Abstract
In many sensory systems, stimulus sensitivity is dynamically modulated through mechanisms of peripheral adaptation, efferent input, or hormonal action. In this way, responses to sensory stimuli can be optimized in the context of both the environment and the physiological state of the animal. Although the gustatory system critically influences food preference, food intake and metabolic homeostasis, the mechanisms for modulating taste sensitivity are poorly understood. In this study, we report that glucagon-like peptide-1 (GLP-1) signaling in taste buds modulates taste sensitivity in behaving mice. We find that GLP-1 is produced in two distinct subsets of mammalian taste cells, while the GLP-1 receptor is expressed on adjacent intragemmal afferent nerve fibers. GLP-1 receptor knockout mice show dramatically reduced taste responses to sweeteners in behavioral assays, indicating that GLP-1 signaling normally acts to maintain or enhance sweet taste sensitivity. A modest increase in citric acid taste sensitivity in these knockout mice suggests GLP-1 signaling may modulate sour taste, as well. Together, these findings suggest a novel paracrine mechanism for the regulation of taste function.
Keywords: glucagon-like peptide-1, gustducin, paracrine signaling, serotonin, sweet, taste
Many of the cellular and molecular mechanisms important for the recognition and transduction of taste stimuli, as well as for the transmission of sensory information from the taste bud to the nervous system, have been elucidated in recent years (Scott 2005; Chandrashekar et al. 2006; Roper 2006). Stimuli of different taste qualities [i.e. sweet, bitter, sour, salty, and umami (glutamate taste)] are detected by distinct receptors on specialized taste cells (TCs) within taste buds (Scott 2005; Chandrashekar et al. 2006; Roper 2006). Type II TCs are narrowly tuned to sweet, bitter, or umami stimuli (Tomchik et al. 2007), and express components of a G protein-coupled transduction cascade (McLaughlin et al. 1992; Rossler et al. 1998; Hoon et al. 1999; Adler et al. 2000; Matsunami et al. 2000; Clapp et al. 2001; Perez et al. 2002; Chandrashekar et al. 2006; Roper 2006). The function of Type III cells is less clear. Most release serotonin (5-hydroxytryptamine; 5-HT) upon depolarization (Huang et al. 2005), and display conventional synapses with afferent nerve fibers (Yang et al. 2000). They have been suggested to integrate taste responses within the taste bud in part because they appear to be broadly tuned to stimuli of all taste qualities (Roper 2006; Tomchik et al. 2007). However, deletion of Type III cells specifically affects acid taste responses (Huang et al. 2006; Kataoka et al. 2008), suggesting that this subset of TCs may be primarily involved in the transduction of sour tastants.
A key function of the gustatory system is to detect nutrients, toxins, and indicators of spoilage, thus providing critical information to the animal about the quality and nutritional value of food before it is ingested (Breslin and Huang 2006). Many of the molecules important for the recognition and transduction of taste stimuli, including T1R (Hoon et al. 1999) and T2R (Adler et al. 2000; Matsunami et al. 2000) taste receptors and the G protein subunit α-gustducin (McLaughlin et al. 1992), also have important roles in nutrient response and assimilation (Wu et al. 2002; Scott 2005; Chandrashekar et al. 2006; Roper 2006; Rozengurt 2006; Bezencon et al. 2007; Jang et al. 2007; Margolskee et al. 2007). We recently reported that α-gustducin and the sweet taste receptor subunit T1R3 (Bachmanov et al. 2001; Kitagawa et al. 2001; Max et al. 2001; Montmayeur et al. 2001; Nelson et al. 2001; Sainz et al. 2001) mediate the glucose-dependent secretion of the incretin hormone glucagon-like peptide-1 (GLP-1) from enteroendorcrine L cells of the gut (Jang et al. 2007). Because of the close cellular and functional relationship of GLP-1, T1R3, and α-gustducin in the gut, we hypothesized that these molecules may also work together in the gustatory system. In this study, we show that functional GLP-1 is expressed in two distinct subsets of TCs: a subset of Type II cells that co-express T1R3, and a subset of Type III cells. We also report that disruption of GLP-1 signaling modulates taste sensitivity in mice. Our results demonstrate that specific taste sensitivities can be regulated by paracrine signaling within the taste bud, and support a model for the conservation of chemosensory mechanisms throughout the alimentary canal.
Materials and methods
Animals and tissue processing
All animal testing procedures were approved by the Animal Care and Use Committee of the National Institute on Aging. GLP-1 receptor (GLP-1R) knockout (KO) mice (maintained on a CD1 background (Scrocchi et al. 1996, 1998); n = 12 of each, 6–10 weeks of age) and their wild-type CD1 counterparts (WT), as well as Sprague—Dawley rats (n = 12, 8–10 weeks of age) were sources of taste, ileal, and pancreatic tissue. Taste tissue was also harvested from a single macaque monkey. Tissue processing was performed as described previously (Yee et al. 2001).
Immunohistochemistry
Immunofluorescence analyses of taste and ileal tissue were performed as described previously (Theodorakis et al. 2006). All immunohistochemical studies of taste tissue were performed on circumvallate papillae (CV). Sources and dilutions of the applied primary antibodies are listed in Table S1. After antigen retrieval with 1x citrate buffer (Biogenex, San Ramon, CA, USA) at 98°C for 20 min, sections were incubated with primary antisera overnight. To visualize these antisera, sections were incubated for 1 h in fluorescent secondary antibodies (Alexa 488 and 568, 1 : 1000 dilution; Molecular Probes, Carlsbad, CA, USA), along with TO-PRO-3 (1 : 7000 dilution; Molecular Probes) in some cases, for nuclear staining. For triple labeling, adjacent serial sections were incubated with either the GLP-1 and α-gustducin antisera or the T1R3 antisera, and the images merged after confocal imaging. For detection of 5-HT-accumulating Type III TCs, mice and rats were injected intraperitoneally with 5-hydroxy-L-tryptophan (0.08 mg/g; Sigma, St Louis, MO, USA) 1 h prior to death and removal of the tongues (Yee et al. 2001). For logistical reasons this was not possible in the monkey. In no cases was fluorescent staining observed when either the primary or secondary antibodies were omitted.
Isolation of tongue epithelium
The dorsal epithelium of rodent tongue, containing both anterior and posterior taste fields, was isolated using a modified protocol ( Behe et al. 1990). The peeled epithelium was stored at -70°C for RNA, and protein preparation and quantification. Rodent ileum was also stored at -70°C for similar procedures.
Protein extraction
Protein extractions were performed as described previously (Schmidt et al. 1991). The extracts were then freeze-dried and reconstituted with assay buffer containing 0.05 M phosphatebuffered saline, pH 6.8, containing proprietary protease inhibitors, with Tween 20, 0.08% sodium azide, and 1% bovine serum albumin.
Bioassay of lingual and ileum extracts: intracellular cAMP determination in CHO/K1 and CHO/GLP-1R cells
We previously stably transfected Chinese hamster ovary (CHO)/K1 cells with rat GLP-1R (CHO/GLP-1R cells) (Wang et al. 2001). We treated both CHO/K1 and CHO/GLP-1R cells with full-length GLP-1 (Bachem, Torrance, CA, USA) as well as lingual and ileal protein extracts. Intracellular cAMP assays were performed as described previously (Theodorakis et al. 2006). After treatment with lingual or ileal protein extract and GLP-1, the cell supernatant was assayed using a cAMP (direct) EIA Kit (Assay Designs, Ann Arbor, MI, USA).
RNA isolation and real-time PCR of taste tissue and ileum
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from lingual epithelium and ileum according to the manufacturer’s instructions. After reverse transcription, the resulting materials were used for PCR amplification using gene-specific primer pairs (Table S2 and Fig. S1) and SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA). For real-time PCR, amplification conditions were 50°C (2 min), 95°C (10 min), and then 40 cycles at 95°C (15 s) and 60°C (1 min) (Lal et al. 2004). The data were normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA. All real-time PCR analyses are represented as the mean ± SEM from at least three independent experiments, each performed in triplicate.
Taste behavioral tests
All taste testing took place during daylight hours. Two-month-old male GLP-1R KO and WT mice (n = 8 per group) were habituated to the laboratory environment for 35 min each day before the initiation of taste testing. All tastants were prepared with distilled water and reagent grade chemicals, and were presented to the animals at 23°C. Test stimuli consisted of various concentrations of sucrose (1, 3, 10, 30, and 100 mM; Fisher Scientific, Atlanta, GA, USA), sucralose (0.001, 0.01, 0.1, 1, and 5 mM; Toronto Chemical, Toronto, ON, Canada), NaCl (30, 100, 200, 300, 600, and 1000 mM), denatonium benzoate (DB: 0.01, 0.05, 0.1, 0.5, 1, and 5 mM; Sigma-Aldrich, St Louis, MO, USA), and citric acid (CA; 0.3, 1, 3, 10, 30, and 100 mM; Fisher Scientific). Brief-access taste testing took place in a ‘Davis Rig’ gustometer (Davis MS-160; DiLog Instruments, Tallahassee, FL, USA), as previously described ( Boughter et al. 2002; Glendinning et al. 2002; Nelson et al. 2003; Dotson and Spector 2004). Brief-access procedures minimize post-ingestive effects that may confound other assays such as intake tests (Nelson et al. 2003). Mice accessed the taste stimuli (presented as a concentration range) or water in sipper bottles through a small opening in the mouse chamber. Before taste testing was initiated, mice were trained to lick a stationary tube of water in the gustometer after being placed on a 23.5 h restricted water-access schedule. Unconditioned licking responses were recorded for later analyses in 25-min brief-access test sessions, during which mice could initiate as many trials as possible in this period. Stimulus presentation order was randomized within blocks. The duration of each trial (5 s) was regulated by a computer-controlled shutter that allowed access to the sipper tube. There was a 7.5 s inter-presentation interval, during which time a stepper motor moved one of up to seven tubes (containing water or a concentration of taste stimulus) in front of the shuttered opening.
Two different testing protocols were used; one for normally preferred stimuli (sucrose and sucralose), and one for normally avoided substances (DB, CA, and NaCl). For sucrose and sucralose, animals received 3 days of testing with the five stimulus concentrations and with purified water. For DB, NaCl, and CA, mice first received 2 days of testing with purified water to increase the number of stimulus trials taken during testing. On the following 3 days, the mice were tested with purified water and six stimulus concentrations. A water rinse presentation (1 s) was interposed between the test trials for the normally avoided stimuli to help control for potential carry-over effects.
Data analysis and statistical methods for behavioral testing
The average number of licks per trial for each stimulus concentration was divided by the average number of water licks per trial, yielding a tastant/water lick ratio. This ratio controls for individual differences in lick rates and for differences in motivational state (Glendinning et al. 2002). The ratios were analyzed with standard ANOVA and t-test. When a genotype × concentration interaction was significant, one-way ANOVA was conducted within each genotype to test for simple effects. The conventional p ≤ 0.05 was applied as the statistical rejection criterion. Curves were fit to the mean data for each group using a two- or three-parameter logistic function of the form:
where x = log10 concentration, c = log10 concentration at the inflection point, and b = slope. For sucrose and sucralose, a = the asymptotic tastant/water lick ratio and d = minimum asymptote of tastant/water lick ratio. For NaCl, DB, and CA, a = 1.0 and d = 0.
Results
To determine if GLP-1 may play a role in modulating gustatory function, we first asked if the peptide is expressed in TCs. We found GLP-1 immunoreactivity in a small subset (∼10%) of TCs in mouse CV taste buds [Fig. 1a; similar results were seen in rat (Fig. S2 and Feng et al. 2008) and in preliminary results from one macaque monkey (Fig. S3)]. Approximately half of all GLP-1-positive cells are immunopositive for the G protein α-gustducin (55.7%; Table 1), and only one-third of α-gustducin-positive TCs express GLP-1 (34.0%; Fig. 1b and Table 1). GLP-1 antibodies also labeled approximately one-quarter of serotonergic Type III TCs in the CV (23.1%; Fig. 1c and Table 1). GLP-1 never co-localized with protein gene product 9.5 (PGP 9.5) in TCs, which has been suggested as a marker of α-gustducin-negative Type II TCs and 5-HT-negative Type III TCs (Yee et al. 2001) (Fig. S2). Together, these results indicate that GLP-1 is expressed in subsets of both Type II and Type III cells of the mouse CV and suggests that the hormone plays a local role in modulating taste bud function.
Fig. 1.
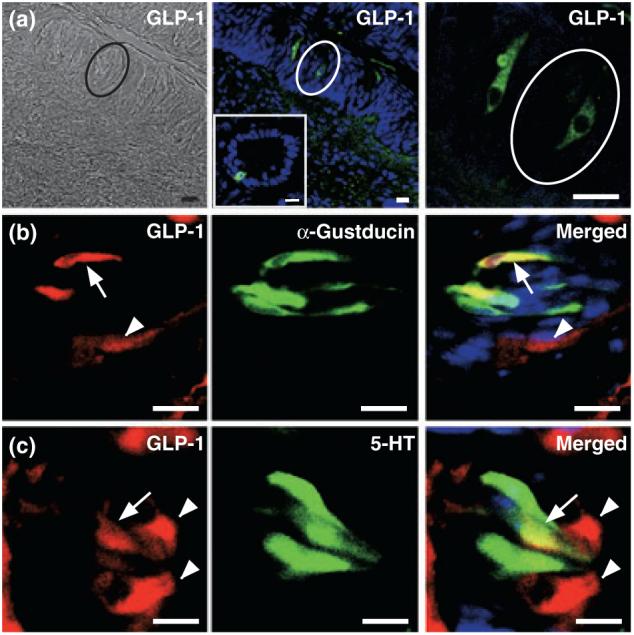
Expression of GLP-1 in taste cells of the mouse circumvallate papillae (CV). (a) Bright-field (left panel) and immunofluorescence images (middle and right panels) of GLP-1-expressing cells (green) within CV taste buds (ovals). GLP-1 staining in ileum is shown (insert, middle panel) as a positive control. (b) GLP-1 (red) and α-gustducin (green) are co-localized in a subset of α-gustducin-positive cells (yellow cells). Arrowhead, cell expressing only GLP-1; arrows, cells expressing both markers. (c) GLP-1 (red) and 5-HT (green) are co-localized in a subset of 5-HT-positive cells (yellow cells). Arrowheads, cells expressing only GLP-1; arrow, cell expressing both markers. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain. Sections are representative from three mice.
Table 1.
Co-localization of antigenic markers in mouse CV taste cells
| Counts of cells expressing one or both markers | ||||
|---|---|---|---|---|
| Marker 1 |
||||
| Marker 2 | α-Gustducin | GLP-1 | T1R3 | 5-HT |
| α-Gustducin | - | 636/1869 | 105/687 | n.d. |
| GLP-1 | 636/1142 | - | 182/353 | 354/802 |
| T1R3 | 105/687 | 182/747 | - | n.d. |
| 5-HT | n.d. | 354/1533 | n.d. | - |
The denominator is the number of cells expressing Marker 2, while the numerator is the number of cells expressing both Marker 1 and Marker 2. We examined 6-18 sections for each double-labeling experiment. CV, circumvallate papillae; GLP-1, glucagon-like peptide 1; 5-HT, 5-hydroxytryptamine; n.d., not determined.
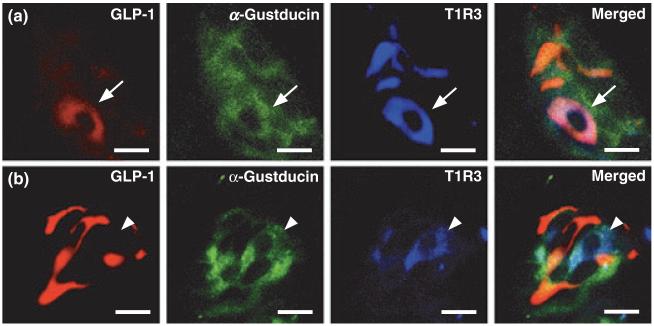
The T1R3 taste receptor subunit co-localizes with α-gustducin in subsets of Type II cells ( Chandrashekar et al. 2006; Roper 2006). As T1R3 mediates sugar-dependent secretion of GLP-1 from enteroendocrine L cells in the gut (Jang et al. 2007), we asked if the GLP-1-positive Type II cells might express this receptor subunit. Consistent with previous reports (Kim et al. 2003; Stone et al. 2007), we found T1R3 expressed in 14.9% of α-gustducin-positive cells (Table 1). T1R3 is expressed in 49.1% of GLP-1-positive cells (Table 1), suggesting that T1R3 is expressed in all GLP-1-positive/α-gustducin-positive cells. Indeed, triplelabel immunohistochemistry showed that all GLP-1-positive/α-gustducin-positive cells in the CV also express T1R3 (Fig. 2a). In mouse CV, T1R3 is almost always co-expressed with the other subunit of the sweet taste receptor, T1R2 (Max et al. 2001; Montmayeur et al. 2001; Nelson et al. 2001; Kim et al. 2003), strongly indicating that GLP-1-positive/α-gustducin-positive/T1R3-positive cells are sweet-sensitive TCs. As expected, T1R3 is also expressed in a separate population of GLP-1-negative cells that appear weakly stained for α-gustducin (Fig. 2b). At any rate, it appears that a subset of T1R3-expressing cells express both α-gustducin and GLP-1.
Fig. 2.
Co-expression of GLP-1 and T1R3 in mouse CV. (a) GLP-1 (red), α-gustducin (green) and T1R3 (blue) are co-localized in a subset of T1R3-positive cells (arrow). (b) Some α-gustducin-positive/T1R3-positive cells are GLP-1-negative (arrowhead). Scale bars, 20 μm. Sections are representative from three mice.
Glucagon-like peptide-1 is produced from proglucagon through enzymatic cleavage by the prohormone convertase (PC) 1/3 (Baggio and Drucker 2007). If GLP-1 is produced in taste buds, it should be co-expressed with PC 1/3. Indeed, immunohistochemical staining shows that PC 1/3 is expressed in all GLP-1-positive TCs of the mouse CV (Fig. 3a), confirming that GLP-1 is produced in TCs. PC 1/3 was also expressed in GLP-1-negative cells (Fig. 3a), suggesting that this enzyme regulates the cleavage of other peptides in the taste bud. PC 2, which cleaves glucagon from proglucagon, is also found in mouse TCs (Fig. 3b), and glucagon co-localizes with GLP-1 (Fig. S3 and data not shown). Therefore, GLP-1 and glucagon are produced in TCs and are present in the same TC population, suggesting a coordinated regulation of their production in taste tissue.
Fig. 3.
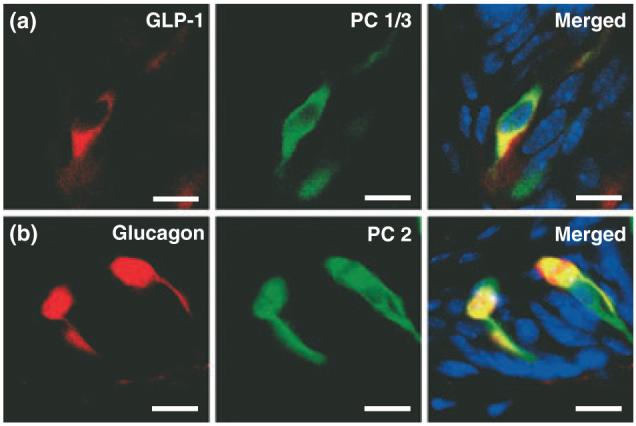
Enzymes required for the cleavage of proglucagon products are in CV taste cells. (a) GLP-1 (red) and PC 1/3 (green) are co-expressed in a subset of PC 1/3-positive cells (yellow). (b) Glucagon (red) and PC 2 (green) are co-expressed in mouse TCs (yellow). Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain.
To determine if the GLP-1 produced in TCs is an active form of this peptide, we measured GLP-1 activity in lingual extracts. We treated both CHO cells transfected with the rat GLP-1R (CHO/GLP-1R cells) cells and control CHO/K1 cells (no receptor) with lingual or ileal protein extracts or with active GLP-1 (7–37) peptide, and quantified intracellular cAMP responses (Doyle et al. 2003). Both ileal and lingual extracts elicited a concentration-dependent increase in cAMP in CHO/GLP-1R cells but not CHO/K1 cells (Fig. 4), demonstrating that GLP-1 derived from lingual tissue is active.
Fig. 4.
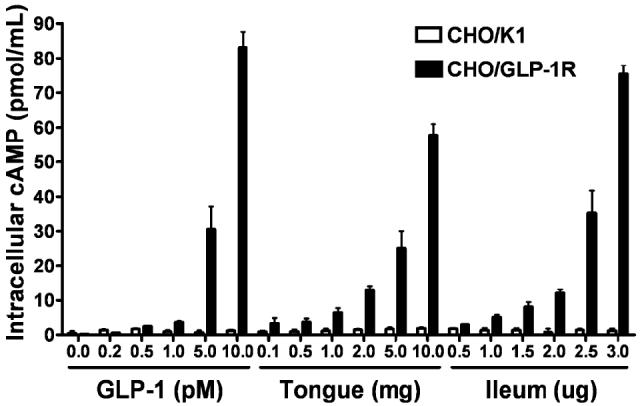
Extracts of lingual epithelium elicit a GLP-1R-dependent increase in intracellular cAMP. GLP-1 and extracts of both lingual (containing taste buds) and ileal epithelium elicit significant concentration-dependent increases of cAMP in CHO/GLP-1R cells, but not in CHO/K1 control cells (cell type × concentration repeated measures ANOVA; GLP-1: cell type, F(1,4) = 5052.3, p < 2 × 10-7; concentration, F(5,20) = 250.15, p < 9 × 10-6; cell type × concentration, F(5,20) = 249.97, p < 2 × 10-5; lingual epithelium: cell type, F(1,4) = 831.18, p < 1 × 10-9, concentration, F(5,20) = 518.93, p < 1× 10-9, cell type × concentration, F(5,20) = 480.85, p < 1 × 10-9; ileal epithelium: cell type, F(1,4) = 627.69, p < 1 × 10-9, concentration, F(5,20) = 311.17, p < 1 × 10-9, cell type × concentration, F(5,20) =313.00, p < 1 × 10-9). Experiments were carried out in triplicate with at least two replications. Values are expressed as mean ± SEM.
Glucagon-like peptide-1 secreted from enteroendocrine L cells of the gut acts distantly on GLP-1Rs in the pancreas and other tissues ( Baggio and Drucker 2007). We asked if GLP-1 in TCs might act on local targets. Taste buds are innervated by PGP 9.5-positive afferent nerve fibers, which communicate taste information from activated TCs to the CNS. We found GLP-1R immunoreactivity on PGP 9.5-positive nerve fibers adjacent to 5-HT-positive TCs (Fig. 5a and b; Figs S3 and S4). The proximity of GLP-1 and its cognate receptor indicates that GLP-1 signaling is local to the taste bud, and suggests that this hormone may modulate taste signaling in a paracrine manner.
Fig. 5.

The GLP-1 receptor is expressed on intragemmal nerve fibers of CV. (a) GLP-1R (red) is expressed on nerve fibers contiguous with TCs expressing 5-HT (green); e.g., *. (b) GLP-1R (red) and PGP 9.5 (green) are co-localized on nerve fibers and cells (yellow). Arrows expressing both markers. (c) GLP-1R is not expressed in CV from the GLP-1R KO mouse. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain.
The small number of TCs expressing GLP-1 begs the question of whether the hormone can reach meaningful concentrations in taste buds. In blood and in ileum, the degradative enzyme dipeptidyl peptidase 4 rapidly cleaves GLP-1 into an inactive form that can no longer stimulate the GLP-1R (Baggio and Drucker 2007). Both immunohistochemical and real-time PCR analysis of CV tissue shows that little or no dipeptidyl peptidase 4 is expressed in taste buds (Fig. 6a and b), though it is present in ileum controls (Fig. 6b and c). Thus, the half-life of GLP-1 in taste tissue should be high, ensuring sufficient concentrations within the taste bud to stimulate the GLP-1R.
Fig. 6.

Dipeptidyl peptidase 4 (DPP4) is not expressed in CV taste buds. (a) No DPP 4 immunoreactivity was observed in taste buds (oval). (b) Quantitative real-time PCR of cRNA from rat tongue and ileum. Experiments were carried out in triplicate and replicated at least twice. Values are expressed as mean ± SEM. (c) DPP 4 (green) was clearly observed proximal to rat ileum enteroendocrine L cells (identified by GLP-1 immunoreactivity, red), as well as in blood vessels (arrow). Scale bars, 20 lm. Blue is TO-PRO-3 nuclear stain.
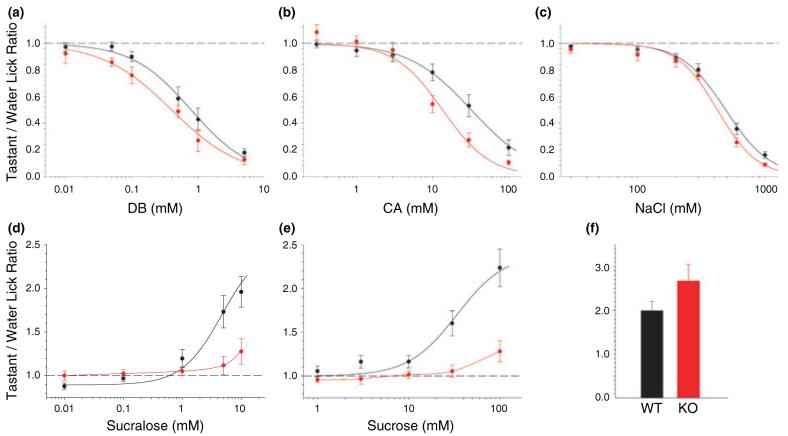
To determine how GLP-1 signaling in the taste bud impacts taste function, we tested the ability of GLP-1R KO mice (Scrocchi et al. 1996) to detect taste stimuli. KO mice showed no gross taste bud abnormalities and expressed GLP-1 in TCs (Fig. S5), though GLP-1R immunoreactivity was absent as expected (Fig. 5c). Mice were tested in a computer-controlled gustometer using a brief-access procedure that minimizes post-ingestive effects (Nelson et al. 2003). Although there were no significant differences between the responses of KO and age-matched WT controls (n = 8 for each genotype) for the bitter-tasting DB, the prototypical salt NaCl or the sour-tasting CA, there was a trend towards hypersensitivity of KO mice to these three aversive stimuli (Fig. 7a, b, and c). This suggests that GLP-1 signaling plays a subtle modulatory role for responses to these taste stimuli, possibly through its expression in broadly tuned Type III cells. Indeed, there was a significant genotype × concentration interaction [two-way ANOVA: F(5,70) = 5.5, p < 0.0003] for CA, with independent t-test indicating that the KO animals showed greater lick avoidance to CA at the 10 and 30 mM levels (both p < 0.03) relative to WT controls.
Fig. 7.
Altered sweet taste responses of GLP-1R KO mice in brief access taste tests. (a—c) Taste responses, expressed as taste/water lick ratios and as a function of stimulus concentration, of GLP-1R KO (red) and WT (black) to denatonium benzoate (DB) (a), citric acid (CA) (b), and NaCI (c). (d and e) Taste responses of GLP-1R KO (red) and WT (black) to sucralose (d) and sucrose (e). (f) GLP-1R KO and WT mice exhibit equivalently robust responses to high concentrations of sucrose (400 mM: no significant effect of genotype). Points are expressed as mean ± SEM. Curves were fit as described in Materials and methods, except for the sucrose WT and KO histograms, which were not fitted to a function.
In contrast, KO mice displayed significantly reduced sensitivity to two sweeteners, sucralose and sucrose, when compared with WT (Fig. 7d and e). KO mice were unresponsive to sucralose up to 10 mM. As analyzed by two-way ANOVA, there was a significant main effect of both genotype [F(1,13) = 6.0, p < 0.03] and concentration [F(4,52) = 23.0, p < 0.00001], as well as a significant interaction [F(4,52) = 9.5, p < 0.00001] for sucralose. Separate one-way ANOVA for each genotype revealed that only the WT mice showed a significant monotonically increasing concentration-response function [F(4,24) = 23.8, p < 0.00001]. For sucrose, there was also a significant main effect of both genotype [F(1,13) = 13.1, p < 0.004] and concentration [F(4,52) = 36.9, p < 0.00001] and a significant interaction [F(4,52) = 12.5, p < 0.00001]. Separate oneway ANOVA for each genotype revealed that both displayed a significant monotonically increasing concentration—response function for sucrose [WT: F(4,24) = 30.9, p < 0.00001; KO: F(4,28) = 5.5, p < 0.003], though only WT mice showed significantly greater lick responses to sucrose than to water (30 mM: t > 4.2, p < 0.006; 100 mM: t > 5.8, p < 0.001). KO mice were not sweet ageusic, as they responded to high concentrations of sucrose as robustly as WT mice (Fig. 7f). Rather, the KO mice exhibit a diminished sensitivity to sweeteners. Together, these results clearly show that GLP-1 signaling plays an important role in modulating taste sensitivity in the gustatory system.
Discussion
We found that GLP-1 is expressed in two populations of TCs: a subset of α-gustducin-expressing/T1R3-expressing cells, and a subset of serotonergic cells (Figs 1 and 2 and Table 1). This divergent expression may provide insights into distinct functional roles of GLP-1 within the taste bud. Indeed, GLP1-R KO mice exhibit reduced taste sensitivity to both nutritive and non-nutritive sweeteners, but display hypersensitivity to CA (Fig. 7). The differential responses of GLP-1R KO mice to preferred (sucrose and sucralose) and aversive (CA) taste stimuli may reflect the differential effects of GLP-1 secreted from subsets of Type II and Type III cells. These two cell types display numerous molecular and physiological differences, including breadth of tuning (Tomchik et al. 2007), and are therefore likely to play distinct roles in peripheral taste coding (Roper 2006). The results described here suggest that Type II and Type III cells may provide distinct sites for modulation of taste coding, as well.
Type II and Type III cells not only differ from each other, but represent heterogeneous cell populations (Roper 2006). The functional diversity of Type II cells correlates reasonably well with the differential tuning of sweet-, bitter-, and umami-sensitive cells: Type II cells appear to exclusively express either T1Rs or T2Rs, only some of which express α-gustducin. Restriction of GLP-1 to a subset of T1R3-positive/α-gustducin-positive Type II cells is consistent with this functional classification, and with the dramatic reduction in taste sensitivity to sweet, but not bitter, stimuli in GLP-1R KO mice. The majority of Type III cells in the mouse and rat CV are serotonergic, though a second subset that does not accumulate 5-HT but expresses PGP 9.5 has been reported (Yee et al. 2001; Ma et al. 2007). Interestingly, all Type III cells express the polycystic kidney disease 2-like 1 (PDK2L1) channel subunit (Kataoka et al. 2008), which is a candidate sour taste receptor (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006). Indeed, nerve responses to acid stimuli are nearly abolished in mice where PKD2L1-expressing cells have been genetically deleted (Huang et al. 2006). However, it remains unresolved if all PKD2L1-expressing TCs are acid sensors. The expression of GLP-1 in only a subset of serotonergic Type III TCs is surprising, and highlights a greater functional diversity in this population than was previously understood. As GLP-1R KO mice appear hypersensitive to CA, it may be that GLP-1-expressing Type III cells play a particularly important role in sour taste.
Many physiological processes are regulated by GLP-1, including insulin secretion and biosynthesis, gastric emptying, neuronal survival, and cardiac contractility (Drucker 2006). While we cannot rule out a contribution of these other GLP-1 signaling systems to the modulation of taste sensitivity, several lines of evidence strongly suggest that the taste phenotype we observed in GLP-1R KO mice results from a deficit in GLP-1 signaling within the taste bud. First, the brief-access behavioral assay minimizes post-ingestive effects (Nelson et al. 2003), indicating that the changes observed are likely ones of orosensory sensitivity, not postoral nutrient response. Second, we observed altered taste sensitivity to stimuli of a preferred (sweet) and an aversive (sour) taste quality (Fig. 7), indicating that there was not an overall effect on taste function. Third, the WT responses of GLP-1R KO mice to bitter or salt stimuli is consistent with specific effects on sweet and sour taste and also clearly demonstrate that the KO mice had no difficulty learning or completing the task. Fourth, GLP-1 is specifically co-localized with putative sweet- and sour-sensitive TCs, consistent with the specific behavioral changes. Fifth, the opposite effects of GLP1-R deletion on sweet (reduced) and sour (enhanced) taste sensitivity shows a specificity of the functional loss depending on cell type. Thus, we conclude that the most parsimonious interpretation of our findings is that GLP-1 signaling within the taste bud modulates taste sensitivity.
The presence of gut hormones in the taste bud highlights an interesting parallel between the gustatory and intestinal epithelia. A number of recent studies in rodent and human have shown that many taste transduction molecules, including T1R and T2R taste receptors, α-gustducin, phospholipase C-β2 and the transient receptor potential M5 channel, are expressed in subsets of cells in the stomach and intestine. Some of these cells have been identified as enteroendocrine L cells, which secrete GLP-1 in a T1R3- and α-gustducin-dependent manner in response to stimulation with sweeteners (Jang et al. 2007). Our observation that a subset of TCs expressing both T1R3 and α-gustducin (as well as phospholipase C-β2 and transient receptor potential M5; Chandrashekar et al. 2006) also express and secrete GLP-1 suggests that they may share other molecular mechanisms or physiological roles.
Other hormones and hormone receptors are expressed in the taste bud, suggesting that the peripheral gustatory apparatus is subject to endocrine and paracrine modulation. The neuropeptides cholecystokinin, vasoactive intestinal peptide, and neuropeptide Y are produced in TCs, where they may act as local transmitters or modulators (Herness et al. 2002; Shen et al. 2005; Zhao et al. 2005). However, while cholecystokinin and neuropeptide Y both modulate K+ conductances in some TCs (Herness et al. 2002; Zhao et al. 2005), their effects on taste transduction, taste coding, and behavior remains unknown. Although leptin is produced outside the taste system, leptin receptors are expressed in TCs (Kawai et al. 2000; Shigemura et al. 2004). Mice lacking these receptors (db/db mice) show enhanced responses to sweet stimuli (Shigemura et al. 2004), and i.p. injection of leptin in lean mice suppressed taste responses to sweeteners (Kawai et al. 2000; Shigemura et al. 2004), suggesting that this hormone may help to suppress ingestive behavior through the peripheral suppression of sweet taste function. Interestingly, the effects of leptin contrast with those of GLP-1. First, GLP-1 is expressed in taste tissue, indicating a paracrine rather than endocrine action. Second, reduced sweet taste sensitivity in GLP-1R KO mice suggests that local GLP-1 signaling normally acts to maintain or enhance sweet taste sensitivity. In this light, the taste bud may serve as an important target for positive and negative modulators of taste sensitivity, thus providing a peripheral mechanism for the regulation of ingestive behaviors in the context of an animal’s metabolic state.
Supplementary Material
Acknowledgements
This work is supported by the Intramural Research Program, NIA/NIH and by Grants from the NIDCD (SDM: DC005786, DC008301). CDD is supported by training grants from the NIDCD (DC000054) and the NIDCR (DE007309).
Abbreviations used
- 5-HT
serotonin or 5-hydroxytryptamine
- CA
citric acid
- CHO
Chinese hamster ovary
- CV
circumvallate papillae
- DB
denatonium benzoate
- GLP-1
glucagon-like peptide 1
- GLP-1R
GLP-1 receptor
- KO
knockout
- PC
proconvertase
- PGP 9.5
protein gene product 9.5
- PKD2 L1
polycystic kidney disease 2-like 1
- TC
taste cell
- WT
wild-type
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem. Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Behe P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J. Gen. Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem. Senses. 2002;27:133–142. doi: 10.1093/chemse/27.2.133. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. Adv. Otorhinolaryngol. 2006;63:152–190. doi: 10.1159/000093760. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem. Senses. 2004;29:489–498. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Theodorakis MJ, Holloway HW, Bernier M, Greig NH, Egan JM. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul. Pept. 2003;114:153–158. doi: 10.1016/s0167-0115(03)00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Feng XH, Liu XM, Zhou LH, Wang J, Liu GD. Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem. 2008;110:151–154. doi: 10.1016/j.acthis.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A highthroughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem. Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J. Neurosci. 2002;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of tastespecific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Using biosensors to detect the release of serotonin from taste buds during taste stimulation. Arch. Ital. Biol. 2005;143:87–96. [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl Acad. Sci. USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl Acad. Sci. USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by Type III taste cells in the mouse. Chem. Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl Acad. Sci. USA. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem. Biophys. Res. Commun. 2003;312:500–506. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem. Biophys. Res. Commun. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- Ma H, Yang R, Thomas SM, Kinnamon JC. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci. 2007;8:5. doi: 10.1186/1471-2202-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl Acad. Sci. USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gust-ducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson TM, Munger SD, Boughter JD., Jr Taste sensitivities to PROP and PTC vary independently in mice. Chem. Senses. 2003;28:695–704. doi: 10.1093/chemse/bjg062. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell. Mol. Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur. J. Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J. Neurochem. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Poulsen SS, Rasmussen TN, Bersani M, Holst JJ. Substance P and neurokinin A are codistributed and colocalized in the porcine gastrointestinal tract. Peptides. 1991;12:963–973. doi: 10.1016/0196-9781(91)90045-q. [DOI] [PubMed] [Google Scholar]
- Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Marshall BA, Cook SM, Brubaker PL, Drucker DJ. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes. 1998;47:632–639. doi: 10.2337/diabetes.47.4.632. [DOI] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds of mice. Chem. Senses. 2007;32:255–262. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein trans-location from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 2001;142:1820–1827. doi: 10.1210/endo.142.5.8128. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl Acad. Sci. USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J. Comp. Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J. Comp. Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc. Natl Acad. Sci. USA. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.