.Chakrapani H, Showalter BM, Kong L, Keefer LK, Saavedra JE. V-PROLI/NO, a prodrug of the nitric oxide donor, PROLI/NO. Org Lett. 2007;9:3409–3412. doi: 10.1021/ol701419a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results from elemental analysis. This material is available free of charge via the Internet at http://pubs.acs.org.