Abstract
We cross-linked scaffolds of electrospun collagen to varying degrees with glutaraldehyde using an ethanol-based solvent system and subsequently defined how the percentage of cross-linking impacts bulk and microscale material properties and fiber structure. At hydration, electrospun fibers underwent coiling; the extent of coiling was proportional to the percentage of cross-linking introduced into the samples and was largely suppressed as cross-linking approached saturation. These data suggest that electrospun collagen fibers are not deposited in a minimal energy state; fiber coiling may reflect a molecular reorganization. This result has functional/structural implications for protein-based electrospun scaffolds. Changes in fiber topology that develop during post-electrospinning processing may alter monomer organization, mask or unmask receptor binding sites, and/or change the biological properties of these nanomaterials. Hydrated scaffolds were mounted into a custom stretching device installed on a microscope stage and photographed after incremental changes in strain. Changes in fiber alignment were measured using the two-dimensional fast Fourier transform method. Fibers in all scaffolds underwent alignment in response to strain; however, the rate and extent of alignment that could be achieved varied as a function of cross-linking. We propose four distinct modes of scaffold response to strain: fiber uncoiling, fiber reorientation, fiber elongation and interfiber sliding. We conclude that bulk material properties and local microscale architecture must be simultaneously considered to optimize the performance of electrospun scaffolds.
Keywords: electrospinning, electrospun collagen, mechanical testing, 2D FFT, anisotropy, fiber alignment, scaffold design
1. Introduction
Electrospinning can be used to process native, synthetic or blended polymers into fibrous mats that show considerable promise as tissue engineering scaffolds [1,2]. Both scaffold and fiber-level properties can be adjusted at several stages in the electrospinning process. Fiber composition and diameter can be tailored by varying the polymer identity and starting concentration. During electrospinning, fiber alignment (anisotropy) can be selectively increased or decreased by adjusting the mandrel’s tangential velocity [3–5]. Once completed, the material properties of a protein-based scaffold can be further modified through various cross-linking protocols [6]. Each of these variables can be manipulated individually or in concert to provide superb control over the chemical, structural and material properties of a tissue engineering scaffold. The functional properties of this unique class of biomaterials can be manipulated by incorporating various growth factors [7], pharmaceuticals [2,8] or cells [9] into a scaffold at the time of fabrication. The control afforded over the electrospinning process theoretically makes it possible to use this fabrication technique to produce complex, multicellular organs [1,10].
We believe that two basic criteria must be satisfied in order to extend the method of electrospinning from the bench top into clinical practice. First, the macroscale material properties and architectural features of the electrospun scaffold must be consistent and mimic the profile of the native tissue of interest. Second, the local microscale environment must provide physiologically relevant binding sites to anchor cells to the surrounding scaffold. It is through these considerations that the resident cells of most organ systems receive the physical and mechanical signals necessary to express the appropriate phenotypic profile [11,12]. The macroscale architecture and material properties of an electrospun scaffold can be manipulated by regulating fiber composition, fiber diameter, fiber cross-linking and the relative degree of anisotropy introduced into a scaffold during the fabrication process. In turn, the physical cues provided by these macroscopic features can be used to modulate cell phenotype. For example, physical cues provided by the introduction of anisotropy into an electrospun scaffold promote fibroblasts [3] and neuronal cells [13] to align in parallel with the primary axis of fiber alignment. These results suggest that it will be possible to use anisotropy to produce constructs composed of highly aligned cells, a cytoarchitectural feature that is fundamental to the structure and function of skeletal muscle, blood vessels and many other hollow organs. Architectural features and scaffold composition also interact to regulate the extent to which a given construct can support the infiltration and migration of local cell populations [14].
In this study we examined how the extent of cross-linking that is introduced into an electrospun collagen scaffold impacts the structure and response of this type of construct to externally applied mechanical loads. At modest degrees of cross-linking, we report that the fibers of these scaffolds undergo extensive coiling when hydrated. This coiling was largely suppressed as the degree of cross-linking was increased. Overall, we believe that strain is distributed throughout these scaffolds by at least four distinct modes. The systematic application of strain induces fiber straightening (uncoiling), fiber reorientation (fiber realignment along the axis of strain), fiber elongation (changes in fiber length) and interfiber sliding (fibers moving with respect to one another). Our results suggest that fiber topology plays a critical role in determining the stress–strain relationships observed in collagen electrospun scaffolds.
2. Materials and methods
2.1. Collagen isolation
type I collagen was acid-extracted from calfskin corium (animals <6 months old; Lampire Biologics, Pipersville, PA). Corium was defrosted at 4°C, diced into 10–20 cm3 squares and placed into ice-cold distilled water at a concentration of 25–50 g of solid per liter of solution. Glacial acetic acid was added to the suspension to bring the molarity to 0.5 M, the solution was stirred and allowed to incubate for 12 h at 4°C. The ice-cold suspension was then homogenized in a commercial grade blender (Waring) in 10–15 s bursts to limit heating. After an additional 48 h of incubation at 4°C under gentle stirring the resulting collagen-rich solution was centrifuged for 12 h at 14,000 g (4°C). Pellets were discarded, and the supernatant was dialyzed against 20 vol of 18 MΩ-cm water. Water was changed 3–4 times while maintaining the dialysis at 4°C. The extract was then frozen to −70°C and lyophilized until dry. A representative 10% sodium dodecyl sulfate (SDS) gel of fractions isolated by this method and used in our electrospinning experiments is illustrated in Fig. 1.
Fig. 1.
Collagen analysis by SDS gel electrophoresis. Composite image of two separate gels with samples run under non-reducing (lanes 2 and 3) and reducing conditions (lanes 4 and 5). Lane 1= mol. Wt. standards; lane 2= Cell Prime type I collagen standard; lane 3= type I collagen isolate from calfskin corium; lane 4= Cell Prime type I collagen standard; lane 5= type I collagen isolate from calfskin corium.
2.2. Electrospinning
Reagents were purchased from Sigma Aldrich (St. Louis, MO) unless noted. Collagen was solubilized in 2,2,2-triflouroethanol (TFE) at 55 mg ml−1 for 1 h on a clinical rotator [15]. Solutions were loaded into 10 ml syringes capped with a blunt tipped needle. The positive lead of a constant voltage power supply (Spellman CZE1000R; Spellman High Voltage Electronics Corporation) was attached by an alligator clip to the needle; electrospinning was conducted at 22 kV. The collagen solution was delivered to the needle at 8–12 ml h−1 using a Harvard profusion pump. A grounded, rectangular, steel mandrel (70mm × 10mm × 5 mm) was placed 20 cm away, and served as the target. The mandrel was set to rotate at less than 200 rpm to ensure the production of scaffolds composed of random elements [4]. Starting conditions were adjusted to produce scaffolds composed of fibers that exhibited an average cross-sectional diameter of 1 μm.
2.3. Gel electrophoresis
Samples (3 μg protein lane−1) of the collagen isolate used in our electrospinning experiments and Cell Prime (type I collagen by Cohesion Technologies) were separated by SDS gel electrophoresis on a 10% polyacrylamide gel. Samples were diluted in Laemmli buffer supplemented with 5% SDS, with or without 10% β-mercaptoethanol. Collagen was separated and gels were stained with Commassie blue overnight.
2.4. Scaffold response to strain
For macroscopic materials testing, electrospun scaffolds were removed from the mandrel and cut into “dog-bone” shaped samples using a die punch (2.67 mm wide, gauge length of 0.295 mm). This configuration is used to control for grip and geometry effects of the samples to be tested. The samples were then immersed in 0, 0.1, 1, 10 or 40 vol.% glutaraldehyde prepared in ethanol for 12 h. After cross-linking, each sample was sequentially washed in 15 min rinses in 5, 10, 25, 50 and 75% phosphate-buffered saline (PBS) prepared in ethanol, followed by two PBS rinses. We have found that this graded series of rinses reduces solvent-induced changes in fiber diameter (swelling).
The thickness of each hydrated dog-bone shaped sample was determined with a Mitutoyo IP54 digital micrometer (Mitutoyo American Corp; Aurora, IL). Scaffolds were uniaxially tested to failure at an extension rate of 10 mm min−1 with a Bionix 200 Mechanical Testing Systems instrument (MTS Systems Corp, Eden Prairie, MN). Data sets were screened by one-way analysis of variance (ANOVA; p<0.01) to test for the effects of cross-linking on material properties. We measured stress at failure and strain at failure, and calculated Young’s modulus of elasticity [5].
For microscopic materials testing we again cut the electrospun scaffolds into dog-bone shaped samples and processed them with varying concentrations of glutaraldehyde as described for the macroscopic materials testing. A schematic of how data was acquired and evaluated in these experiments is illustrated in Fig. 2. To enhance the contrast of the fibrous scaffolds we stained the cross-linked samples with Coomassie blue (0.1% w/v solution in PBS) for 10 min at room temperature. The stained scaffolds were rinsed in PBS, clamped into a custom stretching device and bolted to the microscope stage of a Nikon TE300 as previously described [16]. The scaffolds were then subjected to incremental changes in strain and photographed. Images were captured every 0.5 mm of displacement using a ×40 0.60 n.a. objective brightfield lens with a Nikon DXM 1200 digital camera. All images were captured at 3840 × 3072 pixels and stored and analyzed as TIF files.
Fig. 2.
Schematic of data flow for the analysis of scaffold structure in response to strain. Digital images were captured at intervals as strain was applied to each scaffold. In turn, each image at each incremental change in strain was processed by 2D FFT analysis to determine the relative degree of fiber alignment present.
2.5. 2D fast Fourier transform
To evaluate the impact of strain on scaffold structure we used the two-dimensional fast Fourier transform (2D FFT) to quantify changes in fiber alignment [4]. Scaffold images were converted to 8-bit grayscale and cropped to 2048 × 2048 pixels using Adobe Photoshop (Adobe Systems). An inverted, circular grayscale window mask was used to reduce edge noise in all images (see Refs. [4,16] for a complete discussion of this process). Using ImageJ software (NIH, http://rsb.info.nih.gov/ij), each of the data images was processed with the 2D FFT function. The 2D FFT frequency output plot was rotated 90° counterclockwise to correct for the 90° rotation that is inherent in this analysis. An oval projection was applied to the frequency plots and the pixel intensities for each 1° sector of the projection were summed using the oval profile plug-in (authored by William O’Connell). All data were normalized as a percent increase over the starting alignment value. The 2D FFT alignment scale is arbitrary and can be adapted to different absolute values. Still, various imaging methods, such as brightfield, scanning electron mocroscopy or confocal, generate slightly different alignment ranges, and independent scaling of each imaging modality is recommended [16].
To measure increasing alignment in the direction of stretch, the normalized alignment data within 10° of the axis of stretch was isolated for analysis. The 2D FFT alignment values were then plotted for each image in the stretch sequence. A linear regression for the alignment data for each incremental change in strain was calculated for each scaffold, the slope of this regression analysis representing how rapidly the scaffold aligns in response to stretch.
2.6. Cross-linking assay
Samples of electrospun collagen were cut into 12 mm diameter circles using a dermal punch (average sample mass 1.56 mg). Scaffolds were cross-linked to varying degrees and rinsed in increasing volumes of PBS as previously described. All data are expressed as the percentage of cross-linking present in a sample with respect to the cross-linking present in a dry, un-crosslinked control sample cut from the same sheet of electrospun material as the experimental groups [6]. Samples were placed into 2 ml of a solution containing 2.0% (w/v) of sodium bicarbonate and 0.25% 2,4,6-trinitrobenzenesulfonic acid (TNBS) prepared in distilled water and heated for 2 h at 40°C. Next, 3 ml of 6 M HCl was added to each sample, and the samples were incubated for an additional 1.5 h at 60°C. Aliquots of equal volume from each sample were placed into a 96-well plate and read at 345 nm on a Spectramax Plus microplate spectrophotometer (Molecular Devices). Percent cross-linking was calculated from the formula:
where Absc= absorbance of the controls at 345 nm (dry sheets of electrospun collagen prior to any manipulation); the unit of mass is mg. Absnc= absorbance of the unknowns at 345 nm; again the unit of mass is mg. This assay is colorimetric in nature and detects free amines. As the percentage of cross-linking increases in a sample, there is a parallel decrease in the number of free amines and therefore a decrease in the number of binding sites available for TNBS to interact with on the protein substrate. For statistical analysis, data sets were screened by one-way ANOVA, and a Tukey test was used in pairwise comparison.
3. Results
3.1. Collagen cross-linking
The scaffolds used in this study were composed of fibers that averaged 1 μm in cross-sectional diameter (data not shown). The degree of cross-linking present in the samples of electrospun collagen varied as a function of the initial percent of glutaraldehyde present at the onset of cross-linking (Fig. 3). Our analysis indicated that 43% of the available sites were cross-linked in samples that were immersed in 0% glutaraldehyde/100% alcohol for 12 h. At 0.1% glutaraldehyde, the average cross-linking increased to 52%. Scaffolds processed in 1% glutaraldehyde were 64% cross-linked, scaffolds processed with 10% glutaraldehyde were 69% cross-linked and scaffolds processed in 40% glutaraldehyde were 70% cross-linked. One-way ANOVA indicated that samples cross-linked in 10 and 40% glutaraldehyde were different than samples processed in 0 and 0.1% glutaraldehyde (p<0.050). There was a subjective increase in the amount of cross-linking present in samples processed with 0, 0.1 and 1% glutaraldehyde; however, no statistical differences were detected among these groups (Fig. 2). In conjunction with these experiments, we also examined how changes in fiber diameter might impact the degree of cross-linking that occurs in an electrospun scaffold. For each concentration of glutaraldehyde used in these studies, the degree of cross-linking detected was similar in scaffolds of electrospun collagen composed of fibers that exhibited a wide range of average fiber diameters (0.8–3 μm diameter, data not shown).
Fig. 3.
Analysis of the extent of cross-linking introduced into electrospun collagen as a function of the starting glutaraldehyde concentration. Cross-linking was similar in scaffolds processed with 0 (100% ethanol) and 0.1% glutaraldehyde but different from samples processed in 10 and 40% glutaraldehyde (p<0.05).
3.2. Bulk material properties
The bulk material properties of the electrospun scaffolds varied as a function of the degree of cross-linking present in the samples. Stress at failure in scaffolds processed in 100% ethanol that were cross-linked 43% was 0.143 MPa (Fig. 4A). This value ranged upward as the degree of cross-linking increased in the samples. For example, stress at failure in scaffolds cross-linked 52% was 0.255 MPa, samples cross-linked 64% exhibited a value of 0.283 MPa, samples cross-linked 69% exhibited a value of 0.317 MPa and samples cross-linked 70% exhibited a value of 0.325 MPa. Screening these data sets by one-way ANOVA indicated that stress at failure was similar in scaffolds processed with 10 and 40% glutaraldehyde. These results are not surprising, given the observation that the percentage of cross-linking present in these samples is essentially identical (69 and 70% cross-linked, respectively). Stress at failure was different in scaffolds cross-linked 52, 69 and 70% with respect to scaffolds processed in 100% ethanol and cross-linked 43% (p<0.004).
Fig. 4.
Macroscopic stress at failure (A), strain at failure (B) and the modulus of elasticity (C) in electrospun scaffolds as a function of cross-linking. In conventional materials testing experiments, the stress at failure was similar in all samples (A), with the exception that stress was decreased in scaffolds processed in 100% ethanol with respect to scaffolds processed in 1, 10 and 40% glutaraldehyde (p<0.004). Strain at failure (B) generally decreased as a function of increasing glutaraldehyde concentration and the extent of cross-linking introduced into the constructs (p< 0.005). The modulus of elasticity (C) increased as a function of increased cross-linking (p<0.005).
Strain at failure, as judged by bulk materials testing, also varied as a function of the percentage of cross-linking induced into the samples (Fig. 4B). Scaffolds processed in 100% ethanol and nominally cross-linked 43% exhibited a strain of 233% at failure. Scaffolds cross-linked 52% had an average strain value of 168% at failure, scaffolds cross-linked 64% exhibited an average strain value of 210% at failure, scaffolds cross-linked 69% exhibited an average strain value of 169% at failure and scaffolds cross-linked 70% exhibited an average strain value of 123% at failure. Once again, screening these data sets by one-way ANOVA indicated that strain at failure was similar in scaffolds processed in 10% glutaraldehyde (69% cross-linked) and 40% glutaraldehyde (70% cross-linked). Strain at failure in these scaffolds was different with respect to scaffolds cross-linked 43% (p<0.005). In addition, strain was different in scaffolds cross-linked 70% with respect to scaffolds cross-linked 64% (p<0.005), and scaffolds cross-linked 52% were different from scaffolds cross-linked 43% (p<0.005).
Consistent with these observations, Young’s modulus of elasticity was lowest in scaffolds processed in 100% ethanol with respect to all other conditions assayed (p<0.005) (Fig. 4C). This material property was increased in scaffolds processed in 40% glutaraldehyde (70% cross-linked) with respect to samples processed in 1% glutaraldehyde (52% cross-linked). Together, these data indicate that changes in cross-linking introduce fundamental changes into the structure of an electrospun collagen scaffold. To examine how cross-linking impacts scaffold structure we imaged fiber distribution as we applied varying degrees of strain across the different constructs.
3.3. Scaffold response to strain
In brightfield images all scaffolds were fibrillar in nature, regardless of the percentage of cross-linking present in the sample. In the dry state the scaffolds were composed of straight fibers; however, upon hydration the fibers coiled and developed a helical structure. With the application of strain the fibers of hydrated scaffolds visibly un-coiled and underwent realignment. Individual fibers were also observed to elongate and fail, allowing other fiber populations to continue sliding past one another (Fig. 5). The movement of fibers with respect to one another decreased as the percentage of cross-linking increased in the scaffolds. We used the 2D FFT approach to quantify changes in scaffold structure in response to calibrated degrees of strain.
Fig. 5.
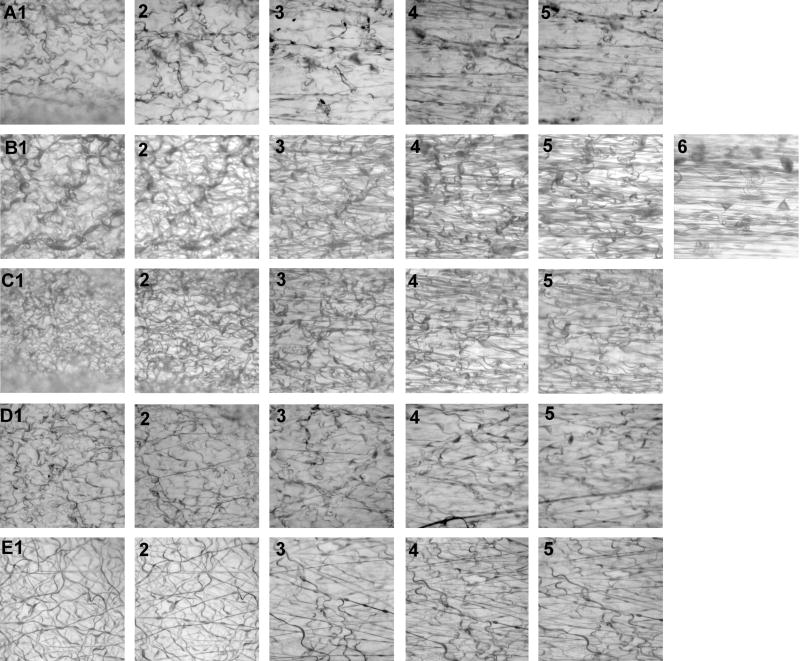
Brightfield images of electrospun scaffolds subjected to differing degrees of strain. Panels A1–A5 (the figure reads from left to right) depict a series of brightfield images of a representative scaffold processed in 100% ethanol and then subjected to varying degrees of stretch: A1=0% stretch; A2=50%; A3=75%; A4=100%, A5=150%. Panels B1–B6, scaffolds processed in 0.1% glutaraldehyde: B1=0% stretch; B2=50%; B3=75%; B4=100%, B5=200%, B6=400%. Panels C1–C5, scaffolds processed in 1% glutaraldehyde: C1=0%, C2=50%; C3=75%; C4=100%, C5=300%. Panels D1–D5, scaffolds processed in 10% glutaraldehyde: D1=0%, D2=50%; D3=75%; D4=100%, D5=250%. Panels E1–E5, scaffolds processed in 40% glutaraldehyde: E1=0%, E2=50%; E3= 75%; E4=100%, E5=200%. Note: hydration of lightly cross-linked scaffolds results in fiber coiling while the fibers of highly cross-linked scaffolds exhibit a much more linear conformation (e.g. A1 vs. E1). Italics indicate approximate strain value immediately prior to failure for a given panel of images.
Scaffolds processed in 100% ethanol (0% glutaraldehyde, 43% cross-linking) exhibited very swollen fibers relative to scaffolds processed with even nominal amounts of glutaraldehyde (Fig. 5A1–A5). The Coomassie blue stain used to enhance contrast for imaging appeared unevenly distributed in these scaffolds. The samples were fragile and several disintegrated as they were being placed into the static stretching device. The response of fibers to the applied strains was very regional in the two scaffolds that we were able to successfully test to failure. Some domains in these samples underwent considerable realignment, while fibers in adjacent domains remained relatively unchanged as strain was applied. The non-homogeneous distribution of strain resulted in abrupt changes in fiber orientation and, as a result, the 2D FFT alignment data varied considerably from one strain interval to the next. Very high alignment values were detected early in the process of applying strain; subsequently, the alignment values decreased (Fig. 6A). This decrease in alignment as a function of increasing strain was, in part, mediated by changes in scaffold structure associated with fiber rupture. As individual fibers underwent failure they were observed to retract and coil; this coiling is detected and reported by the 2D FFT method as an increase in random information in the micrograph. The slope of the line defining the change from the minimum to maximum 2D FFT alignment value in response to strain was 0.0002 (Fig. 6F). Mean strain at failure was 157 ± 87% (mean ± SD; Fig. 7A) and the mean peak 2D FFT value was 0.0852 ± 0.005 (Fig. 7B).
Fig. 6.
Panels A–E illustrates the impact of strain on fiber alignment in scaffolds cross-linked to varying degrees, as indicated. Alignment data become increasing clustered along the regression lines as cross-linking is increased (compare A, B and C with D and E). We believe this indicates that strains are more uniformly distributed throughout an electrospun scaffold of collagen as the extent of cross-linking is increased. Panel F depicts the slopes of the lines defining the change from the minimum 2D FFT alignment value to the maximum 2D FFT alignment value in response to strain for each data set depicted in panels A–E. Data were normalized so that each line passed through the origin to facilitate comparisons. Note: the slope increases as a function of increased cross-linking for samples processed in 1, 10 and 40% glutaraldehyde.
Fig. 7.
Summary data. Panel A illustrates strain at failure, as determined from imaging experiments, in scaffolds processed with differing degrees of cross-linking. Strain at failure was decreased in scaffolds cross-linked 70% with respect to scaffolds cross-linked 64%. Data sets encompassing scaffolds cross-linked 43 and 52% were composed of only two samples each and were excluded from the analysis. Panel B shows the average peak 2D FFT alignment value detected immediately prior to failure as a function of cross-linking. Panel C demonstrates the peak strain at failure as detected by conventional materials testing and imaging experimentation. The values detected for scaffolds cross-linked 64% (p<0.006) and 69% (p<0.005) were higher in the data sets derived from imaging experiments than conventional materials testing.
Scaffolds processed in 0.1% glutaraldehyde (52% cross-linked) were slightly more resilient than samples processed in 100% ethanol, but were still very delicate. The fibers of these samples were less swollen, underwent extensive coiling when hydrated and were stained more evenly with the Coomassie blue (Fig. 5B1–B6) than the fibers of scaffolds processed in 100% ethanol. Again, after repeated attempts we were only able to fully test two samples processed at this level of cross-linking. We again observed evidence that strain was not evenly distributed throughout the fibers of these scaffolds. During experimentation, fibers were observed to slip past one another. Failure occurred in a sequential fashion, individual fibers broke at intervals and fiber slipping allowed the scaffolds to extensively elongate before the final failure point was reached (Fig. 6B). The slope of the line defining the change from the minimum to maximum 2D FFT alignment value in response to strain was 0.00018 (Fig. 6F). Mean strain at failure was 413 ± 167% (Fig. 7A) and the mean peak 2D FFT value was 0.119 ± 0.072 (Fig. 7B).
Scaffolds cross-linked in 1% glutaraldehyde (64% cross-linked) were durable, with intermediate fiber swelling, extensive fiber coiling and an even distribution of Coomassie blue staining (Fig. 5C1–C5). The fibers of these scaffolds responded to the applied loads in a much more uniform fashion than samples processed at lower levels of cross-linking (Fig. 6C). The slope of the line defining the change from the minimum to maximum 2D FFT alignment value in response to strain was 0.00012 (Fig. 6F). Mean strain at failure was 304 ± 46.3% (Fig. 7A) and the mean peak 2D FFT value was 0.898 ± 0.009 (Fig. 7B).
Scaffolds processed in 10% glutaraldehyde were noticeably stiffer during manual manipulation for materials testing than those processed with lower concentrations of glutaraldehyde. Fibers were less swollen and exhibited very even staining with the Coomassie blue (Fig. 5D1–D5). These samples also exhibited less fiber coiling than scaffolds processed with lower degrees of cross-linking. Strain induced incremental changes in alignment in these samples (Fig. 6D). The slope of the line defining the change from the minimum to maximum 2D FFT alignment value in response to strain was 0.00013 (Fig. 6F). Mean strain at failure was 245 ± 23.8% (Fig. 7A) and the mean peak 2D FFT value was 0.087 ± 0.0084 (Fig. 7B).
Scaffolds cross-linked in 40% glutaraldehyde were very robust. As with samples processed in 10% glutaraldehyde, fiber swelling was minimal, and strain was evenly distributed along the fibers (Fig. 5E1–E5). Very little coiling was evident and the coils that were present were lost at very modest degrees of strain (see Fig. 5E1–E3). Strain also induced systematic changes in fiber alignment in these samples (Fig. 6E). The slope of the line defining the change from the minimum to maximum 2D FFT alignment value in response to strain was 0.00019 (Fig. 6F). Mean strain at failure was 176 ± 14% (Fig. 7A) and the mean peak 2D FFT value was 0.091 ± 0.0054 (Fig. 7B).
In summary, the overall patterns of strain at failure in scaffolds cross-linked to varying degrees that were reported by conventional materials testing and our imaging experiments were very similar, with one exception (Fig. 7C). Not surprisingly, the absolute values reported by each technique were not identical; soft tissue is notoriously difficult to evaluate for strain in conventional materials testing experiments. We believe the discrepancies that we detected can be attributed to the manner in which the imaging experiments were conducted. Strains were applied incrementally to allow us to photograph the samples, however, this small time lapse also allowed the fibers to reorganize and relax. Strains were applied in a steady fashion in scaffolds processed for conventional materials testing. Finally, our imaging experiments also revealed that the peak 2D FFT alignment values achieved immediately prior to failure was similar in each of the scaffolds that we tested (Fig. 7B).
4. Discussion
In previous studies we have used the 2D FFT approach to examine how varying degrees of fiber alignment, induced during the electrospinning process, impacts the material properties and functional profile of electrospun scaffolds in a dry state [4,5,13,16]. This body of work, and related experimentation from other laboratories [17], clearly demonstrates that fiber alignment plays a critical role in determining the material properties of an electrospun scaffold. In the present study we have examined the converse circumstance and have used the 2D FFT approach to determine how incremental changes in strain impact fiber alignment in hydrated samples of electrospun collagen. The hydrated scaffold responds to strain in a fundamentally different pattern than similar scaffolds tested in a dry state. The fibers of a dry sample do not undergo as much rearrangement in response to strain as a wet sample and, further, dry constructs composed of electrospun gelatin and electrospun collagen abruptly fracture at a defined site when undergoing failure [16].
In conventional materials testing assays, hydrated scaffolds of electrospun collagen exhibited decreased stress at failure and a decreased modulus of elasticity when compared to dry scaffolds. The hydrated scaffolds also sustained higher strains at failure than dry scaffolds [15]. We believe that hydration-induced changes in fiber structure and interfiber motions facilitated by the aqueous testing environment account for these effects. In randomly oriented electrospun scaffolds, on a very local basis (e.g. over a 1–2 μm2 surface area), fibers of collagen [15] and gelatin [4,14] are typically deposited as relatively linear structures. When hydrated, our experiments demonstrate that electrospun collagen can undergo a pronounced degree of coiling. The helical conformation adopted by the electrospun collagen is observed within the extracellular matrix (ECM) of intact tissues [17,18] and the contribution of this structural pattern in the establishment of material properties within intact tissues was recognized very early [19].
In our samples, coiling developed as the scaffolds were transferred into the 50% ethanol: 50% PBS rinse used in our hydration process. The extent of coiling appeared to be proportional to the degree of cross-linking present in the scaffolds at the time they underwent hydration (see Fig. 5). Scaffolds subjected to the highest degree of cross-linking (>60% cross-linking) exhibited nominal coiling and the fibers retained a nearly linear conformation throughout processing and testing. The gross changes in fiber structure observed with hydration may reflect an alteration in molecular conformation and/or the reorganization of the collagen molecules within the electrospun fibers. It seems clear from these observations that electrospun fibers are not deposited in the lowest possible energy state during the electrospinning process. The potential for a molecular reorganization in these fibers clearly has functional implications. Changes in fiber topology that are induced during the post-electrospinning processing of a protein-based scaffold may mask or unmask receptor binding sites, alter scaffold biological properties, and/or change the antigenicity of these nanomaterials. The extent to which these topographical changes might specifically impact alpha chain organization and fiber surface structure (i.e. 67 nm banding pattern) in a collagen-based scaffold remains to be defined.
Unlike conventional collagen gels (which are prepared by neutralizing a solution of acid-soluble collagen), scaffolds of electrospun collagen are freely soluble in aqueous solutions unless they are subjected to cross-linking. This observation reveals the unique nature of the electrospun collagen fiber. We used ethanol as a carrier solvent; the alcohol precipitates and stabilizes the structure of the electrospun fibers of collagen and allows glutaraldehyde mediated cross-linking to take place. Alcohol precipitation of the electrospun collagen undoubtedly acts to stabilize the fibers by promoting hydrogen bonding; this effect on the evolution of collagen material properties following cross-linking has been examined to some extent and has been found to vary as a function of the dielectric constant of the carrier alcohol [20].
We have detected evidence of cross-linking in scaffolds processed in 100% ethanol and believe this represents an artifact of the alcohol-induced precipitation process; precipitation appears to sequester amines within the structure of the partially stabilized fibers, effectively reducing the number of TNBS binding sites. We note that ethanol-treated scaffolds are stable in an aqueous environment when hydrated, including the bicarbonate/TNBS solution used in our cross-linking assay. In contrast, the dry, electrospun scaffolds that we used to generate baseline values of cross-linking undergo complete dissolution (nearly immediately) when exposed to the bicarbonate/TNBS buffer system used in the cross-linking assay. In theory, dissolution exposes all available sites to the colorimetric agent, thereby “inflating” the total number of binding sites that can be detected by the assay. The putative sequestered sites that we believe are present in the ethanol-processed samples appear to exist in a very stable compartment and do not appear to be particularly susceptible to cross-linking with glutaraldehyde. It is possible that the glutaraldehyde does not effectively penetrate the surface of the electrospun fibers (possibly because the ethanol extracts water from the collagen) in the alcohol-based solvent system, a condition that may lead to the formation of a cross-linked “shell” on the fibers. We believe that as a result of these effects we cannot achieve 100% cross-linking under the conditions used to process our fibers.
Our imaging experiments reveal that the cross-linking conditions and the expression of specific fiber tertiary structure interact to define the material properties of our hydrated scaffolds. Further, these experiments indicate that strain is distributed throughout the scaffolds by at least four different modes: fiber straightening (uncoiling), fiber reorientation (fiber realignment along axis of strain), fiber elongation (changes in fiber length) and interfiber sliding (fibers moving with respect to one another). Similar motions have been described in the connective tissue compartment of the pericardium, arterial walls and other hollow organs (e.g. [18,19,21,22]). In our experiments, evidence of fiber straightening originates with the observation that strain increases the pitch of the helical structures present along a coiled fiber (see Fig. 5). Ultimately, the applied strains substantially eliminate these structures. In tandem with this effect, the fibers are reoriented and drawn in parallel with the principal axis of stretch. This conclusion is supported by 2D FFT analysis, which demonstrates that alignment values increase along the axis of strain as a function of the applied load (Fig. 6).
Once fiber alignment occurred, evidence of fiber elongation was detected. In linear fibers, strain was observed to increase the distance between imperfections that were present along the individual fibers. Little investigation has been done into the structure of electrospun collagen nanofibers and it is impossible to describe with any certainty the molecular events that might underlie this effect. It is known, however, that D-band elongation has been reported to account for a substantial degree of stretch in native collagen [21] and the same mechanisms may also apply to electrospun collagen since fibers of this type of biomaterial can exhibit the 68 nm banding that typifies the native fibril [14,15].
Interfiber movement (sliding) is represented by the movement of fibers with respect to one another along the principle axis of strain. This movement occurred in a non-uniform fashion as strain was applied to the scaffolds; a fiber (or fibers) would move with respect to an adjacent fiber, stop, then subsequently slip and move for a variable distance before stopping once again. This type of interfiber sliding allows the matrix to reorganize and transmit stress to the fibers as a population, and, initially, does not necessarily affect alignment values. This type of motion was most pronounced in scaffolds processed in 0.1% glutaraldehyde. We suspect that cross-linking under these conditions is enough to stabilize individual fibers and does not result in any substantial intrafiber cross-links, allowing fibers an extensive range of motion. With the onset of fiber sliding we also began to observe the gradual breakage of individual fibers. Once broken, these fibers recoiled and adopted a tightly coiled shape. While strain increases fiber alignment, the accumulation of these coiled structures introduces random elements into our 2D FFT analysis. In turn, the accumulation of these random elements generates low frequency pixels into the power spectrum of the 2D FFT output image. As the samples begin to undergo failure, this signature signal degrades the overall alignment value assigned to the sample [16]. This condition undoubtedly underlies our observations that a peak 2D FFT alignment value appears to exist in scaffolds that have been subjected to strain (Fig. 7B). In contrast to our results with hydrated samples, the fibers of dry scaffolds fail to undergo any substantial degree of reorganization in response to strain and fracture abruptly at a defined point in the scaffold.
Modulating the percentage of cross-linking present in a hydrated scaffold served to induce incremental changes in scaffold material properties. Scaffolds processed in 100% ethanol and low concentrations of glutaraldehyde exhibited a very regional response to strain. The hydrogen bonds present in these samples appear to stabilize the structure of individual fibers, however, it is unlikely these bonds can mediate fiber-to-fiber interactions to any great extent, resulting in a nonuniform response to strain. Similar arguments may explain why scaffolds processed in low concentrations of glutaraldehyde (<1%) exhibit this same behavior, especially if the use of an alcohol carrier induces the formation of a structure (“shell”) that largely excludes glutaraldehyde from the interior of the fibers. Strains were uniformly distributed in the scaffolds as the concentration of glutaraldehyde was increased beyond 1%.
The percentage of cross-linking present in the scaffolds did not change to any great extent in samples processed in 1, 10 and 40% glutaraldehyde (e.g. Fig. 3). Despite this result, scaffolds processed under these conditions exhibited subtle differences in material properties. We believe these differences can be attributed to the chemistry of glutaraldehyde and specific structural changes imparted on individual fibers by this cross-linking agent. Glutaraldehyde is described as a zero distance cross-linker and, as such, this agent should primarily impact the structure of individual fibers. However, at high concentrations glutaraldehyde can undergo polymerization, effectively increasing its cross-linking distance [23]. Our observations that strain can induce more abrupt changes in fiber alignment as the degree of cross-linking increases would appear to support this conclusion (steeper slope in the function describing the change in alignment in response to strain; Fig. 6F). Highly cross-linked scaffolds also exhibit less elasticity than scaffolds processed with nominal amounts of cross-linking. Any increase in intrafiber cross-linking can be expected to more directly and uniformly distribute the applied loads across the fibers as a population. Highly cross-linked scaffolds lack the coiled structures that appear to dampen the initial effects of strain on a scaffold that has been subjected to a nominal degree of cross-linking (e.g. “no coils to uncoil”).
In closing, the material properties of electrospun scaffolds can be modulated at several sites in the fabrication process. For example, the introduction of varying degrees of fiber alignment into a scaffold at the time of fabrication (through the use of rotating target mandrels) can be used to modulate the anisotropic material properties of this unique class of nanoscale biomaterials. The extent to which this type of anisotropy can be used to modulate cell shape and to provide guidance cues designed to direct cell migration remains largely unexplored [3,13]. However, cells placed in contact with a polarized matrix undergo elongation in parallel with the principle axis of alignment [2,13], a response that impacts cell shape [2,12], response to strain and the metabolic profile of the effected cells [11,24]. In this study we explored how changes in cross-linking and hydration can be used to modulate the material properties of the electrospun scaffold. Notably, the tertiary structure that individual fibers adopt appears to play an important role in determining how these samples respond to a mechanical challenge. It should be possible to exploit this property to directly tailor these biomaterials to specific applications. For example, the introduction of high degrees of cross-linking into scaffolds composed of highly aligned fibers might be used to produce constructs that mimic relatively inelastic connective tissues like ligaments and tendons. Subjecting a scaffold to a nominal degree of cross-linking allows extensive fiber coiling to take place upon hydration. This type of scaffold can then be subjected to more cross-linking to take advantage of these coiled structures, potentially making it possible to more closely mimic the structure of the extracellular matrix in more complex native tissues like arteries and other hollow organs.
Acknowledgments
This work was supported in part by NIH R01EB003087 (Simpson) and NIH 5R21EB003407. The authors have U.S. and International Patents Issued and Pending concerning the electrospinning process. All image analysis was conducted at the core facilities of the Department of Neurobiology & Anatomy Microscopy Facility at Virginia Commonwealth University, supported, in part, with funding from a NIH-NCRR shared instrumentation grant (1S10RR022495) and a NIH-NINDS Center core grant (5P30NS047463).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Simpson DG, Bowlin GL. Tissue engineering scaffolds – can we re-engineer mother nature? Expert Reviews of Medical Devices. 2006;3(1):9–15. doi: 10.1586/17434440.3.1.9. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241–68. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LY. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 2006;1:79(3):456–63. doi: 10.1002/jbm.a.30870. [DOI] [PubMed] [Google Scholar]
- 4.Ayres CE, Bowlin GL, Henderson SC, Taylor L, Schultz J, Alexander JK, Telemeco TA, Simpson DG. Modulation of anisotropy in electrospun tissue engineering scaffolds: analysis of fiber alignment by the fast Fourier transform. Biomaterials. 2006;27(32):5524–5534. doi: 10.1016/j.biomaterials.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayres CE, Bowlin GL, Pizinger R, Taylor LT, Keen CA, Simpson DG. Incremental changes in anisotropy induce incremental changes in the material properties of electrospun scaffolds. Acta Biomaterials. 2007;3(5):651–661. doi: 10.1016/j.actbio.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes CP, Pemble CW, Brand DD, Simpson DG, Bowlin GL. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Engineering. 2007;7:13(7):1593–1605. doi: 10.1089/ten.2006.0292. [DOI] [PubMed] [Google Scholar]
- 7.Keen C, Wnek G, Baumgarten CM, Newton D, Bowlin GL, Simpson DG. Bioengineered skeletal muscle. In: Wnek GE, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. New York: Marcel Dekker; 2004. pp. 1639–1651. [Google Scholar]
- 8.Kenawy E, Mansfield K, Bowlin GL, Simpson DG, Wnek GE. New drug delivery system: control release of tetracycline hydrochloride as a model drug from electrospun fibers of poly(lactic acid) and poly(ethylene vinyl acetate) In: Wnek GE, Bowlin GL, editors. J Control Release. Vol. 81. pp. 57–64. 200. [Google Scholar]
- 9.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland ED, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagens and elastin for vascular tissue engineering. Frontiers in Biosciences. 2004;1(9):1422–32. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 11.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res Ultrarapid communication. 1999;85:59E–69E. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- 12.Simpson DG, Terracio L, Terracio M, Price RL, Turner DC, Borg TK. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J Cell Physiol. 1994;161:89–105. doi: 10.1002/jcp.1041610112. [DOI] [PubMed] [Google Scholar]
- 13.Chow W, Simpson DG, Bigbee JW, Collelo RJ. Evaluating neuronal and glial growth on electrospun polarizied matrices: bridging the gap in precussive spinal chord injuries. Neuron Glia Biology. 2007;3(2):119–126. doi: 10.1017/S1740925X07000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telemeco TA, Ayres CA, Bowlin GL, Wnek G, Boland G, Cohen NM, Baumgarten CM, Mathews JA, Simpson DG. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomaterialia. 2005;1:377–385. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning collagen nanofibers. J of Macromolecules. 2002;11:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 16.Ayres CE, Shekhar Jha B, Meredith H, Bowman JR, Bowlin GL, Henderson SC, Simpson DG. Measuring fiber alignment in electrospun scaffolds: a user’s guide to the 2D fast Fourier transform approach. J of Biomaterials Science, Polymer edition. 2008;19(5):603–621. doi: 10.1163/156856208784089643. [DOI] [PubMed] [Google Scholar]
- 17.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Liao J, Yang L, Grashow J, Sacks MS. Molecular orientation of collagen in intact planar connective tissues under biaxial stretch. Acta Biomaterialia. 2005;1(1):45–54. doi: 10.1016/j.actbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Burton AC. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954;34(4):619–642. doi: 10.1152/physrev.1954.34.4.619. [DOI] [PubMed] [Google Scholar]
- 20.Gratzer PF, Pereira CA, Lee MJ. Solvent environment modulates effects of glutaraldehyde cross-linking on tissue-derived biomaterials. Journal of Biomedical Materials Research. 1998;31(4):533–543. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micron. 2001;32(3):251–260. doi: 10.1016/s0968-4328(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 22.Freed AD, Doehring TC. Elastic model for crimped collagen fibrils. Journal of Biomechanical Engineering. 2005;127(4):587–593. doi: 10.1115/1.1934145. [DOI] [PubMed] [Google Scholar]
- 23.Jones GJ. Polymerization of glutaraldehyde. J Histochem. 1974;22:911–913. doi: 10.1177/22.9.911. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblasts. Biomaterials. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]