Abstract
Histone H2A.X is an H2A variant present in multicellular organisms that is specifically phosphorylated on the serine in the C-terminal consensus sequence, canonically “SQEY,” in response to DNA damage. We have recently shown the significance of phosphorylation of the penultimate tyrosine for maintenance and processing of the DNA damage response in mammalian cells. Here, we report the identification of distinct H2A.X variants in the eggs and early embryos of the frog Xenopus laevis that contain a C-terminal SQEF, among other changes; we have denoted these proteins as “H2A.X-F.” H2A.X-F is present only in late-staged oocytes, eggs, and premidblastula transition embryos and is not present in somatic cells. Similar unannotated isoforms were identified in other rapidly developing aquatic species, such as Xenopus tropicalis, goldfish, and zebrafish, and in Arabidopsis and chickpea. Furthermore, we demonstrate by mass spectrometry and phospho-specific antibodies that H2A.X-F is phosphorylated in the absence of exogenous DNA damage, in both actively dividing, unperturbed embryos and cell-free egg extract in the absence and presence of DNA damage and S-phase checkpoint conditions. We propose that this isoform may be involved in modulating the cellular response to the rapid early cell cycles in externally developing species.
Keywords: chromatin, histone, variant, DNA damage
In eukaryotes, DNA is dynamically packaged around histone proteins into chromatin, ultimately the physiological genetic material. There are 4 core histone proteins (H2A, H2B, H3, and H4) around which the DNA is wrapped into a nucleosome containing 2 copies of each histone. Additionally, there are numerous variants of the canonical histones present in a variety of contexts (1–4). The histone proteins have been postulated to encode epigenetic information (5, 6). This histone code is used to modulate transcriptional activity, cell-cycle transitions, and DNA damage-dependent activities.
One of the most extensively studied histone variants is the protein H2A.X, which replaces H2A in some nucleosomes in metazoans (1–10% of nucleosomes, depending on the cell type) (2). Intriguingly, the C-terminal motifs in H2A.X appear to have arisen multiple times via convergent evolution (3). H2A.X contains a hallmark C-terminal domain with one or more SQ motifs that are phosphorylation targets for the ATM and ATR checkpoint kinases (7, 8). Phosphorylation of H2A.X has been well-established as an early mark of DNA double-strand breaks (DSBs). This phosphorylation is likely to be involved with chromatin remodeling and repair around the damage site (9–13). H2A.X-deficient mice cells have high levels of genomic instability and are defective in class-switch recombination and spermatogenesis (14), consistent with a role for the protein in DNA DSB repair. The equivalent phosphorylation site in yeast H2A (Ser-129) has been precisely mapped to DSBs and large bordering regions (15).
Furthermore, we recently demonstrated that the phosphorylation of the extreme C-terminal tyrosine (…SQEY) by the WSTF protein is crucial for the maintenance of damage foci during the repair process (16). Other studies have shown a crucial role for that tyrosine residue in binding by the known adapter protein Mdc1 to phosphorylated S139 (17, 18), suggesting that the entire C terminus of H2A.X is important for proper responses to DNA damage.
Xenopus laevis egg extract is useful for study of the cell cycle, DNA replication, and early development (19). The eggs contain a large store of histone proteins (20, 21), in preparation for the rapid early cell cycles of development. Older studies noted the presence of an abundance of the H2A.X variant (50% or more of the H2A) in the early embryos of the frog (21–25). The significance of this observation is unknown.
We have also recently described isolation procedures and characterization of histone variants and histone modifications across many embryonic and somatic cell types in Xenopus (26). In that study we observed a highly abundant H2A isoform in the oocytes, eggs, and early embryos of the frog. In database searching, we discovered that Xenopus contains a distinct H2A.X variant with a C-terminal phenylalanine in place of the canonical tyrosine.
In this article we present the characterization of the H2A.X-F isoforms in X. laevis. We find that its abundance is maximal at egg laying, it is incorporated into early embryo chromatin, and it is no longer present at the tadpole stage. Intriguingly, we observed in parallel that undifferentiated mouse ES cells contain a higher level of H2A.X than do differentiated cells, although we note that mice only contain the canonical SQEY isoform of H2A.X. Using specific antibodies that we developed, we find that H2A.X-F is phosphorylated in the absence of exogenous DNA damage and that the phosphorylation occurs during normal early development.
Results
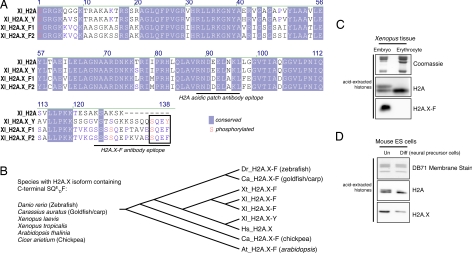
We discovered a number of Xenopus H2A.X genes upon database searching, including a canonical H2A.X, encoding 1 protein with the C-terminal SQEY motif, and 2 distinct genes encoding proteins with an SQEF motif (Fig. 1A). After performing BLAST searches and using a short-protein motif search tool (A. Basu, and C. D. A, unpublished work), we identified a number of other H2A.X isoforms containing a C-terminal SQxF (Fig. S1). These were only present in rapidly developing aquatic species and in a few plant species (Fig. 1B). Mammals only contained canonical SQEY H2A.X proteins. Multiple-sequence alignments suggest that these “F” isoforms evolved by convergence, implying an important role for this amino acid change. We have recently reported a significant role for the phosphorylation of the C-terminal tyrosine in mammalian H2A.X-mediated DNA damage response (16); phenylalanine is hydrophobic but not a phospho-acceptor, suggesting a specific evolutionary loss of a functional side group. We developed antibodies against a peptide from the extreme C terminus of H2A.X-F1 and a doubly serine phosphorylated peptide (Fig. S2).
Fig. 1.
Protein sequence of H2A.X isoforms and H2A.X enrichment in embryonic cells. (A) Multiple sequence alignment of Xenopus H2A, H2A.X-Y, and H2A.X-F1 and F2 proteins as determined by ClustalW. Conserved amino acids are shaded in blue, and amino acids known to be phosphorylated are written in red. The epitopes of the H2A “acidic patch” and the H2A.X-F antibodies are underlined. (B) Species containing an H2A.X with a C-terminal SQxF motif are listed, as is a phylogenetic tree (determined by sequence parsimony). (C) Coomassie-stained and H2A- and H2A.X-F-specific immunoblots of histones from Xenopus early-embryo equivalent pronuclei and erythrocytes. (D) H2A- and H2A.X-specific immunoblots of isolated histones from undifferentiated and differentiated mouse ES cells.
Earlier reports had demonstrated a large abundance of histone H2A.X in early embryos of Xenopus (21). We isolated histones from early-embryo equivalent chromatin and erythrocytes and blotted them for H2A and the H2A.X-F isoform. Because H2A.X is 9 aa longer than canonical H2A, the general H2A antibody can distinguish between both proteins on a high-percentage gel. At least 50% of the H2A in the early embryo is composed of the slower-migrating H2A.X (Fig. 1C). A blot of the same samples with the H2A.X-F-specific antibody demonstrated that the F isoform is limited to the early embryo and is not present in somatic erythrocytes, nor is it present in any other somatic fractions examined (Fig. S3). Furthermore, isolation of the stored egg histones (26, 27) and subsequent reversed-phase fractionation and immunoblotting revealed approximately equivalent quantities of H2A, H2A.X-F1, and H2A.X-F2 (Fig. S4). MS analysis of these fractions identified the proteins as unannotated gene products (Fig. S1). Thorough MS analysis of these fractions and gel slices did not reveal any unique peptide sequence assignable to the H2A.X-Y isoform.
To determine whether the elevated abundance of H2A.X was a general phenomenon, we isolated histones from undifferentiated and differentiated mouse ES cells. As shown in Fig. 1D, ES cells contained a significantly higher level of H2A.X than did the differentiated neural precursor cells. Mice only contain the canonical SQEY isoform of H2A.X, so the embryonic H2A.X abundance may be unrelated to the identity of the penultimate amino acid.
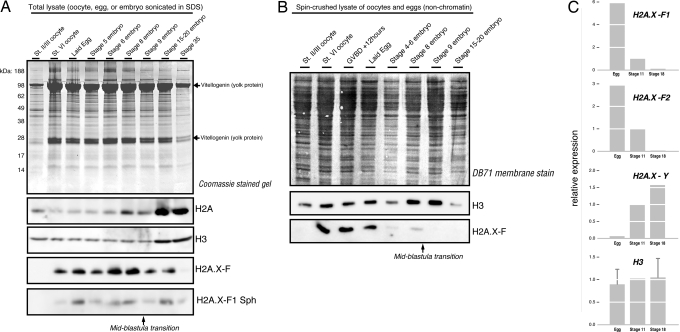
To further explore the developmental timing of H2A.X-F expression we collected staged oocytes, eggs, and staged embryos through the early tadpole stage. We then lysed the frozen embryos directly into SDS loading buffer, sonicated them, and immunoblotted for H2A, H2A.X-F, and phosphorylated H2A.X-F (Fig. 2A). The midblastula transition (MBT), at which time zygotic transcription starts and the maternally deposited embryonic program ends, occurs approximately at stage 8 or 9. After stage 9 we observed a marked increase in total H2A and H3, yet the amount of H2A.X-F substantially decreased. We also observed H2A.X-F phosphorylation during normal, unperturbed development (Fig. 2A). These embryos were flash-frozen and thawed into SDS buffer, so the observed phosphorylation is unlikely to be caused by damage during handling.
Fig. 2.
H2A.X-F expression is limited to early development. (A) Total protein from staged oocytes and embryos were run on a NuPAGE Bis/Tris Mes gel and Coomassie-stained and immunoblotted for H2A, H2A.X-F, and phosphorylated H2A.X-F. (B) Soluble protein (nonchromatin) from staged oocytes, progesterone-treated oocytes (GVBD + 12 h), and staged embryos were immunoblotted for H3 and H2A.X-F. (C) Microarray analysis of gene expression from untreated eggs and stage 11 and 18 embryos. Egg and stage 18 expression were normalized to stage 11, arbitrarily set at 1.0.
We further examined the developmental timing of H2A.X-F by spin-crushing staged oocytes, progesterone-treated oocytes [germinal vesicle breakdown (GVBD)], eggs, and embryos and immunoblotting the soluble material. This approach resolves only the unincorporated histones, because chromatin is pelleted during the centrifugation step. As seen in Fig. 2B, soluble H2A.X-F disappeared rapidly as fertilization/proliferation commenced, consistent with a rapid depletion of the soluble pool as it was deposited into the chromatin. At stages 15–20, at which point we observed the increased total histone content seen in Fig. 2A, we saw a reduced amount of H3, consistent with a shrinking soluble pool of histones post-MBT.
As shown in Fig. 2C, we extracted expression data from a deposited Xenopus developmentally staged microarray analysis (GEO GSE4448) (28). Expression data from the genes encoding H2A.X-F1, H2A.X-F2 (2 copies), H2A.X-Y, and H3 (6 copies) genes from the egg, stage 11, and stage 18 embryos were normalized and then relative values were plotted in a histogram with the stage 11 dataset to 1.0. We observed a substantially enriched relative expression of the H2A.X-F genes in the egg compared with the later stages. The H2A.X-Y gene was barely expressed in the egg whereas it was enriched in the later stages. The average of the H3 genes was approximately consistent across the 3 stages.
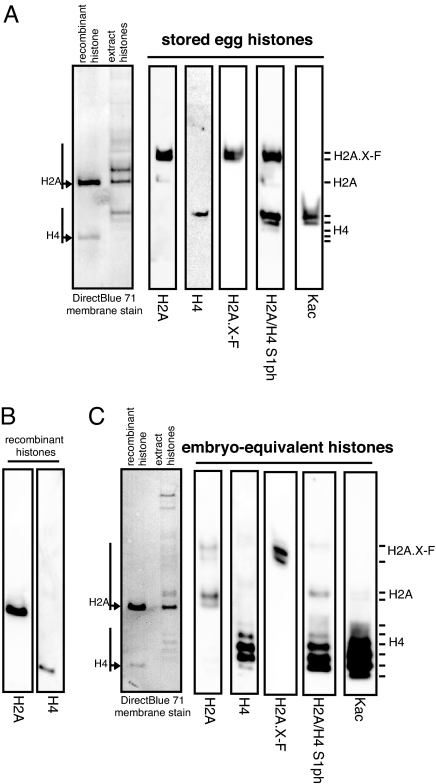
H2A and H4 both are phosphorylated on S1 in a cell-cycle-dependent fashion, primarily during S-phase and M-phase (29), in addition to the H2A.X C-terminal SQ phosphorylation. Therefore, we ran egg and pronuclei (early-embryo equivalent) histones on Triton-acid urea (TAU) gels and immunoblotted them for H2A, H4, H2A.X-F, H2A/H4 S1ph, and lysine acetylation (Fig. 3 A and C, with the position of recombinant H2A and H4 blotted in Fig. 3B). TAU gels separate histones variants and differentially charged histone isoforms. We observed the known shift in H4 acetylation (from fully acetylated, running more slowly in the egg, to less acetylated, running faster in the early-embryo equivalent). Canonical H2A shifted from 1 isoform on the gel in the egg sample to 2 isoforms, consistent with the known acquisition of S1 phosphorylation upon deposition (29). However, using the phospho-S1 specific antibody, we observed H2A.X-F to be preferentially prephosphorylated on S1 in the egg, whereas H2A and H4 become more phosphorylated upon deposition in the early embryo pronuclei. H2A.X did not have a concomitant enrichment of S1 phosphorylation (Fig. 3 A and C and Fig. S3).
Fig. 3.
TAU gel analysis of egg and early-embryo equivalent histones. (A) Isolated egg histones were run on a TAU gel and immunoblotted for H2A, H4, H2A.X-F, and H2A/H4 phosphorylated on S1 and acetyl-lysine. The membrane was stained to show total histone protein, and recombinant H2A and H4 were run in parallel as markers. (B) Immunoblotted recombinant histones H2A and H4 run on a TAU gel. (C) Isolated early-embryo equivalent histones were run on a TAU gel and immunoblotted as above.
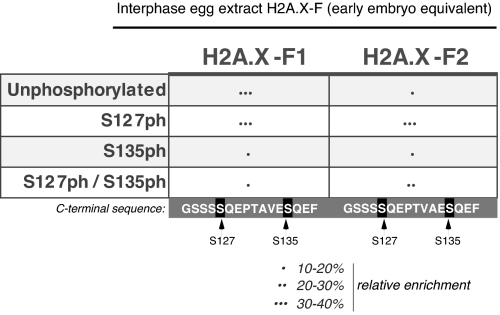
We subjected the isolated H2A.X-F1 and F2 proteins from actively replicating, unperturbed, early-embryo pronuclei to MS analysis to probe posttranslational modifications on the proteins. MS/MS of the C terminus of H2A.X-F1 and F2 revealed extensive phosphorylation on serines S127 and S135, both contained in consensus checkpoint kinase target sequences (SQ) (Fig. 4). Intriguingly, the extent of phosphorylation on both isoforms was different, suggesting perhaps different roles for these 2 proteins. In a noncheckpoint situation, with actively replication chromatin, the majority of H2A.X-F was phosphorylated on the serines.
Fig. 4.
MS/MS analysis of early-embryo H2A.X-F1 and F2 C-terminal tails. Histones H2A.X-F1 and H2A.X-F2 isolated from early-embryo equivalent pronuclei were subjected to MS analysis. Phosphorylated forms of C-terminal tails of H2AX-F1 and H2AX-F2 were observed in the MS analysis of tryptic digests. The relative abundances were calculated as the percentage of the area under the single-ion chromatogram of each form the peptide (all charge states observed) to the total area under the curve of all forms of the peptide.
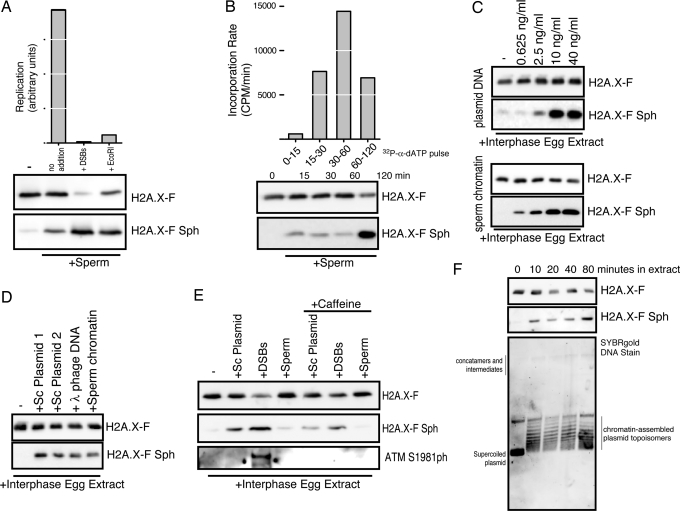
To further characterize the phosphorylation of H2A.X-F during normal cell cycles, we immunoblotted extracts in various contexts. First, we used freshly prepared egg extract (nonfrozen) and added sperm chromatin in the absence or presence of either HaeIII-digested plasmid DNA (DSBs) or EcoRI restriction enzyme (to digest the sperm template). In Fig. 5A, we observe normal levels of DNA replication in the control sample, but completely blocked DNA replication in the presence of DSBs and EcoRI, consistent with the established checkpoint mechanisms (30). When we blotted these extracts for H2A.X-F phosphorylation, we observed extensive phosphorylation in the presence of DSBs and EcoRI phosphorylation in the absence of any damaging agent or checkpoint activating signal in trans. Note that the H2A.X-F general antibody is sensitive to phosphorylation (Fig. S2), so in the presence of DSBs the high level of SQ phosphorylation causes a concomitant reduction in general H2A.X-F antibody signal.
Fig. 5.
H2A.X-F phosphorylation occurs in nondamaged and checkpoint contexts in Xenopus egg extracts. (A) DNA replication of sperm chromatin at 4,000/μL in interphase egg extract with and without DNA damage in trans (DSBs) and damage in cis (EcoRI restriction enzyme) was monitored after 60 min and plotted in arbitrary units. Total extract (0.25 μL) from each condition, incubated in parallel, was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (B) DNA replication of sperm chromatin at 4,000/μL in interphase egg extract was pulse-labeled with 32P-α-dATP for the time periods indicated (0–15, 15–30, 30–60, and 60–120 min). The quantitated incorporation divided by the time produced the plotted rate of incorporation. Total extract (0.25 μL) from each time point, incubated in parallel, was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (C) (Upper) Supercoiled plasmid DNA (≈5 kb pC1-EYFP) was titrated into clarified egg extract and incubated for 60 min. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (Lower) Sperm chromatin was titrated into clarified egg extract and incubated for 60 min. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (D) Supercoiled plasmids pC1-EYFP and pG5ML, lambda phage, sperm chromatin, and DSBs at 40 ng/μL final concentration were incubated in clarified egg extract. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (E) Supercoiled plasmid DNA, HaeIII-digested plasmid (DSBs), and sperm chromatin at 40 ng/μL final concentration were incubated in clarified egg extract. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F, phosphorylated H2A.X-F, and ATM phosphorylated on Ser-1981. (F) Supercoiled plasmid was incubated in clarified egg extract at 40 ng/μL and time points were removed. Isolated DNA was run on an agarose gel at 1.5 V/cm and stained with SYBRgold. Total protein from the same samples was immunoblotted for H2A.X-F and H2A.X-F Sph.
We pulse-labeled the replicating chromatin during a few time periods and immunoblotted a parallel incubation at the end of each time point (Fig. 5B). We observed that the rate of replication was the highest during the 30- to 60-min time window; however, extensive H2A.X-F phosphorylation did not occur until the second hour of DNA replication.
This H2A.X-F phosphorylation was not entirely DNA-replication dependent. Titration of supercoiled plasmid DNA and sperm chromatin into ultracentrifuge-clarified interphase egg extract (competent for chromatin assembly but not DNA replication) resulted in a concentration-dependent phosphorylation of H2A.X (Fig. 5C). Intriguingly, addition of plasmid and sperm DNA to clarified egg extract, while promoting H2A.X-F phosphorylation, did not result in activation of ATM as measured by its autophosphorylation on S1981 (Fig. 5E). In contrast, addition of DSBs did result in more potent H2A.X-F phosphorylation and ATM S1981 phosphorylation (Fig. 5E Bottom). Caffeine partially rescued the H2A.X-F phosphorylation and abrogated the ATM S1981 phosphorylation, consistent with our observations that the H2A.X-F phosphorylation is not entirely concomitant with checkpoint activation (Fig. 5A).
To further control for modest amounts of DNA damage in any of the DNA sources used, we added DNA from a second supercoiled Qiagen-purified plasmid and lambda DNA to egg extract. As seen in Fig. 5D, all of the DNAs induced similar levels of H2A.X-F phosphorylation. Additionally, we did not observe any linearization or other damage of recovered DNA incubated in egg extract over time that did exhibit H2A.X-F phosphorylation (Fig. 5F).
Discussion
We have identified a distinct isoform of histone H2A.X, containing a C-terminal SQEF motif, present in the early embryos of the frog X. laevis and similar isoforms present in Xenopus tropicalis, Danio rerio (zebrafish), and Carassius auratus (goldfish). Additionally, we identified SQD/EF isoforms in a number of plant species, including Arabidopsis thalina and Cicer arietium. Intriguingly, we could not identify a C-terminal tyrosine-containing isoform in either plant species. We also observed that none of the H2A.X-F proteins contain the GKK motif that is absolutely conserved in canonical H2A.X, slightly N-terminal to the SQEY motif (31) (Fig. S1). Because histone lysines are frequently modified, we expect that future studies may observe acetylation, methylation, or other additions to that GKK sequence.
The fact that H2A.X-F is present only during a brief period of early development is intriguing, suggesting that it may play a significant biological role. An earlier study had shown that during initial pronuclei chromatin assembly, newly synthesized H2A and H2B, from maternal mRNA stores, were deposited but no newly synthesized H2A.X was deposited; only stored H2A.X protein was assembled into chromatin (23). Furthermore, H2A.X-F composes at least half of the H2A in the egg and early embryo. Surprisingly, we observed that H2A.X is highly enriched in undifferentiated mouse ES cells and that enrichment is lost upon differentiation. NT2 cells, cancer cells that in many ways mimic ES characteristics, did not exhibit a similar elevation of H2A.X protein, suggesting that H2A.X abundance may be an important indicator of true stem-cell determination (Fig. S5).
It has been long established that the mitotic checkpoint is not active in early embryos of Xenopus (32); in fact, irradiated embryos will keep dividing until the MBT, at which point they will synchronously undergo apoptosis (33). In contrast, the intra-S-phase checkpoint is detectable in interphase-trapped extracts by assaying DNA replication (30). Phosphorylation of mammalian H2A.X C-terminal peptides (with an SQEY motif) in egg extract has been used to measure checkpoint kinase response in different contexts (34).
Phosphorylation of the N-terminal serine of H2A and H4 (note that both H2A and H4 start with the same SGRGK motif) has been linked to ongoing DNA replication and progression through mitosis (29). In yeast, H4 S1 phosphorylation has been shown to occur after DNA damage (35), although this has not been demonstrated to occur in metazoans. We observed a differential phosphorylation of S1 on H2A.X-F compared with canonical H2A; H2A.X-F S1 was phosphorylated during predeposition storage in the egg, whereas H2A, along with H4, became more phosphorylated after deposition (see Fig. 3; note that the egg histones are predeposition and the early-embryo equivalent histones are postdeposition). Intriguingly, H2A.X was shown to be phosphorylated during nucleosome assembly in oocytes and important for proper nucleosome spacing (21). Another study demonstrated that the phosphorylation of H2A.X (probably both on S1 and the C terminus) increased relative to the phosphorylation of H2A (only on S1) as the sperm concentration was increased (see figure 8 in ref. 23); this observation is strikingly similar to the data we present here.
Phosphorylation of the C terminus of H2A.X has been shown to be important for recruitment of other checkpoint proteins and repair factors and for maximum efficiency of the NHEJ and HR repair pathways. Binding studies and crystal structures have demonstrated the extreme importance of the C-terminal tyrosine hydroxyl group in recruitment of the Mdc1 protein (18, 36), the primary recognition adapter of the γ-H2A.X damage signal on chromatin. Therefore, the specific acquisition of an H2A.X isoform in the early embryo that is significantly less capable of recruiting Mdc1 [Stucki et al. (18) report at least a 100 times worse binding constant for Mdc1 to peptides without a C-terminal tyrosine] suggests that the H2A.X-F protein may contribute to the suppression of the checkpoint during the early cell cycles. It is reasonable, therefore, to consider developmentally alternative platforms for assembling chromatin-acting machinery for differential responses in different contexts; the embryonic H2A.X-F isoform could potentially alter the cellular response to chromatin structures encountered during normal cell cycles.
It is formally possible that the phosphorylation of H2A.X-F in cell-free extracts is in part caused by DNA damage accrued during chromatin assembly, during replication, and/or manipulations during preparation. However, fresh extract did exhibit H2A.X-F phosphorylation in clearly noncheckpoint conditions (Fig. 5A) and no obvious DNA damage occurred to plasmid recovered from extract (Fig. 5F). Furthermore, fertilized eggs and developing embryos exhibited H2A.X-F phosphorylation (Fig. 2A) when we used the highly phospho-specific affinity-purified antibody. This observation is consistent with a normal, nonartifactual H2A.X-F phosphorylation during rapid cell cycles.
We hypothesize that during very rapid early-embryo cell cycles transient chromatin conformations occur that result in signaling, perhaps through the checkpoint kinase pathway (37), that do not actually arrest S-phase. This differential activity might therefore occur explicitly because the phospho-SQEF motif does not recruit Mdc1 and perhaps does not nucleate other checkpoint factors, such as the MRN complex. Intriguingly, interactions between Mdc1 and components of the MRN complex have been reported (17, 38, 39), and indeed Nbs1 binding to Mdc1 has recently been shown to be required to maintain MRN at damage sites (40). Because ATM activation in Xenopus egg extracts in response to DSBs has been demonstrated to occur in a 2-step process of recruitment and an MRN-dependent activation (41), complete activation resulting in a checkpoint-blocking replication may occur only in the presence of bona fide damage. Furthermore, we have demonstrated an important role for ATR in regulating ongoing DNA replication (37), and ATR is known to respond to DNA replication intermediates (42), so ATM and ATR may have distinct roles in phosphorylation of H2A.X-F. Further studies are required to distinguish between damage and nondamage responses in egg extracts.
Materials and Methods
Detailed methods and materials are available in SI Text. Briefly, Xenopus interphase egg extract was prepared and used for DNA replication studies as described (37). Pronuclei and histones were isolated from extract, and immunoblotting and MS were performed as described (26, 27).
Supplementary Material
Acknowledgments.
We thank H. Funabiki (The Rockefeller University) for use of the frog colony; N. Levenkova for analysis of the microarray data; A. Goldberg (The Rockefeller University) for samples of mouse ES cells; P. Lewis (The Rockefeller University) for NT2 cells; T. Swigut for initial assistance with embryology; J. A. Kim for comments on the manuscript; and A. Dupre and J. Gautier for initial assistance with oocyte isolation, staging, and extract preparation. D.S. is supported by a Kirschstein fellowship. D.F.H. is supported by National Institutes of Health Grant GM37537, and C.D.A. is supported by National Institutes of Health Grant GM40922.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812207106/DCSupplemental.
References
- 1.Henikoff S, Ahmad K. Assembly of variant histones into chromatin. Annu Rev Cell Dev Biol. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: The histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Biol. 2003;10:882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein E, Hake SB. The nucleosome: A little variation goes a long way. Biochem Cell Biol. 2006;84:505–517. doi: 10.1139/o06-085. [DOI] [PubMed] [Google Scholar]
- 5.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 6.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 7.Lowndes N, Toh G. DNA repair: The importance of phosphorylating histone H2AX. Curr Biol. 2005;15:R99–R102. doi: 10.1016/j.cub.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Thiriet C, Hayes J. Chromatin in need of a fix: Phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–622. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Fillingham J, Keogh M, Krogan N. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84:568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- 10.Franco S, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Furuta T, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 12.Kruhlak M, et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol. 2006;172:823–834. doi: 10.1083/jcb.200510015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riches L, Lynch A, Gooderham N. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis. 2008;23:331–339. doi: 10.1093/mutage/gen039. [DOI] [PubMed] [Google Scholar]
- 14.Celeste A, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukuda T, Fleming A, Nickoloff J, Osley M. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005;438:379–383. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao A, et al. WSTF regulates the H2A.X DNA damage response via a tyrosine kinase activity. Nature. 2008 doi: 10.1038/nature07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukas C, et al. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004;23:2674–2683. doi: 10.1038/sj.emboj.7600269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stucki M, et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Almouzni G, Wolffe A. Nuclear assembly, structure, and function: The use of Xenopus in vitro systems. Exp Cell Res. 1993;205:1–15. doi: 10.1006/excr.1993.1051. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschmidt JA, Fortkamp E, Krohne G, Zentgraf H, Franke WW. Coexistence of two different types of soluble histone complexes in nuclei of Xenopus laevis oocytes. J Biol Chem. 1985;260:1166–1176. [PubMed] [Google Scholar]
- 21.Kleinschmidt JA, Steinbeisser H. DNA-dependent phosphorylation of histone H2A.X during nucleosome assembly in Xenopus laevis oocytes: Involvement of protein phosphorylation in nucleosome spacing. EMBO J. 1991;10:3043–3050. doi: 10.1002/j.1460-2075.1991.tb07855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohsumi K, Katagiri C. Occurrence of H1 subtypes specific to pronuclei and cleavage-stage cell nuclei of anuran amphibians. Dev Biol. 1991;147:110–120. doi: 10.1016/s0012-1606(05)80011-9. [DOI] [PubMed] [Google Scholar]
- 23.Dimitrov S, Dasso M, Wolffe A. Remodeling sperm chromatin in Xenopus laevis egg extracts: The role of core histone phosphorylation and linker histone B4 in chromatin assembly. J Cell Biol. 1994;126:591–601. doi: 10.1083/jcb.126.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitrov S, Wolffe A. Fine resolution of histones by two-dimensional polyacrylamide gel electrophoresis: Developmental implications. Methods. 1997;12:57–61. doi: 10.1006/meth.1997.0447. [DOI] [PubMed] [Google Scholar]
- 25.Dilworth S, Black S, Laskey R. Two complexes that contain histones are required for nucleosome assembly in vitro: Role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987;51:1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
- 26.Shechter D, et al. Analysis of histones in Xenopus laevis. Part I: A distinct index of enriched variants and modifications exists in each cell type and is remodeled during developmental transitions. J Biol Chem. 2008 doi: 10.1074/jbc.M807273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicklay JJ, et al. Analysis of histones in Xenopus laevis. Part II: Mass spectrometry reveals an index of cell-type specific modifications on H3 and H4. J Biol Chem. 2008 doi: 10.1074/jbc.M807274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinner D, et al. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- 29.Barber CM, et al. The enhancement of histone H4 and H2A serine 1 phosphorylation during mitosis and S-phase is evolutionarily conserved. Chromosoma. 2004;112:360–371. doi: 10.1007/s00412-004-0281-9. [DOI] [PubMed] [Google Scholar]
- 30.Costanzo V, Gautier J. Xenopus cell-free extracts to study DNA damage checkpoints. Methods Mol Biol. 2004;241:255–267. doi: 10.1385/1-59259-646-0:255. [DOI] [PubMed] [Google Scholar]
- 31.Li A, Eirin-Lopez JM, Ausio J. H2AX: Tailoring histone H2A for chromatin-dependent genomic integrity. Biochem Cell Biol. 2005;83:505–515. doi: 10.1139/o05-114. [DOI] [PubMed] [Google Scholar]
- 32.Greenwood J, Costanzo V, Robertson K, Hensey C, Gautier J. Responses to DNA damage in Xenopus: Cell death or cell cycle arrest. Novartis Found Symp. 2001;237:221–230. doi: 10.1002/0470846666.ch17. discussion 230–224. [DOI] [PubMed] [Google Scholar]
- 33.Greenwood J, Gautier J. From oogenesis through gastrulation: Developmental regulation of apoptosis. Semin Cell Dev Biol. 2005;16:215–224. doi: 10.1016/j.semcdb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Costanzo V, Paull T, Gottesman M, Gautier J. Mre11 assembles linear DNA fragments into DNA damage signaling complexes. PLoS Biol. 2004;2:E110. doi: 10.1371/journal.pbio.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung WL, et al. Phosphorylation of histone H4 serine 1 during DNA damage requires casein kinase II in S. cerevisiae. Curr Biol. 2005;15:656–660. doi: 10.1016/j.cub.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Edwards R, Thede G, Glover J. Structure of the BRCT repeat domain of MDC1 and its specificity for the free COOH-terminal end of the gamma-H2AX histone tail. J Biol Chem. 2005;280:32053–32056. doi: 10.1074/jbc.C500273200. [DOI] [PubMed] [Google Scholar]
- 37.Shechter D, Costanzo V, Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- 38.Lee A, Fernandez-Capetillo O, Pisupati V, Jackson S, Nussenzweig A. Specific association of mouse MDC1/NFBD1 with NBS1 at sites of DNA damage. Cell Cycle. 2005;4:177–182. doi: 10.4161/cc.4.1.1354. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg M, et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003;421:952–956. doi: 10.1038/nature01445. [DOI] [PubMed] [Google Scholar]
- 40.Chapman J, Jackson S. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupré A, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11–Rad50–Nbs1 complex. Nat Chem Biol. 2008;4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shechter D, Costanzo V, Gautier J. Regulation of DNA replication by ATR: Signaling in response to DNA intermediates. DNA Repair (Amst) 2004;3:901–908. doi: 10.1016/j.dnarep.2004.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.