Abstract
IL-17 is the signature cytokine of recently discovered T helper type 17 (Th17) cells, which are prominent in defense against extracellular bacteria and fungi as well as in autoimmune diseases, such as rheumatoid arthritis and experimental autoimmune encephalomyelitis in animal models. IL-25 is a member of the IL-17 family of cytokines, but has been associated with Th2 responses instead and may negatively cross-regulate Th17/IL-17 responses. IL-25 can initiate an allergic asthma-like inflammation in the airways, which includes recruitment of eosinophils, mucus hypersecretion, Th2 cytokine production and airways hyperreactivity. We demonstrate that these effects of IL-25 are entirely dependent on the adaptor protein CIKS (a.k.a. Act1). Surprisingly, this adaptor is necessary to transmit IL-17 signals as well, despite the very distinct biologic responses these two cytokines elicit. We identify CD11c+ macrophage-like lung cells as physiologic relevant targets of IL-25 in vivo.
Keywords: Transgenic/Knockout Mice, Lung, Cytokines, Signal Transduction
Introduction
Asthma is a chronic condition in which the airways become inflamed and constrict in response to triggers such as allergens. The inflammatory response in lungs is associated with infiltration of CD4+ T helper cells type 2 (Th2) and eosinophils, as well as with excessive production of mucus, IgE antibodies, and airways hyperreactivity, among other pathologies (1). Although the Th2 response and its signature cytokine profile are dominant in allergic asthma, Th17 and possibly even Th1 and their signature cytokines may also contribute to lung inflammation (2-4).
Mouse models of asthma have yielded significant new insights recently. In particular the cytokine IL-25 has been shown to have a role (5); it is a member of the IL-17 cytokine family, a.k.a. IL-17E (6, 7). IL-25 has been reported to promote polarization of naïve CD4+ T cells towards the Th2 phenotype, to enhance cytokine production from polarized Th2 cells, and to induce expression of Th2 cytokines such as IL-4, IL-5 and IL-13 from other cell sources, yet to be clearly defined (8-14). Based on such data, IL-25 was proposed to initiate and maintain Th2 responses. Furthermore, by directly and/or indirectly inducing the expression of in particular IL-13 and IL-5, IL-25 may be responsible for the observed mucus production and lung infiltration of eosinophils during allergic reactions. The chain of inflammatory events may initially be triggered by allergens, which have been suggested to induce IL-25 expression from lung epithelial cells via innate immune sensors, although definitive proof remains to be obtained (8). IL-25 may also be expressed by activated basophils and mast cells (10), and by an unknown subset of T cells present in the cecal patch of the gastrointestinal tract (15). IL-25 appears to have a pivotal role in Th2-mediated host defense against helminthic parasites (15, 16). In animals challenged with the parasite Nippostrongylus brasiliensis, IL-25 was proposed to induce the early production of Th2-like cytokines by an unknown non-T, non-B cell subset present in mesenteric lymph nodes. The induced Th2 cytokines in turn facilitate rapid worm expulsion even in the absence of T cells. Nevertheless, lack of IL-25 does not prevent eventual worm clearance via a delayed Th2 response (16).
The mechanism by which IL-25 signals cells is largely unknown. IL-25 binds to a member of the IL-17 receptor family, IL-17RB (IL-25 R, IL-17 receptor homolog 1 (IL-17Rh1) or EVI27). More is known about signaling by IL-17. This cytokine binds to and signals via IL-17RA (a.k.a. IL-17R); it has recently been reported that IL-17 also binds to IL-17RC and that IL-17’s true receptor may be a heteromeric complex composed of both IL-17RA and RC (17, 18). All members of the IL-17 receptor family encode a SEFIR-like domain in their cytoplasmic tails (6). In the case of IL-17-induced signaling, the adaptor protein CIKS, a.k.a. Act1, is recruited to the receptor via heterotypic SEFIR domain association, as documented recently (19, 20). CIKS was originally cloned as an IκB kinase γ (Nemo)-interacting protein in our laboratory (21), while Act1 was cloned in a genetic screen for NF-κB-activating genes by others (22). The SEFIR domains of the IL-17RA receptor and CIKS/Act1 appear to be necessary for all downstream signaling, including activation of the transcription factors NF-κB, c/EBPβ and c/EBPδ in response to IL-17, and activation of MAPK pathways (19, 20, 23-25). In contrast to these reports, Act1/CIKS-dependent IL-17 signals have been suggested instead to primarily stabilize unstable mRNAs, especially short-lived mRNAs transcriptionally induced via TNFα-mediated NF-κB activation (26). Certainly much remains to be learned about the mechanisms underlying IL-17 signaling and specifically the role of CIKS/Act1. Regardless of the molecular mechanism however, Act1/CIKS is essential not only for IL-17 signaling in vitro but also for aspects of Th17-driven inflammation in vivo. Loss of Act1/CIKS in mice has been reported to ameliorate Th17-dependent inflammatory pathology in experimental autoimmune encephalomyelitis (EAE) and in DSS-induced colitis (20).
In addition to its role in IL-17 receptor signaling, Act1/CIKS has been reported to play a negative role in CD40 and BAFF receptor (BAFF R). Act1 deficient mice were noted to exhibit a pronounced CD40- and BAFF R-dependent B cell hyperactivation, accompanied by spenomegaly, lymphadenopathy and hyergammaglobulinemia, and with age, a lupus-like pathology (27). However, the molecular mechanisms involved and the physiologic significance of these findings remain unclear.
While CIKS is critical for IL-17 signaling, it is not known if IL-25 signals in ways similar to or distinct from IL-17. IL-25 has been conjectured to mediate effects in co-stimulated T cells in vitro via NF-κB as well as MAPKs (28); it may signal in a TRAF6-dependent manner, as determined with cells expressing exogenously introduced IL-25 Receptors (IL17RB) (29). However, efforts to understand IL-25 signaling mechanisms have been hampered by the lack of a readily responsive target cell. IL-17 and IL-25 are the most divergent members of this family, with very distinct biologic activities, suggesting distinct signaling pathways.
Here we investigate IL-25 signal-dependent functions with the help of CIKS-deficient mice generated in our laboratory. These CIKS-deficient mutant mice are impaired in IL-17 signaling, confirming prior observations, but they do not display the hyperactive B cell-dependent phenotypes of Act1-deficient animals cited above. Surprisingly we find that the CIKS adaptor is also absolutely required for all aspects of IL -25-induced pulmonary inflammation in vivo. We identify macrophage-like cells as IL-25-responders in vivo and in vitro.
Materials and Methods
Mice
CIKS deficient (KO) were originally generated and maintained on a 129/SvJ background. Subsequently the CIKS deficiency (KO) was also introduced onto a C57Black/6 (BL/6) background (10 retro-crosses of 129 KO mice to BL/6) and onto a mixed 129/Balb/c background (2 retro-crosses of 129 to Balb/c). Wild-type (WT) mice were obtained from interbreeding of heterozygous animals. Mice were bred and housed in NIAID Institute facilities and all experiments were done with approval of the NIAID Animal Care and Use Committee and in accordance with all relevant institutional guidelines.
B cell analysis
Single cell suspensions prepared from spleens were depleted of erythrocytes with ACK lysis buffer, and 106 cells were incubated with different combinations of antibodies for three or four color fluorescence surface staining. Data were collected in a FACSCalibur flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree star Inc,). The following monoclonal antibodies were used: anti-IgM (clone II/41), anti-B220 (clone RA3-6B2), anti-CD21 (clone 7G6), anti-CD23 (clone B3B4), anti-L-selectin (clone MEL-14), anti- IgD (clone 11-26c.2a). All the antibodies were purchase from BD Bioscience except anti-IgD (Southern Biotechnology Associates, Inc.)
NP-KLH challenge and immunoglobulin measurements
For Immunoglobulin (Ig) baseline detection, sera from naïve WT and KO mice were harvested and analyzed by ELISA (Southern Biotechnology Associates Inc.). For the antigen-specific Ig response, mice were challenged i.p. on days 0 and 7 with 200 μl of 100μg NP-KLH (Biosearch Technology Inc.), adsorbed to 4 mg Imject alum (Pierce Biotechnology). Sera were harvested 1 week after the last challenge and antigen-specific Ig production was measured by ELISA (Southern Biotechnology Associates Inc.).
Western blot for NF-κB2 processing
B cells were isolated from spleens via positive selection. 107 cells were incubated with anti-B220 MACS magnetic beads and sorted by passage through a LS magnetic column (Miltenyi Biotec). Splenic B220+ cells were treated or untreated with BAFF 1μg/ml (Peprotech) for various times. After incubation, B cells were harvested and whole cell protein extracted in lysis buffer containing 0.5% Triton X100. Blots were probed with rabbit polyclonal anti-p52 (directed against amino acids 1-399).
Mouse embryo fibroblasts (MEFs) and RNA isolation
Mouse embryos (E12-E13) were harvested from timed pregnancies. Heads and fetal liver were removed and the remaining tissue was chopped and digested in DMEM media containing collagenase (140 μg/ml), DNase (100 μg/ml) and hyaluranidase (140μg/ml) for 45 min on a shaker. The supernatant containing single cells was transferred to a new tube and the remaining material was subjected to a second round of digestion. After collecting the supernatant the remaining material was digested with trypsin (Invitrogen) for 30 min. All supernatants were combined and cells were spun down (MEFs). MEFs were then grown in DMEM supplemented with 10% FBS, L-glutamine (2mM), Penicillin (100 U/ml) and Streptomycin (100 μg/ml) (Invitrogen) up to 90-95 % confluence. Cells were treated or untreated with recombinant (r) IL-17 (100 ng/ml, R&D Systems) and/or TNFα (2 ng/ml, Peprotech) for 4 and 24h. Cell free supernatants were collected for multiplex cytokine assays (Linco platform, Millipore); in addition, cells were collected for RNA isolation. Cells were lysed in Trizol (Invitrogen) and total RNA was isolated using RNeasy RNA isolation kit (Qiagen) following the manufacturer’s instructions.
Real-Time PCR (RT-PCR)
cDNA was synthesized with oligo-dT using Superscript III (Invitrogen). Expression of GM-CSF, IL-6, KC (CXCL1), LNC2, IL-5, IL-13, IL-4, Eotaxin-2 (CCL24), HPRT and β-actin was quantified with the Taqman Gene Expression Assay kits (Applied Biosystems). Gene expression results are generally expressed as 2-ΔΔCt, where ΔΔCt = (Ct target-Ct HPRT) for IL-25 or IL-17 treatment - (Ct target - Ct HPRT) for PBS treatment. Data are shown as the mean ± s.e.m. In the case of MEF analyses the β-actin gene served as control instead of HPRT.
Cytokine measurements
IL-4, IL-5, GM-CSF, KC (CXCL1) and IL-6 were analyzed via multiplex assays using a LINCO platform (Millipore) or mouse Th1/Th2 kits (Bender MedSystems or BD Biosciences). IL-13 and eotaxin-2 (CCL24) were measured by ELISA (R&D Systems).
Intranasal administration of IL-25 and IL-17
Mice matched by sex and age were anesthetized with isofluorane and then challenged via intranasal (i.n.) inhalation with 40 μl of rIL-25 (5 μg) or rIL-17 (5 μg) (R&D stystems) daily for 4 days. Mice were analyzed 24 h after the last challenge. In additional experiments, mice were treated a 5th time on day 6 and then harvested 24 h later or they were treated only a single time and harvested 24 h later.
Bronchoalveolar lavage fluids (BALFs) and cytometric analysis
24h after the last challenge with rIL-25 or rIL-17, the mice were anesthetized with an i.p. (intra-peritoneal) injection of Avertin. Anesthetized mice were exsanguinated by severing the inferior vena cava and renal artery and then, tracheae were exposed. The lungs and upper airways were lavaged with 0.5ml of PBS. BALFs were centrifuged at 300x g for 5 min and supernatants were stored at -70C for further cytokine analysis. Cells were resuspended in 100 μl of PBS and stained for flow cytometric analysis. Four color staining was performed as follows: after blocking with CD16/CD32 antibodies for 10 min on ice, cells were incubated with FITC-GR-1 (RB6-8C5), PE-SiglecF (E50-2440), PercP-CD4 (L200) and APC-CCR3 (83101) for 15 min on ice. Flow cytometry of stained cells was performed with a FACSCalibur or FACSCanto (BD Biosciences) and data were analyzed using FlowJo software (Tree star Inc.). All antibodies were purchased from BD Biosciences except CCR3 (R&D Systems).
Lung histology
After bronchial lavage, lungs were perfused wih 2ml 10% formalin buffer. Paraffin-embedded 5 micron tissue sections were stained with Hematoxylin and Eosin (H&E), Periodic Acid Schiff (PAS) or Masson’s Trichrome.
Measurement of airways hyper-reactivity (AHR)
For AHR, mice were treated with 5 μg/day of rIL-25, administered on 4 consecutive days and again on day 6, and then analyzed on day 7. AHR was measured by whole body plethysmography (Buxco). Mean Penh was calculated from measurements during a 5 minute period following inhalation of methacholine (Sigma-Aldrich) (3-50 mg/ml) for 2.5 minutes.
Plasmids
Full length of CIKS and IL-17RB were cloned in the Gateway Entry vector. Entry clones were subcloned into pcDNA3.1 HA or Flag Tag destination vectors (pDest-472 and pDest-780) by Gateway LR recombination using the manufacturer’s protocols (Invitrogen) to create the expression clones. Plasmid construction and confirmatory sequencing was performed by the Protein Expression Laboratory, NCI-Frederick. The HA-tagged TNFR1δC construct was a gift from Dr. Richard Siegel (30).
Transfection, immunoblotting and immunoprecipitation
Hela cells (10 cm plates) were co-transfected with expression vectors for Flag-CIKS and either HA-IL-17RB or HA-TNFR1δC (1.5 or 3 μg of each) with the help of lipofectamine 2000 (Invitrogen), following the manufacturer’s instruction. 48h after transfection, cells were washed once with ice-cold PBS and lysed in cell lysis buffer (1% NP-40, 10 mM Tris, 150mM NaCl, 1mM EDTA 50mM NaF, 1mM Na2VO4, 25 mM β-glycerol phosphate, 0.1 mg/ml AEBSF, 10% glycerol and supplemented with protease inhibitors). The cells were incubated at 4 °C for 30 min with lysis buffer. Cell debris and unbroken cells were pelleted by centrifugation (15,000Xg) for 20 min at 4 °C. Supernatant fractions were used directly for Western blots or for immunoprecipitations. For immunoprecipitations, cell lysates were incubated with α-HA or α-Flag antibody-conjugated agarose (Sigma-Aldrich) for 1h at 4 °C, agarose beads were then washed 3 times with lysis buffer and eluted with HA or Flag peptides (Sigma Aldrich) following the manufacturer’s instructions. Eluted protein solutions and whole cell lysate supernatants were denatured with 2xSDS sample buffer for 5 min at 95 °C. For Western blots, the eluted immunoprecipitates or cell lysate supernatants were electrophoresed through 10% SDS-polyacrylamide gels followed by transfer to PVDF (Millipore) membranes. The membranes were blocked overnight in 10% milk in TBST, incubated for 2h with primary α Flag or α HA antibodies, followed by 1h incubation with anti-mouse HRP- or anti-rabbit HRP-conjugated antibodies, respectively. The signal was detected with ECL reagent (GE Healthcare).
Analysis of lung CD11c+ cells
Lungs were perfused with 2ml dispase/collagenase (1 mg/ml) and incubated for 45 min. Then, lungs were finely minced and incubated in 3 ml of DMEM without serum and digested with 100μg/ml DNase for 10 min. Cells were ACK lysed to remove erythrocytes, passed through a 70 μm cell strainer, followed by a 45μm cell strainer and centrifuged at 300xg for 5 min. Total lung cells were first blocked with CD16/CD32 antibodies for 10 minutes and then stained with FITC-CD11c, PE-CD11b, PercP-B220, PE-Cy7-F4/80 and APC-IL-17RB or PE-I-Ab, PercP-CD11b, PE-Cy7-CD11c and APC-IL17RB. Flow cytometric data were acquired in a FACSCanto (BD Bioscience) and analyzed with FlowJo software (Tree star Inc.). All antibodies were from BD Biosciences, except IL-17RB (R&D Systems). The remaining portion of the cell suspension was used to isolate CD11c+ cells using CD11c-MACS beads (Miltenyi Biotech). Briefly, 5×105 -106 cells suspended in 90μl of buffer (PBS with 0.5% BSA and 0.5mM EDTA) were incubated with 10μl of beads during 20 min on ice and then, washed in buffer. Finally CD11c+ cells were separated through a MS magnetic column. CD11c+ cells contained mainly alveolar macrophages based on cytospin analysis. RNA from CD11c+ cells was extracted and used for RT-PCR analyses.
Bone marrow derived dendritic cells (BMDCs) and macrophages (BMDMs)
BMDCs and BMDMs were generated from WT, CIKS KO and Rag1 KO mice as follows: Bone marrow cell suspensions were prepared from one femur and then ACK lysed. 5×106 cells/ml were cultured with 20 ng/ml of GM-CSF and IL-4 (R&D Systems) for 6 days to obtain BMDCs, or they were cultured with 30ng/ml of M-CSF for 5 days to obtain BMDMs. Then, cells were harvested and incubated in fresh media with or without 100 ng/ml of IL-25 for 24h. Then RNA was isolated (see above) and analyzed by RT-PCR analyses.
Depletion of alveolar macrophages with Clodronate-loaded liposomes
To deplete alveolar macrophages in vivo, mice were anesthetized with isofluorane and then treated via i.n. inhalation with 100μl of clodronate or PBS-loaded liposome on day 1, followed by 50μl of clodronate or PBS-lodaded liposomes on day 2 (loaded liposomes were obtained from Encapsula NanoSciences). 24h after the last liposome application, anesthetized mice were treated with 5μg of IL-25 (50μl) or 50μl of PBS via i.n. inhalation. BALFs were harvested 24h later and analyzed as described above. A lung lobule was frozen for subsequent RT-PCR analyses. The remaining lung tissue was digested with collagenase /dispase as described above and the extracted cells were used in FACS analyses.
Results
Generation and initial characterization of CIKS deficient mice
Mice deficient in CIKS (a.k.a. Act1, Traf3ip2) were generated in the 129/Sv background (Fig. S1). We also introduced this deficiency into a 129/Balb/c mixed background (129 backcrossed twice to Balb/c) and into the C57BL/6 (BL/6) background (129 backcrossed 10 times to BL/6). Gross examination of CIKS deficient mice showed no signs of disease, regardless of background. Mutant spleens and lymph nodes were normal-sized, contained normal numbers of cells and their splenic microarchitecture was grossly indistinguishable from that of wild-type controls, as judged by H&E tissue staining (Fig. S2 and data not shown). Further analysis also revealed normal numbers of B cells, with appropriate relative ratios of transitional 1, transitional 2, mature follicular and marginal zone B cells (Fig. 1A). Baseline immunoglobulin IgM and IgG1 levels in sera were essentially normal (Fig. 1B); furthermore, the immunoglobulin response to challenge with the T-dependent antigen NP-KLH did not significantly differ from that of wild-type littermate controls (Fig. 1C). Finally, B cells isolated from CIKS-deficient mice showed normal levels of NF-κB activation in response to anti-CD40 or BAFF, as judged by EMSA analysis, IκB phosphorylation/degradation and/or processing of NF-κB2 p100 to p52 (Fig. 1D and data not shown). These results contrast with those previously obtained with independently generated Act1 (CIKS)-deficient animals on a mixed Balb/c/129 background (27). In that study the mutant mice presented with splenomegaly, lymphadenopathy, increased numbers of B cells, increased basal and T-dependent antigen-induced immunoglobulin (Ig) levels, and hyperactivation of NF-κB in response to αCD40 and BAFF, including enhanced processing of NF-κB2 p100 to p52. Act1 deficient mice also developed a lupus-like autoimmunity accompanied by production of autoantibodies. After partial backcrossing to BL/6 mice, Act1 mutant mice were reported to have a delayed onset of B cell-mediated autoimmune phenotypes (20). It is unclear why the CIKS-deficient animals generated in our laboratory do not present such B cell-associated defects. Environmental factors, such as a distinct microflora, or subtle genetic background differences between the two mutant mouse models could play a role; the absence of Act1 might result in an unresolved immune reaction to a specific biologic agent. Final resolution of this question will have to await further experimentation and direct comparison of the two mutant models.
Figure 1.
CIKS deficient mice (KO) have a normal B cell population and normal immunoglobulin (Ig) levels. (A) Cell numbers of total splenic B cells (B220+), T1 (transitional 1, B220+ CD23- CD21- IgM high), T2 (Transitional 2, B220+ CD23+ IgM high CD21+), M (Mature, B220+ CD23+ IgM low CD21+) and MZ (marginal zone, B220+ CD23- IgM high CD21+) for wild-type (WT) and CIKS deficient (KO) mice. No significant differences were noted between KO and WT mice. Filled-in and empty bars are for splenic B cells from WT and KO mice, respectively. Values are expressed as the mean ± s.e.m. of 5 individual mice. (B) Normal Ig G1 and IgM levels in naïve CIKS KO compared to WT mice. Serum from naïve WT or CIKS KO mice were analyzed by ELISA. Values for individual mice and the means are shown. (C) Normal Ig responses after NP-KLH antigen challenge. Serum was harvested from animals primed with NP-KLH 14 and 7 days before and antigen-specific Igs were determined by ELISA. Values for individual mice and the means are shown. (D) Western blot of splenic B220+ B cells from CIKS KO and WT mice showing BAFF cytokine-induced processing of p100 (NF-κB2) to p52 during an 18 h time course. WT and KO B cells showed similar processing. Non-specific bands are indicated by *.
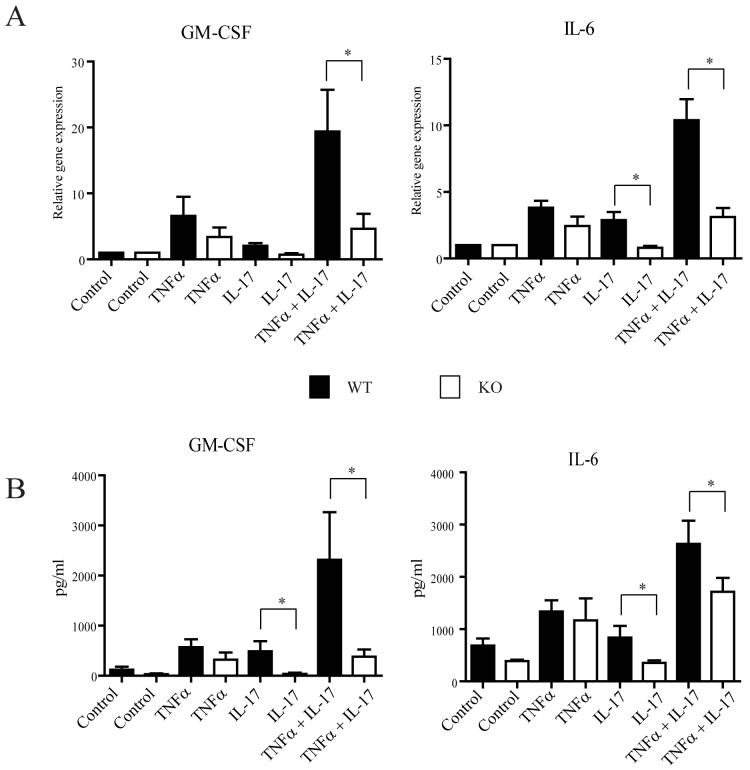
In contrast to the differences observed with respect to B cell hyperactivity, both mutant mouse models are defective in IL-17 signaling. In agreement with prior studies (19, 20), IL-17 was able to induce target genes such as IL-6 and GM-CSF alone and/or in synergy with TNFα in wild-type mouse embryo fibroblasts (MEFs), but not in CIKS-deficient MEFs; this was demonstrated by RT-PCR analysis (Fig. 2A) and by detection of protein secreted into media (Fig. 2B). In the absence of CIKS both the direct effects of IL-17 and its synergy with TNFα were abolished, while the induced expression due to TNF alone was unaffected. Similarly, loss of CIKS had no effect on LPS-induced gene expression (not shown).
Figure 2.
CIKS deficient (KO) mouse embryo fibroblasts (MEFs) do not respond to IL-17. (A) MEFs from WT or KO mice were left untreated or treated with TNFα, IL-17 and TNFα + IL-17 for 4h. RT-PCR analysis for GM-CSF and IL-6 shows KO cells were unresponsive to IL-17 (with or without TNFα), when compared to equivalently treated WT cells (data are shown as means ± s.e.m. for 5 independent experiments; * p<0.05, t-test). (B) MEF WT or CIKS KO cells were treated as described for (A) except that supernatants were harvested after 24 h and analyzed for GM-CSF and IL-6 with multiplex assays. The effects of IL-17 in WT mice were again lost in KO mice (data are shown as means ± s.e.m. for 3 independent experiments; * p<0.05, t-test).
CIKS is necessary for IL-25 induced infiltration of eosinophils into airways
IL-17 and Th17 cells, as well as the IL-17 family member IL-25 and Th2 cells have previously been implicated in inflammation of the airways, including asthma (4, 5, 11, 31, 32). To address the question whether CIKS-mediated signaling has a role in lung inflammatory pathologies, we administered IL-25 or IL-17 directly into the airways of wild-type and CIKS-deficient mice. These cytokines were introduced into lungs via nasal inhalation (i.n.) on four consecutive days and the mice were harvested on the 5th day. Bronchoalveolar lavage fluids (BALFs) of wild-type mice exposed to IL-25 contained profoundly increased numbers of cells over BALFs from control mice exposed to PBS, while those exposed to IL-17 contained more moderately increased cell numbers (Fig. 3A). In contrast, all CIKS-deficient mice exposed to either IL-25 or IL-17 failed to show any increase in BALF cellularity.
Figure 3.
IL-25-induced recruitment of eosinophils into lungs is dependent on CIKS. (A) Cell counts in BALFs from WT and CIKS KO mice treated i.n. with PBS, IL-25 or IL-17. IL-25 and, to a lesser extent, IL-17 increased cell numbers in BALFs of WT but not CIKS KO mice. (B) Differential cell counts in BALFs from WT and CIKS KO mice treated with PBS, IL-25 or IL-17. IL-25 treatment recruited eosinophils, neutrophils and macrophages, while IL-17 recruited some neutrophils and macrophages. No cells were recruited in CIKS KO mice after IL-25 or IL-17 treatment. Differential cell counts are based on FACS analyses, examples of which are shown in Fig. S3. All data are shown as means ± s.e.m; n= 6 mice; * p<0.05; **p<0.005.
Flow cytometric analysis of BALF cells revealed that IL-25 potently recruited SiglecF+, Gr-1-, CCR3+, SSChigh eosinophils into the airways of wild-type mice. IL-25 also recruited lesser amounts of SiglecF-, Gr-1+, CCR3-, SSClow, FSClow neutrophils and increased the numbers of SiglecF+, CCR3-, FSChigh, SSChigh cells; the latter cells are also CD11c+, CD11bint, autofluorescencehigh (the autofluorescence shows up in the Gr-1 channel; cells are Gr-1-) (data not shown) and consist mainly of alveolar macrophage populations that are already well-represented in BALFs from unchallenged mice (Fig. 3B; the data combine numbers obtained from flow cytometric analyses of 6 mice and a representative set of these analyses is shown in Fig. S3). CCR3 is the receptor for the chemokine exotaxin-2 (CCL24), an attractant for eosinophils. In contrast to IL-25, IL-17 failed to recruit any eosinophils, but moderately increased the numbers of neutrophils and macrophages (Fig. 3B). Consistent with the lack of increased cell numbers in BALFs from CIKS deficient mice, neither IL-25 nor IL-17 were able to recruit any individual cell type into the lungs of these mutant mice.
CIKS is required for IL-25 induction of Th2 cytokines, mucus hypersecretion and airways hyperreactivity
We tested for expression of Th2 mediators known to be involved in allergic lung inflammation. Analysis of BALFs after 4 consecutive daily exposures to IL-25 or IL-17 showed that IL-25, but not IL-17, was able to induce the protein production of IL-5, IL-13 and eotaxin-2 (CCL24) in wild type lungs (Fig. 4A). CIKS-deficient mice were completely blocked in IL-25-induced production of these proteins. We also investigated for cytokine/chemokine mRNA expression with RT-PCR analysis of whole lung tissue 24 h after a fifth and final IL-25 exposure (given on the 6th day), which revealed significantly enriched expression of mRNA for IL-4, IL-5, IL-13 and CCL24 (eotaxin-2) in WT mice, but not in CIKS deficient mice (Fig.4B).
Figure 4.
IL-25-induced expression of Th2 cytokines is dependent on CIKS. (A) Multiplex (IL-5) and ELISA analyses (IL-13, eotaxin-2) of BALFs from IL-25, IL-17 or PBS treated WT and CIKS KO mice. Mice were treated on 4 consecutive days and harvested 24 h after the last treatment. (B) RT-PCR analyses of RNA from lung tissue of WT and CIKS KO mice harvested 24 h after a 5th and final treatment with IL-25. Genes analyzed are shown. (C) RT-PCR analysis of RNA from lung tissue from WT and CIKS KO mice harvested 24 h after a single treatment with IL-25 or IL-17. Genes analyzed are shown. Data are represented as the means ± s.e.m. obtained from 9 individual mice in (A) (** p<0.001) or 6 individual mice in (B) (** p<0.005) or 4 individual mice in (C) (* p< 0.05). The indicated p values for (A) and (C) were obtained with one-way Anova analyses and confirmed with the t-test for the effects of IL-25 (or IL-17) on WT versus KO mice. The p values for (B) were obtained with the t-test.
Next we investigated cytokine/chemokine mRNA expression by RT-PCR in lungs 24 h after a single exposure to IL-25 or IL-17. As shown in Fig. 4C, IL-25 caused substantial increases in the levels of mRNAs for IL-5, IL-13 and CCL24 (eotaxin-2) in wild-type mice, but failed to do so in CIKS deficient mice. At these early times after a single exposure to the cytokine, cellular infiltration into lungs of wild-type mice had not yet commenced. In contrast to IL-25, IL-17 failed to induce Th2-related cytokines. Conversely, IL-17, but not IL-25, induced expression of lipocalin-2 and the chemokine KC (CXCL1), two known target genes of IL-17 (Fig. 4C). Induction of CXCL1 is consistent with infiltration of neutrophils upon repeated exposure to IL-17 (see Fig.3B). As predicted by the requirement of CIKS in IL-17 signaling, IL-17 was able to induce genes in wild-type, but not in CIKS deficient mice.
Lung sections from wild-type mice exposed to IL-25 via nasal inhalation showed significant mucus hypersecretion/goblet cell hyperplasia as detected with Periodic Acid Schiff (PAS) stain, while IL-17-treated mice, CIKS deficient mice or PBS-treated control mice failed to develop such pathology (Fig. 5A). Nearly 40% of the bronchioles present in the lung sections stained positive with PAS in IL-25-treated wild-type mice, while none stained positive in similarly treated CIKS deficient mice. Sections were also stained with H&E to more clearly visualize the cellular infiltrations (Fig. 5B) and adjoining sections with Masson’s Trichrome stain to visualize collagen deposition, a feature of airway remodeling associated with asthma (Fig.5C). Infiltration of inflammatory cells and collagen deposition around bronchioles were readily apparent in IL-25-treated wild-type, but not CIKS deficient samples; furthermore cellular infiltration into lung tissue or collagen deposition could not be discerned in PBS- or IL-17-treated mice (not shown), even though IL-17-treated mice had shown a modest increase in cells in BALFs (see Fig. 3A). Given the collagen deposition in IL-25-treated mice, we next measured airways hyperreactivity to methacholine, a critical feature of asthmatic lungs. Wild-type mice pretreated with IL-25 showed significantly increased hyperreactivity to methacholine compared to PBS-treated mice; in contrast, CIKS deficient mice pretreated with IL-25 did not show increased hyperreactivity (Fig. 5D). Based on these data IL-25 alone can induce many symptoms of allergic asthma in wild-type mice, consistent with most, but not all studies (9, 33). Differences in the details of the experimental protocols might account for the apparent discrepancy regarding effects of IL-25 alone in lungs (see Discussion). Importantly, the conclusion of the present experiments is that all of the IL-25-induced effects observed in lungs absolutely required CIKS, given the complete lack of any in vivo responses to IL-25 in the absence of CIKS.
Figure 5.
IL-25-induced mucus hypersecretion, eosionophil recruitment and collagen deposition in lungs depends on CIKS. (A) Periodic Acid Schiff (PAS) staining (mucus hypersecretion within bronchiole stained magenta) of formalin fixed lung sections from WT and CIKS KO mice treated with PBS, IL-25 and IL-17, as shown. Mice were treated on 4 consecutive days and lung tissue was obtained after BALFs were collected. The two panels on the right show a higher magnification of the boxed areas in the left panels (showing the walls of a bronchiole) of IL-25-treated mice. WT IL-25 treated mice: 98 of 259 bronchioles PAS positive; KO IL-25 treated mice: 0 of 200 bronchioles PAS positive. (B) H&E staining (to show cellular infiltrates in blue next to bronchiole) and (C) Masson’s Trichrome staining (to show collagen deposition in light blue next to bronchiole and vessel) of formalin fixed adjoining lung sections from WT and CIKS KO mice treated with IL-25 as in (A). Erythocytes in vessel next to bronchiole are stained red in (B) and (C). (D) Response of WT and CIKS KO mice to methacholine on day 7, after mice were first treated with IL-25 on 4 consecutive days and on day 6. Penh measurements show a significant reduction of airway resistance in KO mice when compared to WT mice. Data are shown as the means of 6 individual mice (2 independent experiments) ± s.e.m. (* p<0.05; two-way Anova analyses).
IL-25 functions as an innate effector cytokine
The rapid induction of cytokines within 24 hours after a single exposure to IL-25 in lungs of wild type mice strongly suggest that this cytokine functioned as an innate effector, independent of T cells. To test whether any T cells were required in our model, we administered IL-25 into lungs of Rag1 deficient mice, which lack all peripheral B and T cells. IL-25 was an even stronger inducer of lung inflammation in Rag1 deficient mice than in wild-type animals. Analysis of BALFs from mice on day 5 after 4 daily exposures to IL-25 showed a profound increase in cellular infiltrates into lungs; this was not seen when PBS was administered instead (Fig. 6A). Cellular infiltrates in IL-25-treated Rag1 deficient mice included significant amounts of SiglecF+, Gr-1-, CCR3+, SSChigh eosinophils (Fig. 6B), similar to what was seen in wild-type mice (see Fig. 3 and Fig. S3). Furthermore, staining of lung tissue sections from IL-25 treated mice revealed mucus hypersecretion/goblet cell hyperplasia (Fig. 6C) and infiltration of SiglecF+ eosinophils (Fig. 6D). Finally, IL-25, but not PBS, also induced Th2 mediators in Rag-1 deficient mice, as shown for IL-5 and eotaxin-2 (CCL24) (Fig. 6E). Therefore T cells are not required for IL-25 induced lung inflammation.
Figure 6.
IL-25 induced inflammation of airways is independent of T cells. (A) Cell counts in BALFs from RAG1 deficient mice treated i.n. with PBS or IL-25. Treatment was as described for Fig. 3. (B) Flow cytometric analysis of BALFs from IL-25 or PBS treated mice. Eosinophils were identified as SiglecF+, Gr-1-, SSChigh, CCR3+ cells as shown in Fig. S3). Data for (A) and (B) are shown as the means ± s.e.m. of 3 individual mice (* p<0.05). (C) PAS staining of formalin fixed lung sections from IL-25 (left panel) or PBS treated (right panel) Rag1 deficient mice (see Fig. 5 for further details). (D) Immunohistochemical staining of frozen sections from IL-25 (left panel) or PBS (right panel) treated mice with SiglecF antibodies, showing SiglecF+ cells, primarily eosinophils, surrounding a bronchiole in IL-25 treated sample. (E) Levels of IL-5 and CCL24 (eotaxin-2) in BALFs isolated from IL-25 or PBS treated RAG1 deficient mice. Data are shown as the means of 3 individual mice ± s.e.m. (* p<0.005).
IL-17RB interacts with CIKS and is present on some CD11c+ cells in lung
IL-25 has been shown to bind to IL-17RB, a member of the family of IL-17 receptors, although what actually constitutes a functional IL-25 receptor and how it may signal remains to be determined. Therefore we first tested whether IL-17RB is able to physically interact with CIKS. We co-transfected HeLa cells with expression vectors encoding HA-tagged IL-17RB and Flag-tagged CIKS, and as negative control, we co-transfected cells with HA-tagged TNFR1δC and Flag-tagged CIKS (TNFR1δC lacks the death domain to avoid cell killing (30)). The cell extracts were then immunoprecipitated with α-HA antibody-conjugated agarose, followed by peptide-specific elution and finally Western analyses with α-Flag antibodies. Immunoprecipitation of HA-IL-17RB brought down Flag-CIKS (Fig. 7A; left top panel; total lysate Westerns shown below). We also performed the reverse co-immunoprecipitation, confirming that CIKS and IL-17RB could form a complex (not shown). In contrast, the negative control experiment failed to show any CIKS in association with the TNFR1δC, even though this receptor was expressed at very high levels (Fi.g 7A; left panels).
Figure 7.
IL-25 promotes complex formation of IL-17RB with CIKS and IL-17RB is expressed on CD11c+ cells that respond to IL-25 in vivo. (A) Extracts from HeLa cells expressing HA-IL-17RB together with Flag-CIKS (left and right panels; twice as much DNA transfected in left panels), or expressing HA-TNFR1δC together with Flag-CIKS (left panel only) were immunoprecipitated (IP) with α-HA antibody (Ab) conjugated agarose, eluted with HA peptide and analyzed in Western blots (WB) with α-Flag antibodies. Western blots of 5% of the total cell lysate inputs are shown below each Co-IP, confirming expression of HA-IL-17RB, HA-TNFR1δC and Flag-CIKS. (Left panel exposure times: IP/WB 5 min; lysate WBs 1 min. Right panel exposure times: IP/WB on’t; lysate WB HA 1h; lysate WB Flag 1 min). The Co-IPs in the right set of panels show a time course of IL-25 stimulation revealing increased complex formation of IL-17RB and CIKS at the 10 min time point. (B) IL-17RB is expressed on some alveolar macrophages. Flow cytometric analysis for IL-17RB expression on cells gated on CD11c+, CD11blow, autofluorescencehigh (alveolar macrophages; gate R1; these cells are F4/80+ as well) revealed a subpopulation of IL-17RB positive cells. Also shown are analyses for IL-17RB expression on monocytes/interstitial macrophages (CD11c-, CD11b+; gate R2) (F4/80+ as well). The isotype control for gate R1 (alveolar macrophages) is shown in the right bottom panel. 6 individual mice were analyzed yielding similar results. (C) RT-PCR analyses of RNA from lung CD11c+ cells treated with IL-25 or PBS in vivo. Select genes were assayed as shown. Data are the means ± s.e.m. of 3 independent experiments comparing the IL-25 response relative to the PBS controls; for each experiment cells were pooled from 3 individual mice (*p<0.05; t-test). (D) Real time PCR analysis of RNA from BMDCs and BMDMs left untreated or treated with IL-25 for 24h. Data are shown as the means of 5 independent experiments (5 different mice) ± s.e.m.; *p<0.05. Loss of CIKS abrogated the IL-25 response.
We investigated whether stimulation with IL-25 might enhance the association of IL-17RB with CIKS. As shown in the right panels of Fig. 7A, IL-25 significantly increased the ability of the α-HA antibody-conjugated agraose (directed at HA-IL-17RB) to co-immunoprecipitate Flag-CIKS after 10 min of stimulation. At later times the complex appears to be dissociated. We conclude that IL-17RB and CIKS can associate and that complex formation is briefly but greatly enhanced upon engagement of the ligand IL-25. The interaction of CIKS and IL-17RB is likely to be mediated by heterotypic protein-protein interactions of the respective SEFIR domains of the two proteins, although this will require formal proof.
Given that CIKS interacts with the IL-25 receptor (IL-17RB), we investigated for the presence of this receptor chain on cells in lung tissue in hopes of identifying potential target cells of IL-25. Alveolar macrophages represent a prominent population of cells in unchallenged lung and they function as guardians of the alveolar-blood interface; they are critical in clearance of particulate matter and in defense against pathogens (34). We investigated alveolar macrophages and other populations for expression of IL-17RB. Flow cytometric analysis of cells from digested lung tissue of unchallenged mice revealed that CD11c+, CD11blow, autofluorescencehigh cells (gate R1), comprised of mainly alveolar macrophages, contained cells that expressed significant levels of IL-17RB on their surface (Fig. 7B) (these cells are also F4/80+, not shown); this was true regardless of whether cells were derived from wild-type or CIKS deficient mice (not shown). In contrast, CD11c-, CD11bhigh cells (also F4/80+) (gate R2), comprised of mostly monocytes and interstitial macrophages, did not contain significant numbers of cells expressing this receptor chain. We obtained too few CD11c+, CD11b+, F4/80-, autofluorescence-, mostly dendritic cells (gate R3), to determine whether they included an IL-17RB+ subpopulation. Together these data implicate CD11c+ alveolar macrophage-like cells as potential targets of IL-25.
IL-25 induces Th2-type mediators in CD11c+ cells in vivo and in vitro
To determine if CD11c+ cells respond to IL-25 in vivo, we administered IL-25 i.n., harvested mice 24h later, isolated CD11c+ cells from digested lung tissue with microbeads, and then tested for expression of cytokines and chemokines by RT-PCR. As shown in Fig. 7C, IL-25 induced the expression of the Th2-type cytokines IL-5 and IL-13 and to a lesser extent the chemokine CCL24 in CD11c+ cells of wild-type mice in vivo. On the other hand, IL-25 was unable to induce the expression of either IL-6 or IFNγ. These findings indicate that a CD11c+ population present in unchallenged lungs responded to IL-25 in vivo; the CD11c+ cells consist of mainly alveolar macrophages, but also include smaller numbers of dendritic cells and possibly some basophils.
Since at least some CD11c+ cells appear to express IL-17RB it was reasonable to hypothesize that they responded directly to IL-25, as opposed to indirectly via induced expression of secondary mediators; however, this remained to be demonstrated. To address the question whether IL-25 can directly induce IL-5 and IL-13, we generated cultures of bone marrow-derived dendritic (BMDC) cells and macrophages (BMDM) in hopes that these cultures contained cells that respond to IL-25 in vitro; if so this would provide some evidence for a direct effect of IL-25. As judged with RT-PCR analyses, IL-25 was able to induce the expression of IL-5 and IL-13 in BMDC and especially in BMDM cultures established from wild-type mice, but failed to do so in similar cultures generated from CIKS deficient mice (Fig. 7D). Th2-type inflammatory cytokines were induced as early as 2 hours after stimulation, which provides further evidence for a direct response to IL-25 (data not shown). Similar results were obtained with bone marrow cultures from Rag1-deficient mice, which rules out the possibility that potentially contaminating T lymphocytes might have been the source of IL-25-induced mediators in our cultures (data not shown). Together, these findings implicate a CD11c+ cell population in lungs in the production of Th2-type inflammatory mediators in direct response to IL-25 stimulation.
Clodronate-sensitive alveolar macrophage-like cells are the major source of IL-25-induced Th2-type cytokines
To determine the physiologic significance of CD11c+ alveolar macrophage-like cells in the IL-25 response in vivo, we investigated the induction of Th2-type cytokines/chemokines in animals significantly depleted in pulmonary macrophages. Clodronate (dichloromethylene diphosphonate; CL2MDP) is a macrophage toxin that can be effectively delivered in a liposome-encapsulated form. The lungs of mice were depleted of macrophages by administering clodronate-loaded liposomes i.n. before challenging with IL-25. The clodronate treatment resulted in a significant reduction of CD11c+ alveolar macrophages, as judged by flow cytometric analyses of BALFs and of collagenase-digested lung tissue, while control mice treated with PBS-loaded liposomes showed no such reduction (Fig. 8A). (This reduction occurred regardless of whether mice were subsequently treated with IL-25 or with PBS; data not shown). When the control mice (pretreated with PBS-loaded liposomes) were challenged with a single dose of IL-25, we observed expression of IL-5, IL-13 and eotaxin-2 (CCL24), as expected. In contrast, when clodronate pretreated animals were challenged with IL-25, the response was significantly impaired (Fig. 8B). Together, these data implicate a CD11c+, clodronate-sensitive alveolar macrophage-like population as the major source of Th2-type cytokines/chemokines in response to IL-25. These findings do not exclude the possibility that additional cell types present in lungs may respond to IL-25 as well (see Discussion).
Figure 8.
Depletion of alveolar macrophages impairs the IL-25 response. (A) I.n. inhalation of Clodronate-loaded liposomes reduced the number of alveolar macrophages in BALF (left panel) and lung (right panel). Data are shown as the means ± s.e.m. obtained from 4 individual mice (* p<0.05). For the controls, mice were treated with PBS-loaded liposomes. Total numbers of CD11c+ were as follows: BALF (Control 3.1× 104± 0.82×104; Clodronate 1×104± 0.13×104); Digested lung (Control 1.8×105± 0.36×105; Clodronate 7×104± 0.9×104). (B) Depletion of macrophages in lung greatly reduced IL-25-induced expression of IL-5, IL-13 and eotaxin-2, as shown. Data are the means ± s.e.m. of 4 individual mice (two-way Anova analyses; ** p<0.001).
Discussion
Little is known about how IL-25 transmits signals in cells or what cell types are targeted by this cytokine. The present findings demonstrate that all aspects of IL-25-induced inflammation in lungs absolutely depend on the adaptor protein CIKS (Act1) and that CD11c+ macrophage-like cells are important targets of IL-25 in lung tissue.
Repeated introduction of IL-25 into lungs via nasal inhalation caused significant pulmonary pathology consistent with allergic inflammation of the airways associated with asthma: IL-25 administration led to recruitment of eosinophils, mucus hypersecretion, Th2-associated cytokine production, collagen deposition and airways hyperreactivity. All of these responses were completely absent in lungs of mice lacking CIKS. Also, IL-25 was able to induce Th2-type cytokines in bone marrow-derived macrophage and dendritic cell cultures from wild-type, but not CIKS deficient mice in vitro. Thus, IL-25 signals are mediated exclusively via CIKS. It is surprising that both IL-17 and IL-25 absolutely require CIKS for signaling, because these cytokines are associated with very distinct T helper cell responses, Th17 and Th2, respectively, and may even cross-inhibit each other’s biologic responses (15, 35, 36). Further investigations will be needed to understand how these two cytokines can evoke such distinct physiologic effects despite their total reliance on the same adaptor protein CIKS.
In this study IL-25 was able to induce strong allergic inflammation of the airways. Previously, the presence of IL-25 in lungs was found to cause similar responses in some, but not all investigations. Direct administration of IL-25 or adenovirus-mediated expression of IL-25 in lungs induced allergic inflammation (9, 14, 37), while this was not observed when IL-25 was expressed via a transgene in Clara cells (33). Excluding possible differences in genetic backgrounds of mice used in these studies, it is conceivable that Clara cell transgene expression was not sufficient to induce significant effects by itself. Nevertheless, IL-25 produced by Clara cells was found to strongly enhance antigen-induced Th2 responses. In our study the responses to IL-25 did not require the presence of T cells, as IL-25 administered to Rag1 deficient mice caused strong allergic airway inflammation; this result is consistent with other studies that also suggested T cell-independent effects of IL-25 (13, 14). In our hands even a single administration of IL-25 was able to induce the expression of Th2-type inflammatory mediators. Therefore, IL-25 can function as an innate effector cytokine, capable of causing strong allergic inflammation in airways.
The CIKS-deficient mice generated in our laboratory did not exhibit the hyperactive B cell phenotype of the previously reported Act1-deficient mice, even though both mutant mouse models were blocked in IL-17 signals. Therefore, the present investigations with our mutant mouse model were free of potentially complicating effects of high levels of antibodies and lupus-like autoimmune pathologies. Future direct comparisons of the two mutant mouse models will be required to resolve why they share the IL-17 signaling defect, but not the B cell defects.
Despite prior efforts to discover physiologically relevant cellular targets of IL-25 in various biologic contexts, these cells remain to be clearly identified. Antigen-receptor activated naïve and Th2-differentiated T cells have been proposed as possible targets of IL-25 (3, 8, 10, 28, 33), although actual mechanisms have not been elucidated and it remains questionable whether these cells are the direct targets of IL-25. Other studies have implicated an unknown accessory non-T/non-B cell type as the primary target of IL-25, while yet others have considered eosinophils, basophils/mast cells, fibroblasts, and epithelial cells (8, 13, 14, 16, 38). Our present analyses show that some CD11c+ alveolar macrophage-like cells express the IL-25 receptor (IL-17RB) and that CD11c+ cells are functionally relevant targets of IL-25 in vivo. The CD11c+ population is comprised of primarily alveolar macrophages, and to a lesser degree, dendritic cells as well as some rare cell types; IL-25 was able to induce the expression of IL-5, IL-13 and CCL24 in cells of this population. These mediators may be sufficient to initiate allergic inflammation of the airways, which may then be amplified and sustained by infiltrating inflammatory cells, such as eosinophils. We also discovered that partial depletion of pulmonary macrophages (consisting mainly of CD11c+ alveolar macrophages) with clodronate significantly impaired induction of Th2-type mediators in response to IL-25 in lungs. Therefore our in vivo data implicate a CD11c+, alveolar macrophage-like cells as the primary responders to IL-25 in vivo. However, these data do not rule out the existence of additional responding cell types. CD11c+ dendritic cells could also contribute to the IL-25 response in vivo. If so it might explain why IL-25 was able to induce Th2-type cytokines (in a CIKS-dependent manner) not only in bone marrow-derived macrophages but to some degree also in bone marrow-derived dendritic cell cultures in vitro, although both cultures could also have harbored the same IL-25-responsive subpopulation. Beyond dendritic cells, other cell types, including epithelial cells, may potentially respond to IL-25, and responsiveness to IL-25 could even be induced during the inflammatory process. In any case, the fact that clodronate was able to largely eliminate the IL-25 response suggests that an alveolar macrophage-like cell is the main initial target in vivo.
IL-25 has been shown to bind to IL-17RB, but the exact composition of the actual receptor for IL-25 is not presently known. It has been reported recently that the receptor for IL-17 is a heteromer composed of IL-17RA and IL-17RC (17, 18). Our data now suggest that IL-25 can recruit CIKS into a short-lived complex with IL-17RB, confirming the functional link between IL-25 and CIKS at the IL-17RB receptor level. Since all members of this receptor family and CIKS share a SEFIR domain, one might predict that these receptors and thus their cytokine ligands share at least some signal transduction mechanisms. However, IL-25 and IL-17 have very divergent biologic effects; they may even oppose each other - IL-25 down-modulates IL-17-fueled inflammation associated with EAE (35), while IL-17 keeps Th2-driven allergic pulmonary pathologies in check (36). One might thus predict that these two cytokines must signal via distinct pathways. It is therefore surprising that present results show signals from both IL-17 and IL-25 to be mediated exclusively via CIKS. It will be of interest to determine if and if so how these cytokines and their receptors transmit unique signals via the same adaptor.
After submission of this manuscript a report appeared in which the receptor for IL-25 was conjectured to be a heteromer containing both IL-17RB and IL-17RA; this was based primarily on the apparent lack of biologic responses to IL-25 in IL-17RA deficient mice (39). While direct physical proof of such a receptor complex remains to be obtained, our results would be fully consistent with such a heteromeric receptor. As CIKS is known to be an essential component of IL-17RA-mediated IL-17 signaling, it stands to reason that this adaptor might then also be involved in IL-25 signaling if its receptor is composed of both IL-17RA and IL-17RB chains.
The importance of CIKS in IL-17 and IL-25 signaling could have biomedical implications. A number of inflammatory diseases have now been shown to involve either Th2- or Th17-type responses, so one may consider potential therapeutic approaches aimed at interfering with IL-25 or IL-17 mediated signaling, respectively. Since CIKS is essential for signaling by both cytokines this adaptor could be a potential therapeutic target for a wide variety of inflammatory diseases, ranging from rheumatoid arthritis to asthma.
Supplementary Material
Supplemental Figure 1. Generation of CIKS deficient mice. (A) The wild-type genomic locus with position of exons, the targeting vector and the recombined allele are shown. The recombined allele lacks exon 2, which is replaced the by the neo cassette. Generation of the recombined allele was confirmed with Southern blots (not shown). (B) Northern blot showing CIKS exon 2 deficient mice have a shortened mRNA, as predicted by loss of exon 2. Northern blot was hybridized with a probe made to parts of exon 4 and 5 (see Supplemental Materials and Methods). (C) cDNAs were generated from RNAs isolated from CIKS deficient B cells and cloned. 5 independent clones were sequenced (see Supplemental Material and Methods). All mutant cDNAs showed that exon 1 was spliced to exon 3, resulting in a sequence that cannot encode a CIKS protein: All potential ATG start codons upstream of exon 3 and located in the correct reading frame are followed by at least 2 stop codons prior to the start of exon 3, so no functional protein can be generated from them. The first ATG present within the functional reading frame downstream of the start of exon 3 is located in exon 5, a long distance from the start of the message. This ATG is unlikely to be recognized by the translational machinery, given the many ATGs that precede it in the other reading frames; in any case, this ATG could only generate a 157 amino acid long fragment from the 3′ end of the 555 amino acids long CIKS protein. Therefore, loss of exon 2 appears to have resulted in a complete loss of CIKS protein expression.
Supplemental Figure 2. H&E staining of sections from formalin fixed WT and CIKS KO spleens. Both the overall spleen size and the architecture appear normal in CIKS KOs, including white and red pulp areas. The spleens were isolated from mice on 129/SvJ and a mixed Balb/c/129 backgrounds.
Supplemental Figure 3. FACS staining of BALFs from mice treated with IL-25, IL-17 or PBS. (A) A set of FACS analyses of mice treated with PBS, IL-25 or IL-17, as shown. Data are representative of 6 different mice for each treatment. IL-25 recruited eosinophils, neutrophils and macrophages (see analyses of the various gates of an IL-25-treated mouse in (B)), while IL-17 recruited some neutrophils and macrophages. Loss of CIKS abrogated cell recruitment by either cytokine. (B) A set of FACS analyses representative of 6 individual mice treated with IL-25. Such analyses were used to identify the various cell populations in BALFs. Eosinophils (gate R2) are SiglecF+, Gr-1-, CCR3+, SSChigh and FCSlow. Neutrophils (gate R1) are SiglecF-, Gr-1+, CCR3-, SSClow and FCSlow. Alveolar macrophages (gate R3) are SiglecF+, Gr-1- (autofluorescencehigh, which is why they appear to have some Gr-1 staining), CCR3-, SSChigh and FCShigh.
Acknowledgements
We would like to thank Dr. A.S. Fauci for continued support. We are indebted to Drs. Helene Rosenberg, Paul Foster, Brian Kelsall and Aymeric Rivollier for discussion and to Richard Siegel for a gift of the TNF receptor I construct. This research was supported by the Intramural Research Program of NIAID, NIH.
Abbreviations used in this paper
- BALF
bronchoalveolar lavage fluid
- KO
gene knockout
- i.n.
intranasal
- i.p.
intraperitoneal
- PAS
Periodic Acid Schiff
- DC
dendritic cell
- BMDC
bone marrow-derived dendritic cell
- BMDM
bone marrow-derived macrophage
- RT-PCR
real time PCR
- MEF
mouse embryo fibroblast
Footnotes
This work was supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases
References
- 1.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara M, Hirose K, Kagami S, Takatori H, Wakashin H, Tamachi T, Watanabe N, Saito Y, Iwamoto I, Nakajima H. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J Allergy Clin Immunol. 2007;119:662–670. doi: 10.1016/j.jaci.2006.12.643. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007;142:265–273. doi: 10.1159/000097357. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhall C, Sjostrand M, Kolls JK, Anderson GP, Linden A. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am J Respir Cell Mol Biol. 2007;36:442–451. doi: 10.1165/rcmb.2006-0020OC. [DOI] [PubMed] [Google Scholar]
- 5.Tamachi T, Maezawa Y, Ikeda K, Iwamoto I, Nakajima H. Interleukin 25 in allergic airway inflammation. Int Arch Allergy Immunol. 2006;140(Suppl 1):59–62. doi: 10.1159/000092713. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam Horm. 2006;74:255–282. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 7.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 8.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, Van GY, Clarkin K, Nguyen HQ, Yu YB, Jing S, Senaldi G, Elliott G, Medlock ES. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 13.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 14.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 15.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 20.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 21.Leonardi A, Chariot A, Claudio E, Cunningham K, Siebenlist U. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc Natl Acad Sci U S A. 2000;97:10494–10499. doi: 10.1073/pnas.190245697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. Act1, an NF-kappa B-activating protein. Proc Natl Acad Sci U S A. 2000;97:10489–10493. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 26.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 27.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, Kotzin BL, Li X. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Wong CK, Li PW, Lam CW. Intracellular JNK, p38 MAPK and NF-kappaB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112:82–91. doi: 10.1016/j.imlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Maezawa Y, Nakajima H, Suzuki K, Tamachi T, Ikeda K, Inoue J, Saito Y, Iwamoto I. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 30.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 34.Suarez CJ, Parker NJ, Finn PW. Innate immune mechanism in allergic asthma. Curr Allergy Asthma Rep. 2008;8:451–459. doi: 10.1007/s11882-008-0085-8. [DOI] [PubMed] [Google Scholar]
- 35.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, Schow P, Gurney AL. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 38.Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J Allergy Clin Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Generation of CIKS deficient mice. (A) The wild-type genomic locus with position of exons, the targeting vector and the recombined allele are shown. The recombined allele lacks exon 2, which is replaced the by the neo cassette. Generation of the recombined allele was confirmed with Southern blots (not shown). (B) Northern blot showing CIKS exon 2 deficient mice have a shortened mRNA, as predicted by loss of exon 2. Northern blot was hybridized with a probe made to parts of exon 4 and 5 (see Supplemental Materials and Methods). (C) cDNAs were generated from RNAs isolated from CIKS deficient B cells and cloned. 5 independent clones were sequenced (see Supplemental Material and Methods). All mutant cDNAs showed that exon 1 was spliced to exon 3, resulting in a sequence that cannot encode a CIKS protein: All potential ATG start codons upstream of exon 3 and located in the correct reading frame are followed by at least 2 stop codons prior to the start of exon 3, so no functional protein can be generated from them. The first ATG present within the functional reading frame downstream of the start of exon 3 is located in exon 5, a long distance from the start of the message. This ATG is unlikely to be recognized by the translational machinery, given the many ATGs that precede it in the other reading frames; in any case, this ATG could only generate a 157 amino acid long fragment from the 3′ end of the 555 amino acids long CIKS protein. Therefore, loss of exon 2 appears to have resulted in a complete loss of CIKS protein expression.
Supplemental Figure 2. H&E staining of sections from formalin fixed WT and CIKS KO spleens. Both the overall spleen size and the architecture appear normal in CIKS KOs, including white and red pulp areas. The spleens were isolated from mice on 129/SvJ and a mixed Balb/c/129 backgrounds.
Supplemental Figure 3. FACS staining of BALFs from mice treated with IL-25, IL-17 or PBS. (A) A set of FACS analyses of mice treated with PBS, IL-25 or IL-17, as shown. Data are representative of 6 different mice for each treatment. IL-25 recruited eosinophils, neutrophils and macrophages (see analyses of the various gates of an IL-25-treated mouse in (B)), while IL-17 recruited some neutrophils and macrophages. Loss of CIKS abrogated cell recruitment by either cytokine. (B) A set of FACS analyses representative of 6 individual mice treated with IL-25. Such analyses were used to identify the various cell populations in BALFs. Eosinophils (gate R2) are SiglecF+, Gr-1-, CCR3+, SSChigh and FCSlow. Neutrophils (gate R1) are SiglecF-, Gr-1+, CCR3-, SSClow and FCSlow. Alveolar macrophages (gate R3) are SiglecF+, Gr-1- (autofluorescencehigh, which is why they appear to have some Gr-1 staining), CCR3-, SSChigh and FCShigh.