Abstract
The jasmonate signal pathway is known to control defenses against herbivores, such as leaf eaters (folivores). Does the reach of the pathway extend to defense against other types of animal? Among the arthropods attracted to seed baits placed below flowering Arabidopsis thaliana plants are 2 largely nocturnal isopod crustaceans generally considered as detritivores: Porcellio scaber and Armadillidium vulgare. Parallel laboratory experiments identified the isopods as being capable of predation on intact plants. Isopod feeding was strongly facilitated in jasmonate-deficient Arabidopsis and rice plants. The feeding activity of isopods revealed potentially detritivore-sensitive, jasmonate-protected Achilles' heels in these architecturally different plants (petioles and inflorescence stems in Arabidopsis, and lower stem and mesocotyl in rice). The work addresses the question of what stops 2 detritivores from attacking living plants and provides evidence that it is, in part, the jasmonate signal pathway. Furthermore, senescent leaves from an Arabidopsis jasmonate mutant were consumed more rapidly than senescent wild-type leaves, suggesting that past activity of the jasmonate signal pathway in leaves may slow carbon recycling through detritivory.
Keywords: detritivory, isopods, jasmonic acid, plant defense, senescence
Herbivory is one of the most important processes in the biosphere, whereby carbon fixed by plants is transferred to higher trophic levels (1, 2). In many cases the largest category of herbivores on the vegetative tissues of plants tends to be insects (3). Additionally, many organisms are known to prey on seeds or otherwise affect seed fate (4). However, herbivores are not the only organisms associated with plants, and only a proportion of the animals associated with plants eat living plant tissues. Another important category of organisms found associated with plants are the detritivores: feeders on dead and dying material (5). Detritivory of plant material is a second major environmental process releasing carbon from plants to higher trophic levels. Like herbivory, this is one of the truly large-scale processes in nature. Detritivores, generally considered apart from herbivores, may actively survey the plant and its vicinity looking for senescent, decaying, or otherwise compromised plant tissues. Furthermore, there is no clear division in nature between detritivory and herbivory, and many arthropods are omnivores feeding at more than one trophic level (6). It is possible that some detritivores, if given the opportunity, might be facultative herbivores. Indeed, it is conceivable that defense barriers against detritivores would be provided by the jasmonate signal pathway, because this pathway operates on the expression of hundreds of wound-inducible defense and survival genes (7) and underlies a major defense system against many herbivores (8).
Through field observations we first identified arthropods potentially capable of devouring Arabidopsis seeds. These were early-stage juveniles of the nocturnal dermapteran (earwig) Forficula sp., the beetle Amara aenea, and the pavement ant Tetramorium caespitum. Interestingly, 2 isopod crustaceans, Porcellio scaber and Armadillidium vulgare, were also captured on seed baits. Laboratory feeding experiments with Arabidopsis seeds, and subsequently with intact Arabidopsis and rice plants, were conducted to further investigate the potential for the 2 isopods to act as herbivores. Feeding experiments with plants defective in jasmonic acid (JA) biosynthesis were conducted and revealed that jasmonate-deficient plants were strongly preferred over wild-type plants by the isopods. The isopods attacked jasmonate-deficient plants in a distinct manner, often selecting weak spots in the plant.
Results
Isolation and Observations of Seed Predators.
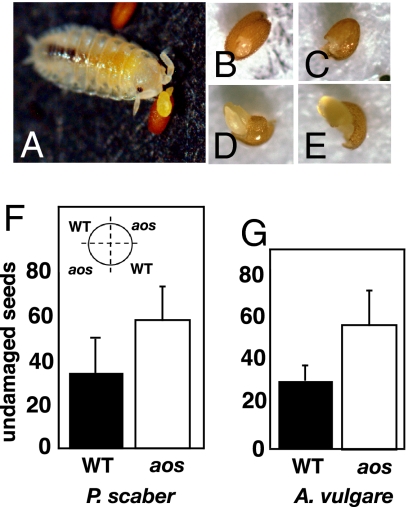
Field experiments to identify organisms attracted to seed baits were conducted within wild populations of Arabidopsis thaliana growing in Lausanne, Switzerland. These experiments identified several insects (Fig. 1 A–C), as well as the pillbug A. vulgare (Fig. 1D) and the woodlouse P. scaber (Fig. 1 E and F), as nocturnal seed predators. In parallel, soil from the compost heap was maintained in the laboratory and used as a source of organisms capable of predation on Arabidopsis seeds. This revealed that both A. vulgare and P. scaber present in the compost were responsible for seed removal (Fig. 2A). Naive P. scaber that had been fed only on Arabidopsis leaves rapidly recognized and consumed Arabidopsis seeds. First, a piece of testa is consumed, making a window that exposes part of the embryo (Fig. 2B), which often is damaged (Fig. 2 C–E). P. scaber sometimes expels the embryo from the testa/endosperm, most or all of which is then eaten. The naked embryo is often severed and abandoned. During this time, opportunists (typically collembolae and mites) feed before the isopods return to devour the embryo.
Fig. 1.
Field observation of organisms drawn to baits of 50–100 seeds deposited in a natural population of A. thaliana. (A) Juvenile earwigs (Forficula sp.). (B) The beetle Amara aenea. (C) Ants (T. caespitum) removing seed. (D) Two isopod crustacea. The upper individual was tentatively identified as A. vulgare. (E) the isopod P. scaber and an earthworm (yellow arrowhead; not observed to remove seeds). (F) P. scaber visiting seed bait near a compost heap.
Fig. 2.
Laboratory observation of seed predation by P. scaber. (A) P. scaber juvenile feeding on WT seed. (B–E) Stages in seed consumation. (F and G) Effect of jasmonates on seed predation by small isopods in the laboratory. In both cases, feeding on WT and homozygous aos seeds (produced by hand-pollinating aos plants with pollen from other aos plants sprayed with MeJA to rescue male fertility) was compared to an experimental design shown in the Inset for F. The circle diameter was 2.3 cm, and 50 seeds were scattered evenly in each sector (2 sectors for each genotype, total of 100 seeds per genotype). (F) Feeding of small (4–6.5 mm) P. scaber on WT and aos seeds (results from 8 filters; paired t test: t = 3.87, df = 7, P = 0.006). (G) Similar experiments with small (3.5–5 mm) A. vulgare; results were from 3 filters (paired t test: t = 4.6, df = 2, P = 0.044). The results in F and G show that aos seeds were damaged or consumed more readily than WT seeds by the small isopods.
Effect of the Jasmonate Pathway on Seed Predation.
Seed feeding assays were used to determine whether the presence of jasmonates could affect the defense of seeds against P. scaber and A. vulgare. These isopods, when larger than 6 mm in length, devoured seeds very rapidly and were difficult to use in the assays. Smaller individuals (≤6 mm) were used in these tests. To address the question of whether jasmonates contribute to seed defense, a mutant for the ALLENE OXIDE SYNTHASE (AOS) gene, which is necessary for JA biosynthesis, was used to block JA production. The aos mutation renders plants male sterile, but viable pollen can be obtained by spraying flowers with methyl jasmonate (MeJA) (9). To examine the effect of the aos mutation on seed consumption, homozygous aos plants were first hand pollinated with aos pollen generated by spraying flowering homozygous aos plants with MeJA. We found that aos seeds generated in this way were heavier than seeds from the WT. When weighed, 200 WT seeds yielded an average weight of 15.66 μg per seed. However, 200 aos seeds gave an average of 29.39 μg per seed. One hundred seeds of each genotype displayed on replicated filters (Fig. 2F Inset) were offered to P. scaber (4–6 mm in length). After feeding, undamaged remaining seeds were scored. Seeds from the WT plant were damaged/eaten more rapidly than seeds from the aos plants (Fig. 2F). Similar results were obtained with A. vulgare of 3.5 to 5 mm in length (Fig. 2G). Finally, when 4-day-old germinated seedlings (both WT and aos) that lacked the first true leaves were fed to P. scaber, they were rarely consumed, and no effect of the jasmonate pathway was observed.
Isopod Damage to Living and Dying Vegetative Tissues.
If starved for a period of 1 to 2 days, both isopod species (P. scaber and A. vulgare) will attack Arabidopsis WT plants or detached leaves. These behaviors were not observed in the field. P. scaber attempts to feed directly on leaf tissue cutting irregularly, mostly at the leaf tips. Sometimes, holes are made in the lamina. Also observed was scraping of cells from the abaxial epidermis. The adaxial epidermis was rarely damaged. Woodlice can climb onto plants (Fig. 3A) and sever inflorescence stems or, more frequently, petioles (Fig. 3B). Often, part of the severed leaf is consumed soon after excision, but sometimes the lamina is consumed up to 24 h later, leaving only the petiole/midrib. Feeding on aos is greatly accelerated relative to feeding on WT, and even midribs were consumed (Fig. 3 C and D). By using a quantitative assay, the relative rate of feeding of P. scaber on intact WT Arabidopsis plants and plants lacking the ability to produce jasmonates (aos) was compared. The aos genotype was consumed rapidly (Fig. 3E) compared with the WT.
Fig. 3.
Damage patterns inflicted by isopods on Arabidopsis plants. (A) P. scaber climbing on the underside of a cauline leaf from a WT plant. Note a severed inflorescence stem (yellow arrowhead). (B) Petiole-severing activity observed on a WT Arabidopsis plant (yellow arrowhead). (C and D) Comparison of feeding on WT Arabidopsis plants and aos plants compromised in jasmonate production. Arabidopsis was grown for 5 weeks and then transplanted into fresh soil. Plants were photographed before the addition of P. scaber that had been starved for 2 days. Seven days later the plants were again photographed. P. scaber consumed all aboveground tissues of aos, even destroying the leaf midribs. Note that plants were in pairs. (E) Arabidopsis plants (WT and aos) were grown for 6 weeks in the same container before introducing P. scaber. Leaf damage was monitored.
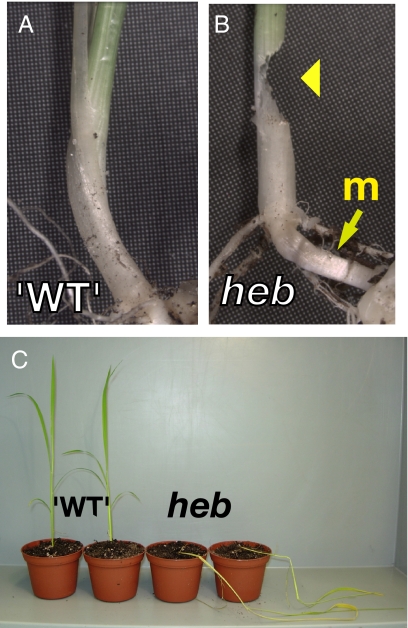
When offered to isopods, the stems of small WT rice plants (at the 2- to 3-leaf stage) growing in soil were rarely severed, and the damage inflicted by both P. scaber and A. vulgare was usually restricted to the upper leaves. However, the rice jasmonate-deficient mutant hebiba (10) was attacked. Typically, hebiba plants grown in soil were severed just above the junction separating the mesocotyl and the base of the coleoptile (Fig. 4 A and B). Another behavior consisted of burrowing into the soil and severing the base of the mesocotyl. This behavior is cryptic and easily overlooked since the plants usually remain upright for days after being severed. During this time the mesocotyl is often consumed. These behaviors were rarely observed when P. scaber was offered uprooted rice plants placed on the soil surface.
Fig. 4.
Damage patterns inflicted by isopods on rice plants. (A and B) Seven-day-old rice plants were transferred for 24 h into soil containing A. vulgare. Arrowhead indicates damage to stem of the homozygous hebiba (heb) mutant but not to plants that did not segregate for the homozygous mutation (WT). The center of the elongated mesocotyl (m) in hebiba is indicated with an arrow. (C) Pairs of 5-leaf-stage rice plants that did not segregate for the hebiba phenotype (WT) and the hebiba phenotype plants (heb) plants after culture with P. scaber. P. scaber felled the hebiba plants.
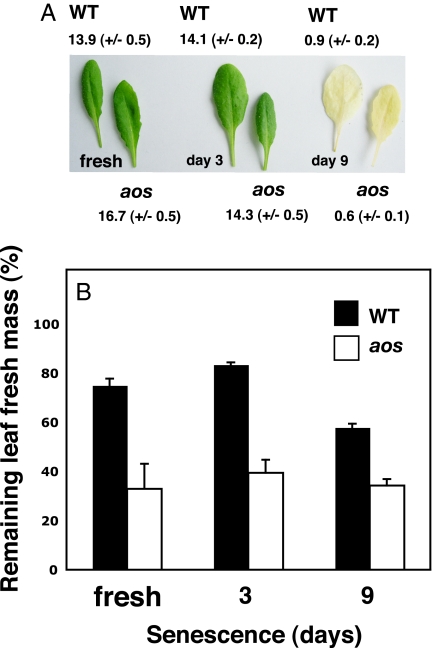
The ability of the woodlice to distinguish between freshly detached leaves and senescing leaves of the 2 genotypes (WT and aos) was then tested on freshly detached leaves and leaves allowed to senesce for 3 or 9 days before feeding to isopods. Quantitative chlorophyll measurements indicated that both leaf genotypes had lost ≈95% of their chlorophyll at day 9 (Fig. 5A). P. scaber consumed the freshly detached leaves of aos more rapidly than detached leaves from the WT. The same was true for leaves allowed to senesce in the dark on humid soil for 3 or 9 days (Fig. 5B).
Fig. 5.
Jasmonate synthesis restrains feeding by an isopod crustacean on dying leaves. (A) Chlorophyll measurements on representative batches of detached and senescing leaves (9 to 10) used in feeding experiments (B). The photo shows representative leaves of both genotypes (WT and aos), with WT on the left of each pair. Chlorophyll values are in micrograms per milligram dry weight (±SD) and are shown for freshly detached leaves (day 0) and for leaves 3 and 9 days after incubation on humid soil in the dark at 23–24°C. (B) Leaves (7–8) from 4.5- to 5-week-old WT and aos plants were detached and either offered immediately to P. scaber (fresh) or allowed to senesce for 3 or 9 days before feeding. Experiments (fresh, 3 days, and 9 days) were performed separately. Standard deviations were calculated from n = 3 (day 0), n = 4 (day 3 and day 9). In all cases, the leaves of aos were more susceptible to P. scaber than were WT leaves. Approximately 8 woodlice per liter of soil were used for the assays.
Discussion
Insects and Isopod Crustaceans Can Prey on Arabidopsis Seed Baits.
Although specialist weevils that feed on Arabidopsis inflorescences have been identified (11), there is less information on predators of postdehiscent Arabidopsis seeds. In baiting experiments, seeds were often discovered by pavement ants (Tetramorium caespitum), with maximum activity 30 min to 2 h after sunrise and just before sunset. Other organisms that preyed on seed baits during the night included beetles, earwigs, and 2 isopods: P. scaber and A. vulgare. The repeated observation of these isopods feeding on Arabidopsis seed baits described here suggests that they might be facultative predators of small seeds in nature.
Seed defenses and their relationship to the jasmonate pathway were tested by comparing the consumption of near WT seeds and homozygous AOS-deficient seeds (in a similar genetic background) by the isopods. Analysis of the results (Fig. 2F) showed that the WT seeds were consumed more quickly than the aos seeds by small isopods. This effect was not seen with larger isopods, which eat both seed types rapidly, sometimes displaying a statistically weak preference for aos (data not shown). We attribute the results with small isopods to differences in seed size. WT seeds were substantially smaller (by approximately a factor of two) than the aos seeds, and were thus more easily consumed by the small isopods. The presence of the homozygous aos mutant gene has a strong effect on seed size. Interestingly, a strong mutation that reduced jasmonate perception in tomato resulted in fewer, smaller-than-WT seeds, in part because of maternal effects on seed maturation (12). In summary, in our experiments, seeds from plants having an active jasmonate pathway did not have increased defense against small isopods. The results led us to test whether jasmonate production could help protect later-stage vegetative tissues from attack by detritivores.
Jasmonates Protect Potential Achilles' Heels in Plants.
Starved P. scaber and A. vulgare attempt to consume mature living tissues of 2 evolutionarily distant plants, rice and Arabidopsis. In this respect, the isopods behave as extremely broad-spectrum generalists, and this behavior is accentuated when the jasmonate pathway is compromised, making both plant species vulnerable to attack. The extent of protection conferred by the jasmonate pathway was revealed with quantitative assays. This revealed that living Arabidopsis plants lacking the ability to produce jasmonates were an effective food source for the isopods. This finding is reminiscent of field observations on wild tobacco, where reducing the activity of the oxylipin/jasmonate biosynthesis pathway caused these plants to attract herbivores not normally associated with the tobacco (13). Furthermore, plants lacking triunsaturated fatty acids, the precursors of JA, were susceptible to saphrophagous dipterans (14).
How does isopod feeding on plants parallel that of other plant-associated crustaceans? Observations of isopod feeding revealed parallels with the feeding strategies of some terrestrial crabs, one of which is even listed as a crop pest (15). Some crabs climb plants and feed on leaves (16), and others procure leaves by cutting them (17). We observed similar behaviors with the isopods (Figs. 3 A and B and 4B). The feeding strategies of isopods observed in the laboratory give us a glimpse at how these ancient organisms that are poorly adapted to folivory do their best to try to breach the formidable defenses in the vegetative tissues of modern angiosperms.
The isopods frequently severed petioles in Arabidopsis. Having long petioles may therefore be a potential risk in the presence of isopods. Moreover, a well-known attack-triggered growth response in Arabidopsis is to produce leaves with reduced petiole length, a response that depends on the activity of the jasmonate pathway (18, 19). It is well known that the density of defensive structures (such as spines and trichomes) in plants can be controlled through attack-stimulated activity of the jasmonate pathway (20, 21), and jasmonate-slowed growth may allow resource diversion to defense (22). Our observations with isopods now suggest that some of the changes commonly observed in response to attack (e.g., production of leaves with short petioles) might specifically affect plant architecture so as to reduce the vulnerability of potential weak spots to opportunistic attackers.
Rice plants have a very different architecture to dicotyledons like Arabidopsis. Not surprisingly, P. scaber and A. vulgare confront the rice plant differently and use tissue-severing activity to a greater extent than they do on Arabidopsis. Tissue severing at the bases of WT rice plants is rare, although damage to leaf tips is more frequent. In contrast, the rice hebiba mutant, in which jasmonate production is impaired, is typically severed through the base of the stem or, alternatively, through the base of the mesocotyl. A common characteristic of the petioles of Arabidopsis and the mesocotyls of rice is that their development is both light- and jasmonate-dependent. Our observations show that these structures are potential Achilles' heels in jasmonate-deficient plants. In fact, it is during the life stages of Arabidopsis in which organ development is most critically dependent on light that the jasmonate pathway exerts a strong effect in protecting against detritivore feeding. This effect is not pronounced at the seed stage and was not detectable in our experiments during a window during germination, hypocotyl elongation, and cotyledon expansion that occurred before the appearance of the first leaves. These observations are consistent with the fact that light quality can strongly affect plant–insect interactions (23).
Finally, the experiments revealed effects of the jasmonate pathway that were still measurable in senescing leaves even after ≈95% chlorophyll loss (Fig. 5B). This suggests that the after-effects of jasmonate activity might affect the rate at which detritivores sequester carbon from dying plant tissues.
Conclusion
By using combined field and laboratory observations, this work suggests that the boundary between herbivory and detritivory might, at least in part, be determined by the jasmonate pathway. Classic work has shown that selection for certain defense capacities in plants may depend more on suites of herbivores than on individual herbivores (24). The present work suggests the possibility that independent suites of detritivorous arthropods (perhaps with detritivores from other phyla) could also add to the pressure on the plant defense system. Moreover, experiments with detached leaves suggest that the past activity of the jasmonate pathway could potentially affect the speed with which carbon in fallen leaves is made available to higher trophic levels. Operating at a nexus in many plant–animal interactions, the activity of the jasmonate pathway may not only influence the performance of herbivores, but may also constrain certain other important groups of organisms to feeding on dead or dying plant material. We speculate that the jasmonate pathway may first have evolved to stop detritivores feeding on living plant tissues.
Materials and Methods
Plants and Seed Staining.
A. thaliana Columbia-0 (Col-0) accession (WT) was used in laboratory experiments with allene oxide synthase (aos) mutants from Park et al. (9). This mutant is in the Col-6 background and does not have trichomes. These aos plants were first backcrossed 5 times to Col-0 to restore trichomes. Some experiments used seeds produced by spraying aos plants 3 times per week for 2 weeks during flowering with water containing 0.01% (vol/vol) MeJA in 0.01% (vol/vol) Tween 20. WT plants were treated identically. WT plants sprayed with MeJA produced lighter-colored seeds than unsprayed WT plants. The rice hebiba mutant was from Riemann et al. (10).
Field Observations and Isolation of Seed and Leaf Predators.
Populations of A. thaliana growing in Dorigny, Lausanne, Switzerland (6°34′41.59″E and 46°31′26″N) were observed over a 3-year period (2004–2006). Most flowering occurred in April through to early June. The majority of siliques were produced by the second week of May, and seed release was observed until the end of June. For baiting experiments, seeds (≈50–100) from wild A. thaliana plants were deposited within 10 cm of silique-bearing Arabidopsis plants and observed. Separately, seeds were placed near a compost heap. Invertebrates attracted to the seeds were photographed. The presence of the observer may have precluded observation of vertebrate granivores. Care was taken not to confuse A. thaliana with Erophila verna.
Parallel to field observations, garden compost was used as a second source of organisms capable of feeding on Arabidopsis seeds. Compost (40 L) was maintained in the dark at 23–26°C. Moistened filter papers (9-cm diameter) carrying ≈50–100 Arabidopsis Col-0 seeds were placed on the soil surface in the evening, and seed loss was observed the next morning. Invertebrates >5 mm in length were: Myriapoda: Diplopoda: Glomeris marginata; Chilopoda: Polydesmus angustus, one unidentified member of the order Polydesmida, and one unidentified order Iulida; Isopod crustacea: P. scaber and A. vulgare; Coleoptera, Carbidae, Amara aenea; Dermaptera, Forficula sp. Earthworms were not observed removing seeds and did not survive culture. Only P. scaber and A. vulgare were Arabidopsis seed predators in this system. To find organisms capable of leaf predation, a mixture of beech (Fagus sylvatica) leaf compost (15 L) and general garden compost (15 L) was incubated in the dark at 22–26°C. The culture was supplied with WT Arabidopsis plants placed on the soil surface. Four months after the initiation of feeding, the container was placed in the light (90 μmol·m−2·s−1 and 22–26°C under a 10-h light/14-h dark photoperiod). Six-week-old Arabidopsis plants were transplanted into the culture soil. After 1 week, plants were found to be damaged. Arthropods >5 mm in length were: Myriapoda: Chilopoda: Lithobius forficatus; Myriapoda: Diplopoda: Polydesmidae, one unidentified species; Order Craspedosomatida, one unidentified species; Crustacea: Isopoda: P. scaber, A. vulgare. One earthworm species survived in this soil culture but was not identified. The arthropod groups were separately fed WT Arabidopsis plants. Only P. scaber and A. vulgare damaged Arabidopsis leaves.
Isopod Culture and Preparation.
P. scaber and A. vulgare were cultured in plastic boxes (internal dimensions: 33 × 51 cm, 22 cm high) with 6 cm soil (10 L). Soil was never allowed to dry and had access to air. Between assays, a 20 × 20 × 1 cm wooden board was placed on the soil surface, facilitating yearly (P. scaber) or sporadic (A. vulgare) reproduction. Every 2 weeks the crustaceans were fed with pesticide-free Arabidopsis plants placed under the wooden plates. Four times a year, isopods were given approximately 0.2 mg aquarium fish food (Vitakraft). Before assays, woodlice were starved for a period of 1–5 days. For detached organ assays with leaves placed on soil containing the isopods, the soil was also watered and turned 24–48 h before the assays. The 3 densities of woodlice often used are: low density, 1–3 per liter of soil; medium density, 4–6 per liter of soil; and high density, 7–15 per liter of soil.
Seed Predation Assays.
Dry Arabidopsis seeds were placed on moist filter paper and introduced into the assay using a low to medium density of woodlice. Although the larvae of P. scaber were not observed to feed on seeds, all other life stages (≥4 mm) were voracious seed predators, but only P. scaber 4–6 mm long were used in seed feeding assays. Once a group of seeds was located, P. scaber often ceased exploring until all seeds were consumed. Thus, for some experiments, designs allowing seeds to be offered as mixtures were conceived. To assess feeding, the number of intact seeds with undamaged coats was recorded as remaining uneaten seeds. It is essential that seeds from different plants to be tested are produced simultaneously and stored under identical conditions. The presence of even small quantities of agarose, often used to help pipette seeds, interferes with the assay. Seed surface sterilization did not affect the outcome of the assays.
Whole-Plant Predation Assays.
Arabidopsis: Four WT plants and 4 aos mutant plants were grown in one box filled to a depth of 6 cm with soil in a 12-h light/12-h dark photoperiod (100 μE·m−2·s−1). Three independent boxes were used as biological replicates. The plants were placed equidistantly and in alternate positions to randomize position effects. When plants reached 6 weeks of age, 80 starved P. scaber were placed in each box (time 0). The assay was conducted under 8 h light/16 h dark (100 μE·m−2·s−1) for 120 h. At different times, digital images were obtained from each individual plant. A numerical scale was placed next to the plant to calculate area values. The digital images were used to convert pixel values into leaf area values using the ImageJ (National Institutes of Health) and Photoshop (Adobe) software. The initial leaf area was considered to be 100%. Under these conditions, and in the absence of woodlice, growth over 120 h contributed to approximately a 5% increase in leaf area.
Rice.
Seeds from a segregating population of rice plants containing the recessive hebiba mutation were germinated in the dark for 4 days, and then homozygous hebiba mutants were selected based on their long mesocotyl phenotype (10). The plants were transferred to soil and grown for 5 to 10 days at 26°C in 14 h light/16 h dark (160 μE·m−2·s−1, 60% relative humidity) before use in bioassays, where their defensive capacity was compared to plants that did not segregate for the homozygous mutation. These assays were carried out in darkness over 3-day periods.
Detached Organ Assays and Chlorophyll Measurement.
The pattern of feeding on detached organs can differ from feeding on growing plants. Leaves, flowers, etc. were covered with a perforated Plexiglas sheet (3-mm diameter holes spaced at 3-cm intervals) raised 2–4 mm off the soil surface. Leaves were placed adaxial side down in darkness or in dim red light. With high densities of woodlice, the assay can be conducted rapidly (within 3 h), provided that limited numbers of leaves are used. For the data in Fig. 5B, controls (data not shown) were performed to ensure that water loss from detached WT and aos leaves in the absence of isopods was similar. Chlorophyll was measured according to Arnon (25).
Acknowledgments.
E.E.F. thanks Gustavo Bonaventure (Max Planck Institute of Chemical Ecology, Jena, Germany) who performed the experiment for data in Fig. 3E while at the University of Lausanne. P. Nick (University of Freiburg, Freiburg, Germany) kindly provided rice seeds. We thank N. Geldner, C. Gutjahr, A. Chételat, V. Rodriguez, I. Acosta, A. Gfeller, S. Stolz, P. Vittoz, L. Keller, C. Randin, and J. Goudet (University of Lausanne, Lausanne, Switzerland) for discussions and/or help with experiments. N. van Dam (Netherlands Institute of Ecology, Heteren, The Netherlands) provided helpful comments on the manuscript. A. Freitag, D. Cherix, and J.-L. Gattolliat (Cantonal Zoology Museum, Lausanne, Switzerland) are thanked for identifying arthropods. This work was supported by the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cyr H, Face ML. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993;361:148–150. [Google Scholar]
- 2.McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature. 1989;341:142–144. doi: 10.1038/341142a0. [DOI] [PubMed] [Google Scholar]
- 3.Root RB, Cappuccino N. Patterns in population change and the organization of the insect community associated with goldenrod. Ecol Mono. 1992;62:393–420. [Google Scholar]
- 4.Forget PM, Lambert JE, Hulme PE, Vander Wall SB, editors. Seed Fate: Predation, Dispersal and Seedling Establishment. Wallingford, UK: CABI Publishing; 2005. [Google Scholar]
- 5.Wardle DA. Communities and Ecosystems: Linking the Aboveground and Belowground Components. Princeton: Princeton Univ Press; 2002. [Google Scholar]
- 6.Coll M, Guershon M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Ann Rev Entomol. 2002;47:267–297. doi: 10.1146/annurev.ento.47.091201.145209. [DOI] [PubMed] [Google Scholar]
- 7.Reymond P, et al. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe GA, Jander G. Plant immunity to insect herbivores. Ann Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, et al. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002;31:1–12. doi: 10.1046/j.1365-313x.2002.01328.x. [DOI] [PubMed] [Google Scholar]
- 10.Riemann M, et al. Impaired induction of the jasmonate pathway in the rice Mutant hebiba. Plant Physiol. 2003;133:1820–1830. doi: 10.1104/pp.103.027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arany AM, Jong TJ, Meijden E. Herbivory and abiotic factors affect population dynamics of Arabidopsis thaliana in a sand dune area. Plant Biol. 2005;7:549–555. doi: 10.1055/s-2005-865831. [DOI] [PubMed] [Google Scholar]
- 12.Li L, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: Induced plant defenses and insect populations. Science. 2004;305:665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- 14.McConn M, Creelman RA, Bell E, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burggren WW, McMahon BR. Biology of the Land Crabs. Cambridge, UK: Cambridge Univ Press; 1988. [Google Scholar]
- 16.Vannini M, Ruwa RK. Vertical migrations in the tree crab Sesarma leptosoma (Decapoda, Grapsidae) Marine Biol. 1994;118:271–278. [Google Scholar]
- 17.Hicks J, Rumpff H, Yorkston H. Christmas Crabs. 2nd Ed. Christmas Island, Australia: Christmas Island Natural History Association; 1990. [Google Scholar]
- 18.Cipollini D. Interactive effects of lateral shading and jasmonic acid on morphology, physiology, seed production, and defense traits in Arabidopsis thaliana. Int J Plant Sci. 2005;166:955–959. [Google Scholar]
- 19.Yan Y, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heil M, et al. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traw MB, Bergelson J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 2003;133:1367–1375. doi: 10.1104/pp.103.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavala JA, Baldwin IT. Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Cell Environ. 2006;29:1751–1760. doi: 10.1111/j.1365-3040.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 23.Caputo C, Rutitzky M, Ballaré CL. Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): Impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia. 2006;149:81–90. doi: 10.1007/s00442-006-0422-3. [DOI] [PubMed] [Google Scholar]
- 24.Maddox GD, Root RB. Structure of the selective encounter between goldenrod (Solidago altissima) and its diverse insect fauna. Ecology. 1990;71:2115–2124. [Google Scholar]
- 25.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidases in Beta Vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]