Abstract
IL-17-producing CD4+ T cells have been recognized as key players in organ-related autoimmune disease; however, the parameters that govern their development are yet to be elucidated fully. By using both in vivo and in vitro systems, we have investigated the role of antigen dose, pathogen-associated molecular patterns, and CD40–CD40 ligand (CD40L) cross-talk in Th17 differentiation. We found that the strength of antigenic stimulation critically influenced the extent of Th17 differentiation, because high, but not low or intermediate, antigen concentrations led to IL-17 production. Strong antigenic stimulation of T cells up-regulated CD40L expression, which in concert with certain microbial stimuli (i.e., cytosine phosphate guanine, curdlan, and zymosan) synergistically increased dendritic cell (DC) IL-6 production and Th17 polarization. CD40-deficient DCs exhibited reduced cytokine release and failed to drive Th17 development in vitro. These results were confirmed in vivo where the absence of CD40–CD40L cross-talk was found to prevent the expansion of IL-17-producing cells and accordingly the development of experimental autoimmune encephalitis. Our data demonstrate that CD40–CD40L cross-talk is important for Th17 development by translating strong T cell receptor and microbial stimuli into IL-6 production.
Keywords: IL-6, Th17 cells
IL-17-producing CD4+ T cells, referred to as Th17 cells, have been identified as key players in several mouse models of autoimmune diseases, including experimental autoimmune encephalitis (EAE) (1–3), collagen-induced arthritis (4), and experimental autoimmune myocarditis (5, 6). Moreover, IL-17 production has been associated with human autoimmune and inflammatory diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel diseases, and psoriasis (7–10). Several cytokines have been implicated in the differentiation of Th17 cells including TGF-β, IL-6, IL-1, and TNF-α (1, 11–15). IL-23, although not absolutely required for the differentiation of naïve CD4+ T cells to Th17, has been shown to be critical for the expansion and/or survival of human and mouse Th17 effectors (1–3, 15–17). The Th1 and Th2 prototypic cytokines appear to exert inhibitory effects upon Th17 differentiation, because enhanced Th17 development has been reported in IFN-γ- and IL-12-deficient mice or upon neutralization of IL-12, IFN-γ, IFN-α/β, or IL-4 (1, 12, 18, 19). Paradoxically, Th17 differentiation in vivo is paralleled by development of Th1 cells (1, 12, 20), and in models of EAE effector CD4+ T cells have been shown to produce both IFN-γ and IL-17 (21). Thus, it remains to be clarified exactly how Th1, Th2, and Th17 responses are related and counter regulated.
Antigen-presenting cells (APCs) have been proposed to be the main source of cytokines required for Th17 differentiation. Bone marrow-derived dendritic cells (BMDCs) in mouse and peripheral blood monocytes in humans have been shown to promote differentiation of naïve CD4+ T cells toward Th17 upon triggering of pattern-recognition receptors (PRRs) by the corresponding pathogen-associated molecular patterns (PAMPs). The nature of the stimuli required for Th17 development is still debated and might vary according to the type of APCs and consequently their particular PRR expression profile. Indeed, mouse BMDCs have been reported to induce Th17 polarization on stimulation by the Toll-like receptor (TLR)-4 agonist LPS (14) or by the dectin-1-agonist curdlan (22), whereas human monocytes can promote Th17 differentiation upon exposure to LPS or to the TLR2 agonist peptidoglycan (PG) (11, 16). In addition, Th17 development in vivo has been shown to occur after immunization with complete Freund's adjuvant (CFA) or zymosan, a fungal cell wall component, as adjuvants (20).
It is well recognized that the strength of signal provided by antigen dose and costimulatory molecule expression can regulate the differentiation of Th1 and Th2 cells. Specifically, high antigen doses have been shown to promote Th1 polarization, whereas low antigen doses favor Th2 differentiation (23–25). Similarly, cross-talk between CD40 on dendritic cells (DCs) and CD40 ligand (CD40L) on T cells has been reported to influence Th1 and Th2 polarization and is known to be a crucial factor in the pathogenesis of several autoimmunity models, including EAE, collagen-induced arthritis, and experimental autoimmune myocarditis (26–30). Notably, the relevance of antigen dose and CD40–CD40L signaling in Th17 differentiation has yet to be addressed.
We have examined the role played by several parameters, including antigen dose and the effects of PRR and CD40 ligation on DC, in Th17 differentiation in vitro and in vivo. We found that the strength of antigenic stimulation critically influences Th17 development by modulating CD40–CD40L interactions. Upon exposure to high antigen doses, T cells markedly up-regulated CD40L, thus providing efficient costimulation to antigen-presenting DCs for their release of inflammatory cytokines. Simultaneous PRR stimulation of DCs further supported Th17 differentiation by synergistically enhancing the release of IL-6. CD40–CD40L cross-talk played a central role in translating the strength of signal provided by high antigen dose into a cytokine environment that promoted Th17 development, because the absence of CD40 impaired Th17 differentiation in vitro and in vivo. Our data show that optimal CD40–CD40L interaction, favored by high antigen concentrations, is central to the development of Th17 responses.
Results
High Antigen Concentrations Promote Th17 Differentiation in the Absence of Exogenous Cytokines.
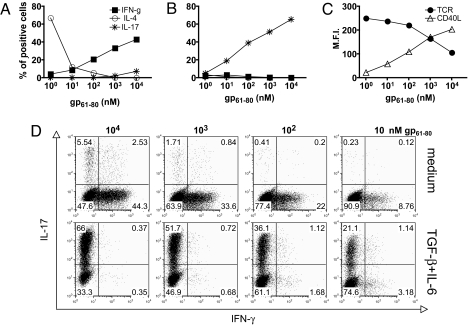
It is well established that supplementing T cell cultures with TGF-β and IL-6 leads to the differentiation of Th17 cells, but the role of antigen concentration has not been investigated. To address this issue specifically, we cultured naïve CD4+ T cells specific for the lymphocytic choriomeningitis virus (LCMV) glycoprotein gp61–80 from Smarta-2 T cell receptor (TCR) transgenic mice (31) with spleen-derived DCs and titrated concentrations of the specific peptide in the absence or presence of TGF-β and IL-6. The extent of T cell proliferation, as evaluated by carboxyfluorescein succinimidyl ester (CFSE) dilution, was proportional to the antigen doses, and it was comparable between cultures performed in the absence or presence of exogenous cytokines (supporting information (SI) Fig. S1). After 5 days of culture, T cell cytokine production was evaluated. At low antigen doses T cells differentiated primarily toward IL-4-secreting Th2 cells, whereas at high antigen doses Th1 differentiation was dominant, as reported (25, 32). Notably, at the highest concentration of peptide tested (i.e., 10 μM), IL-17-producing cells developed even in the absence of exogenous polarizing cytokines (Fig. 1 A and D). As expected, the addition of TGF-β and IL-6 to the cultures suppressed Th1 and Th2 polarization and induced Th17 development (Fig. 1 B and D). The extent of Th17 polarization increased proportionally with the antigen dose (up to 66 ± 0.2% of cells at 10 μM antigen) and could be improved further by the addition of anti-IFN-γ-, anti-IL-4-, and IL-12-neutralizing antibodies (data not shown). Phenotypic analysis of stimulated CD4+ T cells revealed TCR down-regulation (up to 60% of basal level) and CD40L up-regulation occurring at high antigen doses as a consequence of strong TCR engagement (Fig. 1C). Thus, the acquisition of a Th17 phenotype in vitro is favored by strong antigenic stimulation.
Fig. 1.
Cytokine-driven Th17 differentiation requires strong TCR engagement. Naïve TCR transgenic CD4+ T cells specific for the LCMV glycoprotein gp61–80 from Smarta-2 mice were cultured with spleen-derived DCs in the presence of the indicated antigen doses in the absence of exogenous cytokines (A and D Top) or in the presence of TGF-β (5 ng/ml) and rIL-6 (20 ng/ml) (B and D Bottom). After 5 d, cytokine production by expanded T cells was assessed by intracellular cytokine staining after PMA/ionomycin restimulation. Shown are the percentages of IFN-γ-, IL-4-, or IL-17-producing CD4+ T cells (A and B) and the expression levels (mean fluorescence intensity, M.F.I.) of TCR and CD40L assessed by flow cytometry after 5 and 20 h of culture, respectively (C). SD ≤2 for all points depicted. (D) Dot plot of IL-17+ and IFN-γ+ CD4 T cells cultured at the indicated antigen concentrations in the absence or presence of TGF-β and IL-6.
High Antigen Concentrations and the Presence of Pathogen-Associated Molecular Patterns Synergistically Enhance Th17 Polarization.
Certain microbial products have been shown to induce Th17 polarization in cultures of CD4+ T cells and mouse BMDCs or human peripheral blood monocytes. We sought to investigate the role of various PAMPs on primary splenic DC in the differentiation of naïve T cells to the Th17 subset by using TCR transgenic T cells and specific antigens. Exposure of splenic DCs to the TLR9 agonist cytosine phosphate guanine (CpG), and to the dectin-1 ligands zymosan and curdlan induced development of IL-17-producing CD4+ T cells (Fig. 2A). In contrast, stimulation of DC by the TLR4 agonist LPS, the TLR7 agonist imiquimod, the TLR3 agonist polyinosinic-polycytidylic acid sodium salt (polyI:C), or by the TLR2 agonists lipoteichoic acid (LTA) and PG failed to drive Th17 differentiation (Fig. 2A).
Fig. 2.
Th17 differentiation is induced synergistically by high antigen doses and stimulation of primary DC with certain microbial stimuli. (A) Naïve transgenic Smarta-2 CD4+ T cells were cultured with spleen-derived DCs in the presence of gp61–80 peptide (1 μM) and the microbial stimuli CpG (1000 or 100 nM), imiquimod (IQ, 5 or 1 μg/ml), LPS (100 or 10 ng/ml), polyI:C (pIC, 5 or 1 μg/ml), LTA (5 or 1 μg/ml), PG (10 or 2 μg/ml), zymosan (Zym, 300 or 100 μg/ml), and curdlan (Cdl, 500 or 100 μg/ml). (B–D) Naïve transgenic Smarta-2 CD4+ T cells were cultured with spleen-derived DCs in the presence of the indicated doses of antigen and CpG (B), curdlan (C) or zymosan (D). After 5 d, IL-17 production by expanded T cells was assessed by intracellular cytokine staining after PMA/ionomycin restimulation. *, P < 0.05, compared with culture without microbial stimuli.
The effect of the antigen dose on DC-mediated Th17 polarization then was evaluated. Naïve CD4+ T cells and DCs were cultured in the presence of titrated doses of antigen and of CpG, curdlan, or zymosan. The extent of Th17 differentiation increased in parallel with the antigen concentration and was further promoted at high doses of PAMPs (Fig. 2 B–D). These data indicate that optimal Th17 differentiation is favored by both strong TCR stimulation on T cells and PRR ligation on antigen-presenting DCs.
CD40 Ligation on DCs Boosts Pattern-Recognition Receptor-Induced Cytokine Production.
The capacity of microbial stimuli to enable DCs to promote Th17 differentiation may rely on their ability to trigger DC maturation and cytokine production. Therefore, we analyzed in detail the effects of CpG, curdlan, and zymosan on spleen DC phenotype and cytokine secretion. All stimuli induced a dose-dependent up-regulation of the expression of costimulatory molecules including CD40, CD86, CD80, and, to a lesser extent, MHC class II (Fig. 3A and data not shown). CpG-stimulated DC released IL-12p35 (up to 360 ± 30 pg/ml) and high amounts of IL-6 (up to 12.8 ± 4 ng/ml) (Fig. 3 B and C), whereas curdlan- and zymosan-stimulated DCs produced low levels of IL-6 (up to 1.1 ± 0.03 and 2.9 ± 0.06 ng/ml, respectively) and negligible amounts of IL-12p35 (Fig. 2 B and C). The addition of agonistic anti-CD40 antibodies markedly amplified the level of cytokine production (up to 5-fold increase of IL-6 production upon all stimulatory conditions and up to 7-fold increase of IL-12p35 production upon CpG stimulation), indicating that CD40- and PRR-mediated signals synergize in boosting cytokine release by DCs. All of the stimuli also induced secretion of high amounts IL-12p40 (430 ± 70 ng/m upon CpG, 85 ± 8 on curdlan, and 54 ± 3 ng/ml on zymosan stimulation), which were increased further ≈5-fold after the addition of anti-CD40 antibodies. In contrast, no significant IL-23, TNF-α, or IL-1β production was detected under any of the stimulatory conditions, irrespective of the presence or absence of anti-CD40 antibodies (data not shown). Microbial stimuli that poorly induced Th17 differentiation, such as LPS, PG, polyI:C, imiquimod, and LTA, induced IL-12p35 release by DCs to various extents but failed to trigger IL-6 production in high amounts (Fig. S2).
Fig. 3.
CD40 ligation on DCs boosts PRR-induced cytokine production. (A–C) Spleen-derived DCs were incubated with the indicated doses of CpG, curdlan, or zymosan in the absence or presence of 2 or 10 μg/ml of anti-CD40 antibody. After 20 h, surface molecule expression was analyzed by flow cytometry (mean fluorescence intensity, M.F.I.) (A), and the cytokine content in supernatants was determined by ELISA (B and C). *, P < 0.05 compared with cultures in the absence of anti-CD40 antibodies. (D) Spleen-derived DCs were incubated with CpG (100 nM), curdlan (500 μg/ml), or zymosan (300 μg/ml) in the presence of naïve Smarta-2 CD4+ T cells (DC:T cells = 1:5) and the indicated antigen doses. After 20 h the cytokine content in supernatants was determined by ELISA. *, P < 0.05.
Under physiological conditions, activated T cells express CD40L that acts to promote DC maturation through CD40 stimulation. Because CD40L was markedly up-regulated on CD4+ T cells after stimulation with high (but not with low) antigen doses (Fig. 1C), we tested the ability of CD4+ T cells to influence cytokine production by PRR-activated DCs. Antigen-exposed CD4+ T cells enhanced the extent of PRR-induced DC cytokine production proportionally to the strength of antigenic stimulation. The CD40–CD40L stimulatory pathway was central to this phenomenon, because CD40-deficient (CD40−/−) DCs exhibited only a marginal increase of cytokine production (Fig. 3D). Taken together, these data indicated that by inducing CD40L expression on T cells, strong antigenic stimulation synergistically enhances cytokine production by PRR-activated DCs.
CD40–CD40L Interaction Is Critical for Th17 Differentiation in Vitro and in Vivo.
We next evaluated whether this CD40-CD40L-mediated DC activation influenced Th17 differentiation. Naïve transgenic CD4+ T cells were primed at high antigen concentration by WT or CD40−/− DCs, in the presence of exogenous IL-6 or different Th17-promoting PAMPs, and cytokine production was determined after 5 d of culture. In the presence of TGF-β and IL-6, WT and CD40−/− DCs led to the expansion of comparable proportions of IL-17-producing CD4+ T cells. In contrast, upon CpG stimulation CD40−/− DCs exhibited an impaired capacity to drive development of Th17, but not Th1, cells (Fig. 4A). The addition of exogenous IL-6 to the culture completely restored the Th17-polarizing capacity of CpG-stimulated CD40−/− DCs (Fig. 4 A and B). Similar results were obtained by using curdlan and zymosan for DC stimulation, although in the latter case exogenous IL-6 only partially restored Th17 polarization in cultures with CD40−/− DC (Fig. 4B). These results clearly indicate that simultaneous PRR and CD40 engagement on antigen-presenting DCs is required for optimal secretion of IL-6 and the promotion of Th17 development.
Fig. 4.
CD40–CD40L interaction is required for efficient Th17 differentiation in vitro and in vivo. (A) Naïve transgenic Smarta-2 CD4+ T cells were incubated with WT or CD40−/− spleen-derived DCs and 1 μM antigen in the presence of TGF-β and IL-6, CpG (1000 nM), or CpG (1000 nM) and IL-6. After 5 d, cytokine production by expanded T cells was assessed by intracellular staining after PMA/ionomycin restimulation. (B) Smarta-2 CD4+ T cells were incubated with WT or CD40−/− spleen-derived DCs in the presence of 1 μM antigen and the indicated doses of CpG, curdlan, or zymosan in the absence (Top) or presence (Bottom) of rIL-6. After 5 d cytokine content in supernatants, following antigenic stimulation of expanded T cells, was determined by ELISA. (C) C57BL/6 WT or CD40−/− mice were immunized s.c. with the indicated doses of gp61–80 peptide in CFA and boosted after 7 d. Six days after the boost, draining lymph node cells were restimulated in vitro with 1 μM of gp61–80 peptide, and cytokine production by CD4+ T cells was assessed by intracellular cytokine staining.
We next assessed the role of the CD40–CD40L pathway in Th17 development in vivo. Naïve WT or CD40−/− mice were immunized s.c. with different doses of gp61–80 peptide in CFA, and Th1/Th17 development was monitored ex vivo by intracellular cytokine staining of draining lymph node cells. Upon immunization with low antigen doses, CD40−/− mice showed reduced development of both Th1 and Th17 cells. Immunization with higher antigen concentrations, however, led to expansion of comparable fractions of Th1 cells in WT and CD40−/− mice but did not rescue Th17 differentiation (Fig. 4C).
Similar results were obtained when antigen was administered together with zymosan as adjuvant (Fig. S3). Thus, in vivo development of Th17 critically depends on CD40–CD40L cross-talk.
CD40–CD40L Interaction Is Required for the Development of Encephalitogenic Th17 Cells During Experimental Autoimmune Encephalitis.
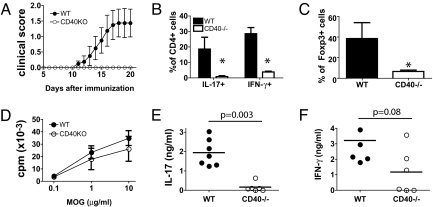
Given the central role of Th17 cells in autoimmunity, we investigated the function of CD40–CD40L cross-talk in Th17 differentiation in a model of EAE. Naïve WT and CD40−/− mice were immunized s.c. with myelin oligodendrocyte glycoprotein (MOG 35–55) peptide in CFA and monitored for the appearance of clinical signs of EAE up to day 20 after immunization. Starting from day 11, WT mice developed progressive disease, whereas CD40−/− mice were completely protected (Fig. 5A), as reported (33). In WT mice analysis of CNS-infiltrating cells revealed a considerable infiltration of CD4+ T cells (up to 32 ± 9% of total cells), which included significant fractions of IL-17- or IFN-γ-producing cells and of Foxp3+ cells. In contrast, CNS of CD40−/− mice contained few CD4+ cells (up to 3.5 ± 1%) that were neither effector nor regulatory cells (Fig. 5 B and C). Interestingly, upon in vitro restimulation with MOG35-55, spleen-derived T cells from CD40−/− mice exhibited only a slightly decreased proliferative response compared with cells from WT mice (Fig. 5D), indicating that T cell priming had occurred in the absence of CD40 ligation. However, when cytokine production was assessed, cells from WT mice released substantial amounts of IL-17 and IFN-γ, consistent with the expected development of MOG-specific Th17 and Th1 cells, respectively. In contrast, although IFN-γ secretion was detected in 3 of 6 CD40−/− mice, IL-17 production was not detectable (Fig. 5 E and F). Thus, CD40 is critical for the development of pathogenic Th17 cells in vivo.
Fig. 5.
CD40–CD40L cross-talk is required for differentiation of self-reactive Th17 cells. C57BL/6 WT (n = 7) or CD40−/− mice (n = 6) were immunized s.c. with MOG35–55 (150 μg) in CFA at days 0 and 7. (A) Longitudinal clinical score (mean ± SD). (B and C) CNS-infiltrating lymphocytes were isolated from WT and CD40−/− mice. Shown are the percentages of CD4+ cells that were IL-17+ and IFNγ+ after 4 h PMA/ionomycin stimulation (B) and the percentages of Foxp3+ cells (C) assessed by flow cytometry after intracellular staining. *, P < 0.05. (D–F) Spleen cells were harvested at day 20 and restimulated for 48 h with titrating concentrations (D) or 10 μM MOG35–55 (E and F). (D) Values show 3[H] thymidine uptake at indicated antigen concentrations (averages ± SD) and (E and F) cytokine concentrations measured by ELISA.
CD40–CD40L Signaling Is Dispensable for Development of Protective Th1 Response to Listeria monocytogenes.
To assess further the relative contribution of CD40–CD40L signaling to Th17 and Th1 development in vivo, we investigated Th1 and Th17 differentiation on infection with an intracellular pathogen, such as Listeria monocytogenes. Infection of WT mice resulted in predominant generation of IFN-γ-producing CD4 T cells and a low frequency of IL-17-producing CD4 T cells (Fig. S4). CD40−/− mice showed normal Th1 responses on restimulation with either heat-killed Listeria or phorbol myristate acetate (PMA)/ionomycin, whereas frequencies of specific Th17 cells were severely compromised. Thus, CD40 appears to be more important for the generation of Th17 cells than of Th1 cells.
CD40–CD40L Interaction Is Not Required for Th17 Differentiation of Gut Lamina Propria Lymphocytes.
Gut lamina propria lymphocytes (LPL) contain a fraction of IL-17-producing CD4+ T cells developing under steady-state conditions (21). We assessed LPL cytokine production in WT and CD40−/− mice. Percentages of IL-17+ cells were not significantly decreased in CD40−/− compared with WT mice (Fig. S5A). Similarly, no major differences were observed in percentages of Foxp3+ CD4+ T cells (Fig. S5B). Thus, CD40 signaling does not appear to be required for steady-state levels of Th17 cells in LPL.
Discussion
A number of studies have begun to unravel the nature of the stimuli governing Th17 differentiation. Although it is clear that cytokines, in particular IL-6 and TGF-β, drive the differentiation of Th17 cells (1, 12, 14), the signals that culminate in their production have yet to be elucidated fully. By using an antigen-specific system, we show that Th17 differentiation requires a strong antigenic stimulation that by up-regulating CD40L expression on T cells results in increased DC activation and the production of IL-6. This TCR-driven signal together with PRR-ligation on DCs acts to enhance the development of Th17 responses.
Antigen dose emerged from our study as a key parameter in the differentiation of Th17 cells; specifically, the proportion of Th17 cells increased relative to the increase in the concentration of antigen. In a number of systems, low concentrations of antigen have been shown to favor Th2 differentiation, whereas high concentrations favor Th1 (23–25). Now, Th17 differentiation can be included in this scheme, seeming to develop under the strongest inflammatory conditions. Many levels of redundancy support Th1 and CTL activation (34, 35), which may be a distinguishing characteristic between such responses and Th17 responses. Th17 cells do not seem to have the luxury of such redundancies, requiring the collective signals of strong antigen stimulation and full DC maturation through PRR agonists and CD40–CD40L cross-talk.
The influence exerted by different degrees of CD40–CD40L ligation on Th1 and Th2 polarization has been described by our group (25). Here, we show that the extent of CD40 ligation on antigen-presenting DCs also modulates the balance between Th1 and Th17 polarization. T cells activated in the presence of high antigen dose delivered strong CD40-mediated costimulation to DCs that led to the release of high amounts of IL-6 and the differentiation of Th17 cells. Such results also were validated in vivo. We demonstrated that CD40-deficient mice showed impaired Th17 but not Th1 development in response to immunization with high-dose antigen, whereas both Th1 and Th17 responses were affected by immunization with lower antigen doses (Fig. 4C and Fig. S3). Moreover, Th17 but not Th1 differentiation was reduced in CD40-deficient mice infected with Listeria monocytogenes (Fig. S4). CD40-deficient mice were protected from MOG-induced EAE, which was associated with absence of Th17, Th1, and regulatory T cells in the CNS. Interestingly, proliferation of MOG-specific CD4 T cells appeared to be normal in the spleen of CD40-deficient mice, but IL-17 production was abolished, and IFN-γ production was partially reduced. In contrast, the frequency of Th17 cells in gut LPL was normal in CD40−/− mice (Fig. S5). The requirements for development of inflammatory Th17 cells upon immunization or infection versus steady-state Th17 cells in LPL has not been clarified, although both have been shown to require IL-6 (21). It is possible that enterocytes or other cells in gut produce IL-6 independent of CD40 (36), in contrast to DC in lymph nodes and spleen.
In our study, the ability of the different stimuli to drive Th17 polarization was correlated with their capacity to trigger cytokine production by DCs. In particular, IL-6 secretion seemed to be a major determinant of Th17 polarization, consistent with previous reports (1, 12, 14). Indeed, PAMPs such as LPS, PG, polyI:C, imiquimod, and LTA, which poorly induced IL-6 release by DCs, also failed to promote Th17 differentiation (Fig. 2A and Fig. S2). Moreover, Th17 polarization was abrogated completely when IL-6-deficient DCs were used as APCs (data not shown). Furthermore, the inability of CD40−/− DCs to promote Th17 development could be overcome by the addition of exogenous IL-6 to cultures (Fig. 4 A and B). Notably, upon T cell priming by zymosan-exposed CD40−/− DCs, Th17 expansion was rescued only partially by exogenous IL-6 (Fig. 4B), suggesting that in these stimulatory conditions cytokines other than IL-6 might support the differentiation and/or the expansion of IL-17-producing effectors. IL-23, in particular, has been shown to be critical for survival and/or expansion of mouse and human Th17 cells (1, 14–17). We could not detect significant IL-23 production in any of the conditions tested, and Th17 development was decreased marginally (< 10%) by using IL-23p19-deficient DC (data not shown), suggesting a minor role for IL-23 in the early phases of Th17 development. Furthermore, spleen DCs also failed to release detectable amounts of TNF-α and IL-1β on stimulation with the PRR agonists used in our study (data not shown). When BMDCs were tested in parallel, significant TNF-α production in response to zymosan and curdlan (up to 7 ± 0.5 and 8 ± 2 ng/ml, respectively) and IL-1β secretion on exposure to zymosan (up to 20 ± 3 ng/ml) was observed (data not shown). Thus, depending on the type of APCs used, identical stimuli may mediate the release of distinct types of inflammatory cytokines and contribute differentially to Th17 polarization. Different APC populations, including mouse BMDCs, human monocytes, and monocyte-derived DCs, have been shown to drive the expansion of Th17 cells in response to distinct types of PRR agonists. We have analyzed the ability of spleen-derived DCs to drive Th17 differentiation of naïve T cells, in response to a panel of PRR agonists. The TLR9 agonist CpG and the dectin-1 ligands zymosan and curdlan proved the most effective stimuli, in line with data recently reported for mouse BMDCs (22). In contrast, LPS, which has been shown to promote Th17 development on stimulation of BMDCs (14), was marginally effective on spleen DCs (Fig. 2A); however, this result is not unexpected, given that splenic DCs are known to be less responsive than BMDCs to TLR4 triggering (37).
Taken together, our data demonstrate that high antigen concentrations synergize with PRR-mediated activation of DC to drive differentiation of IL-17-producing CD4+ T cells by fostering CD40–CD40L cross-talk.
Materials and Methods
Mice.
C57BL/6 mice were obtained from Charles River Laboratories, Germany. Smarta-2 transgenic mice, encoding a transgenic TCR specific for LCMV glycoprotein gp61–80 peptide (31) and CD40−/− mice (38) have been described and were kindly provided by Dr. M. Bachmann (Cytos Biotechnology AG). All mice were bred and maintained under specific pathogen-free conditions in our animal colony (BioSupport, Schlieren, Switzerland). Mice used in experiments were between 8 and 10 weeks old. All experiments were performed with permission from the Zürich Animal Ethics Committee.
Reagents.
Human recombinant TGF-β type I was purchased from Sigma-Aldrich. Human recombinant IL-6 (cross-reacting with mouse species) was kindly provided by Dr. L. Aarden (University of Amsterdam). Phosphorothioate CpG (TCCATGACGTTCCTGATGCT) was synthesized by Microsynth AG. LPS from Escherichia coli (0111:B4), zymosan A from Saccharomyces cerevisiae, and curdlan from Alcaligenes faecalis were purchased from Sigma-Aldrich. PolyI:C, imiquimod (R837), LTA, and peptidoglycan from Streptococcus aureus were obtained from InvivoGen. Gp61–80 peptide (GLNGPDIYKGVYQFKSVEFD) was purchased from NeoMPS and MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) from Anawa Trading SA. Pertussin toxin was obtained from List Biological Laboratories Inc.
DC Stimulation and T Cell Differentiation in Vitro.
Spleens from WT and CD40−/− mice were incubated with collagenase-D (Worthington). CD11c+ DCs from cell suspensions were positively selected by sorting with anti-CD11c antibody-conjugated magnetic beads (Miltenyi Biotec). Naïve transgenic CD4+ T cells were isolated from spleens of Smarta-2 mice upon sorting with anti-CD4 antibody-conjugated magnetic beads (Miltenyi Biotec) and in some experiments were labeled with 2 μM CFSE (Molecular Probes) before stimulation. DCs were cultured in the presence of different PRR ligands, alone or in the presence of purified Smarta-2 CD4+ T cells (ratio 1:5) and different doses of gp61–80. DC cytokine production and cell surface molecules were measured by ELISA and flow cytometry, respectively, after 20 h of incubation. TCR and CD40L expression on T cells was evaluated by flow cytometry after 5 h and 20 h, respectively. T cell cytokine production was assessed after 5 d of culture by intracellular cytokine staining after PMA stimulation or by measuring cytokine contents in supernatants by ELISA 24 h after restimulation with 1 μM antigen.
Analysis of Surface Marker Expression and Intracellular Cytokine Detection.
DCs were stained with allophycocyanin-conjugated anti-CD11c antibody and FITC-conjugated anti-CD40, CD80, CD86, and I-Ab antibodies. T cells were stained with allophycocyanin-conjugated anti-CD4 antibody and FITC-conjugated anti-TCRvα2 or phycoerythrin (PE)-conjugated anti-CD40L antibodies. For intracellular cytokine detection, T cells were incubated with PMA (10–7 M), ionomycin (1 μg/ml), and monensin (3 μM) for 4 h, then fixed, permeabilized, and stained by FITC-anti-IFN-γ, PE-anti-IL-4, and allophycocyanin-anti-IL-17 antibodies. All antibodies were purchased from eBioscience.
Cytokine Detection by ELISA.
IL-12p35, IL-12p40, IL-6, TNFα, IL-1β, and IL-23p19 contents in supernatants from DC cultures and IFN-γ and IL-17 contents in supernatants from T cell cultures were measured by sandwich ELISA. All antibodies used were from eBioscience.
T-Cell Differentiation in Vivo.
WT or CD40−/− mice were immunized s.c. and 7 days later were boosted with the indicated doses of gp61–80 in CFA or in incomplete Freund's adjuvant plus 500 μg of zymosan. Seven days after the boosting, cells from draining lymph nodes were collected and restimulated in vitro with 1 μM of the specific antigen. After 24 h, cytokine contents in the supernatants were determined by ELISA.
Induction and Assessment of Experimental Autoimmune Encephalitis.
WT or CD40−/− mice were immunized s.c. with 150 μg of MOG 35–55 peptide in CFA and 400 ng of pertussis toxin. Starting from day 8, mice were monitored daily for the appearance of clinical signs of disease. The following clinical score was used: 0, no detectable signs of EAE; 0.5, distal limp tail paralysis; 1.0, complete limp tail paralysis; 1.5, limp tail and hind limb weakness; 2.0, unilateral partial hind limb paralysis; 2.5, bilateral partial hind limb paralysis; 3.5, complete hind limb paralysis and unilateral front limb paralysis; 4.0, total paralysis of fore and hindlimbs; 5.0, death. Mice were killed 20 d after immunization. CNS-infiltrating lymphocytes were isolated as described in ref. 39, and frequencies of CD4+ and Foxp3+ cells were determined by staining with specific antibodies (Ebioscience). The frequency of CD4+ cells expressing IL-17 and IFN-γ was assessed by intracellular staining after 4 h of PMA/ionomycin stimulation. Splenocytes were restimulated with titrated doses of MOG 35–55 peptide. After 48 h, cell proliferation was measured by 3H-thymidine incorporation, and cytokine contents in supernatants were assessed by ELISA.
Listeria monocytogenes Infection.
WT and CD40−/− mice (n = 4) were infected i.v. with 1000 cfu of Listeria monocytogenes (strain 10403 S). After 7 d, spleen CD4+ T cells were restimulated with heat-killed Listeria or PMA/ionomycin. After 5 h, frequencies of IFN-γ+ or IL-17+ CD4+ cells were determined by intracellular cytokine staining.
Analysis of Lamina Propria Lymphocytes.
LPL were isolated from WT and CD40−/− mice as described in ref. 21. Cells were evaluated for Foxp3 expression and assessed for cytokine production on PMA/ionomycin restimulation.
Supplementary Material
Acknowledgments.
This project has been supported by research grants from the Swiss National Foundation (3100A0–100233/1) and from Eidgenössische Technische Hochschule Zurich (TH-18/04–3).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810769106/DCSupplemental.
References
- 1.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 5.Rangachari M, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonderegger I, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36(11):2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 7.Chabaud M, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42(5):963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 16.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104(43):17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27(4):660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7(11):1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 22.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 23.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182(5):1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182(5):1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J Immunol. 2000;30(7):2056–2064. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson U, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9(12):1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 27.Gerritse K, et al. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93(6):2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grewal IS, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273(5283):1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 29.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 30.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukocyte Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: Effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28(1):390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200(2):181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193(8):967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nembrini C, Abel B, Kopf M, Marsland BJ. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J Immunol. 2006;176(12):7180–7188. doi: 10.4049/jimmunol.176.12.7180. [DOI] [PubMed] [Google Scholar]
- 35.Skokos D, Nussenzweig MC. CD8-DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204(7):1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritts T, et al. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia—role of transcription factors and regulation by the stress response. American Journal of Surgery. 2002;183(4):372–383. doi: 10.1016/s0002-9610(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 37.Boonstra A, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: Dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197(1):101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabe T, et al. The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 39.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38(7):1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.