Abstract
All described feathers in nonavian theropods are composite structures formed by multiple filaments. They closely resemble relatively advanced stages predicted by developmental models of the origin of feathers, but not the earliest stage. Here, we report a feather type in two specimens of the basal therizinosaur Beipiaosaurus, in which each individual feather is represented by a single broad filament. This morphotype is congruent with the stage I morphology predicted by developmental models, and all major predicted morphotypes have now been documented in the fossil record. This congruence between the full range of paleontological and developmental data strongly supports the hypothesis that feathers evolved and initially diversified in nonavian theropods before the origin of birds and the evolution of flight.
Keywords: Early Cretaceous, filament, display
Feathers have been documented in most nonavian coelurosaurian theropod groups, based mainly on recent discoveries of exceptionally well-preserved specimens from the Early Cretaceous Jehol Group of western Liaoning, China (1–4). The feathers present in these specimens can be categorized into several morphotypes, but all of them are composite structures formed by multiple slender filaments (5–7). Here, we report on a morphotype, present in the basal therizinosaur Beipiaosaurus (8), in which each feather comprises a single broad filament.
Description of the Specimens
STM31-1 (housed in the Shandong Tianyu Museum of Nature, China), collected from the Early Cretaceous Yixian Formation (9) of Jianchang, western Liaoning, is represented by a fully articulated skeleton missing the posterior half. STM31-1 is clearly a therizinosaur based on the presence of a suite of features unique to the Therizinosauroidea, including a lateral ridge on the dentary that forms a horizontal shelf, and leaf-shaped teeth with cylindrical roots (10). Elements that are present in both this specimen and the Beipiaosaurus inexpectus holotype (IVPP V11559, housed in the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing) are nearly identical in morphology (8), and STM31-1 is therefore identified here as Beipiaosaurus sp.
In STM31-1, feathers are preserved along the skull and neck, and trunk, around the forelimbs (Fig. 1A). These structures can be divided into two categories: short, slender filamentous feathers that are similar to those reported in many other nonavian theropods (1, 5–7, 11–13), and elongated broad filamentous feathers (EBFFs) that are novel. The EBFFs are preserved ventral to the posterior half of the mandible, dorsal to the posterior portion of the skull and the anterior portion of the neck (Fig. 1B), and dorsal to the posterior half of the trunk and are oriented at ≈45° to the long axes of the bones. Further preparation of the B. inexpectus holotype has revealed the presence of EBFFs along the tail. Here, the EBFFs overlap extensively and form dense patches that are difficult to interpret in detail, but several single EBFFs that are displaced from the patches provide more extensive morphological information (Fig. 1C).
Fig. 1.
EBFFs in Beipiaosaurus. (A) Beipiaosaurus sp. STM31-1. Arrows point to EBFFs. (B) Close-up of EBFFs in STM31-1. Arrow points to the basal end of a single EBFF. (C) Tail of the B. inexpectus holotype. Arrows point to separately preserved EBFFs, whereas most tail feathers overlap extensively. It should be noted that nearly all tail feathers are incompletely preserved in IVPP V11559 because of damage during preparation by an amateur collector. [Scale bar: 50 mm (A), 15 mm (B), and 10.5 mm (C).]
The EBFFs differ significantly in morphology from any other feathers that have so far been reported in nonavian theropods (1, 2). First, each EBFF is a single, unbranched filament (Figs. 1 B and C, and 2 A and B), whereas the filamentous feathers in other nonavian coelurosaurian specimens are composite structures formed by multiple filaments either diverging from a common base or branching from along the length of a main central filament (Figs. 2 C–E) (5, 6). Although it is possible that feathers represented by single filaments are present in other reported feathered coelurosaurian specimens, they have not yet been positively identified (5). Second, the EBFFs are proportionally longer than the filamentous feathers in other reported specimens (Fig. 2). They are ≈100–150 mm long, with the longest EBFFs being slightly shorter than the skull and approximately half the length of the neck in STM31-1. Filamentous feathers in other nonavian coelurosaurian specimens (5, 8, 12, 13) are much shorter, relative to the associated skeletal elements [Fig. 2 C–E and supporting information (SI) Table S1]. For example, the longest filamentous feathers associated with the neck are only ≈15% of the neck length in Sinosauropteryx prima (5). Finally, the EBFFs are proportionally very broad, ≈2 mm wide along their nearly entire length in STM31-1 and ≈3 mm wide in B. inexpectus holotype. For comparison, integumentary filaments in S. prima are at most 0.2 mm wide and in many cases are considerably narrower than 0.1 mm (5). In Dilong and Sinornithosaurus, the integumentary filaments are only slightly wider than in S. prima. Because the EBFFs are preserved as dark carbonized impressions, their relatively great width makes them appear planar. In contrast, the individual filaments of all reported composite feathers are fairly slender, hair-like (Fig. 2 C and D), and subcircular in cross-section (5). Other distinctive features of the EBFFs include their probable flexural stiffness and the presence in each of a distally rounded slight basal expansion (Fig. 1B). Flexural stiffness can be inferred from the lack of examples of curved or bent EPFFs, as is particularly evident in the B. inexpectus holotype (Figs. 1C and 2B).
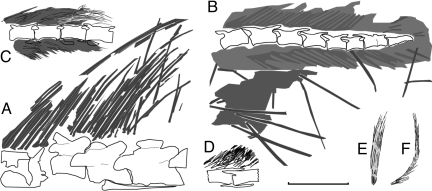
Fig. 2.
Comparative line drawings of filamentous feathers in several nonavian theropods. (A) EBFFs dorsal to the posterior portion of the skull and the anterior portion of the neck in STM31-1. (B) EBFFs near the end of the tail in IVPP V11559. (C) Filamentous feathers along the tail of S. prima (IVPP V12415). (D) Filamentous feathers along the tail of Dilong sp. (IVPP V11579). (E and F) Isolated filamentous feathers in the Sinornithosaurus millenii holotype (IVPP V12811). (C) E and F are modified from Fig. 3 in ref. 12 and Figs. 3 and 4 in ref. 6. [Scale bar: 40 mm (A, B, and D), 30 mm (C and F), and 15 mm for (E).]
Discussion
All reported feathers in nonavian theropods are composite structures formed by multiple slender filaments (1, 5–7), which are morphologically congruent with relatively advanced stages in feather evolution predicted by recently proposed developmental models of the evolutionary origin of feathers (14, 15). However, no adduced fossil evidence has unambiguously demonstrated the presence of monofilamentous feathers in a nonavian theropod, even though such feathers would correspond to the phylogenetically most basal feather morphotype (stage I) predicted by these developmental models (14, 15). Although the EBFFs in the basal therizinosaur Beipiaosaurus differ from the predicted stage I morphology in some features, such as their somewhat planar form, their discovery nevertheless documents an unbranched feather morphology in nonavian theropods and thus completes the array of fossil evidence for the morphological series predicted by the developmental models.
The planar form of the EBFFs, as they are preserved on the slab, deserves special note. Because feathers are essentially tubular structures, the EBFFs are probably hollow, at least basally. Their relatively great width indicates, however, that they are unlikely to be subcircular in cross-section. It is more probable that the filaments developed as stiff large tubes with an elliptical cross-section. Interestingly, not only are modern feathers planar in their overall form, but their individual rami and barbules are also somewhat flattened (16). In this respect, avian feathers differ significantly from hairs, which represent the other major category of filamentous integumentary structures in tetrapods and have a subcircular cross-section. However, the undifferentiated, somewhat planar EBFFs are unlikely to represent the plesiomorphic condition for feathers because the feathers of more basal coelurosaurs are all made up of slender filaments that are subcircular in cross-section (5). Based on the ontogeny of feathers in modern birds (14), it is probable that the composite feathers of a typical feathered coelurosaur each grew from a collar of epidermal tissue that became differentiated into multiple barb ridges, each of which produced one of the filaments. By contrast, each EBFF on the body of Beipiaosaurus presumably grew from an epidermal collar that never underwent differentiation, so that subsequent growth produced a monofilamentous feather. The cross-section of the epidermal collar was probably elliptical, rather than subcircular, a condition that would explain the planar appearance of the preserved EBFFs of Beipiaosaurus. The presence of EBFFs could represent an autapomorphy of Beipiaosaurus or a slightly more inclusive clade.
It is difficult to infer the primary function of the EBFFs, as is the case for many other structures known only in extinct animals, but flight and thermoregulation can be excluded from the list of possibilities given the morphology of the feathers and their distribution on the body. The third most common function for feathers, visual display, is more acceptable for the EBFFs of Beipiaosaurus because of their relatively great length, apparent flexural stiffness, and localized distribution. The EBFFs are much longer than normal filamentous feathers (Table S1) and are inferred to have been very flexurally stiff. They occur only on portions of the head, neck, and tail, body areas that commonly bear integumentary display structures in extant birds (16) and mammals (17). These features suggest that the EBFFs might have been used in display, whereas other types of filamentous feathers in nonavian theropods are more likely to have functioned in thermoregulation (2, 11, 12). Integumentary display using highly modified pennaceous feathers has been documented in basal avians (18) and even in nonavian maniraptorans (19), but no filamentous feathers have been shown to function in display. The EBFFs of Beipiaosaurus provide the first evidence supporting the occurrence of integumentary display using filamentous feathers among nonavian theropods. Because pennaceous feathers have not been reported in therizinosaurs and more basal coelurosaurian groups, EBFFs are thus inferred to have appeared phylogenetically at a more basal point than did feathers of modern aspect. This indicates that integumentary display using feathers evolved at an earlier stage than flight, which is associated with pennaceous feather types.
Finally, the EBFFs share some striking similarities with the filamentous integumentary structures seen in the ornithischian Psittacosaurus (20) and some pterosaurs (21–23), including unbranched morphology, thickness, and apparent flexural stiffness. These points of resemblance suggest a potential primary homology among the integumentary features of these three disparate taxa. This reinforces the intriguing possibility, mentioned by some recent studies (1), that monofilamentous integumentary structures might have evolved early in archosaurian evolution and might characterize a clade far more inclusive than the Coelurosauria. The known ornithodiran fossil record would thus likely push the origin of monofilamentous integumentary structures into the Middle Triassic at least (24).
Supplementary Material
Acknowledgments.
We thank two anonymous referees for critical comments and valuable suggestions, C. Sullivan for commenting on and editing the manuscript, and H.-J. Wang for preparing the specimen. This work was supported by the Chinese Academy of Sciences, the National Natural Science Foundation of China, and Major Basic Research Projects of the Ministry of Science and Technology, China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810055106/DCSupplemental.
References
- 1.Xu X. Feathered dinosaurs from China and the evolution of major avian characters. Integ Zool. 2004;1:4–11. doi: 10.1111/j.1749-4877.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 3.Norell M, Xu X. Feathered dinosaurs. Annu Rev Earth Planet Sci. 2005;33:277–299. [Google Scholar]
- 4.Zhou Z-H. The origin and early evolution of birds: Discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften. 2004;91:455–471. doi: 10.1007/s00114-004-0570-4. [DOI] [PubMed] [Google Scholar]
- 5.Currie PJ, Chen P-j. Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can J Earth Sci. 2001;38:1705–1727. [Google Scholar]
- 6.Xu X, Zhou Z-H, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- 7.Ji Q, Norell MA, Gao K-Q, Ji S-A, Ren D. The distribution of integumentary structures in a feathered dinosaur. Nature. 2001;410:1084–1088. doi: 10.1038/35074079. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Tang Z-L, Wang X-L. A therizinosauroid dinosaur with integumentary structures from China. Nature. 1999;399:350–354. [Google Scholar]
- 9.Swisher Carl C, III, Wang Y-q, Wang X-l, Xu X, Wang Y. Cretaceous age for the feathered dinosaurs of Liaoning, China. Nature. 1999;400:58–61. [Google Scholar]
- 10.Clark JA, Maryanska T, Barsbold R. In: The Dinosauria. 2nd Ed. Weishampel DB, Dodson P, Osmolska H, editors. Berkeley: University of California Press; 2004. pp. 151–164. [Google Scholar]
- 11.Chen PJ, Dong ZM, Zhen SN. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–152. [Google Scholar]
- 12.Xu X, et al. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature. 2004;431:680–684. doi: 10.1038/nature02855. [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer MH, et al. β-Keratin-specific immunological reactivity in feather-like structures of the Cretaceous alvarezsaurid, Shuvuuia deserti. J Exp Zool. 1999;285:146–157. [PubMed] [Google Scholar]
- 14.Prum RO. Development and evolutionary origin of feathers. J Exper Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- 15.Brush AH. Evolving a protofeather and feather diversity. Am Zool. 2000;40:631–639. [Google Scholar]
- 16.Lucas AM, Stettenheim PR. Avian Anatomy: Integument. Washington, DC: U.S. Department of Agriculture; 1972. [Google Scholar]
- 17.Macdonald D, editor. The Encyclopedia of Mammals. London: Greenwich Editions; 1984. [Google Scholar]
- 18.Zhou ZH, Zhang FC. Mesozoic birds of China: A synoptic review. Vertebr Palasiatica. 2006;44:74–98. [Google Scholar]
- 19.Zhang FC, Zhou ZH, Xu X, Wang XL, Sullivan C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 2008;455:1105–1108. doi: 10.1038/nature07447. [DOI] [PubMed] [Google Scholar]
- 20.Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang XL, Zhou ZH, Zhang FC, Xu X. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and “hairs” from Inner Mongolia, northeast China. Chin Sci Bull. 2002;47:226–230. [Google Scholar]
- 22.Ji Q, Yuan CX. Discovery of two kinds of protofeathered pterosaurs in the Mesozoic Daohugou Biota in the Ningcheng region and its stratigraphic and biologic significances. Geol Rev (China) 2002;48:221–224. [Google Scholar]
- 23.Bakhurina NN, Unwin DM. In: Sun AL, Wang YQ, editors. Sixth Symposium on Mesozoic Terrestrial Ecosystems and Biota, Short Papers; Beijing: China Ocean Press; 1995. pp. 79–82. [Google Scholar]
- 24.Irmis RB, et al. A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science. 2007;317:358–361. doi: 10.1126/science.1143325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.