Abstract
Purpose
Treatment with anti-CD3 antibody has been shown to ameliorate and reverse an existing immunopathological condition by inducing tolerance. The purpose of this study is to assess the therapeutic potential of non-Fc receptor (FcR) binding anti-CD3 monoclonal antibody (mAb), CD3F(ab′)2, for the treatment of herpes simplex virus (HSV)-induced stromal keratitis (SK).
Methods
Balb/c and C57BL/6 mice were ocularly infected with HSV-1 strain RE (HSV-1RE). Infected animals were treated with CD3F(ab′)2. Development of SK starting from day 5 postinfection (p.i.), infiltration of inflammatory cells into the corneas and the generation of the immune response were compared with untreated animals using slit-lamp biomicroscopy, flow cytometry, and ELISA.
Results
In vivo administration of CD3F(ab′)2 resulted in significant reduction in the severity and incidence of SK in the infected animals compared to untreated counterparts. Infiltration of fewer pathogenic CD4+ T cells into the cornea, along with a lower percentage of cells that could be induced to express IFN-γ, occurred with anti-CD3F(ab′)2 treatment. Similar observations were noted in the secondary lymphoid tissues. Additionally, an increase in the frequency of CD4+Foxp3+ regulatory T cells was noticed in both cornea and lymphoid tissues of treated animals compared to untreated animals. Treatment with CD3F(ab′)2 also reduced the number of SSIE-FARL peptide-specific CD8+IFN-γ+ T cells in the secondary lymphoid tissues. Furthermore, use of this reagent was moderately effective in limiting lesions in mice with established lesions.
Conclusions
Taken together, these results show that non-FcR binding anti-CD3 treatment could be useful in limiting SK lesions.
Infection with viruses triggers multiple inflammatory and immune responses that are involved in clearing the virus from the site of infection. Sometimes these host-induced responses to the pathogen result in tissue-damaging lesions at the site of infection. One such example is herpes simplex virus (HSV)-induced lesions in the corneal stroma, a condition called stromal keratitis (SK). Therapeutic strategies for SK currently involve the use of antivirals in addition to corticosteroids.1 Long-term treatment with such reagents might result in drug resistance,2–4 and prolonged use of corticosteroids can have unwanted side effects.5,6 Current knowledge of the inflammatory mechanisms and pathways involved in the pathogenesis of SK should result in new approaches that could replace or complement existing methods to control SK lesions. Previous reports from our group and others have shown SK to be mainly a Th1 CD4+ T cell– orchestrated immunopathological lesion.7 Accordingly, mice lacking the capacity to generate an effective T-cell response do not develop SK.8 Thus, diminishing the function of CD4+ T cells represents one logical strategy to limit lesion severity. One potential approach to achieve this is the administration of monoclonal antibodies (mAbs) against anti-CD3.9
Anti-CD3 mAbs have been used as immunosuppressive reagents in several models, including autoimmunity and some cases of transplantation.10–13 However, the therapeutic potential of such antibodies was limited because of nonspecific cytokine production and initial T-cell activation caused by cross linking via Fc receptor binding.14–16 Recently, non-mitogenic non-FcR binding anti-CD3 mAbs were developed by altering their binding to FcR. Such anti-CD3 mAbs were shown to induce tolerance in sensitized Th1 CD4 T cells by several mechanisms, such as modulation of the CD3-T-cell receptor (TCR) complex including its internalization,17 depletion of T cells,18,19 induction of apoptosis resulting in clonal deletion,20 T-cell unresponsiveness,21 and induction of regulatory T cells (Treg).10,22 Although the complete biochemical mechanisms involved in T-cell suppression by non-FcR binding anti-CD3 mAbs are not completely understood, the process likely involves a partial TCR activation that is translated into an off signal that additionally includes a calcium signal.16
In vivo administration of non-mitogenic anti-CD3 mAb was shown to be useful in inducing permanent engraftment of vascularized grafts,12,23 prevention and reversal of virus-induced and insulin-dependent autoimmune diabetes,13 and in the therapy of pathologic lesions such as experimental auto-immune encephalomyelitis (EAE).11 From the above observations, it is conceivable that the immunomodulation by non-FcR binding anti-CD3 antibody CD3F(ab′)2 treatment could be effective in preventing and treating HSV-induced lesions in the cornea.
The present study shows that in vivo administration of CD3F(ab′)2 antibody post-ocular infection resulted in significantly reduced lesion severity as well as a reduced incidence of SK, both in Balb/c and B6 HSV ocularly infected animals. Infiltration of fewer pathogenic CD4+ T cells into the cornea and the inflamed trigeminal ganglia occurred with CD3F(ab′)2 antibody treatment. Similarly, reduced IFN-γ production by CD4+ T cells was noted in the infected cornea as well as cervical draining lymph nodes and spleen of these animals. A three- to fourfold reduction in the frequency and the absolute number of CD4+ T cells expressing VLA4 and CD69 surface markers and an increase in the frequency of CD4+Foxp3+ regulatory T cells were observed in both corneal and lymphoid tissues of treated animals compared to untreated control animals. Additionally, the generation of SSIEFARL peptide specific CD8+IFN-γ+ T cells in the secondary lymphoid tissues was also significantly diminished in the treated group, indicating the influence of CD3F(ab′)2 on the generation of HSV-specific immune responses. Furthermore, use of this reagent was also effective in the resolution of established SK lesions. In summary, our results show that non-FcR binding anti-CD3 treatment could be useful in treating HSV-induced ocular immunopathology.
Materials and Methods
Reagents
Non-mitogenic non-FcR binding CD3F(ab′)2 mAb24 (Bio Express, West Lebanon, NH) was kindly provided by Mathias Von Heraath (La Jolla Institute for Allergy and Immunology, La Jolla, CA). The reagent was stored at −80°C until use. For treatment, four 50-μg doses were injected intravenously (IV) on alternate days.
Mice
C57BL/6 (B6) and Balb/c mice were obtained from Harlan Sprague–Dawley, (Indianapolis, IN) and were maintained in the animal facility of University of Tennessee. To prevent bacterial superinfection, all mice received prophylactic treatment with sulfamethoxazole/trimethoprim (Biocraft, Elmond Park, NY) at the rate of 5 mL/200 mL drinking water after virus infection. All experimental procedures followed the guidelines of the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. The animal facilities of the University of Tennessee (Knoxville) are fully accredited by the American Association of Laboratory Animal Care.
In Vitro Cultures in the Presence of Anti-CD3 and CD3F(ab′)2
For in vitro experiments, lymph node cells were harvested from the spleens of naïve Balb/c animals. Cells were cultured for 48 to 72 hours in 96 well plates in the presence of varying concentrations of anti-CD3 antibody or CD3F(ab′)2 mAb with or without anti-CD28 antibody. For proliferation experiments, 1 μCi/well [3H]TdR was added to the culture for the final 18 hours. Surface staining for CD3, CD4, CD8, CD25, and CD69 markers, and intra-nuclear Foxp3 staining were performed at 48 hours. Culture supernatants for cytokine ELISA were collected at 48 hours and the level of IFN-γ was measured by sandwich ELISA. In some of the experiments, splenocytes were pretreated with CD3F(ab′)2 for 2 hours and the cells were stimulated with anti-CD3 antibody (1 μL/mL) for 24 hours. After stimulation, cells were stained with CD3, CD4, CD8, CD25, and CD69 surface markers. To study the effect of CD3F(ab′)2 on apoptosis, cells were treated with different concentrations (0.5 to 8 μL/mL) of CD3F(ab′)2. Staining for annexin-V (BD Pharmingen, San Diego, CA) was performed along with CD4 and CD3 surface markers.
Corneal Infection and Lesion Scoring Posttreatment with CD3F(ab′)2 mAb
HSV-1 strain RE (HSV-1RE), obtained from Robert L. Hendricks (School of Medicine, University of Pittsburgh, Pittsburgh, PA) was used in all procedures. HSV-1RE, which gives >75% lesions, was used for Balb/c animals. HSV-1RE, obtained from Robert N. Lausch (University of South Alabama College of Medicine), which gives >75% lesions in B6, was used for B6 animals. The viruses were grown in Vero cell monolayer (American Type Culture Collection, Manassas, VA; Cat. no. CCL81) by standard protocol, titrated, and stored in aliquots at −80°C until used. Corneal infection was conducted under deep anesthesia induced by intra-peritoneal injection (Avertin; Sigma-Aldrich, St. Louis, MO). The mice were scarified on their corneas with a 27-gauge needle and infected with a 3-μL drop containing 105 plaque-forming units (pfu) of HSV-1 which was applied to the eye and gently massaged with the eyelids. Non-FcR binding anti-CD3 antibody, anti-CD3F(ab′)2, was administered intravenously (50 μg/dose) on every alternate day starting from day 5 p.i. Control animals were treated with PBS or irrelevant monoclonal antibody. The eyes were examined on different days p.i. for the development of clinical lesions by slit-lamp biomicroscopy (Kawa Co., Nagoya, Japan), and the clinical severity of keratitis of individually scored mice was recorded as described previously.25,26
Histopathology
For histopathologic analysis, eyes from both groups of mice were extirpated at the indicated time point p.i. and snap-frozen in OCT compound (Miles Laboratories, Elkhart, IN). Sections (6-μg thick) were cut, air dried in a desiccation box, and stained with hematoxylin and eosin (Richard Allen Scientific, Kalamazoo, MI).
Purification of Cells from Peripheral Blood
For purification of cells, peripheral blood from naïve CD3F(ab′)2-treated (24 hours posttreatment) and untreated Balb/c animals was collected by retro-orbital bleeding in tubes containing sodium citrate. Cells were isolated from the blood using histopaque (Sigma-Aldrich). Surface staining with anti-CD3, -CD4, -CD8, and -CD25 was performed to determine the change in the frequencies of lymphocytes in the blood posttreatment.
Flow Cytometry
For flow cytometry measurement of the infiltrating cells, four corneas per time point per group were collected by dissecting the corneal buttons above the limbus with a scalpel. Similarly, two trigeminal ganglias (TGs) were collected per time point. Corneas or TGs were digested in a blend of purified collagenase enzymes (Liberase; Roche Diagnostics Co., Indianapolis, IN) for 45 minutes at 37°C. Cervical draining lymph nodes (DLNs) and spleens were also collected from individual animals. Single cell suspension was prepared as described elsewhere.27 The Fc receptors were blocked with unconjugated anti-CD16/32 (BD Pharmingen) for 30 minutes. Samples were incubated with FITC-labeled anti-Gr-1, anti-CD25 (BD Pharmingen), PerCp-labeled anti-CD8, APC-labeled anti-CD11b, and anti-CD4 (BD Pharmingen) antibody for 30 minutes. For intracellular cytokine staining, PE-labeled anti–IFN-γ and FITC-labeled anti–TNF-α antibodies were used (BD Pharmingen). Intracellular staining for TNF-α and IFN-γ was performed using a kit (Fixation/Permeabilization Solution Kit with BD GolgiPlug; BD Biosciences). For intracellular cytokine staining, cells were stimulated for 18 hours with anti-CD3 (1 μg/mL) and anti CD28 (1 μg/mL) or UV inactivated HSV (2 multiplicity of infection [MOI]). Brefeldin A (10 μg/mL) was added to the culture for the final 5 hours. Foxp3 staining was performed using a mouse regulatory T-cell staining kit (eBioscience, San Diego, CA). All samples were collected (FACScan; BD Biosciences) and data were analyzed (Cell Quest 3.1 software; BD Biosciences).
SSIEFARL Peptide-Specific CD8+IFN-γ+ Response in B6 Mice
To determine the number of IFN-γ–producing CD8+ T cells in the infected DLN and spleens of B6 animals, intracellular cytokine staining was performed. Single-cell suspension of infected DLN and spleens were prepared and 106 cells were cultured in 96-well U-bottom plates. Cells were left untreated and stimulated with SSIEFARL peptide (1 μg/mL) for 5 hours at 37°C in 5% CO2. Brefeldin A (10 μg/mL; BD Pharmingen) was added to the culture for the intracellular cytokine accumulation. Cell surface staining was performed followed by intra-cellular cytokine staining for IFN-γ (Cytofix/Cytosperm kit; BD Pharmingen). All samples were collected (FACScan; BD Biosciences) and data were analyzed (Cell Quest 3.1 software; BD Biosciences).
Quantification of IFN-γ Production by ELISA
To measure the level of Th1 cytokine production by infected splenocytes and lymphocytes, cells (1 × 106 cells/well) were restimulated in vitro with syngeneic stimulators pulsed with 3 MOI UV-inactivated HSV-KOS (5 × 105 cells/well) and incubated for 56 hours at 37°C. The supernatants were analyzed for IFN-γ cytokine production by sandwich ELISA protocol. Coating antibody against IFN-γ, and biotinylated anti-IFN-γ antibody were purchased from BD Pharmingen. The color reaction was developed (ABTS; Sigma-Aldrich) and measured with an ELISA reader (Spectramax 340; Molecular Devices, Sunnyvale, CA) at 405 nm. Quantification was performed (Spectramax ELISA reader, software version 1.2; Molecular Devises).
Statistical Analysis
Statistical significance was determined by Student’s t-test. P < 0.05 was regarded as a significant difference between the groups.
Results
Non-FcR-Binding Anti-CD3F(ab′)2 Do Not Activate CD4+ T Cells In Vitro
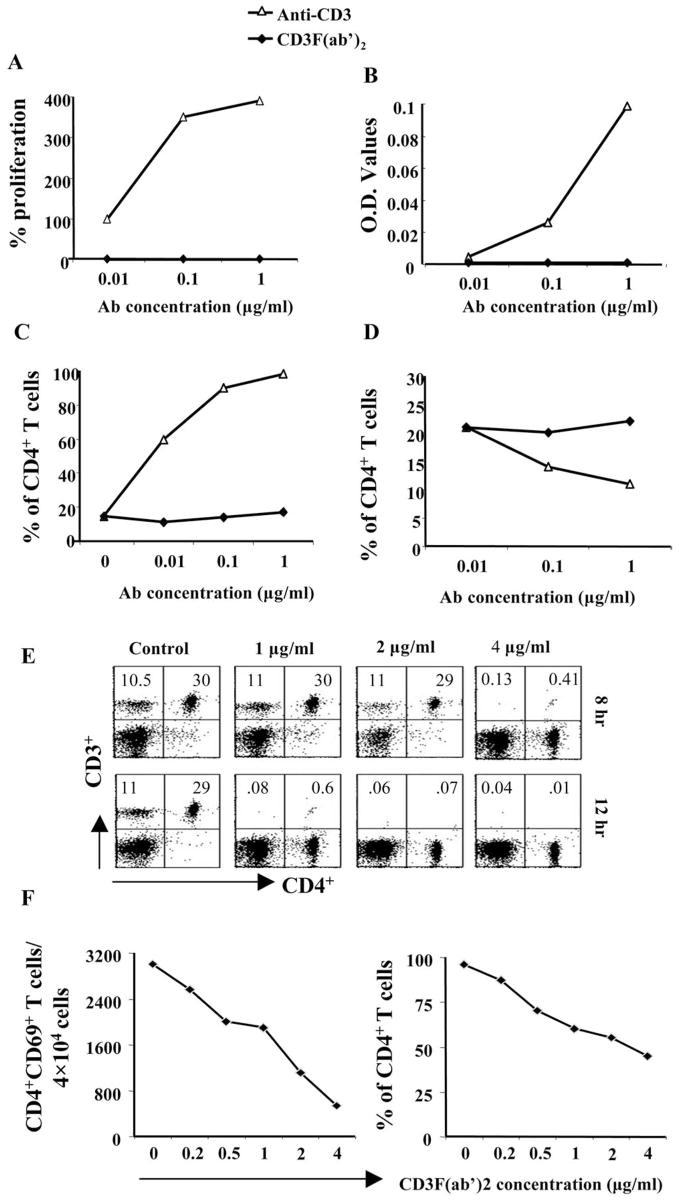
The use of non-mitogenic anti-CD3 mAb is believed to deliver a partial signal through the TCR, which may result in the production of nonspecific proinflammatory cytokines and chemokines from CD4+ T cells.14,16 Before using this reagent for therapy in SK, we tested the activity of this antibody both in vitro and in vivo. Cells isolated from spleens of naïve Balb/c mice were stimulated with different concentrations of soluble anti-CD3 and CD3F(ab′)2 mAb with or without CD28. As shown in Figure 1A, CD3F(ab′)2 failed to cause proliferation of cells in contrast to anti-CD3 antibody. A similar pattern was observed in the production of cytokines, as measured by the concentration of IFN-γ in the 48-hour culture supernatant (Fig. 1B). Additionally, as shown in Figure 1C, in contrast to anti-CD3 stimulation, there was no increase in the CD69 expression on the CD4+ T cells in the presence of CD3F(ab′)2. Interestingly, the proportion of CD4+Foxp3+ among the total CD4+ population in the presence of CD3F(ab′)2 remained almost the same, whereas it decreased in a dose-dependent manner in the presence of anti-CD3 (Fig. 1D). Others have shown that CD3F(ab′)2 mAb treatment causes internalization of CD3 and the TCR complex and may even result in apoptosis.28 To test whether this occurred in our system, splenocytes from naïve mice were treated with several doses of CD3F(ab′)2. As shown in Figure 1E, within 12 hours of culture, complete loss of surface CD3 expression was noticed in the CD3F(ab′)2-treated wells, confirming previous reports.28 Moreover, within this time frame, a dose-dependent increase in apoptosis was also observed in CD3F(ab′)2-treated cells, particularly at 3 hours poststimulation (Table 1). Furthermore, as is evident in Figure 1F, pretreatment of cells with CD3F(ab′)2 before stimulation with anti-CD3 resulted in a reduced number (left panel) as well as a proportion (right panel) of CD4+CD69+ T cells in the cultures, indicating that the CD3F(ab′)2 could modulate the activation of CD4+ T cells.
Figure 1.
Splenocytes harvested from naïve Balb/c animals were cultured in the presence of different concentrations of soluble anti-CD3 antibody or CD3F(ab′)2 mAb. (A) The line diagram represents the percentage of proliferation in the presence of both antibodies compared to media control. (B) Concentration of IFN-γ was measured in the 48 hours culture supernatant by sandwich ELISA. The line diagram represents the IFN-γ concentration as analyzed from the OD values. (C) Cells were stained with CD4 and CD69 surface markers. The line diagram represents the percentage of CD4+CD69+ T cells compared to the frequencies of CD4+ T cells. (D) Intranuclear staining for Foxp3 was performed. The line diagrams denote the CD4+Foxp3+ Treg to CD4+ T cell ratio. The plots show the results of two similar sets of experiments. (E) Splenocytes harvested from naïve Balb/c animals were treated with different concentrations of CD3F(ab′)2. Cells were stained with CD3, and CD4 surface markers after 1, 3, 8, and 12 hours posttreatment. Dot plots on the upper and the lower panels show the CD4+CD3+ T cells in the culture after 8 hours and 12 hours, respectively. The numbers on the upper left and upper right corners denote the percentages when gated on lymphocytes. (F) In a separate experiment, splenocytes were pretreated with various concentrations of CD3F(ab′)2 for 2 hours before stimulation with anti-CD3 antibody. After 24 hours, cells were stained with CD4 and CD69 surface markers. The line diagrams on the left and right panels denote the number of CD4+CD69+ T cells/ 4 × 104 cells and the percentage of CD4+ T cells expressing CD69 marker.
Table 1.
Percentage of CD4+ Annexin-V+ T Cells
| Concentration of Anti-CD3F(ab′)2 (μg/mL)
|
||||
|---|---|---|---|---|
| Time (h) | 0 | 1 | 2 | 4 |
| 1 | 2.25 | 4.0 | 4.0 | 4.35 |
| 3 | 3.15 | 5.4 | 6.1 | 10.7 |
| 8 | 3.5 | 7 | 6.6 | 6.24 |
Splenocytes isolated from naïve Balb/c mice were treated with various doses of anti-CD3F (ab′)2 in vitro and annexin-V staining was performed at the indicated time points posttreatment. The numbers in the table show the percentage of CD4+ annexin-V+ T cells when gated on CD4+ lymphocyte population.
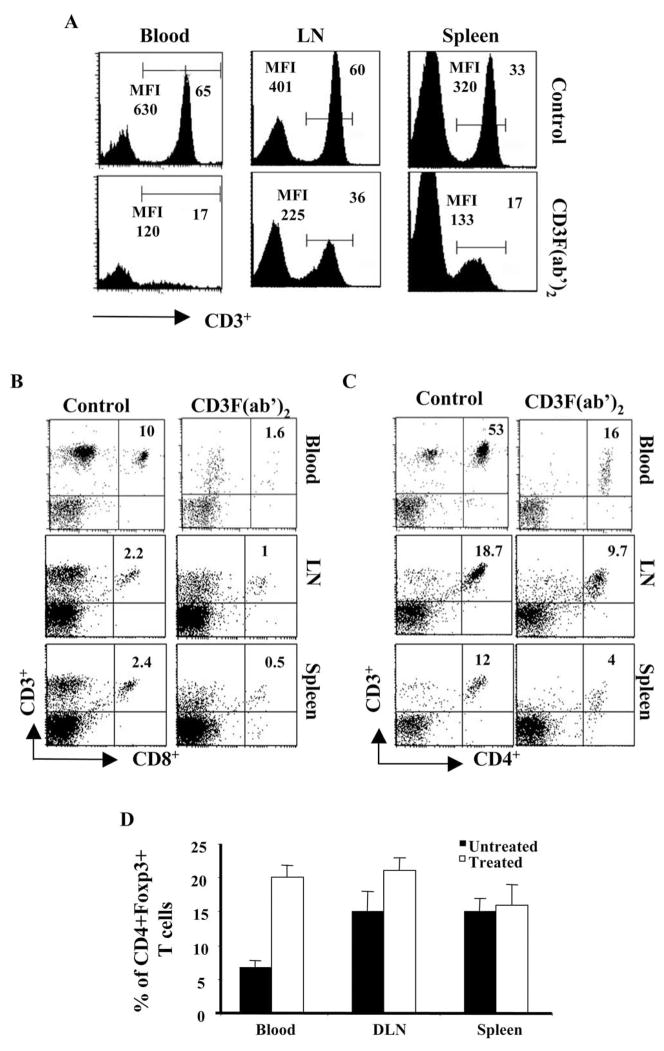
We next wanted to study the in vivo activity of CD3F(ab′)2 antibody. As has been shown previously, systemic administration of non-FcR anti-CD3 mAb could result in transient depletion of lymphocytes from the system.18 As shown in Figure 2A, one single dose (50 μg, IV) of CD3F(ab′)2 administration resulted in the reduction of CD3 expressing cells in blood, LN, and spleen of naïve animals within 20 hours of injection. The frequencies of both CD4+ and CD8+ T cells in the peripheral blood and lymphoid tissues were also significantly reduced at this time point (Figs. 2B, 2C). The cell number returned to normal in the circulation by 6 days p.i., although at this time depletion was still evident in the spleen of CD3F(ab′)2-treated animals (not shown), as also noted by others.28 Interestingly, as is evident in Figure 2D, an increase in the proportion of CD4+CD25+ T cells was noted in the blood and LN of treated animals. These cells also expressed the Foxp3 marker, indicating an increase in natural regulatory T cells in the treated animals. Taken together, the above in vitro and in vivo data indicate that CD3F(ab′)2 does not transmit an activation signal through the TCR. Additionally, this could result in downregulation of CD3 expression as well as transient depletion of lymphocytes from the system.
Figure 2.
Cells from the peripheral blood, spleen, and cervical lymph nodes of naïve Balb/c animals treated with CD3F(ab′)2 (24 hours posttreatment) were harvested. Surface staining with anti-CD3, anti-CD4, anti-CD8, and anti-CD25 surface markers was performed to determine the changes in the frequencies of lymphocytes post administration. Cells isolated from the respective tissues of untreated naïve Balb/c mice were used as a positive control. (A) The histograms show the intensity of CD3 expression in the blood, LNs, and spleens of untreated (upper panel) and CD3F(ab′)2-treated (lower panel) animals. (B, C) The dot plots show the CD8+CD3+ and CD4+CD3+ cells, respectively, in the blood, LNs, and spleens of untreated (left) and CD3F(ab′)2-treated (right) animals (gated on lymphocytes). The number on the upper right quadrant represents the percentage of cells when gated on lymphocytes. (D) The bar diagram shows the percentages of CD4+CD25+ T cells among the CD4+ T cells in blood, LNs, and spleens of untreated and treated Balb/c animals.
Modulation of SK Severity by Anti-CD3F(ab′)2 Antibody Treatment
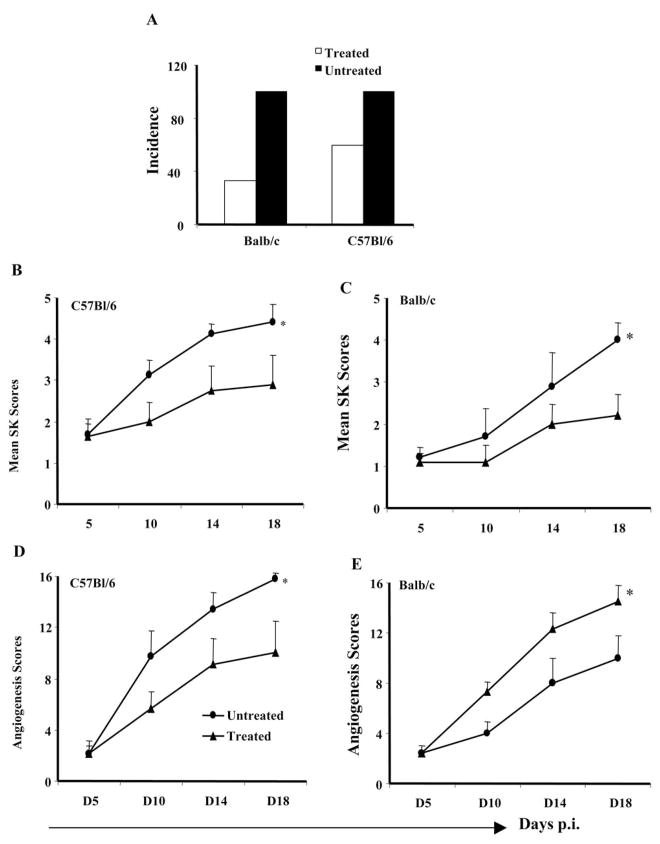
To assess the potential of CD3F(ab′)2 antibody in SK therapy, both Balb/c and B6 animals were ocularly infected with HSV-1RE (5 × 105 pfu/eye). When treatment with anti-CD3F(ab′)2 antibody was started on day 0 p.i., treated animals showed symptoms of encephalitis and had to be terminated (not shown). This was likely the consequence of impaired ability of T cells to control the virus in the central nervous system.29,30 Therefore, for all subsequent experiments, treatment with CD3F(ab′)2 antibody (50 μg, IV on alternate day) was started on day 5 p.i. and lesion development in the treated groups was followed and compared with the untreated control animals for a 18-day test period. Different patterns of SK lesion development were noticed in the treated animals compared to untreated controls. Treatment with CD3F(ab′)2 resulted in a reduced incidence of SK (score ≥ 3) in both Balb/c and B6 animals (Fig. 3A). Thus, at day 18 p.i., 95% to 100% of the untreated animals had SK lesion scores ≥ 3 whereas approximately 40% to 50% of treated animals developed such scores. As is evident in Figures 3B and 3C, significantly reduced SK lesion severity (P ≤ 0.05) was also observed both in B6 and Balb/c animals in those that received anti-CD3F(ab′)2 antibody treatment compared to untreated counterparts. As evident in Figures 3B and 3C, a trend of slower lesion development was observed immediately after the treatment was started in both treated Balb/c and B6 animals. A similar pattern was also noticed in the development of neovascularization in the CD3F(ab′)2-treated animals (Figs. 3D, 3E). Interestingly, although the treated animals had lower lesion scores, some of these animals showed signs of encephalitis and died (data not depicted).
Figure 3.
Reduced incidence and SK lesion in the CD3F(ab′)2-treated mice compared to untreated controls. (A) The bar diagram demonstrates the percentage severity of both Balb/s and C57Bl/6 mice at day 18 p.i. The treated animals show reduced incidence of SK compared to untreated animals. (C, D) The line diagrams denote the mean SK and angiogenesis lesion scores, respectively, in CD3F(ab′)2-treated and untreated C57Bl/6 mice infected with 5 × 105 pfu HSV-1RE at different time points p.i. (E, F) The line diagrams denote the mean SK and angiogenesis lesion scores, respectively, in CD3F(ab′)2-treated and untreated Balb/c mice infected with 5 × 105 pfu HSV-1RE at different time points p.i. *Statistically significant differences compared to untreated control (P ≤ 0.05).
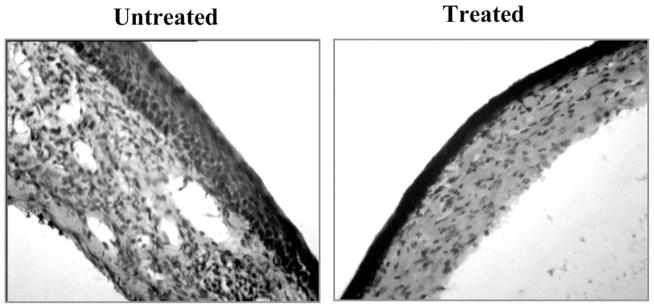
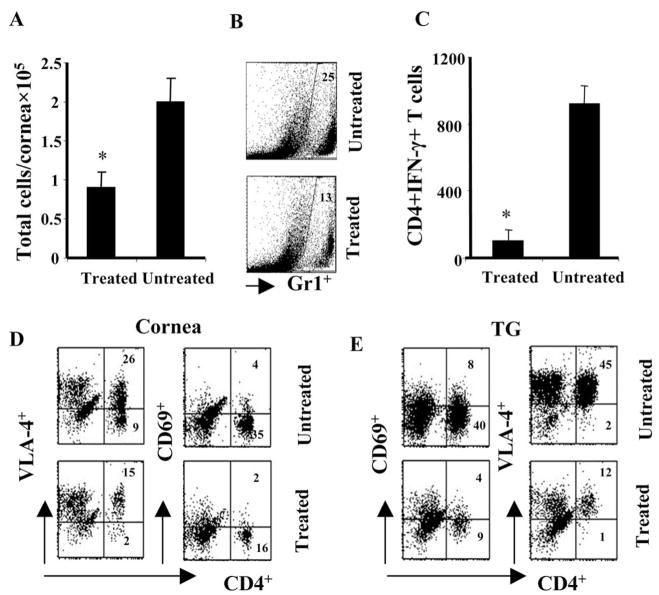
We also measured the inflammatory response in the infected corneas of both groups of animals. Corneal sections were examined by histopathology on day 17 p.i., when experiments were terminated. As shown in Figure 4, reduced corneal pathology and cellular infiltration was observed in the CD3F(ab′)2-treated animals. In a separate experiment, flow cytometric analysis was performed with corneal cells isolated from infected animals at day 13 p.i. As is evident in Figure 5A, there was a twofold reduction in the total viable inflammatory cell count in the corneas of treated animals compared to the control group. Reduced infiltration of inflammatory cells such as polymorphonuclear leukocytes (PMNs) (Gr1+ cells) and pathogenic CD4 T cells was associated with reduced lesion scores in the treated animals (Fig. 5B). Fewer numbers of IFN-γ producing pathogenic CD4+ T cells were present in the corneas of treated animals compared to untreated controls (Fig. 5C). Similarly, the number of CD4+ T cells expressing the activation marker CD69 and a critical integrin VLA4 was also reduced in the corneas of treated animals compared to untreated controls (Fig. 5D). A similar effect was also noticed in the TG, another inflammatory site associated with ocular HSV infection (Fig. 5E). These data suggest that reduced lesion severity in the CD3F(ab′)2-treated animals could be due to the reduced infiltration and activity of pathogenic Th1 cells in the cornea.
Figure 4.
Reduced inflammation and cellular infiltration in the corneas of CD3F(ab′)2-treated compared to untreated animals. (A) Mice were infected with 5 × 105 pfu HSV-1RE. Mice were terminated at day 18 p.i., and eyes were processed for cryosection. Hematoxylin and eosin staining was carried out on 6-μm sections. Original magnifica-tion, ×100.
Figure 5.
Cellular changes in the corneas and TGs of CD3F(ab′)2- treated compared to untreated animals. Single-cell suspensions of the tissues (cornea, TG) from infected animals of both groups were analyzed at day 13 p.i. Flow cytometric analysis of surface markers were performed. (A) The bar diagram shows the absolute number of viable cells per cornea in both groups of animals at day 13 p.i. (B) The corneal cells were labeled for Gr1+ cells. The dot plots represent the Gr1+ cells in the untreated (upper plot) and treated (lower plot) corneas. (C) The bar diagram shows the absolute number of CD4+IFN-γ+ T cells present per cornea in both groups of mice. (D, E) The dot plots denote the CD4+VLA-4+ and CD4+CD69+ T cells in the cornea and TG of both groups. The number on the dot plots denotes the percentage of cells expressing VLA-4 or CD69 when gated on lymphocytes. *Statistically significant differences compared to untreated control (P ≤ 0.05).
Diminished Th1 Type Immune Response and Activation Pattern of Lymphocytes Posttreatment
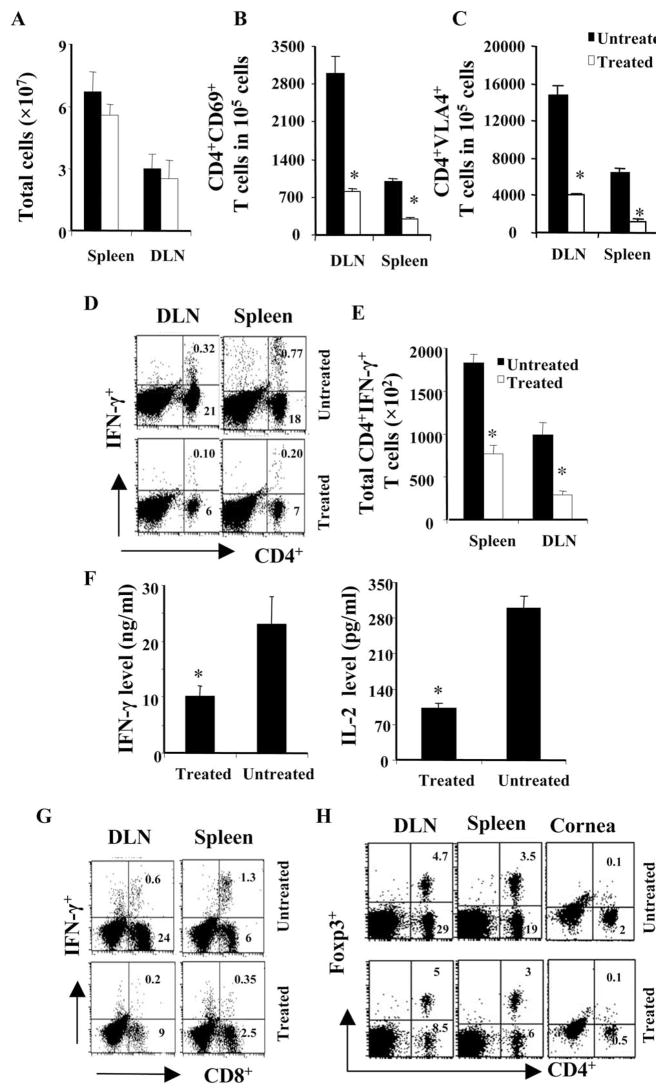
To understand the mechanisms that resulted in diminished lesion expression in CD3F(ab′)2-treated animals, the phenotype and the activity pattern of Th1 cells isolated from both treated and untreated animals was compared. As shown in Figure 6A, at day 13 p.i., treatment did not cause a significant reduction in the number of total cells isolated from lymphoid tissues. Interestingly, the number of CD4+ T cells expressing the activation marker CD69 was reduced compared to controls (Fig. 6B). In addition, CD4+ T cells expressing the VLA-4 marker, critical for the cells that home to the eye,31 were also reduced in the lymphoid tissues of treated animals (Fig. 6B). Next, the cytokine-producing CD4+ T cell population in both the groups was also measured. As shown in Figures 6D and 6E, the frequency and absolute number of IFN-γ+ CD4+ T cells was significantly less in the cervical DLN and spleens of CD3F(ab′)2-treated animals. Similarly, fewer numbers of TNF-α+ CD4+ T cells were observed in the lymphoid tissues of the animals that were treated with antibodies against CD3 (not shown). To determine the activity of the isolated splenocytes ex vivo, such cells were cultured with stimulators pulsed with UV-HSV. As is evident in Figure 6F, cells isolated from CD3F(ab′)2-treated animals at day 13 produced less IFN-γ and IL-2 in the 56 hours cultures compared to the cells taken from untreated infected animals.
Figure 6.
(A) The bar diagram shows the total number of viable cells recovered from the spleen and DLN of both groups of mice at day 13 p.i. (B, C) The bar diagram denotes the numbers of CD4+CD69+ and CD4+VLA-4+ T cells, respectively, in 1 × 105 lymphocytes or splenocytes of both groups of animals. (D) The dot plots in the figure represent the frequencies of CD4+IFN-γ+ cells (cells were stimulated with anti-CD3 and anti-CD28 antibody, 1 μg/mL). The numbers on the upper right quadrant represent the percentage of CD4+IFN-γ+ T cells in the gated population of lymphocytes. The plots are representative of four animals analyzed per group and the numbers shown are the mean of four individual animals. (E) The bar diagram denotes the numbers of CD4+ IFN-γ+ T cells per 1 × 105 lymphocytes or splenocytes of both groups of animals. (F) The splenocytes from the infected animals of both groups were stimulated in vitro with syngeneic stimulators pulsed with 3 MOI UV inactivated HSV. The supernatants were analyzed for IFN-γ and IL-2 cytokine production at 56 hours by sandwich ELISA. The bar diagrams demonstrate the increase in the IFN-γ (left) and IL-2 (right) protein level in the culture supernatant. (G) The dot plots in the figure represent the frequencies of CD8+IFN-γ+ cells (cells from B6 animals were stimulated with SSIEFARL peptide). The numbers on the upper right quadrants represent the percentage of CD8+IFN-γ+ T cells in the gated population of lymphocytes. (H) Change in CD4+Foxp+ T cell ratio posttreatment. Intra-nuclear staining for CD4+Foxp3+ Tregs was performed with the cells isolated from lymphoid tissues and cornea of both the groups. The dot plots represent the cells expressing CD4 and Foxp3 markers. The numbers on the upper right quadrant represent the percentage of CD4+Foxp3+ when gated on lymphocytes. The plots are representative of four animals analyzed per group and the numbers shown are the mean of four individual animals. *Statistically significant differences compared to untreated control (P ≤ 0.05).
Previous observations have shown that CD3F(ab′)2 treatment can affect the generation and distribution of antigen-specific CD8 T cells.13 Therefore, the effect of CD3F(ab′)2 treatment on the generation of HSV-specific CD8 T-cell response was measured. As is evident in Figure 6G, the frequencies of SSIEFARL peptide–specific CD8+IFN-γ+ T-cell number was significantly reduced in the lymphoid tissues of CD3F(ab′)2-treated animals. Thus, our results show that treatment with CD3F(ab′)2 could also influence the virus-specific immune response generation.
As treatment with non-mitogenic anti-CD3 was shown to induce regulatory T cells in treated animals,9,10 it was of interest to determine the frequencies of CD4+Foxp3+ T cells in both groups of animals. As evident in Figure 6F, at day 13 p.i., although the total number of Treg was lower in the treated animals, the proportion of these cells compared to CD4+Foxp3+ T cells in the diseased corneas and lymphoid tissues was higher in the treated animals (1:3) compared to untreated (1:6) animals. Similar results were also obtained at day 18 p.i. (not shown).
Treatment of Established SK with CD3F(ab′)2
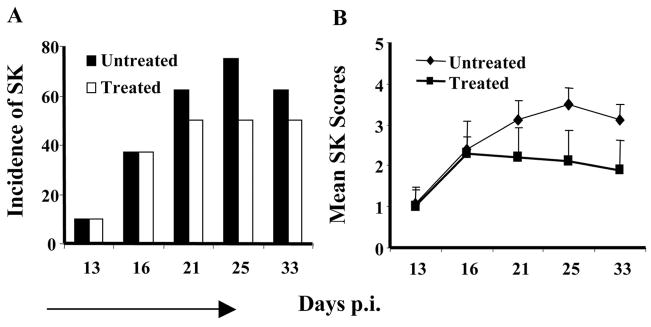
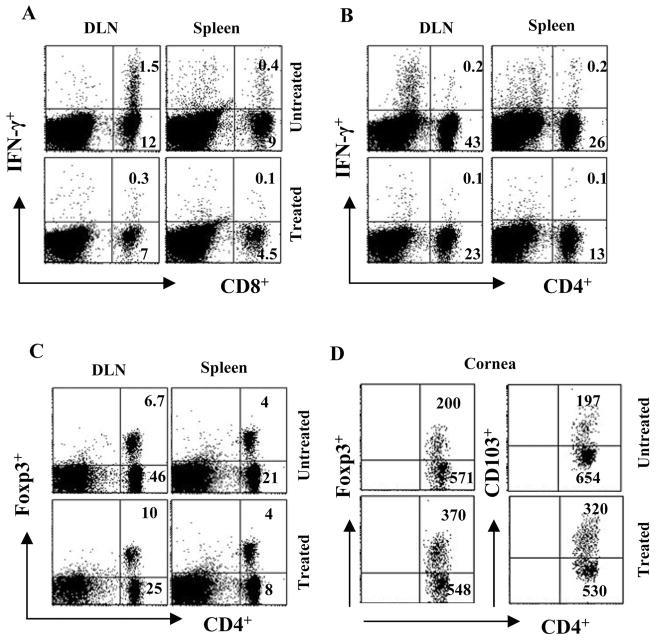
The above results indicate that CD3F(ab′)2 treatment could reduce the development of SK and angiogenic lesions. However, it would be more relevant if the approach also acted to reduce the severity of already established SK. Balb/c mice were ocularly infected with HSV-1RE and were allowed to develop SK lesions until day 15. One group of mice received CD3F(ab′)2 IV treatment from day 15 p.i. until day 21 p.i.. SK lesion scores were monitored until day 33 p.i. As shown in Figures 7A and 7B, there was no increase in the lesion scores or in the incidence of SK in the treated group of mice. The lesion scores of the eyes of both groups of mice were similar before treatment, but as shown in Figure 7B, on day 33 p.i., eyes in the untreated group had higher lesion scores compared to the treated group of animals. Resolution of ulcers and scars was more rapid in the treated group (not shown). Additionally, as shown in Figures 8A and 8B, similar to acute phase treatment with polyclonal stimulation, there was significantly diminished cytokine production by CD4+ and CD8+ T cells isolated from the lymphoid tissues of treated animals compared to the untreated counterparts on day 33 p.i. These results indicate that administration of CD3F(ab′)2 was also effective in clearing the activated CD4+ T cells from the system, an activity that might contribute to diminishing lesion severity. Interestingly, even with this treatment strategy, the proportion of Treg compared to non-Treg CD4+ T cells both in the lymphoid tissues (Fig. 8C) and corneas (Fig. 8D, left panel) were significantly higher in the CD3F(ab′)2-treated animals compared to untreated. In the corneas of CD3F(ab′)2-treated animals, higher numbers of T cells expressed CD103 (integrin αEβ7) marker (Fig. 6D, right panel) that is associated with the trafficking of regulatory T cells to the inflammatory sites.32 These results might mean that treatment with CD3F(ab′)2 could be beneficial both at the acute and chronic phase of the disease.
Figure 7.
Effect of CD3F(ab′)2 treatment on established SK lesions. (A) The bar diagram demonstrates the percentage severity of both groups of mice infected with 5 × 105 pfu HSV-1RE at different time p.i. (B) The line diagram denotes the mean SK scores in treated and untreated animals at different time points p.i.
Figure 8.
DLN, spleen, and cornea cells were harvested at 33 days p.i. from both CD3F(ab′)2-treated and untreated animals and were analyzed using flow cytometry. (A, B) The dot plots in the figure represent the frequencies of CD8+IFN-γ+ cells (A) and CD4+IFN-γ+ cells (B) (cells were stimulated with anti-CD3 and anti-CD28 antibody, 1 μg/mL) in the spleen and DLN. The numbers on the upper right quadrant represent the percentage of CD8+IFN-γ+ and CD8+IFN-γ+ T cells in the gated population of lymphocytes in the DLN and spleens. The plots are representative of four animals analyzed per group (n = 4) and the numbers shown are the mean of four animals. (C) Intra-nuclear staining for CD4+Foxp3+ Tregs was performed with the cells isolated from lymphoid tissues of both groups. The dot plots represent the frequencies of cells expressing CD4 and Foxp3 markers when gated on lymphocytes. (D) Cells from the infected corneas of both groups of animals were stained for Foxp3, CD4, and CD103 markers. The dot plots in the figure represent the CD4+ T cells expressing Foxp3 (left panel) and CD103 (right panel) in the cornea. The numbers represent the cell counts/ 2 × 105 corneal cells in gated CD4+ population.
Discussion
The chronic inflammatory lesion in the cornea that results from HSV infection appears to be mainly the consequence of a T-cell–mediated immunopathological reaction, although the identity of target antigens for the inflammatory T cells remains largely unknown.33 Controlling the severity of SK should be achievable if the proinflammatory function of the lesion-orchestrating T cell is constrained. One approach that achieves this is the use of a non-mitogenic anti-CD3 mAb, which has proven to successfully halt the progression of some autoimmune diseases after a single phase of treatment.9 We have applied this approach to control the severity of SK lesions. Our results show that a course of treatment with anti-CD3F(ab′)2 early during the syndrome resulted in significantly reduced lesions, with some animals remaining lesion-free. Additionally, there was a marked reduction in the number of T cells in the cornea and, of those present, a lower percentage were IFN-γ producers and more had the Foxp3+ phenotype than in controls. In lymphoid tissues, the frequency of cells with the activation phenotype was reduced three- to fourfold as well as cells that expressed VLA-4, the integrin that is expressed by most cells that infiltrate into the cornea.31 The magnitude of the HSV-specific CD4+ and CD8+ T-cell response in treated animals was significantly diminished when measured several days after the treatment ended. Although less effective, CD3F(ab′)2 treatment begun when lesions were fully evident resulted in a significant reduction of lesion severity compared to controls, but it did not cause complete resolution. Accordingly, anti-CD3 proved to be an effective means of reducing SK lesions, but as a means to control established lesions it may be necessary to combine the anti-CD3 approach with another method to achieve maximal levels of resolution.
Monoclonal antibody to CD3 that is non-FcR binding and that serves to suppress the function of T cells has been valuable in the treatment of a variety of inflammatory lesions, although not (to our knowledge) herpetic stromal keratitis.9 Although it is far from clear how the approach works at the molecular level, the outcome of treatment is that T-cell function is modulated, with cells becoming anergic along with some cells, especially activated cells, succumbing to apoptosis.11,16,34 The outcome is suppression of the number and function of cells that normally orchestrate the inflammatory reaction. Additionally, it appears that some types of T cells, such as Foxp3 regulatory cells, are less affected by the treatment, so their representation may increase and they may then contribute to suppression of the proinflammatory response.9 However, some observations have not supported any role for regulatory T cells as involved in the therapeutic effect of anti-CD3.11,35 In the SK model, we observed a marked reduction in CD4 T cells, particularly those of interferon gamma producers, thought to be the main orchestrators of lesions.36 Apart from acting on the T cells in the circulation and lymphoid tissues where the lesion-causing T cells are primed, this reagent might be able to access the corneal tissues directly through the leaky and highly permeable pathologic blood vessels in the SK corneas and might even act on the CD4+ T cells present at the lesion site. An additional effect that was noted in the treated animals was an increase in the frequency of Foxp3+ T cells. This was evident both at the lesion site and in the draining lymph node. We have not formally established whether these Foxp3+ Tregs contributed to lesion control in anti-CD3 treated animals, but previous studies did demonstrate that SK lesions become more severe when the natural regulatory T-cell response is inhibited.37
In our study, anti-CD3 treatment was most effective when begun early in the syndrome. The control that was achieved persisted at least for the relatively short time that lesions were studied. With murine diabetes, the efficacy of a single round of treatment can be extremely long lasting, exceeding one year or more.9 This issue of duration of effect needs to be further investigated. However, there are likely to be some critical differences in the pathogenesis of disease between SK and diabetes that may have an impact on efficacy. Accordingly, with experimental diabetes some have reported that treatment can reverse the quite severe stages of disease, since islet cell regeneration readily occurs and normal function ensues once the T-cell destruction effects are blunted.38 In the SK model, however, once the corneal stroma has been damaged by the inflammatory reaction, significant repair is difficult to achieve. For example, the extensive new blood vessel formation that typifies SK can be shrunken, but their removal appears to be problematic. In our studies, we noted that if treatment was begun when lesions were fully developed, some control was achieved but never to the point of full resolution. It would seem from these studies that other means of treatment would need to be combined with the anti-CD3 approach to achieve more complete levels of repair.
One such approach we are exploring is to combine topical treatment with fibroblast growth factor, which previous studies have shown to be useful in facilitating the repair of epithelial ulcers.39 It might also be appropriate to enhance the frequency and function of regulatory cells, especially those that produce TGF-β, a cytokine involved in tissue repair.40,41 As previously mentioned, one outcome of anti-CD3 therapy is that the frequency of Foxp3+ T cells increases because they are less affected by treatment. We and others have discovered ways of converting Foxp3+ T cells from conventional precursors, which may then contribute to the control of inflammatory lesions.42,43 One such way is to use a fungal metabolite drug (Fingolimod [FTY720]; Calbiochem) which, if given at the time of antigen stimulation, can cause some antigen-stimulated cells to express Foxp3 and exhibit a regulatory function.44 We are currently in the process of evaluating the efficacy of combination therapy with anti-CD3 given along with FTY720.
Finally, a word of caution about the use of anti-CD3 as a therapeutic modality for SK. Our results show that use of the reagent at the time when the virus is still present (starting treatment at day 0 p.i.) can result in uncontrolled spread of the virus which, in the case of HSV infection, may result in encephalitis that is usually lethal (not shown). A few animals treated five days p.i. developed signs of encephalitis with some death, but treating SK animals at day 15 when lesions were fully manifested and replicating virus cleared from the eye had no apparent clinical effect. In contrast, treatment with anti-CD3 one day p.i. invariably led to encephalitis and death. Accordingly, it may be wise to combine treatment with anti-CD3 with antiviral therapy. In some clinical trials in humans, reactivation of a herpes virus (Epstein Barr virus) has been observed 10 to 20 days after a course of treatment.45 It is not known whether the drug can additionally result in HSV reactivation in either human or mouse model. Such studies are currently underway in our laboratory.
It is also conceivable that the anti-CD3 treatment could render recipients more susceptible to many infectious agents. This is because the approach results in a general suppression of the T-cell response, with lymphopenia being evident for up to two or three weeks after a course of treatment. In our own studies, after treatment at day 15 p.i., we observed notable suppression of cytokine producing CD4+ and CD8+ T cells on polyclonal stimulation around two weeks after treatment ended. In contrast, the humoral response remained unaffected by this treatment (not shown). Fortunately, immune function did return to normal, as shown by some long-term studies performed by other groups. Nevertheless, given these potential problems if anti-CD3 is used in clinical situations to counteract SK, it would be wise to use antivirals and perhaps other antimicrobials as part of the therapy.
Conclusions
Our study shows that non-mitogenic non-FcR binding anti-CD3 treatment could prove effective in SK therapy due to its ability to alter the activation and migration pattern of the pathogenic T cells, thus diminishing the progression of an ongoing immunopathological lesion. The ability of CD3F(ab′)2 to remove the pathogenic CD4+ T cells and to increase the frequencies of TGF-β–producing Tregs at the same time makes it a more preferred candidate for SK therapy compared to other immunosuppressive reagents. Possible side effects might be encountered, but these could be minimized by controlling any microbial infections. Use of anti-CD3 in the clinic may also be most efficacious when combined with other forms of therapy.
Acknowledgments
The authors thank Susmit Suvas and Amol Suryawanshi for their invaluable help in this work.
Supported by National Eye Institute Grant EY05093.
Footnotes
Disclosure: P.P. Sarangi, None; B. Kim, None; B.T. Rouse, None The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007604. [DOI] [PubMed] [Google Scholar]
- 2.Mitsuya Y, Liu TF, Rhee SY, Fessel WJ, Shafer RW. Prevalence of darunavir resistance-associated mutations: patterns of occurrence and association with past treatment. J Infect Dis. 2007;196:1177–1179. doi: 10.1086/521624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vartian CV. Acyclovir and overuse of antibiotics. Ann Intern Med. 1984;100:463. doi: 10.7326/0003-4819-100-3-463_1. [DOI] [PubMed] [Google Scholar]
- 4.Biswas S, Jennens L, Field HJ. Single amino acid substitutions in the HSV-1 helicase protein that confer resistance to the helicase-primase inhibitor BAY 57–1293 are associated with increased or decreased virus growth characteristics in tissue culture. Arch Virol. 2007;152:1489–1500. doi: 10.1007/s00705-007-0964-7. [DOI] [PubMed] [Google Scholar]
- 5.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Kolbe L, Kligman AM, Schreiner V, Stoudemayer T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res Technol. 2001;7:73–77. doi: 10.1034/j.1600-0846.2001.70203.x. [DOI] [PubMed] [Google Scholar]
- 7.Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci. 2000;41:453–459. [PubMed] [Google Scholar]
- 8.Mercadal CM, Bouley DM, DeStephano D, Rouse BT. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 10.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 11.Kohm AP, Williams JS, Bickford AL, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 12.Plain KM, Chen J, Merten S, He XY, Hall BM. Induction of specific tolerance to allografts in rats by therapy with nonmitogenic, non-depleting anti-CD3 monoclonal antibody: association with TH2 cytokines not anergy. Transplantation. 1999;67:605–613. doi: 10.1097/00007890-199902270-00020. [DOI] [PubMed] [Google Scholar]
- 13.von Herrath MG, Coon B, Wolfe T, Chatenoud L. Nonmitogenic CD3 antibody reverses virally induced (rat insulin promoter-lymphocytic choriomeningitis virus) autoimmune diabetes without impeding viral clearance. J Immunol. 2002;168:933–941. doi: 10.4049/jimmunol.168.2.933. [DOI] [PubMed] [Google Scholar]
- 14.Ferran C, Sheehan K, Dy M, et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20:509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- 15.Ferran C, Bluestone J, Bach JF, Chatenoud L. In vivo T lymphocyte activation induced in mice following the injection of anti-CD3 monoclonal antibody. Transplant Proc. 1990;22:1922–1923. [PubMed] [Google Scholar]
- 16.Smith JA, Tso JY, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med. 1997;185:1413–1422. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parlevliet KJ, Chamuleau ME, Yong SL, Raasveld MH, ten Berge IJ, Schellekens PT. Effects of anti-CD3 monoclonal antibodies on functional activity of lymphocytes: studies in vivo and in vitro. Clin Exp Immunol. 1995;99:155–159. doi: 10.1111/j.1365-2249.1995.tb05526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch R, Gress RE, Pluznik DH, Eckhaus M, Bluestone JA. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989;142:737–743. [PubMed] [Google Scholar]
- 19.Thistlethwaite JR, Jr, Cosimi AB, Delmonico FL, et al. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation. 1984;38:695–701. doi: 10.1097/00007890-198412000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Chai JG, Lechler RI. Immobilized anti-CD3 mAb induces anergy in murine naive and memory CD4+ T cells in vitro. Int Immunol. 1997;9:935–944. doi: 10.1093/intimm/9.7.935. [DOI] [PubMed] [Google Scholar]
- 21.Williams ME, Lichtman AH, Abbas AK. Anti-CD3 antibody induces unresponsiveness to IL-2 in Th1 clones but not in Th2 clones. J Immunol. 1990;144:1208–1214. [PubMed] [Google Scholar]
- 22.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 23.Nicolls MR, Aversa GG, Pearce NW, et al. Induction of long-term specific tolerance to allografts in rats by therapy with an anti-CD3-like monoclonal antibody. Transplantation. 1993;55:459–468. doi: 10.1097/00007890-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol. 2002;219:108–118. doi: 10.1016/s0008-8749(02)00601-9. [DOI] [PubMed] [Google Scholar]
- 26.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande S, Zheng M, Lee S, et al. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J Immunol. 2001;167:2902–2910. doi: 10.4049/jimmunol.167.5.2902. [DOI] [PubMed] [Google Scholar]
- 28.Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Jiang S, Manczak M, Sugden B, Adamus G. Phenotypes of T cells infiltrating the eyes in autoimmune anterior uveitis associated with EAE. Invest Ophthalmol Vis Sci. 2002;43:1499–1508. [PubMed] [Google Scholar]
- 32.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas PS, Rouse BT. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 35.Chen G, Han G, Wang J, et al. Essential roles of TGF-{beta} in anti-CD3 antibody therapy: reversal of diabetes in nonobese diabetic mice independent of Foxp3+ CD4+ regulatory T cells. J Leukoc Biol. 2008;83:280–287. doi: 10.1189/jlb.0707498. [DOI] [PubMed] [Google Scholar]
- 36.Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4(+) T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol. 1999;29:3674–3682. doi: 10.1002/(SICI)1521-4141(199911)29:11<3674::AID-IMMU3674>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 38.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim B, Lee S, Kaistha SD, Rouse BT. Application of FGF-2 to modulate herpetic stromal keratitis. Curr Eye Res. 2006;31:1021–1028. doi: 10.1080/02713680601038824. [DOI] [PubMed] [Google Scholar]
- 40.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 41.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 42.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sehrawat S, Rouse BT. Antiinflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J Immunol. doi: 10.4049/jimmunol.180.11.7636. In press. [DOI] [PubMed] [Google Scholar]
- 45.Renard TH, Andrews WS, Foster ME. Relationship between OKT3 administration, EBV seroconversion, and the lymphoproliferative syndrome in pediatric liver transplant recipients. Transplant Proc. 1991;23:1473–1476. [PubMed] [Google Scholar]