Abstract
Fragile X, an inheritable form of mental retardation, is caused by the inactivation of a gene on the X chromosome, FMR1 which codes for an RNA binding protein, Fragile X Mental Retardation Protein. Loss of this protein is associated with reduced complexities of neuronal dendrites and alterations in spine morphology in a number of cortical brain regions, and these deficits may underlie the cognitive impairment observed in fragile X patients. Among the many symptoms of fragile X are altered motor functions, although the neuronal basis for these remains unclear. In this study we investigated whether knockout of Fmr1 in the mouse model of fragile X altered dendrite morphology in developing spinal cord motor neurons. We find that Fmr1 knockout leads to modest alterations in the distribution of dendritic arbor across the span of the motor neuron dendritic tree in two week and four week old mice, compared to wild-type controls, consistent with slower rates of extension and abnormal pruning of intermediate dendritic segments. These studies suggest that some motor deficits in fragile X patients may be due to abnormal maturation of dendritic patterning within spinal motor neurons, and suggest that strategies aimed at preventing motor impairment in fragile X patients may be targeted at motor functions during early development.
Fragile X syndrome is the most prevalent heritable cause of genetic mental retardation, affecting approximately 1 in 4,000 males and 1 in 8,000 females (Bagni and Greenough, 2005; Garber et al, 2006). The disease is caused by an expansion of a CGG repeat (>200 repeats) within the untranslated region of the FMR1 gene, encoding the Fragile X Mental Retardation Protein (FMRP), resulting in methylation and silencing of the gene (Sutcliffe et al., 1992). While the intellectual impairment observed in patients with fragile X syndrome is usually the most debilitating symptom, the syndrome represents a constellation of symptoms which include hyperactivity and autistic behaviors, movement disorders, and a variety of physical features such as large ears, elongated face, hyperextensible joints, and macroorchidism (Jin and Warren, 2003; Hagerman and Hagerman, 2004; Restivo et al., 2005; Schneider et al., 2008); together these symptoms suggest that FMRP has widespread importance in development.
FMRP is an RNA binding protein which in neurons appears to be important in localized dendritic translation of various proteins (Bagni and Greenough, 2005; Garber et al., 2006; Zalfa et al., 2006), although the specific targets of FMRP are as yet unclear. A model for the function of FMRP (Bagni and Greenough, 2005) involves its translocation to the nucleus via a nuclear localization signal, where it is complexed with ribonucleoproteins, mRNAs and various other proteins, prior to active transport to neuronal dendrites. FMRP appears to suppress synthesis of a number of proteins involved in dendrite plasticity, candidates for which include some glutamate receptor subunits, and several proteins associated with synapse function, structure and plasticity (Brown et al., 2001; Miyashiro et al., 2003). Accordingly, loss of FMRP in humans (Hinton et al., 1991; Irwin et al., 2001) and in a mouse model (Comery et al., 1997; Nimchinsky et al., 2001; Irwin et al., 2002; Grossman et al., 2006) is associated with morphological changes in neocortical dendritic spines, structures that receive the majority of afferent synaptic input within cortical neurons, and which undergo substantial remodeling during development (Harris, 1999; Dunaevsky et al., 1999). Since dendritic morphology is a determinant of neuronal firing patterns (Purves and Hume, 1981; Schaefer et al., 2003; Vetter et al., 2001), changes in FMRP expression in fragile X may influence activity within neural networks on a large scale.
While cognitive deficits represent a major focus for therapeutic intervention in fragile X, FMRP is transcribed widely in the developing nervous system, including the spinal cord (Hanzlijk et al., 1993), and it is likely that the loss of FMRP has important implications for other regions of the nervous system. Similar to neurons within other regions of the developing central nervous system, motor neurons of the spinal cord undergo wide-scale refinement of dendritic arbor during the postnatal period, via an activity-dependent mechanism involving activation of glutamate receptors (Kalb, 1994; Hebbeler et al., 2002; Inglis et al., 1998). Notably, infants with fragile X have been observed to make more simplified, repetitive movements, indicative of subtle deficits in motor functions (Baranek, 1999; Hagerman and Hagerman, 2004; Baranek et al., 2005). Recently, further evidence for the importance of FMRP in motor function has arisen through the identification of a separate syndrome, in which patients display progressively worsening ataxia, intention tremor, peripheral neuropathy, parkinsonism and dementia (Hagerman and Hagerman, 2004; Amiri et al., 2008). This syndrome, known as Fragile X Associated Tremor/Ataxia Syndrome (FXTAS), results from a premutation in which the region of CGG repeats is expanded (50-200 repeats) but not silenced, resulting in an apparently toxic gain-of-function of FMRP (Amiri et al., 2008). Patients with FXTAS are reported to have slower motor neuron conduction velocities than controls (Soontarapornchai et al., 2008), suggesting that motor neuron pathology may be a key component of FXTAS. Thus, expression of FMRP at an appropriate level appears to be critical for the development and maintenance of normal motor functions, and motor circuitry is an important target for therapies aimed at ameliorating the symptoms of FMR1-related syndromes.
Since FMRP appears to be involved in maturation of dendritic morphology elsewhere in the central nervous system, we investigated whether FMRP was required for the normal maturation of motor neuron dendritic arbor during the postnatal period. Using Fmr1 knockout mice, we find that the absence of FMRP leads to discrete alterations in the morphology of motor neuron dendrites. Our results suggest that FMRP participates in the maturation of motor circuitry during the postnatal period, and suggests a possible locus for clinical intervention in children with fragile X.
EXPERIMENTAL PROCEDURES
Animals
All procedures involving animals were performed according to protocols approved by the Tulane University Institutional Animal Care and Use Committee, and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Breeding pairs of Fmr1 knockout (FVB.129P2-Fmr1tm1Cgr/J strain; Comery et al., 1997) and congenic wild-type mice were obtained from Jackson Laboratories (Bar Harbor, ME), and housed in climate controlled conditions on a 12/12 hour light/dark cycle, with unlimited access to food and water. Since motor neuron dendrites undergo substantial activity-dependent refinement of motor neuron arbor during the postnatal period (Kalb, 1994; Hebbeler et al., 2002; Inglis et al., 1998; Inglis et al., 2000), detailed morphological studies were performed in neurons collected from litters at 14 and 28 days of age (P14 and P28). Pups were collected at P14 and P28, anesthetized deeply with pentobarbital, and sacrificed by intracardiac perfusion fixation with 0.1M phosphate buffered saline (PBS), followed by 4% paraformaldehyde dissolved in PBS (PFA). Following half an hour, the spinal cord and attached ventral roots were dissected from the spinal column, and maintained in PFA for approximately 1 week.
DiI labeling and imaging of spinal motor neurons
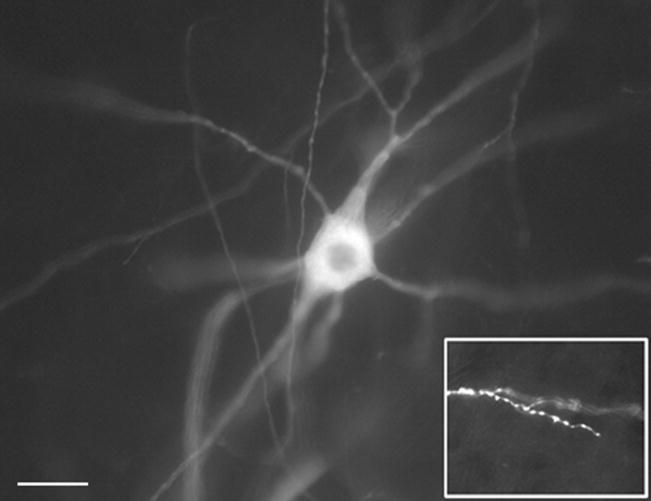
Motor neurons of the ventral spinal cord were labeled with the lipophilic fluorescent dye, DiI (Molecular Probes, Eugene, OR), applied to the ventral roots via a microelectrode, as described previously (Inglis et al., 1998). The ventral roots contain axons of the motor neurons, allowing diffusion of DiI throughout the cell membrane of motor neurons, delineating the cell body, axon and dendritic trees (Figure 1). Following application of DiI, spinal cords were incubated at 37°C for two to four weeks. Spinal cords were then sectioned on a vibratome at 80μm, and wet-mounted onto glass slides. Fluorescent-labeled motor neurons were visualized microscopically (Axiovert 200M; Carl Zeiss, Thornwood, NY), with a 594 nm filter at a 20X objective. Images of each neuron were taken sequentially along the Z-axis with 1.95 μm between each frame, using Axiovision software (Zeiss). Motor neurons were drawn from at least 5 animals per genotype.
Figure 1.
The fluorescent dye DiI delineates neuronal morphology of motor neurons in the ventral spinal cord. Neurons were labeled via application of DiI to the ventral roots. Note that fine terminal dendrites can be easily defined within these neurons (inset). Scale bar represents 40μm; inset is 4X main image.
Morphological Analyses
Z-stacks of labeled motor neurons were traced using a digitized camera lucida system (Neurolucida, MBI Biosciences, Colchester, VT) (Fig. 1), in order to make detailed measurements of several parameters indicative of dendrite complexity and length. From each neuron, we calculated the number of primary dendrites per cell, the number of branch-points and branch tips, the total amount of dendrite per cell, and the average segment length. Together, these parameters provide a good indication of the extent and complexity of dendritic arbor (Inglis et al., 1998; Prithviraj et al., 2008).
We also investigated whether there were discrete alterations in arbor that were limited to a particular part of the tree, using two methods: first, to determine whether there were intrinsic alterations in the geometry of dendrites, we measured the dendritic arbor of each cell according to the branch order. For these analyses, a primary dendrite emanates directly from the cell body; once it bifurcates, two secondary dendrites are formed, and so on. The number of segments of each branch order, the total arbor of each branch order, and the average segment length per branch order were calculated for each cell. Second, to determine whether there were spatial alterations in regions of the dendritic tree, we performed Sholl analyses, in which we measured the total length and the number of branch points within concentric 20μm radii extending outward from the cell body. Comparisons were made between genotypes within each age group.
Statistics
All statistics were performed using Statview 5 (SAS). For Sholl analyses, and analyses of neurons by branch order, groupwise comparisons were made using Repeated Measures Analysis of Variance (ANOVA). Post-hoc comparisons between groups were made using Scheffé’s F test. All other statistical comparisons were performed using Student’s t-test (two-tailed, unpaired).
RESULTS
As with previous studies (Inglis et al., 1998; Inglis et al., 2000), labeling the ventral roots of the spinal cord with DiI allowed us to delineate the entire dendritic tree of motor neurons within the ventral horn, including fine dendritic processes, permitting unambiguous measurements of dendrite morphology. Within P14 animals, we were able to label fewer cells than within the older age group (Tables 1 and 2); this is likely a function of the difficulty in fixation of immature tissues, due to the relatively low abundance of surface proteins within the spinal cord at this age (Hockfield et al., 1990), leading to diffuse labeling with DiI; and smaller numbers of easily identifiable neurons within these tissue samples. Despite differences in the ability to fix tissues within different age groups, there were no differences in the ability to label wild-type and Fmr1 knockout mice within each age group.
Table 1.
Comparisons of key architectural parameters of spinal motor neurons in 14 day old wild-type and Fmr1 knockout mice
| Dendrite Parameter | Wildtype | Fmr1 knockout | t, p |
|---|---|---|---|
| Number of Primary Dendrites | 5.3 ± 0.264 | 5.2 ± 0.34 | t = -0.667; p = 0.512 |
| Number of Branch Points | 9.4 ± 1.19 | 9.7 ± 1.01 | t = 0.148; p = 0.884 |
| Number of Branch Tips | 15.1 ± 1.92 | 15.1 ± 1.05 | t = -0.038; p = 0.970 |
| Total dendritic arbor (μm) | 1767 ± 364 | 1390 ± 116 | t = -1.270; p = 0.218 |
| Average segment length (μm) | 65 ± 8.2 | 57 ± 4 | t = -0.912; p = 0.512 |
Values represent mean ± s.e.m. of 7 neurons from wild-type mice, and 16 neurons from Fmr1 knockout mice. No significant differences were observed between treatment groups with any parameter indicated (Student’s t-test).
Table 2.
Comparisons of key architectural parameters of spinal motor neurons in 28 day old wild-type and Fmr1 knockout mice
| Dendrite Parameter | Wildtype | Fmr1 knockout | t, p |
|---|---|---|---|
| Number of Primary Dendrites | 5.3 ± 0.3 | 5.4 ± 0.3 | t = 0.075; p = 0.940 |
| Number of Branch Points | 6.2 ± 0.8 | 8.3 ± 1.0 | t = 0.146; p = 0.149 |
| Number of Branch Tips | 11.6 ± 0.9 | 13.7 ± 1.1 | t = 1.339; p = 0.186 |
| Total dendritic arbor (μm) | 1397 ± 139 | 1601 ± 125 | t = 1.063; p = 0.293 |
| Average segment length (μm) | 80 ± 5 | 75 ± 4 | t = -0.708; p = 0.482 |
Values represent mean ± s.e.m. of 21 neurons from wild-type mice, and 33 neurons from Fmr1 knockout mice. No significant differences were observed between treatment groups with any parameter indicated (Student’s t-test).
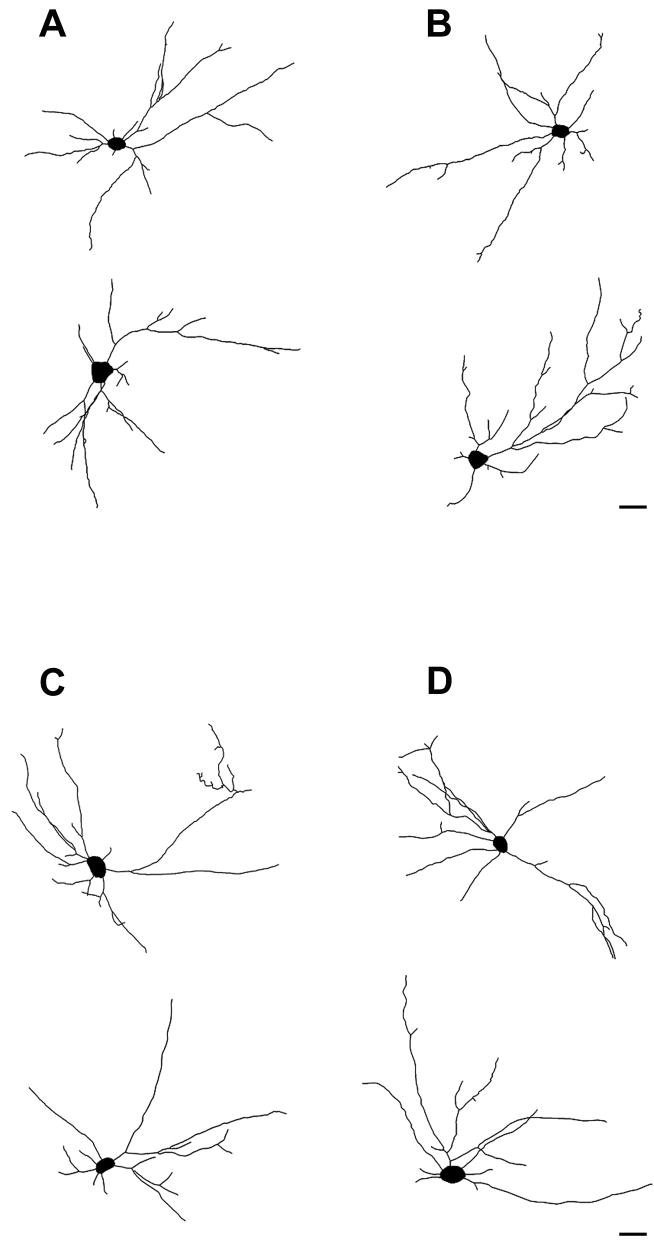
In agreement with previous studies (Kalb, 1994), we found that the length and complexity of dendritic arbor in motor neurons from wild-type mice were greater at P14 compared with P28 (Tables 1 and 2), suggesting that dendritic arbor undergoes pruning over the course of postnatal development. Examination of camera lucida images of motor neurons drawn from P14 and P28 mice did not reveal gross differences between dendritic morphologies of wild-type and Fmr1 knockout mice (Figure 2). Likewise, statistical comparisons of dendritic parameters calculated per cell and compared across age-matched genotypes revealed no differences in dendritic arbor within the parameters employed. Thus, in both P14 (Table 1) and P28 mice (Table 2), the total amount of dendrite, and the complexity of dendrites (as measured by numbers of branch-points) remained unaltered in Fmr1 knockout animals, compared to age-matched controls, when neurons are compared using cell totals.
Figure 2.
Representative digitized camera lucida images of neurons from (A) wild-type and (B) Fmr1 knockout mice at postnatal day 14; and (C) wild-type and (D) knockout mice at postnatal day 28. No gross abnormal morphological differences were observed between neurons within these genotypes. Scale bar represents 40μm.
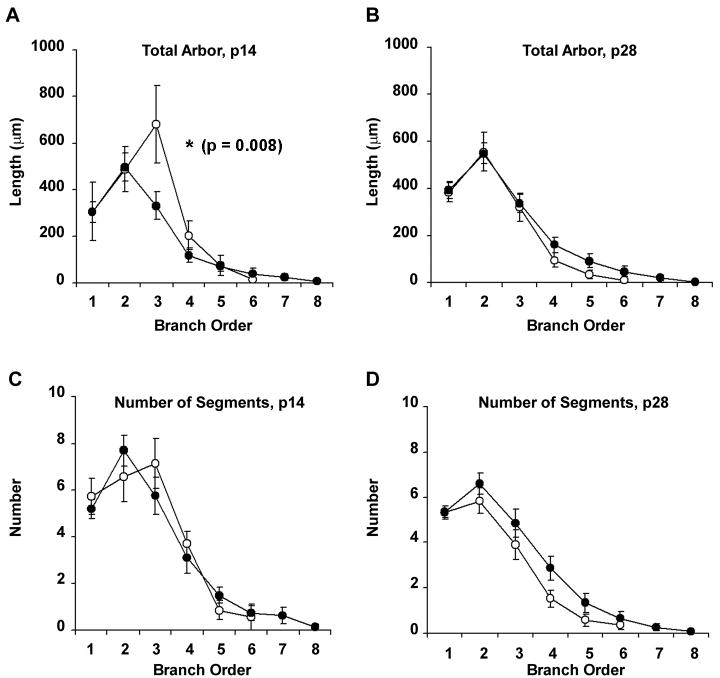
When neurons from P14 animals were analyzed using methods to examine the distribution of dendritic arbor, however, we observed modest differences in the distribution of dendritic arbor between wild-type and Fmr1 knockout animals. We first analysed neurons by their branch order, because these analyses provide information about the intrinsic geometry of the dendrite and how arbor is distributed across the span of the dendritic tree. When dendrites were analyzed according to branch order, we observed that, although there was no overall difference in amount of arbor between groups (F7,147 = 1.612; p = 0.963), arbor was distributed differently between genotypes (Group × Order interaction, F7,147 = 2.857; p=0.008). This difference in distribution is due specifically to a reduced length of dendritic arbor found in third order branches in the neurons in Fmr1 knockout mice (Figure 3), compared to wild-type controls (p=0.02, Scheffé post-hoc test). The redistribution of length according to branch order was not accompanied by numbers of segments at this order (F7,147 = 1.050; p=0.399), suggesting that intermediate branch segments were not less numerous, but were shorter, in Fmr1 knockout mice. These results suggest that the lack of Fmr1 may inhibit elongation of branch segments that usually extend during dendrite development. In contrast to the results observed at P14, there were no differences in distribution of the amount of dendritic arbor (Group × Order, F7,364=77.44; p=0.948), or the number of segments of each order (F7,364=112.2; p=0.356) in neurons from P28 animals (Figure 3). Of interest, the amount of dendritic arbor decreases over time in wild-type animals, such that neurons taken from P14 mice have greater amounts of arbor within interstitial segments than P28 mice (Figure 3A and B). In contrast, the amount of dendritic arbor in Fmr1 knockout mice appears to remain constant over the postnatal period; thus, the transient differences in the distribution of dendritic arbor between knockouts and wild-types is likely to be due to the lack of normal outgrowth and pruning that has been shown to exist in motor neurons during the postnatal developmental period (Kalb, 1994).
Figure 3.
Line graphs illustrating the total amount of arbor (A-B) as a function of branch order, and the total number of dendritic segments (C-D) of each branch order, of p14 and p28 animals. Unfilled circles represent wild-type neurons (n=7 for p14 neurons; n=21 for p28 neurons); filled circles represent neurons from Fmr1 knockout animals (n=15 and 32 for p14 and p28 neurons respectively). Data are presented as average ± s.e.m. ANOVA revealed significant differences in distribution (Group × Radius) of dendritic arbor for p14 (A), but not for p28 animals (B), and found no differences in the numbers of branch segments of each order for each group (C-D).
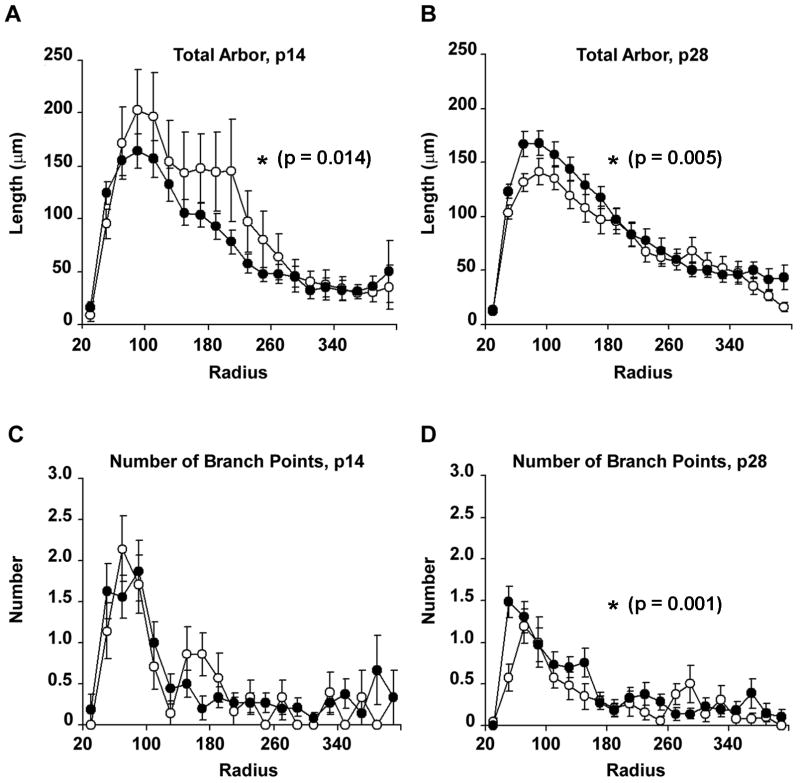
To provide information regarding the spatial distribution of neuronal dendrites, neurons from wild-type and Fmr1 knockout mice were further analyzed using Sholl analyses; these analyses measure the amount of arbor and the number of branch-points in concentric radial bins of 20μm outwards from the cell body. In neurons from P14 animals, we observed a significant Group × Radius effect (F19,380=1.889; p=0.014) when comparing total arbor within the two genotypes across the radial distance from the cell body, such that neurons from Fmr1 knockout animals displayed less arbor at radial distances of between 100 and 240μm from the cell body (Figure 4). The reduction in dendritic arbor at this distance is most likely due to the lower amounts of arbor found in third order dendrites, as reported above. While these results are statistically similar, however, we observed a large inter-subject variability between cell dendrites at this age; for example, wild-type neurons displayed between 500 and 3400 μm of total arbor, whereas knockout cells were measured to have between 600 and 2400 μm of total dendritic arbor; since P14 represents a period in which growth and retraction of dendritic arbor is highly dynamic, we interpret these data with some caution regarding significance.
Figure 4.
Line graphs illustrating the total amount of arbor (A-B) and total numbers of dendritic segments (C-D) as a function of radial distance from the cell body (Sholl analyses), in p14 and p28 animals. Unfilled circles represent wild-type neurons (n=7 for p14 neurons; n=21 for p28 neurons); filled circles represent neurons from Fmr1 knockout animals (n=15 and 32 for p14 and p28 neurons respectively). Data are presented as average ± s.e.m. ANOVA (Group × Radius) revealed significantly less arbor distributed close to the cell body in p14 Fmr1 knockout animals, and significantly greater amounts of dendritic arbor in p28 knockout animals, compared with wild-type controls. Branch-points were not distributed differently among groups.
When similar Sholl analyses were performed on P28 wild-type and knockout animals, we observed that neurons of Fmr1 knockout mice had significantly greater amounts of arbor (F19,931=2.282; p=0.005) at between 80 and 160μm from the cell body (Figure 4). This redistribution of dendritic arbor was accompanied by a significant redistribution in the number of branch-points within each radial bin (F19,931=2.122; p=0.001), so that greater numbers of branch-points were situated closer to the cell body in Fmr1 knockout mice, compared with wild-type controls. Again, the amount of arbor in the dendrites of P28 wild-type mice was lower than those of P14 mice, whereas the amount of arbor in neurons from knockout mice remained similar between P14 and P28. These results suggest that Fmr1 knockout is associated with deficient pruning mechanisms, such that arbor that would normally be lost during the postnatal period is maintained, leading to abnormal redistribution of dendritic length within intermediate segments. Together, these findings imply that whereas dendritic complexity as a whole does not change with respect to the presence or absence of FMRP, loss of FMRP is associated with abnormalities in the spatial distribution of dendritic arbor of motor neurons; and further, that these abnormalities develop over the course of the postnatal period. These observations suggest that FMRP may be an important contributor to the maturation of motor neuron morphology during the critical developmental period in which activity-dependent development is known to shape mature dendritic architecture (Kalb, 1994; Inglis et al., 2000).
DISCUSSION
During normal development, the number of branch-points in spinal motor neurons is normally about maximal in the second postnatal week, and over the ensuing two weeks, activity-dependent rearrangement of dendritic arbor occurs such that many branch segments are eliminated, whereas others are elongated (Kalb, 1994). Our main findings are that the lack of FMRP alters dendrite morphology of spinal motor neurons in a discrete but significant manner, such that at two weeks of age, the absence of FMRP is associated with a reduction in the length of intermediate branch segments, compared with neurons from wild-type animals. In contrast, while the amount of dendritic arbor decreased in motor neurons from wild-type animals over the postnatal period, the extent of the dendritic tree in neurons from Fmr1 knockout animals does not appear to change. Indeed, at 28 days, motor neurons of Fmr1 knockout animals were observed to have greater amounts of dendritic arbor close to the cell body, and in an increase in the number of branch-points. Together these observations suggest that FMRP is required for maturation of the normal motor neuron phenotype, and that in the absence of FMRP, normal dendritic extension and pruning is deficient.
Our results are in accordance with studies of other regions in the central nervous system which imply that loss of FMRP results in changes in dendritic morphology that are characteristic of immature neurons. For example, dendritic spines are more numerous in the cortex of fragile x patients (Hinton et al., 1991; Irwin et al., 2001) and of Fmr1 knockout mice (Comery et al., 1997; Nimchinsky et al., 2001). The long, thin nature of spines in the absence of FMRP is characteristic of immature spines and filopodia, which are normally replaced during development by shorter and stubbier spines (Harris, 1999; Dunaevsky et al., 1999; Yuste and Bonhoeffer, 2004). The presence of immature-looking spines in Fmr1 knockout mice has been reported to be transient, corresponding to the time-course for normal synaptogenesis (Nimchinsky et al., 2001), and implying that the time-course for maturation of spines and synapses is delayed in the absence of FMRP. While most researchers have focused on spine density and morphology, recent evidence suggests that alterations in dendrite complexity are also a feature of neurons in the absence of FMRP. For example, neurons from the visual cortex in Fmr1 knockout mice are reported to have fewer branch segments than wild-type mice (Restivo et al., 2005), although a similar study did not report altered dendritic morphology (Irwin et al., 2002), possibly due to strain differences and retinal degeneration associated with the FVB strain (discussed in Restivo et al., 2005). Other investigators have demonstrated that the number and length of neurites is reduced in both the fmr1 knockout mouse and in tissue from a human Fragile X embryo, suggesting an early role for Fragile X in determining dendrite morphology (Castrén et al., 2005). It is possible that loss of dendritic segments in cortical neurons of Fmr1 knockout mice is due to failure of spine maturation, since filopodial protrusions have been suggested to represent precursors to dendritic branches (Niell et al., 2004). Alternatively, reductions in synaptic density due to reduced numbers of mature spines may result in loss of dendritic segments through an activity-dependent mechanism (Katz and Shatz, 1996); since the pattern of dendrites determines the firing patterns of a neuron, (Purves and Hume, 1981; Schaefer et al., 2003; Vetter et al., 2001), alterations in the distribution of dendritic arbor as a result of the loss of FMRP may have profound effects on network properties within neuronal circuitry. Unlike cortical pyramidal neurons, motor neurons of the spinal cord are aspiny, and therefore changes in the number of synaptic contacts are more likely to be manifest as alterations in the length of the dendritic shaft. Thus, the alterations in dendrite morphology we observed in motor neurons from Fmr1 knockout mice may be predictive of altered synaptic connectivity and network activity. In this regard, our results are significant, in that they indicate that the effect of Fmr1 knockout is not solely limited to dendritic spines.
While the current study focused on the morphology of motor neurons in the absence of FMRP, our results also have important implications for patients with FXTAS, a syndrome which features progressive neurological impairment of late onset, including loss of motor functions and the emergence of neuropathy (Amiri et al., 2008). This syndrome appears in carriers of a fragile X “pre-mutation” in which moderately increased numbers (100-200) of CAG repeats are not silenced, but instead are thought to lead to a toxic gain-of-function of FMRP (Hagerman and Hagerman, 2004). In pre-mutation carriers, FMR1 mRNAs have been shown to be elevated (Tassone et al., 2000), possibly leading to sequestration of RNA-binding proteins, and consequent disruption of normal translational mechanisms within the nucleus (Amiri et al., 2008; Ranum and Cooper, 2006). FMRP has been shown to interact with the protein “survival of motor neuron” (SMN), a protein essential for assembly of RNA splicing machinery (Piazzon et al., 2008), and the absence of which causes motor neuron degeneration. Thus, normal levels of FMRP would appear to be required for maintaining motor function throughout the life of patients.
How might FMRP mediate normal maturation of dendrite morphology in developing motor neurons? While the precise targets of FMRP are unknown in this regard, it is likely that this process involves activation of glutamate receptors. Recently, it has been shown that loss of FMRP accentuates the cellular effects of Group 1 metabotropic glutamate receptors (mGluR1/mGluR5; Huber et al., 2002), and enhances long-term depression (LTD), a synaptic plasticity mediated partly by internalization of ionotropic glutamate receptors (Snyder et al., 2001). For these reasons, drugs that block Group 1 mGluRs are potential therapeutic candidates for patients with fragile X (Catania et al., 2007). Glutamate receptor activity has been shown in many neuronal systems to mediate outgrowth and refinement of dendritic arbor during a key developmental period (Cramer and Sur, 1995; Katz and Shatz, 1996; Cline, 2001; Wong and Ghosh, 2002). In motor neurons, activation of both AMPA (Inglis et al., 2002; Prithviraj et al., 2008) and NMDA glutamate receptor subtypes (Kalb, 1994; Hebbeler et al., 2002; Inglis et al., 1998) is critical for normal maturation of dendrite complexity and length. In this regard, it is interesting to note that reduced levels of GluR1 are found in the cortex of Fmr1 knockout mice (Li et al, 2002). It is possible, therefore, that disrupted glutamatergic receptor firing results in abnormal activity-dependent maintenance or elimination of dendritic segments during the developmental critical period in motor neurons, resulting in immature dendrite morphology. In this scenario, excessive activation of Group 1 mGluRs may lead to alterations in dendrite reorganization during development via reduced activation of glutamate receptors and their intracellular counterparts.
Our results have important implications for the treatment of children with fragile X. In a recent study, it has been shown that rearing animals in an enriched environment overcomes morphological abnormalities of cortical dendrites in FMR1 knockout mice, and promotes behavioral recovery of function (Restivo et al., 2005). Since the dendrites of motor neurons undergo refinement only within a critical period of postnatal development, the window for preventing abnormalities in motor neuron morphology – and consequently in activity throughout motor networks – may be limited. In this light, promoting complex motor activity during the postnatal period, combined with pharmacological intervention, may prove useful in preventing long-term motor deficits in fragile X patients.
Acknowledgments
This work was supported by the National Science Foundation (0446168); NIH/NIGMS CoBRE (1 P20 RR 15637) and the Louisiana Board of Regents (LEQSF (2003-2006)-RD-A-24). We are grateful to Ranjini Prithviraj for her help in preparing the manuscript.
List of Abbreviations
- AMPA
A-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid
- ANOVA
Analysis of Variance
- DiI
1,1’-dioctadecyl-3,3,3’,3’- tetramethylindocarbocyanine perchlorate
- FMR1
Fragile X mental retardation 1 [Homo Sapiens]
- Fmr1
Fragile X mental retardation 1 [Mus Musculus]
- FMRP
Fragile X mental retardation protein
- FXTAS
Fragile X-associated tremor/ataxia syndrome
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-D-aspartate
- p14, p28
postnatal day 14, postnatal day 28
- SMN
survival of motor neuron protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amiri K, Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome: an aging face of the fragile X gene. Arch Neurol. 2008;65:19–25. doi: 10.1001/archneurol.2007.30. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. J Autism Dev Disord. 1999;29:213–24. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Danko CD, Skinner ML, Bailey DB, Jr, Hatton DD, Roberts JE, Mirrett PL. Video analysis of sensory-motor features in infants with fragile X syndrome at 9-12 months of age. Arch Neurol. 2005;65:19–25. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–87. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Castrén M, Tervonen T, Kärkkäinen V, Heinonen S, Castrén E, Larsson K, Bakker CE, Oostra BA, Akerman K. Altered differentiation of neural stem cells in fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:17834–9. doi: 10.1073/pnas.0508995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania MV, D’Antoni S, Bonaccorso CM, Aronica E, Bear MF, Nicoletti F. Group I metabotropic glutamate receptors: a role in neurodevelopmental disorders? Mol Neurobiol. 2007;35:298–307. doi: 10.1007/s12035-007-0022-1. [DOI] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr, Opin, Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KS, Sur M. Activity-dependent remodeling of connections in the mammalian visual system. Curr Opin Neurobiol. 1995;5:106–111. doi: 10.1016/0959-4388(95)80094-8. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. Fmr1 and the fragile X syndrome: Human genome epidemiology review. Genet Med. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96:13438–43. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature- appearing profile of dendritic spines. Brain Res. 2006;1084:158–64. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagermanm RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–16. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlik AJ, Osemlak-Hanzlik MM, Hauser MA, Kurnit DM. A recombination-based assay demonstrates that the fragile X sequence is transcribed widely during development. Nat Genet. 1993;3:44–8. doi: 10.1038/ng0193-44. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–8. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–94. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–14. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Furia F, Zuckerman KE, Strittmatter SM, Kalb RG. The role of nitric oxide and NMDA receptors in the development of motor neuron dendrites. J Neurosci. 1998;18:10493–10501. doi: 10.1523/JNEUROSCI.18-24-10493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Zuckerman KE, Kalb RG. Experience-dependent development of spinal motor neurons. Neuron. 2000;26:299–305. doi: 10.1016/s0896-6273(00)81164-2. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostoo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jin P, Warren ST. New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem Sci. 2003;28:152–8. doi: 10.1016/S0968-0004(03)00033-1. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;3:417–31. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–60. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–46. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzon N, Rage F, Schlotter F, Moine H, Branlant C, Massenet S. In vitro and in cellulo evidences for association of the survival of motor neuron complex with the fragile X mental retardation protein. J Biol Chem. 2008;283:5598–610. doi: 10.1074/jbc.M707304200. [DOI] [PubMed] [Google Scholar]
- Prithviraj R, Kelly KM, Espinoza-Lewis R, Hexom T, Clark AB, Inglis FM. Differential regulation of dendrite complexity by AMPA receptor subunits GluR1 and GluR2 in Motor Neurons. Dev Neurobiol. 2007;68:247–264. doi: 10.1002/dneu.20590. [DOI] [PubMed] [Google Scholar]
- Purves D, Hume RI. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci. 1981;1:441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259–77. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:11557–62. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003;89:3143–3154. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Robertson MM, Rizzo R, Turk J, Bhatia KP, Orth M. Fragile X syndrome associated with tic disorders. Mov Disord. 2008 doi: 10.1002/mds.21995. in press. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–85. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Soontarapornchai K, Maselli R, Fenton-Farrell G, Tassone F, Hagerman PJ, Hessl D, Hagerman RJ. Abnormal nerve conduction features in fragile X premutation carriers. Arch Neurol. 2008;65:495–8. doi: 10.1001/archneur.65.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxem D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–37. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: Dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Achsel T, Bagni C. mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol. 2006;16:265–9. doi: 10.1016/j.conb.2006.05.010. [DOI] [PubMed] [Google Scholar]