Abstract
Background
Inequalities in the use of new medications may contribute to health disparities. We analyzed socioeconomic gradients in the use of tiotropium for chronic obstructive pulmonary disease (COPD).
Methods
In a cohort of adults with COPD aged ≥55 years identified through population-based sampling, we elicited questionnaire responses on demographics, socioeconomic status (SES; lower SES defined as high school education or less or annual household income <US $20,000), and medication use and other clinical variables. In a subset we obtained pulmonary function testing. We used multiple logistic regression analysis to estimate the associations between SES and tiotropium use in COPD, adjusting for disease severity measured by a COPD Severity Score.
Results
Of 427 subjects, 44 (10.3%) reported using tiotropium in 2006. Adjusting for COPD severity, lower SES was associated with reduced odds of tiotropium use (OR 0.3; 95% CI 0.1–0.7; p = 0.005). Among the subset with lung function data (n = 95), after including COPD Global Obstructive Lung Disease (GOLD) Stage ≥2 in the model, lower SES remained associated with reduced odds of tiotropium use (OR 0.03; 95% CI < 0.001–0.7; p = 0.03). Including forced expiratory volume in one second in the model as a continuous variable instead of GOLD Stage ≥2 yielded similar results for lower SES (OR 0.1; 95% CI < 0.001–0.5; p = 0.02).
Conclusion
There was a strong SES gradient in tiotropium use such that there was less use with lower SES. To the extent that this is an efficacious medication for COPD, this gradient represents a potential source of health disparities.
Keywords: socioeconomic status, COPD, tiotropium, medication, health gradients
Introduction
The introduction of novel and efficacious medications can be a major factor in reduced disease morbidity and mortality on a population-wide basis. The salutatory health impact of such innovations, however, depends on availability and use of the drug among those for whom such use is medically indicated and a benefit is anticipated. Health disparities in disease prevalence and severity remain a major public health issue in the US, despite an enormous expenditure of resources on health care generally and prescription pharmaceuticals in particular (Warren and Hernandez, 2007; Adler and Rehkopf 2008). The role that socioeconomic status (SES) may play in potential health disparities related to the use of newly approved prescription drugs could be especially relevant to this question, but has not been well studied.
Tiotropium, a long-acting analogue of the inhaled anticholinergic medication ipratropium bromide introduced in the US in 2004, is one example of a relatively new pharmaceutical with rapid dissemination in prescribing (Anon 2004). The approved indication for tiotropium use is chronic obstructive lung disease (COPD), a relatively common and potentially life-threatening condition. COPD is causally associated with cigarette smoking and with adverse working conditions, both of which, in turn, manifest strong SES gradients (Mannino and Buist 2007). It can be anticipated that many of those most likely to benefit from this medication’s introduction are of lower SES. Thus any SES gradient in tiotropium use in treating COPD could be especially relevant to the broader question of new pharmaceutical-related SES gradients in health.
SES gradients in tiotropium use were examined among our COPD cohort. Using data from 2006 interviews after tiotropium was relatively new in the market but its use was well established, we analyzed the association between SES and tiotropium use, taking into account disease severity.
Methods
Overview
We used structured interview data from a single wave of a longitudinal study in a cohort of older adults with airway disease. Although the total cohort includes persons with other airway disease diagnoses, we limited this analysis to those who reported receiving at least one of three COPD-defining physician diagnoses: COPD, emphysema, or chronic bronchitis. Interviews assessed demographics and extensive health-related data, including a detailed respiratory medication check-list. A subset of subjects also participated in home visits in which spirometry measurements were obtained. The a priori goals of the parent study from which we drew are data were to assess occupational and nonoccupational impairments and health outcomes in lung disease. Because one wave of interviews were carried out following the introduction of a new COPD prescription medication in the United States, we saw an opportunity to examine factors associated with the use of this drug. Specifically, we analyzed self-reported use of tiotropium in relation to demographics and COPD-related covariates. All key analyses were carried out for all COPD subjects together and then repeated excluding subjects who only reported chronic bronchitis, in order to evaluate whether exclusion of this COPD-related diagnosis affected our risk factor estimates. The study was approved by the University of California SF Committee on Human Research.
Subject recruitment and retention
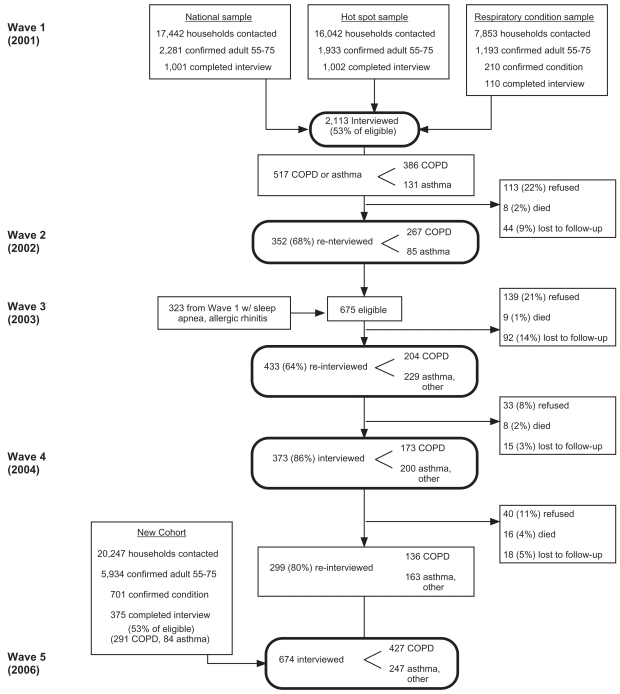
We used cross-sectional data from the 2006 interview Wave 5 only of a population-based, multi-wave longitudinal cohort study of US adults with airways disease that includes a subset of persons with COPD. The details of subject recruitment and retention over five waves of interview history from 2001 through 2006 are summarized in Figure 1. Analyses based on earlier interview waves have been reported previously (Trupin et al 2003; Blanc et al 2004; Chen et al 2006). The 427 interviews used in this analysis included 136 persons with COPD retained through four previous interview waves and 291 persons with COPD newly recruited.
Figure 1.
Recruitment and retention of study subjects.
Interview structure and content
The same questionnaire was administered to all subjects in the 2006 interviews used in this analysis. Structured interviews were performed by trained personnel using computer-assisted telephone interviewing (CATI) software. Interviews queried demographics, smoking, and respiratory condition variables such as symptoms, health care utilization (including subspecialty care by a pulmonary, allergy, or otolaryngology provider in the previous 12 months), insurance coverage for medication costs (none, some, or all costs covered), supplemental oxygen use, and an extensive checklist of respiratory medications used at any time in the pervious two weeks. These medications included various specific brands and generic forms of corticosteroids (oral and inhaled), short- and long-acting beta agonists, corticosteroid-beta agonist combinations, ipratropium bromide, and tiotropium. Where appropriate to efficiently elicit interview responses, brand names were queried. This included “Spiriva®” for the generic medication tiotropium.
We used interview data to calculate COPD Severity Score values for each subject (Eisner et al 2005, 2007). This validated measure uses a weighted algorithm to score multiple components: recent symptoms, current medications, home supplemental oxygen use, past systemic corticosteroid and antibiotic use, and prior hospitalizations and mechanical ventilatory support for respiratory disease. The possible range is 0 to 35 points; standard deviation is approximately 8 points in magnitude; and higher scores indicate greater COPD severity. Because the standard COPD Severity Score includes a one point weighting for tiotropium use, for this analysis we omitted that component so that COPD severity could be analyzed independent of such use.
Medication verification and spirometric assessment
A subset of subjects participated in home visits during which medications were confirmed by direct inspection and spirometry was assessed. To be eligible for home visits, subjects must have resided in northern California at the time of their telephone interviews. From among those geographically eligible, we attempted to recruit an approximate 50% sample for Wave 5 home visits. There were 101 (24%) of 427 subjects with COPD who ultimately participated in spirometry testing carried out in home visits following their Wave 5 interviews. The time interval elapsed between interview and spirometry averaged 5.2 ± 2.3 weeks (maximum 13 weeks). Medication use was confirmed by requesting that subjects show to the home study team all respiratory medications.
Spirometry was performed using an EasyOne™ Spirometer (ndd Medical Technologies, Chelmsford, MA). This instrument has been used widely in field studies of COPD (Menezes et al 2005; Buist et al 2007). Spirometry measurements, including the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were performed conforming to American Thoracic Society guidelines (Miller et al 2005). Subjects did not perform testing following study-administered bronchodilators; they were told to take their normal bronchodilator medicines according to their routine schedule on the day of the home visit. We used standard predictive equations to calculate age, height, and sex predicted FEV1 % predicted values (Hankinson et al 1999). We used Global Obstructive Lung Disease (GOLD) criteria (although not post-bronchodilator values) to define COPD as Stage II or above (FEV1 % predicted <80%; FEV1 to FVC ratio <70%) (GOLD 2006).
Data analysis
We tested differences in subject characteristics between tiotropium users and nonusers using the chi-square, Fisher’s exact test, or Student’s t-test. We used multiple logistic regression analysis to test the associations between low SES (using the measures of educational achievement of a high school education or less [HS]) or HS or an annual household income of less than US $20,000) and the use of tiotropium. All models also included age, sex, and indicator variables for each of up to three reported physician’s COPD condition diagnoses (COPD, emphysema, chronic bronchitis; overlapping, multiple reported diagnoses were possible). In addition, subjects could also report asthma as a condition if this was concomitant with at least one of the COPD diagnoses (those with asthma alone were excluded from this analysis). Therefore, we also included asthma as a separate condition indicator variable.
Additional models included COPD Severity Score. All key analyses were rerun excluding subjects who reported chronic bronchitis as their sole COPD condition. As another test of possible confounding we also re-analyzed our main COPD Severity Score models adding the following additional variables: race-ethnicity (White non-Hispanic vs. all others); regional U.S. geographic location (Northern California vs. all others); access to subspecialty care (visit to a pulmonary, allergy or otolaryngology specialist in the previous 12 months); and any insurance coverage of medication costs (any vs. none). We retested multiple logistic regression models including all of these predictors in the subset of subjects for whom spirometry data were available. We did this in two ways: adding to the previous models FEV1 % predicted as a continuous independent variable and, alternatively, entering GOLD Stage II or above COPD as a dichotomous predictor variable.
Results
Table 1 shows the distribution of key demographic and clinical variables, for the entire study cohort and after excluding 207 subjects who reported chronic bronchitis alone without either concomitant COPD or emphysema. Even among the latter group, tiotropium use was reported by fewer than 1 in 5 subjects. The two measures of lower SES of interest (HS education or less and annual household income <$20,000) were well represented among study subjects and were inter-related. Among 405 with income data, 51 (19%) of 275 with more than a HS education reported annual household incomes <$20,000, compared with 55 (42%) of 130 with HS or less (p < 0.001). Altogether 189 (44%) of subjects had either attained HS or less education or reported <$20,000 income (14 subjects with greater than HS education were in the missing income group and were dichotomized as higher SES by this measure).
Table 1.
Demographic and medical characteristics among study cohort
| Characteristic | COPD, emphysema or chronic bronchitis [n = 427] | COPD or emphysema* [n = 240] |
|---|---|---|
| Female gender | 266 (62%) | 139 (58%) |
| Age >65 Years | 227 (53%) | 147 (61%) |
| White, non-Hispanic | 366 (86%) | 205 (85%) |
| High school education or less (HS) | 138 (32%) | 95 (40%) |
| Annual household income <$20,000† | 106 (26%) | 65 (29%) |
| HS or income <$20,000 | 189 (44%) | 120 (50%) |
| Lives in northern Californian region | 305 (71%) | 175 (73%) |
| Cigarette smoking status | ||
| Former | 240 (56%) | 150 (63%) |
| Current | 94 (22%) | 66 (28%) |
| Never | 93 (22%) | 24 (10%) |
| Respiratory diagnosis‡ | ||
| COPD | 146 (34%) | 146 (61%) |
| Emphysema | 166 (39%) | 166 (69%) |
| Chronic bronchitis | 304 (71%) | 117 (49%) |
| Concomitant asthma‡ | 215 (50%) | 123 (51%) |
| Home oxygen use | 72 (17%) | 65 (27%) |
| Inhaled or oral corticosteroid use | 103 (24%) | 78 (33%) |
| Long-acting beta agonist use | 108 (25%) | 85 (35%) |
| Some medication insurance coverage | 389 (91%) | 216 (90%) |
| Medical subspecialist treatment¶ | 130 (30%) | 91 (38%) |
| Tiotropium use | 44 (10%) | 41 (17%) |
Notes: Bronchitis alone without concomitant COPD or emphysema excluded;
Income data missing for 22 of 427 subjects in entire study group; 13 of 240 subjects in COPD/emphysema stratum;
Multiple diagnoses can be reported. For asthma, a concomitant diagnosis of COPD, emphysema, or chronic bronchitis must be reported;
Treatment in the past 12 months by a pulmonary, allergy, or otolaryngology specialist.
Abbreviation: COPD, chronic obstructive pulmonary disease.
In unadjusted analyses (Table 2), the only demographic variable statistically significantly associated with tiotropium use was older age: 13% of subjects over age 65 reported tiotropium use compared to 7% of those 65 or younger (p = 0.05). Tiotropium use was related to each co-morbid respiratory diagnosis, except for chronic bronchitis. Home supplemental oxygen (home O2) was strongly associated with tiotropium use. Even among home O2 users, only one-third reported using tiotropium. Long-acting beta agonist (LABA) use had a similar pattern to that of home O2 with tiotropium. Those treated by subspecialists were more likely to report tiotropium use (p = 0.002). As shown in the lower portion of Table 2, among the subset of subjects reporting COPD or emphysema (n = 240; excludes subjects with chronic bronchitis as their sole COPD-defining condition), tiotropium use did not differ statistically by demographic variables or by any respiratory condition. In this subset of subjects, however, home O2 and LABA use remained statistically associated with tiotropium use, while subspecialty care was less strongly associated in this subset (p = 0.08).
Table 2.
Subject demographics, clinical status, and tiotropium use
| COPD, emphysema, or chronic bronchitis (n = 427) | |||
|---|---|---|---|
| Subject characteristic | Tiotropium use (%) if Subject characteristic…
|
P value | |
| Present | Absent | ||
| Female gender | 11% | 9% | 0.49 |
| Age >65 years | 13% | 7% | 0.05 |
| White, non-Hispanic | 11% | 7% | 0.37 |
| High school education or less (HS) | 11% | 10% | 0.92 |
| Annual household income <$20,000* | 7% | 11% | 0.26 |
| HS or household income <$20,000 | 9% | 11% | 0.53 |
| Respiratory diagnosis† | |||
| COPD | 19% | 6% | <0.001 |
| Emphysema | 20% | 4% | <0.001 |
| Chronic bronchitis | 9% | 14% | 0.18 |
| Concomitant asthma | 13% | 7% | 0.04 |
| Home supplemental oxygen use | 32% | 6% | <0.001 |
| Inhaled or oral corticosteroid use | 16% | 9% | 0.07 |
| Long-acting beta agonist use | 29% | 4% | <0.001 |
| Medical subspecialist treatment‡ | 18% | 7% | 0.002 |
| COPD or emphysema† (n = 240) | |||
| Subject characteristic |
Tiotropium use (%) if Subject characteristic…
|
P value | |
| Present | Absent | ||
| Female gender | 19% | 14% | 0.34 |
| Age >65 years | 20% | 13% | 0.23 |
| White, non-Hispanic | 18% | 11% | 0.48 |
| High school education or less (HS) | 15% | 19% | 0.54 |
| Annual household income <$20,000* | 9% | 19% | 0.10 |
| HS or household income <$20,000 | 12.5% | 22% | 0.09 |
| Respiratory diagnoses† | |||
| COPD | 19% | 14% | 0.37 |
| Emphysema | 20% | 11% | 0.12 |
| Chronic bronchitis | 21% | 14% | 0.23 |
| Asthma | 21% | 13% | 0.12 |
| Home supplemental oxygen use | 34% | 11% | <0.001 |
| Inhaled or oral corticosteroid use | 18% | 17% | 0.95 |
| Long-acting beta agonist use | 34% | 8% | <0.001 |
| Medical subspecialist treatment‡ | 23% | 13% | 0.08 |
Notes: All proportions are presented as row percentages;
Income data missing for 22 of 427 subjects in entire study group; 13 of 240 subjects in COPD/emphysema stratum;
Multiple respiratory conditions can be reported; for asthma, a concomitant COPD condition must be reported to be included;
Treatment in the past 12 months by a pulmonary, allergy, or otolaryngology specialist.
Abbreviation: COPD, chronic obstructive pulmonary disease.
COPD Severity Score, a measure including use of supplemental home O2, LABA, steroid use, and other medications, as well as symptoms, differed by tiotropium status: 6.9 (SD 5.9) (nonusers) compared to 14.2 (SD 5.7) for tiotropium users (p < 0.001). COPD Severity Score also differed significantly by tiotropium status in the COPD or emphysema subset: 8.8 (SD 6.6) v. 14.1 (SD 5.6) (p < 0.001). Comparing those with SES defined by either HS education or lower income to all others, lower SES was associated with greater COPD severity (mean score difference = 2.8 [SE 0.6]; p < 0.001). Among the COPD/emphysema subset, this difference was similar (mean score difference = 2.7 [SE 0.9]; p < 0.001).
Table 3 shows the association between tiotropium use and lower SES by two measures (HS education alone and HS and/or lower income). Multivariate models are presented for the entire study group and re-estimated among the COPD/emphysema subset. In Model One (unadjusted for COPD Severity Score), selected respiratory condition diagnoses are associated with tiotropium use while lower SES is not. When COPD Severity Score is added (Models Two and Three), lower SES is associated with decreased odds of tiotropium use. Those with lower SES, taking disease severity into account, have one third to half the odds of reporting tiotropium use. Adjusting for severity, which was strongly associated with tiotropium use, also blunted the respiratory condition effects seen in Model One. In this model, emphysema was associated with a three- to four-fold increased odds of tiotropium use.
Table 3.
Tiotropium use in relation to reported respiratory diagnoses, disease severity, and socioeconomic status (SES): Multiple logistic regression analysis
| COPD, emphysema, chronic bronchitis (n = 427) | COPD or emphysema† n = (240) | |||
|---|---|---|---|---|
| MODEL ONE¶ | Tiotropium use | |||
| OR | 95% CI (p value) | OR | 95% CI (p value) | |
| Respiratory conditions* | ||||
| COPD | 2.6 | 1.3–5.3 (0.007) | 1.7 | 0.7–4.0 (0.21) |
| Emphysema | 5.9 | 2.7–13 (<0.001) | 3.4 | 1.4–8.7 (0.01) |
| Chronic bronchitis | 1.1 | 0.5–2.3 (0.80) | 1.4 | 0.7–3.0 (0.36) |
| Concomitant asthma | 2.1 | 1.0–4.4 (0.05) | 1.9 | 0.9–4.0 (0.11) |
| ≤HS education | 0.7 | 0.3–1.4 (0.29) | 0.6 | 0.3–1.3 (0.17) |
| MODELTWO¶ | Tiotropium use | |||
| OR | 95% CI (p value) | OR | 95% CI (p value) | |
| Respiratory conditions* | ||||
| COPD | 1.7 | 0.8–3.7 (0.16) | 1.2 | 0.5–3.1 (0.63) |
| Emphysema | 4.5 | 1.9–10.4 (<0.001) | 2.9 | 1.1–7.8 (0.03) |
| Chronic bronchitis | 0.8 | 0.4–1.8 (0.64) | 1.1 | 0.5–2.6 (0.88) |
| Concomitant asthma | 1.6 | 0.7–3.5 (0.25) | 1.4 | 0.6–3.2 (0.39) |
| COPD Severity Score | 2.9‡ | 1.9–4.6 (<0.001) | 2.6‡ | 1.6–4.1 (<0.001) |
| ≤HS education | 0.5 | 0.2–1.04 (0.06) | 0.4 | 0.2–0.96 (0.04) |
| MODELTHREE¶ | Tiotropium use | |||
| OR | 95% CI (p value) | OR | 95% CI (p value) | |
| Respiratory conditions* | ||||
| COPD | 1.6 | 0.9–4.5 (0.20) | 1.1 | 0.5–2.9 (0.78) |
| Emphysema | 4.2 | 1.8–9.7 (<0.001) | 2.7 | 1.0–7.2 (0.05) |
| Chronic bronchitis | 0.8 | 0.4–1.7 (0.54) | 1.0 | 0.5–2.3 (0.98) |
| Concomitant asthma | 1.6 | 0.7–3.5 (0.27) | 1.4 | 0.6–3.3 (0.39) |
| COPD Severity Score | 3.3‡ | 2.1–5.4 (<0.001) | 3.0‡ | 1.8–5.0 (<0.001) |
| ≤HS education or income <$20,000 | 0.3 | 0.1–0.7 (0.005) | 0.3 | 0.1–0.6 (0.002) |
Notes: Each respiratory condition entered as a separate dummy variable. Condition diagnoses may overlap;
Bronchitis alone without concomitant COPD or emphysema excluded;
OR expressed per 8 point (1 SD) severity score;
Model Two adds COPD Severity Score to the variables in Model One. Model Three substitutes ≤HS education or income <$20,000 for ≤HS education alone. All multivariate models also include age and gender.
Abbreviations: COPD, chronic obstructive pulmonary disease; CI, confidence interval; HS, high school; Income, annual household income; OR, odds ratio.
In order to take into account other potential confounders, we reanalyzed the data adding to model three variables for race-ethnicity, geographic region, subspecialty care, and at least some medication cost coverage through insurance. The covariates for respiratory condition, COPD Severity Score, age, and gender were also included. None of the added variables was statistically associated with tiotropium use either among the entire group (n = 427) or in the subset excluding chronic bronchitis alone (n = 240). The association between lower SES and tiotropium in these models was not substantively different from the results from the simpler model (Model Three) as shown in Table 3: for the whole group, OR 0.3, 95% CI 0.1–0.7 (p = 0.004); for the subset without chronic bronchitis alone, OR 0.2, 95% CI 0.1–.6 (p = 0.002).
There were home visit data for 101 of these subjects. Of 12 subjects with home visits who reported use of tiotropium in the telephone interview, this was confirmed by direct inspection of medications among 9 subjects. There were also 3 additional subjects who did not report use in the interview but did have tiotropium among their medications. Using medication inspection as the gold standard, the interview checklist for tiotropium had a sensitivity of 75% and a specificity of 97%. Excluding the six respondents with discordant tiotropium data, among the remaining 95 subjects the mean FEV1 % predicted for tiotropium users was 62.4% (SD 20.6%) compared to 86.1% (SD 18.6%) among the nonusers (p < 0.001). Analyzed by COPD stage GOLD II or more, tiotropium use also differed significantly. Of 35 with this COPD severity, 7 (20%) used tiotropium compared to 2 (3.3%) of the remaining 60 subjects (p = 0.01).
Table 4 presents the results of multiple logistic regression modeling testing SES in relation to tiotropium including either FEV1 % predicted or GOLD ≥ Stage II COPD along with all of the other independent variables previously modeled. Taking either FEV1 % predicted or GOLD severity into account, lower SES (HS or less or annual income <$20,000) was strongly associated with decreased odds of tiotropium use. Although the confidence intervals were wide, their upper end is consistent with a 20%–50% decreased odds of tiotropium use associated with lower SES.
Table 4.
Tiotropium use in relation to SES: Multiple logistic regression analysis in 95 subjects
| Odds of tiotropium use in both interview and home visit
|
||||
|---|---|---|---|---|
| Multivariate model includes FEV1 % predicted* | Multivariate model includes ≥COPD GOLD II* | |||
| OR | 95% CI (p value) | OR | 95% CI (p value) | |
| FEV1 % predicted† | 0.5 | 0.3–0.9 (0.02) | NA | ------ ------ |
| ≥COPD GOLD II | NA | ------ ------ | 7.2 | 0.7–72.6 (0.10) |
| COPD Severity Score‡ | 7.9 | 1.3–48.0 (0.02) | 6.7 | 1.4–32.2 (0.02) |
| HS or income <$20,000¶ | 0.01 | 30.001–0.5 (0.02) | 0.03 | 0.001–0.8 (0.03) |
Notes: Multivariate models also include age, gender, and 4 overlapping respiratory disease diagnoses (COPD, emphysema, asthma, and chronic bronchitis). Nine subjects using tiotropium; six other subjects with discordant interview compared to home visit data for tiotropium excluded from this analysis;
OR express per 10% change (increase) in FEV1 % predicted; higher % predicted associated with decreased odds of tiotropium use;
OR expressed per 8 point (1 SD) increase in severity score; higher severity associated with greater odds of tiotropium use;
Lower SES associated with decreased odds of tiotropium use.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in one second; HS, high school; SD, standard deviation; SES, socioeconomic status; OR, odds ratio.
Discussion
We observed an SES health gradient in which lower SES was strongly linked to decreased odds of using tiotropium, a COPD medication introduced in recent years. This association was consistently observed adjusting for age, sex, respiratory condition diagnoses and, most importantly, severity of disease. Testing additional potential confounders, including subspecialty care and race-ethnicity did not affect the estimated SES association. Taking COPD severity into account is particularly critical because those with greater disease severity were more likely to report tiotropium use. Because lower SES was also linked to greater COPD severity in this cohort, raw differences in tiotropium use in comparisons unadjusted for severity were modest and not statistically significant until this confounding factor was taken into account. An association between lower SES and COPD has been recognized by others, although disease-specific severity has not been well studied. (Mannino and Buist 2007).
The SES association with tiotropium that we observed may reflect multiple factors. Although the majority of subjects included in these analyses were aged 65 or older and thus eligible for Medicare coverage, health care access may nonetheless have affected medication use. Since Medicare at the time of our survey provided no outpatient medication benefits, the cost of tiotropium may have led to decreased use among those with low SES without supplemental medication coverage, with insurance formulary restrictions, or with high medication co-payments. We did not have specific data on the amounts of insurance co-pays for medication or medication out-of-pocket costs and thus could not include this in our analysis, although we did not find that absence of any medication insurance coverage was related to tiotropium use. Because most of our subjects did have some coverage, we may not have had sufficient power to study this question. A recent study found that the Medicare Modernization Act Prescription Drug Benefit (Part D) was associated with a very modest increase in utilization and savings in out-of-pocket costs (Yin et al 2008).
The deterrent effect of out-of-pocket costs is consistent with the observation that elderly patients with COPD who are reimbursed only for generic medications (compared to those with greater coverage) have an 80% greater odds of stopping a medication in order to reduce out-of-pocket expenses (Spence et al 2006). Tiotropium is not available as a generic drug. A study of inhaled steroid use in childhood asthma, however, has shown that there is an SES gradient in use even with full medication coverage (Kozyrskyj et al 2001). In the US as of 2007, tiotropium costs for 30-days of treatment were less than that of the approved-for-COPD concentration of combination fluticasone/salmeterol and more than salmeterol alone ($134, $185, and $120, respectively) (Anon 2007).
The SES gradient that we observed was apparent even against a background of an overall low penetration rate for tiotropium use, consistent with our survey taking place at a time when medication was relatively new on the market. At the time of our study only a minority of persons with COPD diagnoses (COPD, emphysema, or chronic bronchitis) were using the medication. In a population near to or post-retirement age, estimation of SES using survey research methods is challenging. In this analysis, we used both educational achievement and education combined with income as SES surrogates. We recognize, for example, that lower educational achievement is associated with, but does not predetermine lower SES. Further, relying on household income after retirement may attenuate SES gradients associated with differences in earnings among younger individuals. Moreover, COPD may lead to income loss. We did not assess wealth or assets in this study, which might better reflect SES.
Our study has other limitations. Analysis of tiotropium was not a primary endpoint of the cohort study from which these data were drawn. In addition, we studied this question in a cross-sectional analysis of survey data from a time frame relatively soon after the introduction of tiotropium. We cannot presume that the SES effect we observed will become attenuated or more marked with time as the medication becomes more widely prescribed over time. Pharmacoeconomic analyses have supported the cost effectiveness of tiotropium, although recognizing that this may depend on willingness-to-pay criteria. (D’Souza et al 2006; Rutten-van Mölken et al 2007) The question of prospective trends in tiotropium penetration and its potential relationship to SES can only be addressed through longitudinal follow-up. Lung function was available only for a subset of subjects and we depended on report of a physician’s diagnosis of COPD. This may account, in part, for the association we observed between tiotropium and the reported diagnostic label of emphysema, which may simply be a surrogate marker for severe COPD rather than emphysema clearly established by physiological or radiographic criteria.
In our primary analyses, medication use was based entirely on self-report and was not confirmed by pharmacy records. Thus it is possible that subjects with lower SES preferentially had poorer recall of tiotropium use, even in response to a specific medication checklist. Nonetheless, this survey approach demonstrated acceptable sensitivity and specificity against direct confirmation via home medication inspection. The SES health gradient for tiotropium, wherein those with lower SES may be less likely to use the medication, could lead to under-use among certain groups of patients who could particularly benefit from such therapy. It may also suggest that this SES gradient may be relevant to other medications as they are introduced. To the extent that such new therapies are especially efficacious, such gradients could serve to widen existing health disparities.
Footnotes
Disclosure
Research supported by HL067438 (U.S. National Heart Lung and Blood Institute, NIH) and the Flight Attendants Medical Research Institute (FAMRI).
References
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Ann Rev Public Health. 2008;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Anon Tiotropium (Spiriva) for COPD. Med Lett Drugs Ther. 2004;46:41–2. [PubMed] [Google Scholar]
- Anon Aformoterol (Brovana) for COPD. Med Lett Drugs Ther. 2007;49:53–4. [PubMed] [Google Scholar]
- Blanc PD, Eisner MD, Trupin L, et al. The association between occupational factors and adverse health outcomes in chronic obstructive pulmonary disease. Occup Environ Med. 2004;61:661–7. doi: 10.1136/oem.2003.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Eisner MD, Katz PP, et al. Measuring disease-specific quality of life in obstructive airway disease: validation of a modified version of the airways questionnaire 20 (AQ20) Chest. 2006;129:1644–52. doi: 10.1378/chest.129.6.1644. [DOI] [PubMed] [Google Scholar]
- D’Souza AO, Smith MJ, Miller LA, et al. An appraisal of pharmacoeconomic evidence of maintenance therapy for COPD. Chest. 2006;129:1693–708. doi: 10.1378/chest.129.6.1693. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Trupin L, Katz PP, et al. Development and validation of a survey-based COPD severity score. Chest. 2005;127:1890–7. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Katz P, Iribarren C, et al. Measurement of COPD severity: further validation of a survey-based instrument [Abstract] Eur Resp J. 2007;30:629S. [Google Scholar]
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [online] [Accessed on January 14, 2008];2006 URL: http://goldcopd.org.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Kozyrskyj AL, Mustard CA, Simons FE. Socioeconomic status, drug insurance benefits, and new prescriptions for inhaled corticosteroids in schoolchildren with asthma. Arch Pediatr Adolesc Med. 2001;155:1219–24. doi: 10.1001/archpedi.155.11.1219. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- Menezes AMB, Perez-Padillo R, Jardim JRB, et al. Chronic obstructive pulmonary disease m five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Rutten-van Mölken MPMH, Oostenbrink JB, Miravitlles M, et al. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipatropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ. 2007;8:123–35. doi: 10.1007/s10198-007-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence ME, Hui R, Chan J. Cost reduction strategies by elderly patents with chronic obstructive pulmonary disease to cope with a generic-only pharmacy benefit. J Manag Care Pharm. 2006;12:377–82. doi: 10.18553/jmcp.2006.12.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22:462–9. doi: 10.1183/09031936.03.00094203. [DOI] [PubMed] [Google Scholar]
- Warren JR, Hernandez EM. Did socioeconomic inequalities in morbidity and mortality change in the United States over the course of the twentieth century? J Health Soc Behav. 2007;48:335–51. doi: 10.1177/002214650704800401. [DOI] [PMC free article] [PubMed] [Google Scholar]