Abstract
Many currently used and candidate vaccine adjuvants are particulate in nature, but their mechanism of action is not well understood. Here, we show that particulate adjuvants, including biodegradable poly(lactide-co-glycolide) (PLG) and polystyrene microparticles, dramatically enhance secretion of interleukin-1β (IL-1β) by dendritic cells (DCs). The ability of particulates to promote IL-1β secretion and caspase 1 activation required particle uptake by DCs and NALP3. Uptake of microparticles induced lysosomal damage, whereas particle-mediated enhancement of IL-1β secretion required phagosomal acidification and the lysosomal cysteine protease cathepsin B, suggesting a role for lysosomal damage in inflammasome activation. Although the presence of a Toll-like receptor (TLR) agonist was required to induce IL-1β production in vitro, injection of the adjuvants in the absence of TLR agonists induced IL-1β production at the injection site, indicating that endogenous factors can synergize with particulates to promote inflammasome activation. The enhancement of antigen-specific antibody production by PLG microparticles was independent of NALP3. However, the ability of PLG microparticles to promote antigen-specific IL-6 production by T cells and the recruitment and activation of a population of CD11b+Gr1− cells required NALP3. Our data demonstrate that uptake of microparticulate adjuvants by DCs activates the NALP3 inflammasome, and this contributes to their enhancing effects on innate and antigen-specific cellular immunity.
Keywords: Caspase-1, IL-1, microparticle
Vaccination with most purified vaccine antigens does not elicit strong adaptive immune responses unless adjuvants are included to promote innate immunity. Although many substances have been investigated for their potential to enhance vaccine-specific immunity, the most common adjuvants in clinical use are insoluble aluminium salts, generically referred to as alum (1). Alum comprises crystalline aluminum oxyhydroxide as small primary particles (2), which form aggregates of 1–20 μm in diameter (1). The ability of particulates to promote vaccine-specific immunity has been recognized for more than 80 years (3, 4). Because alum is not optimal for all protein antigens and is relatively ineffective at promoting cell-mediated immunity, other particulate adjuvants, including biodegradable poly(lactide-co-glycolide) (PLG) microparticles and emulsions are under investigation (5, 6). These particulates are distinguished from adjuvants, such as Toll-like receptor (TLR) and nucleotide-binding oligomerization domain-like receptor (NLR) ligands, which are sensed directly by pathogen recognition receptors (7).
Activation of innate immunity is required to induce and direct adaptive immune responses to vaccines. Dendritic cells (DCs) are central in this respect because they are the most potent antigen-presenting cells and are specialized for uptake of antigens, migration to lymph nodes, and activation of naïve T cells (8). The interaction of pathogen-derived or “danger”-associated factors, including TLR and NLR ligands, with DCs leads to “maturation,” which increases their efficiency in promoting T cell activation (8).
Alum can enhance antigen-specific antibody responses in mice deficient in the TLR adaptors MyD88 and TRIF (9–11), although alum-mediated induction of antigen-specific T cell responses relies on DCs and is dependent on MyD88 and uric acid (12). Currently, the mechanism of action of particulate vaccine adjuvants is not fully understood (13), and this is particularly the case for polymeric microparticles.
Interleukin-1β (IL-1β) is a potent proinflammatory cytokine that exerts multiple effects on the immune system (14), which include promoting the differentiation of Th17 cells (15). The production of IL-1β requires 3 steps: (i) priming: the production of pro-IL-1β; (ii) processing of pro-IL-1β by caspase 1 to generate the 17-kDa “mature” IL-1β; and (iii) release of mature IL-1β from the cell (16). Processing of pro-IL-1β requires assembly and activation of inflammasomes (17), which are cytoplasmic multiprotein complexes that contain an NLR and caspase 1 (18). The NALP2/NALP3 inflammasome contains NALP2 or NALP3, the adaptor proteins CARDINAL and ASC, and caspase 1 (19). NALP3 is present in the cytoplasm of DCs (20) and associates with ASC, leading to the recruitment of caspase 1, which is autocatalytically cleaved to produce an enzymatically active heterodimer of 2 p20 and 2 p10 chains (21). A number of factors can activate the NALP3 inflammasome, including microbial nucleic acids (22), muramyl dipeptide, toxins (23, 24), asbestos, silica (25), alum (11), and the endogenous stimuli, ATP, (24) monosodium urate, and calcium pyrophosphate dihydrate crystals (26). However, the role of this pathway in microparticle-induced immune responses has not been determined, and there have been conflicting reports on the role of NALP3 in adjuvanticity (27). Therefore, we investigated the role of NALP3 in the induction of innate and adaptive immune responses by microparticulate vaccine adjuvants.
Results
Particulate Vaccine Adjuvants Promote the Secretion of IL-1β by DCs.
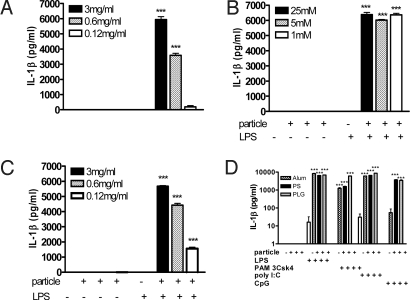
LPS was a weak stimulus for IL-1β secretion in DCs. However, when LPS was added to cells 1 h after biodegradable PLG or polystyrene (PS) microparticles or alum, secretion of IL-1β was significantly enhanced (Fig. 1 A–C). The ability of particulate adjuvants to enhance the secretion of IL-1β was not restricted to LPS, nor was it restricted only to TLR agonists that interact with extracellular TLRs, because the particulate adjuvants also enhanced IL-1β production by the TLR2 agonist PAM3Csk, the TLR3 agonist poly I:C, and the TLR9 agonist CpG (Fig. 1D).
Fig. 1.
Particulate vaccine adjuvants dramatically enhance the secretion of IL-1β by DCs. DCs were incubated with PLG (1 μm) microparticles (A), alum (B), or PS (430 nm) microparticles either alone or for 1 h before the addition of LPS (5 ng/mL) (C). Supernatants were collected after 24 h, and IL-1β was determined by ELISA. Particles plus LPS vs. LPS only: ***, P < 0.001. (D) DCs were incubated with PLG microparticles (3 mg/mL), PS microparticles (3 mg/mL), or alum (25 mM) for 1 h before addition of PAM3Csk (10 ng/mL), LPS (5 ng/mL), CpG (10 ng/mL), or poly I:C (50 μg/mL) to the cells. Supernatants were collected after 24 h and assayed for IL-1β. Particles plus TLR agonist vs. TLR agonist only: ***, P < 0.001.
Uptake of Particulate Adjuvants Is Required for Their Ability to Promote IL-1β Secretion.
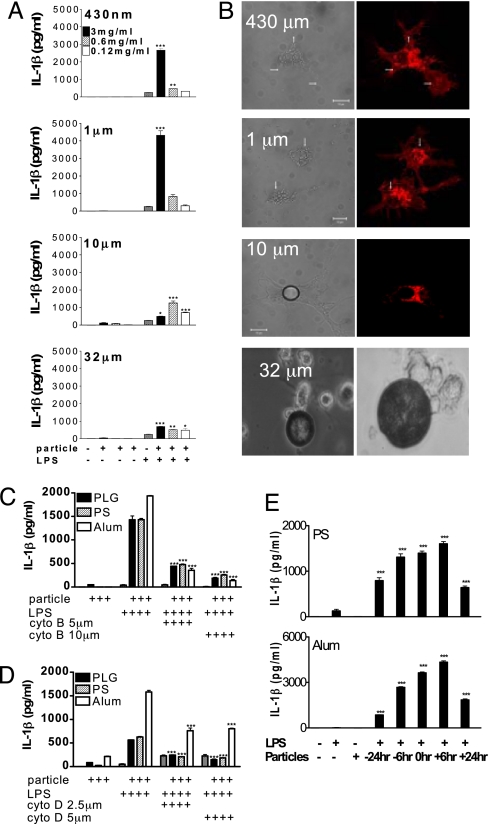
PS microparticles of 430 nm, 1 μm, 10 μm, and 32 μm were used to establish the relationship between particle size and enhancement of IL-1β secretion. The 1-μm particles were most potent in promoting IL-1β, followed by 430-nm particles, 10-μm particles, and 32-μm particles (Fig. 2A). Enhancement of IL-1β secretion by PLG microparticles was similarly dependent on particle size (data not shown). PS particles of 430 nm and 1 μm in size were efficiently taken up, whereas there was limited uptake of 10-μm particles, and 32-μm particles were not endocytosed (Fig. 2B). Preincubation of DCs with cytochalasin B and cytochalasin D, which inhibit actin filament assembly and phagocytosis, abrogated the enhancement of IL-1β secretion by the particulates (Fig. 2 C and D).
Fig. 2.
Particle uptake is required for particulate adjuvants to promote IL-1β secretion. (A) DCs were incubated with PS microparticles (430 nm, 1 μm, 10 μm, 32 μm) alone or for 1 h before addition of LPS. Supernatants were collected 24 h later and assayed for IL-1β. Particles plus LPS vs. LPS only: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) DCs (labeled with Alexa Fluor-conjugated WGA; red) were incubated with PS microparticles for 2 h, and uptake was assessed by confocal microscopy. Images show WGA-labeled DCs, along with bright-field/phase-contrast images. (Magnification, 63×.) The photos relating to 32-μm particles are bright-field images. Arrows point to internalized microparticles. DCs were pretreated with (C) cytochalasin B or (D) cytochalasin D for 1 h before the addition of particles, and LPS (5 ng/mL) was added 1 h later. After 8 h, supernatants were collected, and IL-1β was determined. Particles plus LPS vs. particles plus LPS plus cytochalasin B or D: ***, P < 0.001. (E) DCs were incubated with PS microparticles (0.6 mg/mL) or alum (5 mM) or LPS (5 ng/mL) alone or with particles 6 or 24 h before, at the same time, or 6 or 24 h after LPS. After 24 h, supernatants were collected, and IL-1β was determined. Particles plus LPS vs. LPS only: ***, P < 0.001.
To establish whether the promotion of IL-1β secretion required the particle and TLR ligand to be presented simultaneously to DCs, particles and LPS were added to cells at the same time or separated by 6 or 24 h. Enhancement of IL-1β secretion was greatest when particles were added to cells between 6 h before and 6 h after the TLR agonist, but was detectable even when the addition of the particles and TLR ligand was separated by 24 h (Fig. 2E).
Particulate Vaccine Adjuvants Promote the Processing and Secretion of IL-1β and Caspase 1.
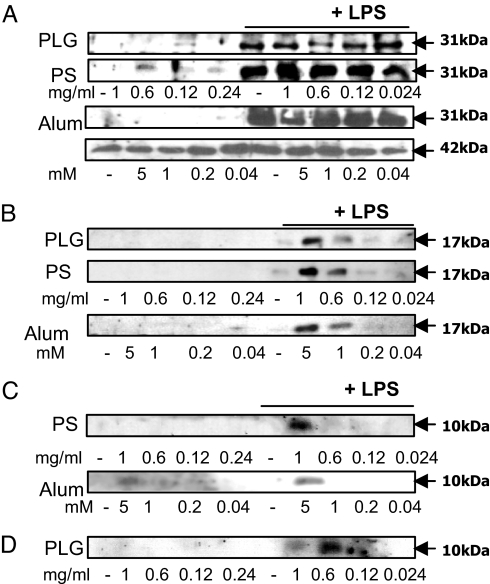
Neither PLG microparticles nor PS microparticles nor alum induced synthesis of pro-IL-1β (31 kDa) in DCs (Fig. 3A). Additionally, the particulates did not enhance the levels of pro-ILβ in cells when added together with LPS. When cells were stimulated with both particulates and LPS, processed IL-1β (17 kDa) was detected in supernatants (Fig. 3B). Because caspase 1 processes pro-IL-1β into mature IL-1β, we determined whether the particles promoted IL-1β processing by activating caspase 1. The particulates synergized with LPS to promote caspase 1 activation but had no effect on their own (Fig. 3C). In addition, incubation of DCs with PLG microparticles and LPS promoted the secretion of processed caspase 1 (Fig. 3D).
Fig. 3.
Uptake of particulates by DCs does not induce pro-IL-1β, but it promotes the processing and secretion of mature IL-1β and activation of caspase 1. DCs were incubated with PLG or PS microparticles or alum, LPS, or with particles for 1 h before LPS and incubated for 24 h. (A) Pro-IL-1β (31 kDa) and β-actin (42 kDa) in cell lysates. (B) Mature IL-1β (17 kDa) in supernatants. (C) Processed caspase 1 (p10) in lysates. (D) Processed caspase 1 (p10) in supernatants.
Particle-Mediated Enhancement of IL-1β Secretion Is Caspase 1-, NALP3-, and Cathepsin B-dependent.
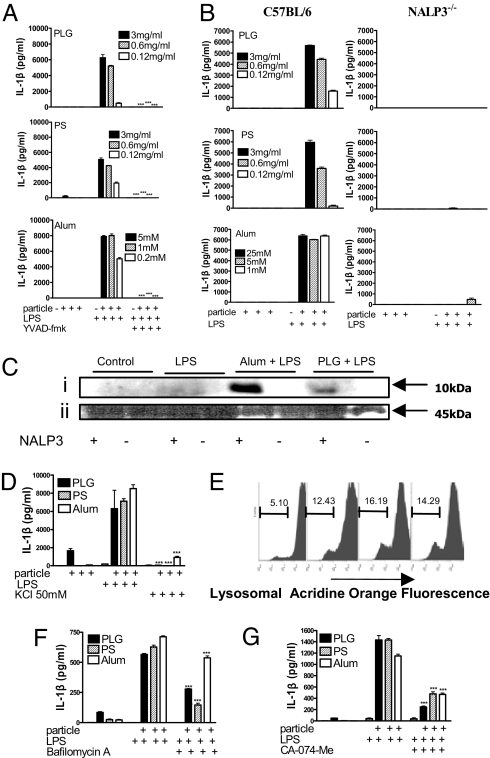
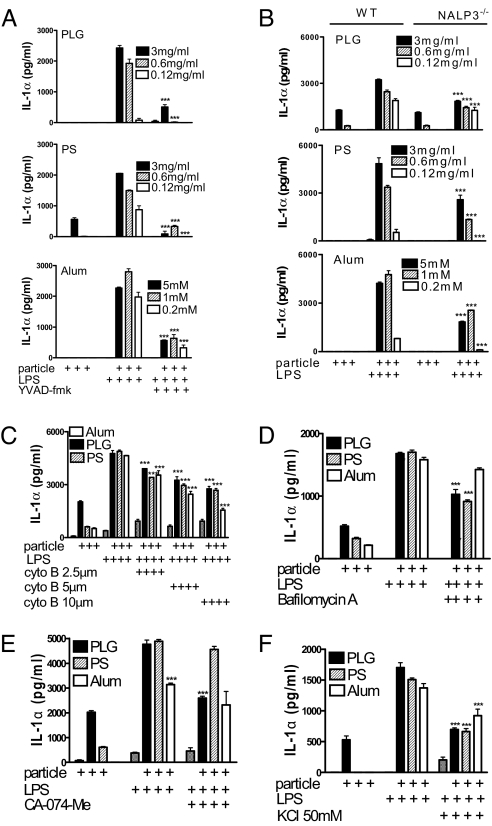
Pretreatment with the caspase 1 inhibitor, YVAD-fmk, completely inhibited IL-1β secretion in response to LPS and microparticles or alum (Fig. 4A). Furthermore, microparticle-mediated enhancement of IL-1β secretion (Fig. 4B) and caspase 1 activation (Fig. 4C) was detected in DCs from wild-type but not NALP3−/− mice. Microparticles also enhanced the secretion of IL-18, and this was NALP3-dependent [supporting information (SI) Fig. S1]. In contrast, production of IL-6 and TNF-α in response to LPS or LPS and particles was comparable in WT and NALP3−/− DCs, demonstrating that there is no global defect in the production of proinflammatory cytokines in these cells (Figs. S2 and S3).
Fig. 4.
NALP3 is required for particulate adjuvants to promote IL-1β processing and secretion and caspase 1 activation. (A) DCs were incubated with or without the caspase 1 inhibitor YVAD-fmk (40 μM) and with particulates for 1 h before LPS (5 ng/mL). Supernatants were collected after 24 h, and IL-1β was determined. Particles plus LPS plus YVAD-fmk vs. particles plus LPS only: ***, P < 0.001. (B) DCs from C57BL/6 or NALP3−/− mice were incubated with particulates for 1 h before the addition of LPS. IL-1β concentrations were determined in supernatants after 24 h. (C) DCs were incubated with PLG microparticles or alum for 1 h before the addition of LPS, and caspase 1 was detected in cell lysates by Western blot. (i) Caspase 1 p10. (ii) Pro-caspase 1. (D) DCs were incubated with complete RPMI medium alone or supplemented with KCl (50 mM) for 30 min before addition of particulates either alone or for 1 h before LPS. Supernatants were collected after 24 h, and IL-1β was determined by ELISA. KCl-supplemented medium vs. complete RPMI: ***, P < 0.001. (E) Flow cytometry of DCs stained with acridine orange and incubated with particulates (left to right: control, PLG, PS, alum, respectively) for 3 h. Numbers refer to percentages of cells with loss of lysosomal staining with acridine orange. DCs were pretreated with (F) bafilomycin A (250 nM) or (G) the cathepsin B inhibitor CA-074-Me (10 μM) for 1 h. Particles were then added to the cells for 1 h before addition of LPS (5 ng/mL). Supernatants were removed after 8 h and analyzed for IL-1β. Particles plus LPS plus inhibitor vs. particles plus LPS: ***, P < 0.001.
Activation of caspase 1 by monosodium urate crystals (28) but not intracellular pathogens (29) required K+ efflux. We found that KCl concentrations of greater than 50 mM inhibited IL-1β secretion in response to LPS and particulate adjuvants (Fig. 4D).
The dye, acridine orange, is cationic and concentrates in acidic compartments, leading to the formation of dimers and the appearance of red fluorescence (30). Uptake of microparticles by DCs resulted in a reduction in red fluorescence, indicating a loss of lysosomes (Fig. 4E). Pretreatment of cells with bafilomycin A, an inhibitor of the vaculolar H+ ATPase, significantly reduced microparticle-mediated enhancement in IL-1β secretion (Fig. 4F). Lysosomal acidification is required for activation of proenzymes, including cathepsin B. Inhibition of cathepsin B with the specific inhibitor CA-074-Me significantly reduced secretion of IL-1β in response to particulate adjuvants (Fig. 4G).
Particulate Vaccine Adjuvants Promote the Secretion of IL-1α from DCs in a Caspase 1-Dependent Manner.
Coincubation of DCs with LPS and microparticles strongly enhanced IL-1α secretion. This effect was significantly suppressed when cells were pretreated using a caspase 1 inhibitor, but it was not totally abrogated (Fig. 5A). However, although particle-mediated IL-1α secretion was significantly reduced in DCs from NALP3−/− mice, this reduction was much less than in the case of IL-1β (Fig. 5B). Moreover, particle-mediated enhancement of IL-1α secretion was only partly dependent on particle uptake (Fig. 5C), lysosomal acidification (Fig. 5D), and cathepsin B activity (Fig. 5E), and it was not completely blocked by inhibiting K+ efflux (Fig. 5F).
Fig. 5.
Particulate vaccine adjuvants enhance the secretion of IL-1α by DCs. (A) DCs were incubated with or without the caspase 1 inhibitor YVAD-fmk (40 μM) before addition of particulates either alone or for 1 h before LPS (5 ng/mL). Particles plus LPS plus YVAD-fmk vs. particles plus LPS only: ***, P < 0.001. (B) DCs from C57BL/6 or NALP3−/− mice were incubated with particulates for 1 h before the addition of LPS, and IL-1α concentrations were determined after 24 h. NALP3−/− vs. C57BL/6: ***, P < 0.001. (C) DCs were pretreated with cytochalasin B for 1 h before the addition of particles. After a further 1 h, LPS (5 ng/ml) was added, and cells were incubated for 8 h. DCs were incubated with (D) bafilomycin A or (E) the cathepsin B inhibitor CA-074-Me (10 μM) for 1 h before particle addition. After 1 h, LPS (5 ng/mL) was added to the cells and left to incubate for 8 h. Particles plus LPS plus inhibitor vs. particles plus LPS only: ***, P < 0.001. (F) DCs from C57BL/6 mice were incubated with complete RPMI medium or with medium supplemented with KCl for 30 min before addition of PLG or PS microparticles or alum either alone or for 1 h before the addition of LPS. Supernatants were collected after 24 h. KCl-supplemented medium vs. complete RPMI: ***, P < 0.001.
NALP3 Is Not Required for the Enhancement of Antibody Responses by Microparticles but Is Involved in Promoting Cell Recruitment and Antigen-Specific Cellular Immunity.
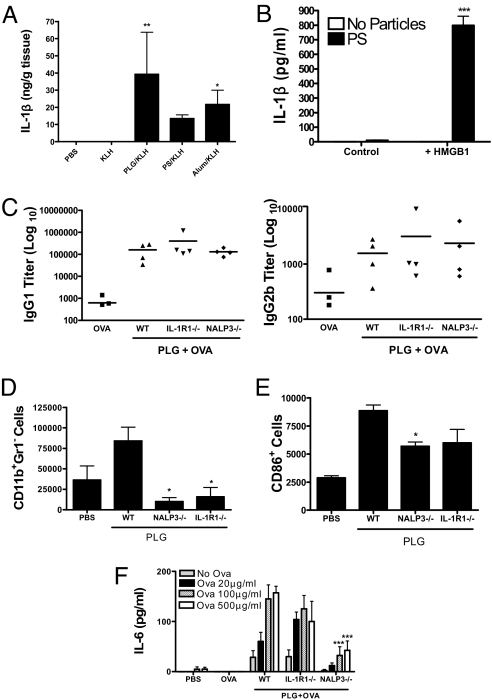
Our data indicate that in vitro, a TLR ligand is required in combination with particulate adjuvants to promote IL-1β secretion. However, these adjuvants are normally injected in the presence of TLR agonist-free antigens. We injected mice with either PBS or with keyhole limpet hemocyanin (KLH) alone or together with particulates. IL-1β was not detected in mice injected with PBS or KLH alone, but it was detectable when KLH was injected together with particulates (Fig. 6A). These data suggest that the ability of particulate vaccine adjuvants to promote the induction of IL-1β in vivo does not require pathogen-derived TLR ligands. In support of this, we found that high-mobility group box 1 (HMGB1), which is released from necrotic cells (31), can synergize with particulate adjuvants to promote IL-1β secretion by DCs (Fig. 6B).
Fig. 6.
NALP3 is required for enhancement of cellular immune responses to PLG microparticles but not for enhancement of antibody responses. (A) C57BL/6 mice were injected subcutaneously with PBS, KLH (5 μg), or KLH and 2.5 mg of PLG or PS microparticles or alum. After 7 days, the injection site was excised and homogenized, and IL-1β was determined by ELISA. (B) DCs were incubated with PLG microparticles either alone or for 1 h before the addition of HMGB1 (40 μg/mL). Supernatants were collected after 24 h. Particles plus HMGB1 vs. HMGB1 only: ***, P < 0.001. (C) NALP3−/−, IL-1R−/−, or WT mice were immunized intraperitoneally with OVA (50 μg) alone or with OVA and PLG microparticles (2.5 mg) on days 1 and 14. Serum was assayed for OVA-specific IgG1 and IgG2b 7 days later. (D) CD11b+ Gr1− cell numbers in the blood and (E) CD11b+Gr1−CD86+ cells in the peritoneal lavage of NALP3−/−, IL-1R−/−, and WT mice 18 h after intraperitoneal injection of PLG microparticles. WT vs. NALP3−/− or IL-1R−/− mice: *, P < 0.05. (F) NALP3−/−, IL-1R1−/−, and WT mice were immunized subcutaneously with OVA (50 μg) alone or with OVA and PLG microparticles (2.5 mg) on days 1 and 14. Antigen-specific IL-6 production was measured from splenocytes isolated from mice 7 days later. PLG plus OVA WT vs. PLG plus OVA NALP3−/−, IL-1R−/−: ***, P < 0.001.
Intraperitoneal injection of mice with ovalbumin (OVA) in the presence of PLG microparticles induced significantly higher antigen-specific antibody responses than antigen alone. This was not NALP3-dependent, because comparable titers of OVA-specific IgG1 and IgG2b were detected in NALP3-deficient and IL-1R1-deficient mice (Fig. 6C). However, 20 h following injection there was a significant increase in the numbers of CD11b+Gr1− cells in the blood of mice injected with PLG microparticles compared with PBS, and this response was significantly reduced in NALP3−/− mice (Fig. 6D). In addition, there was a significant increase in expression of the costimulatory molecule CD86 on CD11b+Gr1− cells in the peritoneal lavage of mice injected with PLG compared with PBS, and this was significantly reduced in NALP3−/− mice (Fig. 6E). Furthermore, subcutaneous immunization with OVA and PLG microparticles significantly enhanced antigen-specific IL-6 production by spleen cells compared with injection of OVA alone, and this was abrogated in NALP3−/− mice (Fig. 6F). There was no difference in antigen-specific IgG1 antibody titers in sera from these mice (data not shown).
Discussion
Particulates, including biodegradable PLG microparticles, emulsions, alum, and calcium phosphate, are the most widely used vaccine adjuvants (32). However, the means by which these adjuvants stimulate innate immunity are unclear (13, 32). Here, we demonstrate that particulate vaccine adjuvants promote activation of the NALP3 inflammasome and secretion of IL-1β, IL-18, and IL-1α. The ability of alum to enhance IL-1β secretion (33, 34) via NALP3 (11, 35) has been described, but our data indicate that this is not specific to alum but is a general property of all particulate adjuvants. Indeed, similar responses were seen in DCs with chitosan, aluminium phosphate, and calcium phosphate (data not shown). Our finding that PS microparticles promote NALP3 inflammasome activation in DCs is in contrast to a recent report on human macrophages (25). The latter study used the beads at a concentration of 0.5 mg/mL, but we consistently found PS particles (from a number of commercial sources) to be a potent stimulus for IL-1β secretion at concentrations greater than 0.6 mg/mL, and particularly at 3 mg/mL.
Recent studies indicate that induction of antigen-specific antibody responses by particulate vaccine adjuvants, including alum and Freund's complete and incomplete adjuvants, did not require signaling through TLRs (10, 36). However, induction of antigen-specific Th1 responses (by Freund's complete adjuvant) but not Th2 responses (by alum) was compromised in MyD88-deficient mice (9). Adjuvants such as Freund's complete adjuvant contain TLR agonists and may be reliant on TLR-mediated signaling for induction of Th1 responses, whereas particulate adjuvants that are free of pathogen-derived TLR/NLR ligands may not require these pathways. This may explain why such adjuvants are less effective in promoting Th1-type responses. However, the induction of cellular immunity by alum did require MyD88 and uric acid (12), and the acute inflammatory response to silica and alum required IL-1R1 (30), so MyD88 does play a role in the innate response to alum. It was shown recently that enhancement of humoral and cellular (11) adaptive immunity by alum was NALP3-dependent, although later reports suggested that NALP3 was dispensable for alum-driven antibody responses (35, 37). Our data demonstrate that the ability of PLG microparticles to potentiate antigen-specific antibody responses was intact in NALP3-deficient mice, but cellular immunity was compromised. Specifically, we found that injection of PLG microparticles promoted recruitment and activation of a population of CD11b+Gr1− cells and enhanced antigen-specific IL-6 production. Both of these effects were reduced in NALP3−/− mice.
Activation of the NALP3 inflammasome by microparticles is dependent on particle uptake, lysosomal acidification, and cathepsin B activity. These data support recent findings that silica crystals, alum (30), and amyloid fibrils (38) activate the NALP3 inflammasome by a process requiring lysosomal acidification and rupture and cathepsin B activity. In addition, we found that elevation of the extracellular K+ concentration abrogated the ability of particulate vaccine adjuvants to enhance IL-1β secretion. Low intracellular potassium concentration promotes activation of the NALP3 inflammasome (28), whereas physiological concentrations of K+ block recruitment of caspase 1 to ASC, possibly because of NALP3 oligomerization (28).
Microparticulate adjuvants also strongly enhanced IL-1α secretion by DCs, and this was dependent on caspase 1. This supports a recent report on regulation of an unconventional secretion pathway for proteins, including IL-1α by active caspase 1 (39). Additionally, although IL-1α secretion was reduced in NALP3-deficient cells, there was still a significant particle-mediated enhancement in IL-1α secretion and, in contrast to IL-1β, neither lysosomal acidification nor cathepsin B activity was required.
Our data demonstrate that particulate adjuvants are potent activators of the NALP3 inflammasome in the presence of a TLR agonist. However, when injected as vaccine adjuvants, no TLR adjuvant is generally included. The second stimulus required to promote inflammasome activation in vivo may derive from an endogenous source. It has been proposed that induction of tissue damage is an effective means to activate immune responses (40), and dying cells may release endogenous danger signals that can amplify and sustain T cell-dependent immune responses. Indeed, it was proposed that the efficacy of alum in promoting activation of DCs and adaptive immunity required the release of uric acid (12). However, we did not find a role for uric acid in the ability of microparticles to promote IL-1β secretion in vitro (Fig. S4). IL-1β and IL-1 α may also act as endogenous danger signals (41). Injection of either PLG or PS microparticles or alum led to increased concentrations of IL-1β at the injection site, suggesting that endogenous factors can synergize with the adjuvants to drive IL-1β production. We found that the endogenous danger signal HMGB1 (31), which is released from necrotic cells, can synergize with microparticles to promote IL-1β secretion. Although this does not prove that HMGB1 is required for the immunomodulatory effects of PLG microparticles in vivo, it does show that endogenous factors can replace pathogen-derived stimuli as a second stimulus, in conjunction with microparticles, to promote IL-1β production by DCs.
Our finding that NALP3 is not required for PLG microparticles to promote antigen-specific antibody responses is in line with recent reports on alum-driven antibody responses (37, 35), but is in contrast to other studies (11, 42). However, our data indicate that the recruitment and activation of innate immune cells and the induction of antigen-specific IL-6 production by microparticles require NALP3. This supports a recent report that the influx of inflammatory cells and activation of adaptive cellular immunity by alum are dependent on NALP3 (37). In conclusion, we show that polymeric microparticle adjuvants activate the NALP3 inflammasome, and this contributes to activation of innate and antigen-specific cellular immunity.
Materials and Methods
Materials.
PLG(RG503) with a copolymer ratio of 50:50 was from Boehringer Ingelheim; polyvinyl alcohol (Mr 15,000) was from MP Biomedicals; and dioctyl sodium sulfosuccinate sucrose, mannitol, and all other chemicals were from Sigma. Acetone was from EMD Chemicals. PLG nanoparticles and microparticles were prepared by the solvent displacement method (43). PS nanospheres (430 nm) were from Corpuscular, and alhydrogel [2.0% Al(OH)3]; alum was from Brenntag Biosector. TLR agonists were obtained from Invitrogen, YVAD-fmk was from Backem AG, and bafilomycin A and CA-074 were from Sigma.
Cell Culture.
Female C57BL/6 mice were obtained from Harlan Olac and were used at 8–16 weeks of age. NALP3−/− mice were bred in the Trinity College Dublin Bioresources Unit. Animals were maintained according to the regulations of the European Union and the Irish Department of Health. Bone marrow-derived DCs were generated as described previously (44). Cells were plated out on day 10 and stimulated with particulates and TLR agonists on day 11. All in vitro experiments were carried out a minimum of 3 times.
Determination of IL-1 and Caspase 1.
DCs (5 × 105/mL) were cultured at 37 °C for 30 min to 24 h with particulates alone, TLR agonists (LPS, CpG-ODN, poly I:C, PAM3Csk) alone, particulates and TLR agonists together, or medium alone. In certain experiments, DCs were incubated with YVAD-fmk, bafilomycin A, cytochalasin B or D, CA-074, or KCl (50 mM) before addition of particulates and TLR agonists. Cytokine concentrations in supernatants were determined by immunoassay (IL-1β detection limit 8 pg/mL; R&D Systems). Supernatants mixed with and cells lysed in Laemmli sample buffer were boiled and separated by SDS/PAGE. Pro-IL-1β and mature IL-1β were determined in cell lysates and supernatants by Western blotting using a sheep anti-IL-1β antibody (National Institute for Biological Standards and Control) at a dilution of 1:200, followed by an anti-sheep HRP conjugate (Sigma) at 1:1,500. Caspase 1 was detected by using a rabbit anti-caspase 1 p10 antibody (Santa Cruz Biotechnology) diluted 1:400, followed by anti-rabbit HRP (Sigma) at 1:1,000. Blots were developed by using enhanced chemiluminescence.
Innate and Adaptive Immunity to Injected PLG Microparticles.
Female C57BL/6 mice were immunized subcutaneously in both flanks with PBS, KLH (5 μg; endotoxin content 5 pg/mg; Calbiochem) alone, or KLH adjuvanted with 2.5 mg of alum or PS or PLG microparticles. After 7 days, injection sites were excised and homogenized in 500 mM NaCl/50 mM Hepes, pH 7.4, containing 0.1% Triton X-100, protease inhibition mixture (Sigma), and 0.02% NaN3. Homogenates were sonicated and centrifuged at 6,000 × g for 20 min at 4 °C. IL-1β was measured in the supernatants by ELISA. Protein concentrations were determined by BCA assay (Thermo Scientific). For intraperitoneal immunization, mice were injected on days 0 and 10 with 50 μg of OVA (grade V; LPS purified; Sigma) alone or mixed with 2.5 mg of PLG microparticles. Antigen-specific serum IgG1 and IgG2b titers were determined by ELISA (BD PharMingen). Spleen cells were cultured with OVA or medium only for 3 days, and cytokines were determined by using ELISA kits (R&D Systems) or antibody pairs (BD Biosciences). Additionally, mice were injected intraperitoneally with PBS only or with 2.5 mg of PLG microparticles, and blood (following lysis of erythrocytes with ammonium chloride) and peritoneal lavage cells, collected after 20 h, were analyzed by flow cytometry.
Confocal Microscopy.
DCs (5 × 105/mL) were cultured on glass coverslips at 37 °C for 18 h with PS microparticles (0.6 mg/mL; Corpuscular). Cells were fixed with 2% paraformaldehyde for 20 min and stained with Alexa-Fluor 594-conjugated wheat germ agglutinin (WGA; 10 μg/mL) for 30 min. Cells were mounted onto glass slides with Dakomation mounting medium (Dako) and analyzed on a Zeiss LSM 510 microscope with LSM 5 software (version 4) and Adobe Photoshop.
Flow Cytometry.
Cells were incubated for 15 min with acridine orange (1 μg/mL), washed, and stimulated with microparticles alone or with LPS. Lysosomal rupture was determined as a loss of emission at 600–650 nm (30). Peritoneal lavage and blood cells were stained with antibodies to CD11b, Gr1, and CD86 to determine cell recruitment and activation. A CyAN (Dako) flow cytometer was used, and data were analyzed by FlowJo software (Tree Star).
Statistics.
Cytokine concentrations were compared by 1-way ANOVA. Where significant differences were found, the Tukey–Kramer multiple-comparison test was used to identify differences between individual groups.
Supplementary Material
Acknowledgments.
We thank M. Coleman, C. Sutton, C. Brereton, and S. Miggin for helpful discussions and advice on Western blotting; O. Hanrahan and D. Nolan for help with confocal microscopy; and B. Moran for assistance with flow cytometry. We thank Prof. S. Martin and I. Afonina for providing HMGB1. The project was supported by the Irish Research Council for Science, Engineering and Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804897106/DCSupplemental.
References
- 1.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6:685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 2.Romero Mendez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–833. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Ramon G. Sur l'augmentation anormale de l'antitoxine chez les chevaux producteurs de serum antidiphterique. Bull Soc Centr Med Vet. 1925;101:227–234. [Google Scholar]
- 4.Glenny A, Pope C, Waddington H, Falacce U. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:31–40. [Google Scholar]
- 5.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 6.O'Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM. Dendritic cells: Versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 9.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 10.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25:1361–1366. doi: 10.1038/nbt1207-1361. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 17.Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Agostini L, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 20.Kummer JA, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 21.Yamin TT, Ayala JM, Miller DK. Activation of the native 45-kDa precursor form of interleukin-1-converting enzyme. J Biol Chem. 1996;271:13273–13282. doi: 10.1074/jbc.271.22.13273. [DOI] [PubMed] [Google Scholar]
- 22.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 23.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 25.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 27.De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: Unraveling a century old mystery. Eur J Immunol. 2008;38:2068–2071. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 28.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 30.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 32.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27:687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–4585. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 35.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441:E4. doi: 10.1038/nature04875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kool M, et al. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 38.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 41.Dinarello CA. Mutations in cryopyrin: Bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum. 2007;56:2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh M, et al. Anionic microparticles are a potent delivery system for recombinant antigens from Neisseria meningitidis serotype B. J Pharm Sci. 2004;93:273–282. doi: 10.1002/jps.10538. [DOI] [PubMed] [Google Scholar]
- 44.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.