Abstract
Objective
Design, implement, and evaluate a standardized approach to teaching clinical toxicology for pharmacy students.
Design
An extensive review of the literature was conducted and a standardized approach to teaching Clinical Toxicology, a 1-credit-hour elective course, was designed, consisting of 9 key questions to address any clinical poisoning. A patient-oriented and problem-solving approach to clinical toxicology was adopted throughout the course.
Assessment
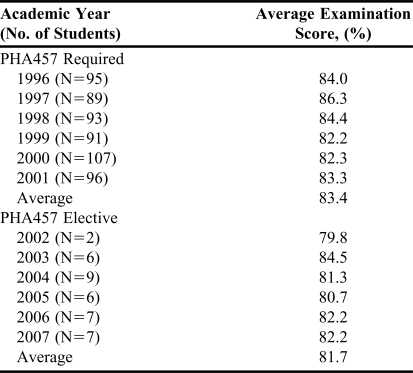
Average performance on course examinations for the academic years 1996-2001 (N=604) when the course was required and the academic years 2002-2007 (N=37) when the course was changed to an elective was 83.4% and 81.7%, respectively.
Conclusions
A standardized, patient-oriented approach to teaching clinical toxicology allowed successful conversion of this material from a required course to an elective and facilitated students' exposure and interest in this important area of practice.
Keywords: clinical toxicology, teaching methods, curriculum, elective, toxicology
INTRODUCTION
Clinical toxicology is a discipline that focuses on diseases that are caused by or are uniquely associated with toxic substances.1,2 Pharmacists are knowledgeable of the principles of clinical toxicology including median lethal dose (LD50), median effective dose (ED50), margin of safety, therapeutic index (TI), risk assessment, spectrum of effects from a drug, regulatory agencies related to handling of drugs and chemicals, types of toxic reactions, types of interactions between chemicals, and basic concepts in the prevention and treatment of poisoning from drugs. Clinical toxicologists treat patients who are poisoned by drugs and other chemicals and develop new techniques for the diagnosis and treatment of such intoxications.1,2 Pharmacists can play a major role since they have an in-depth understanding of drug action and drug adverse effects. In addition, since poisonings do and will occur, pharmacists as the most accessible health care professionals can potentially serve as first responders in the community. With the proper training, this can become a part of their professional responsibility in delivering pharmaceutical care. Career opportunities for pharmacists in this area include working in poison control centers (PCC), emergency departments/care units and intensive care units. Through membership in the American Academy of Clinical Toxicology, pharmacists can seek credentialing through the American Board of Applied Toxicology (ABAT).
There are currently no data available on the number of colleges and schools that offer courses in clinical toxicology. However, a literature search of the last 20 years resulted in a few articles and abstracts related to clinical toxicology courses for pharmacy students.3-9 A description and documentation of the effectiveness of a standardized approach to treating poisoned patients as part of a clinical toxicology course is presented. The overall course objective was to emphasize to the students the important role that pharmacists can play in poison control prevention and treatment, and in the process, stimulate student interest in career opportunities in this important area of pharmacy practice and how they can potentially incorporate knowledge from this discipline into their practice. The standardized approach to each poisoning is the tool used to help the students relate to key questions to address a poisoning. In this manuscript, acetaminophen poisoning is discussed as an example of how this approach is utilized to meet the overall course and lesson objectives.
DESIGN
PHA457, Clinical Toxicology, is 1-credit hour elective course that originally was a required course (1996-2001) taught to third-year pharmacy students at Creighton University. Since 2002, it has been offered as an elective course in the fall semester for both our campus and distance students.12 When it was required, the course was taught in a classroom. As an elective course, students accessed the course via the course Web site, which was authored in Microsoft FrontPage. Students communicated with the instructor via e-mail, online discussion folders for each lesson, and online and on-campus conferences. Students who enrolled in PHA457 had completed the first semester of their second-professional year, the curriculum for which included medicinal chemistry, pharmacology, and patient assessment.
Lesson objectives for PHA457 are linked to the schools educational outcomes and to Bloom's Taxonomy of Learning.10,11 They are based on 9 key objectives and are as follows for all poisonings:
Discuss the incidence of the chemical poisoning in the United States and provide a historical perspective and outcomes based on the American Association of Poison Control Centers (AAPCC) annual report.
Explain the mechanism of the chemical toxicity and its clinical implications.
Analyze how the chemical toxicity would be influenced if your patient for example, has prior exposure to inducers of microsomal enzymes, chronic alcohol/tobacco consumption, or inhibitors of cytochrome (cyp) enzyme system; or is an adult, an older patient, or a child.
Evaluate the critical pharmacokinetic properties of the chemical influencing toxicity/poisoning.
List the stages (if any) or general signs and symptoms of the poisoning and describe the clinical indicators of poisoning/toxicity with the chemical.
Explain the association (if any) between serum concentration of the chemical and the risk of toxicity.
Develop a treatment plan for your patient.
Evaluate the outcome(s) of the chemical poisoning.
Discuss the pharmacist role in preventing/treating the chemical poisoning.
The clinical toxicology course Web site had links to all the course lessons, primary literature articles, selected textbook chapters, and links to helpful Web sites on poisoning prevention and treatment. The first 4 lessons of the course transitioned students from the general knowledge concepts/principles in clinical toxicology to how to apply these concepts/principles in their role as pharmacists. Students were told that the course was not meant to make them experts but rather to stimulate their interest in this important area of clinical pharmacy practice and to pursue higher training and certification. The most common poisonings occurring in the local area and nationally were addressed, including aspirin, iron, tricyclic antidepressants, alcohol, opiates, sedative hypnotics, and pesticides.
All lesson handouts were intentionally written to be comprehensive, descriptive, conversational, and reinforcing. The handout was divided into 9 sections based on the learning objectives above. A thorough understanding of all sections was required to be able to apply the knowledge on the examination. An extensive search of clinical toxicology textbooks was conducted,13-16 and primary literature and the local PCC resources were consulted in creating the final content. With the exception of Goldfrank's Toxicological Emergencies textbook,14 none of the other textbooks reviewed presented a patient-oriented, problem-solving approach to clinical toxicology. However, Goldfrank's content structure changes from chapter to chapter, making it difficult to find information quickly.
Students were encouraged to review their Patient Assessment course notes to help them in recognizing clinical signs and symptoms that are common/typical in a poisoned patient. Also, since there was not a clinical chemistry course in our curriculum, students were given a handout with some of the key laboratory values and their clinical significance, especially those needed to understand the clinical findings for poisonings discussed in the course. This was important to help the students hone in on critical signs and symptoms and combine that with clinical chemistry findings to confirm and appropriately treat the poisoning. Our standardized approach was intended to help students become more comfortable with key principles in poison prevention and treatment and apply them as they encountered different poisonings in real patient scenarios.
Appendix 1 is a sample case study that was obtained from the local PCC. Cases were chosen to highlight the key clinical issues for each poisoning discussed. Another sample case or review article was provided on the course Web site for the students to review on their own.17 Key application questions from the PCC and the review article were introduced on course evaluations to challenge the students. Following the introduction of the case, students were asked to address the 9 key objectives, keeping in mind their patient in the case study.
Course Content
Incidence of Acetaminophen Poisoning.
The incidence of each poisoning, the demographics of the poisoned individuals, and documented outcomes are key information to know about a poisoning. Strategies for the prevention of a poisoning are better developed with an understanding of the above information and its implications for clinicians, including practicing pharmacists. Acetaminophen has been utilized as an analgesic and antipyretic since the mid-1950s and became more prominently recognized as a potential hepatotoxin in overdose situations in the early 1970s.17,18 Based on the 2006 American Association of Poison Control Centers (AAPCC) Annual Report, acetaminophen is now the single most common cause of poisoning requiring hospital admission and medical management.19 Approximately 48,210 cases of acetaminophen (only) ingestion were reported to PCCs in 2006 in the United States, of which 15,556 were evaluated in a health care facility. Several factors contribute to this including increased usage and availability of acetaminophen and increased use in children due to possible development of Reye's syndrome when salicylates are used. Also, many physicians seem unaware of the poor anti-inflammatory activity of acetaminophen.17,18
The actual mortality rate for acetaminophen overdose is remarkably low. Children less than 9 years of age have lower incidence of hepatotoxicity after an acute overdose than do adults due to early spontaneous emesis, differences in metabolic pathways, Cyp enzyme system playing a smaller role in metabolism, increased turnover rate of glutathione, and increased antioxidant activity.17,18
Sixty-two percent of the cases of acetaminophen poisonings occurred in children <6 years of age, 20% in children 6 to 19 years of age, and18% in people >19 years of age.19 The negative outcomes of exposures to the adult formulation were classified as none or minor in 89% of cases; moderate in 8%; and major in 3%, including 45 cases resulting in death. In comparison, less than 1% of exposures to the pediatric formulation were classified as major and no deaths were reported. The report also indicated that 21% of the cases were intentional.
There were 37,701 cases of poisonings involving acetaminophen in combination with another drug (aspirin, codeine, hydrocodone, oxycodone, propoxyphene, and other narcotics) reported in the 2006 AAPCC Annual Report.19 In general, the percent of moderate to major outcomes was higher (18%), with death occurring with all combinations except aspirin. Poisonings involving combination products cause more dramatic acute symptoms.19
(Typical student assignment following coverage of this material: Review the AAPCC and local PCC Annual Report regarding acetaminophen poisoning and provide any practical implications for you as a local or national future pharmacist.)
Mechanism of Toxicity of Acetaminophen.
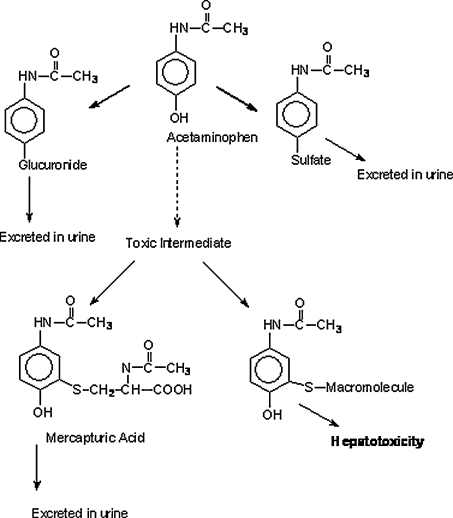
From prior knowledge in pharmacology and medicinal chemistry, students were encouraged to reflect on the importance of knowing the mechanism of action of a drug and how this can help in understanding therapeutic use as well as potential side effects and adverse effects. As important, students learned how to prevent and protect against toxicity. Figure 1 provides a schematic explanation for how acetaminophen metabolism contributes to toxicity. Key concepts that students need to come away with include:
Figure 1.
Mechanism of acetaminophen toxicity.
A maximum therapeutic daily dose of acetaminophen is 4 grams in adults and 150 mg/kg for children.
Acetaminophen is metabolized 98% by the liver through conjugation to inactive glucuronide (42%) and to inactive sulfate metabolites. Both pathways are saturable in adults.
Approximately 3% to 8% is metabolized by Cyt-p450 to highly reactive alkylating metabolite that is inactivated by conjugation with glutathione (GSH) and is excreted in the urine as cysteine and mercapturic acid conjugate.
In overdose/poisoning, the glucuronidation and sulfation pathways become saturable and metabolism by Cyt-p450 is increased, resulting in increased formation of the reactive intermediate, N-acetyl-p-benzoquinoneimine (NAPQI.).
Accumulation of NAPQI. results in rapidly depleting the natural protective stores of GSH. When 70% of GSH stores is depleted, NAPQI. binds covalently with the protein macromolecules in the hepatocytes resulting in cell death and destruction of liver structure.
(Typical student assignment following coverage of this material: Based on your understanding of the mechanism of action of acetaminophen, discuss the clinical implications for the patient following ingestion.)
How specific factors influence acetaminophen toxicity.
Equipped with an understanding of the mechanism of toxicity of acetaminophen, students were challenged to predict how toxicity would be influenced by several predictable and nonpredictable factors. Examples included prior exposure to inducers of microsomal enzymes, chronic alcohol consumption, prior exposure to inhibitors of Cyp enzyme system, and whether the patient was an adult, an older patient, a child, malnourished, or had depleted GSH stores. Studies from the literature that clearly address the impact of any of the above or other factors on the extent and outcome of toxicity were discussed, such as chronic alcohol abuse as an independent factor for mortality and development of hepatic encephalopathy.20-22 Tobacco use is another independent factor for development of severe toxicity, liver failure, and death.23 (Typical student assignment following coverage of this material: Identify and discuss the factors that may have worsened the outcome for the patient.)
The critical pharmacokinetic properties of acetaminophen influencing toxicity/poisoning.
Acetaminophen is rapidly and almost completely absorbed from the gastrointestinal tract by 4 hours. Students should be able to predict that absorption will be prolonged for sustained release formulations and when acetaminophen is co-ingested with drugs that decrease gastric emptying such as diphenhydramine. Acetaminophen is relatively uniformly distributed throughout most body fluids and binding to plasma proteins is variable (20%-50%). The highest concentration is seen in the peripheral zone of the liver and renal medulla. Hence, the potential toxicity for these organs is predictable. The metabolism and its implication are discussed above. As for excretion, only 2% of an acetaminophen dose is excreted unchanged in urine, with an elimination half life 1-3 hours. However, excretion may exceed 12 hours in acute overdose. (Typical student assignment following coverage of this material: Discuss the implications for NZA based on the pharmacokinetics of the acetaminophen preparation ingested.)
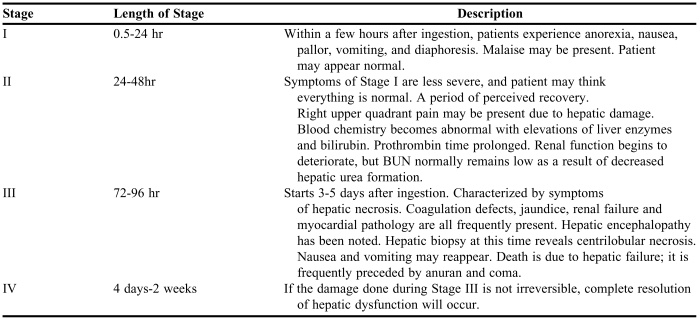
Stages and general signs and symptoms of poisoning from acetaminophen.
Student familiarity and ability to predict the manifestation of toxicity following chemical exposure was important. Some of the poisons have identified stages following the toxicity while others may not and require a differential diagnosis to help identify the causative agent. Table 1 is a summary of the stages of acetaminophen poisoning. The signs and symptoms provide key information that is important to monitoring poisoned patients, such as liver function tests, renal function tests, prothrombin time, total bilirubin, international normalized ration (INR), serum creatinine, blood urea nitrogen, urinalysis, hemoglobin, and electrocardiogram (EKG). (Typical student assignment following coverage of this material: Identify what signs and symptoms of acetaminophen toxicity with which the patient presented. What stage of acetaminophen did she arrive at the hospital with?)
Table 1.
Stages of Acetaminophen Poisoning
The association between serum concentration of acetaminophen and the risk of toxicity.
In the case of acetaminophen, the Rumack-Matthew (R-M) nomogram is reliable for interpreting a single plasma level obtained 4-24 hours after a single acute ingestion. Levels above the lower or treatment line predicts risk for delayed hepatotoxicity and need for full N-acetylcysteine (NAC) treatment. Since the acetaminophen level is critical for determining potential for toxicity, factors that may influence the level should be considered before final decisions are made regarding treatment. These factors include making sure that the laboratory method measures plasma concentration of free acetaminophen, measuring levels for liquid preparations at 2 hours post ingestion, and treating if the levels are greater than or equal to 225 mg/dl, treating patients “near” the treatment line in the R-M nomogram in the case of multiple ingestion since pharmacokinetics are altered by the other drugs in such cases, drawing additional levels if ingestions occurs with food or drugs such as diphenhydramine (eg, Tylenol PM), opioids, or other drugs that delay absorption. (Typical student assignment following coverage of this material: What is the importance of acetaminophen level on day 3 for the patient?)
Developing a treatment plan for a patient.
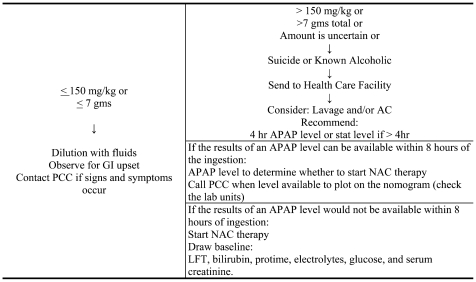
Students were given general recommendations such as if the patient is unconscious or not breathing, call 911 immediately, and if the patient is awake and breathing without symptoms, call the local PCC or the AAPCC (800-222-1222). Students are also asked to recall the general principles of managing the overdosed patient, including that treatment of acute poisoning must be prompt and aimed at treating the patient not the poison. In addition, the first goal should be to maintain the patient's vital functions if impairment is imminent. The second goal is to keep the concentration of the poison in the crucial tissues as low as possible by preventing absorption and enhancing elimination. Finally, the third goal is to combat the pharmacological and toxicological effects at the affected sites (ie, treat with an antidote if one is available).24 Specific recommendations based on the local PCC and major reference textbooks were summarized for the students. Recommendations for pre-hospital treatment and hospital treatment were addressed. For example, Table 2 is a summary based on a review of clinical toxicology textbooks, the literature,13-18 and the local PCC guidelines, and it clearly indicates the recommendation based on the amount of ingestion of less or more than 150 mg/kg of 7 gm total.
Table 2.
Students were challenged to address and research all aspects of drugs used in the treatment such as the time window for administration and effectiveness of activated charcoal, appropriate dosing, and proper administration. Practical issues such as whether activated charcoal should be administered if the patient is receiving oral or intravenous (IV) NAC therapy were also discussed.
NAC is the antidote for toxic acetaminophen overdose. It can be given orally or intravenously. Important information for oral treatment includes knowing the types of preparations on the market, which preparation to use in a pediatric patient, and the importance of mixing it with juice or other flavorings to mask its foul odor. For administration, if the patient can not take it by mouth, a tube may be placed through the mouth and into the stomach to help administer it. However, if oral administration is not possible, IV therapy is warranted. Other important considerations related to NAC therapy include determining the need for therapy based on levels, how to interpret levels less than 4 hours after ingestions, the importance of not delaying therapy for lack of levels, and to not discontinue therapy if the initial level is above the treatment line and subsequent levels fall below the treatment line (Table 2). Appropriate dosing of the oral and IV preparation is also critical. Practical tips such as repeating the oral dose if vomiting occurs within 1 hour of administration, how to prepare the loading and maintenance dose of the IV, and what to watch for as potential signs of toxicity from the IV dose are essential for pharmacists to know when treating an overdosed person in order to contribute to a team effort. Further, the role of antiemetics is also important to discuss in acetaminophen overdose. Metoclopramide, ondansetron and diphenhydramine can be used and appropriate dosing is addressed. Phenothiazines are generally not recommended since they tend to decrease the seizure threshold. (Typical student assignment following coverage of this material: Evaluate and discuss the treatment plan provided to the patient.)
Evaluating the outcome(s) of acetaminophen poisoning.
A thorough review of the clinical literature is essential to better understand the big picture related to any poisoning. Much practical information is gathered to help clinicians individualize the therapy and be familiar with standards of practice for treatment of a poisoning. Students were encouraged to contribute to this section by doing their own readings/research. Information shared with the students included:
If the poisoning is taken care of properly and in a timely manner, the patient will escape major organ damage. However, a high acetaminophen level combined with lack of proper and timely treatment will result in organ damage and even death.
In chronic overdose, most adult cases have compounding factors such as chronic alcohol abuse, starvation, or concomitant use of enzyme-inducing drugs, and hepatotoxicity can not be attributed solely to acetaminophen overdose.
A study of acute acetaminophen overdose during pregnancy in 60 cases concluded that pregnant women who take an acetaminophen overdose and have a potentially y toxic serum level should be treated with NAC as early as possible. In 8 out of 10 cases where the pregnant mother was treated within 10 hours post-ingestion, the mothers delivered normal infants.25 (Typical student assignment following coverage of this material: Discuss the recent recommendations regarding syrup of ipecac stocking at home, and home use.)
The pharmacist role in preventing/treating acetaminophen poisoning.
One of our main goals was to encourage students to develop practical strategies to use as pharmacists in the prevention and treatment of a specific poisoning. General strategies for the prevention and treatment of poisoning were summarized for the students early in the semester and they were challenged in the classroom, through online discussions, and on course examinations to build on those strategies.
Two examinations containing essay (40%-50% of an examination) and multiple-choice questions were administered to students in the course. All questions were application questions and the majority of the essay questions and some of the multiple-choice questions were based on actual case studies and challenged the students to think like pharmacists. Example questions from the acetaminophen lesson and other lessons are presented in Appendix 2.
Student performance on course examinations, students' post-class interest in clinical toxicology, and student perceptions based on course evaluation were utilized to assess whether students met the course objectives. All course evaluations were conducted electronically in IDEA (The IDEA Center, http://www.theideacenter.org/, Manhattan, KS) or QuestionMark (Questionmark Computing Limited, http://www.questionmark.com/us/index.aspx) and student input remained confidential.
ASSESSMENT
Since 2002, 37 students have taken the course including 12 distance students.12 Average performance on course examinations in the academic years 1996-2001 (N=571) when the course was required and the academic years 2002-2007 (N=37) when the PHA457 course was changed to an elective was 83.4% and 81.7%, respectively (Table 3). In general, students' performance on the essay component of the examinations demonstrated an in-depth understanding of the content and an ability to apply it to different patient scenarios based on the cases introduced on the examination. Students' answers to questions on how they would establish a prevention program for a particular poisoning have consistently resulted in innovative, pertinent, and practical suggestions. The following are some examples:
Table 3.
Comparison of Pharmacy Student Performance in a Clinical Toxicology When Administered as a Required Course and as an Elective
Counsel patients/family members on the importance of properly storing acetaminophen and other medications out of the reach of children and in child-resistant containers, dosage forms available, on the necessity of complying with dosage recommendations and provide lessons to teach parents immediate first aid steps.
Educate parents/patients on how to calculate dose based on a child's weight especially based on the concentration of liquid preparations.
Increase patient awareness through posters and pamphlets, and poison prevention programs.
Communicate with local or national PCC specialists on the latest recommendations for prevention and treatment of specific poisonings in an outpatient setting.
Establish strategies for poison prevention education by obtaining and examining local epidemiologic characteristics of exposure and by developing intervention techniques.
As a poison control specialist, emergency room or inpatient hospital pharmacist, review the primary literature for the latest recommendations for treatment of specific poisonings, including appropriate dosing of an antidote and other drugs.
Identify and be familiar with references and information database programs on poisonings and being able to communicate and apply the information in the practice setting to help the medical team attend to a patient with a poisoning.
Advise patients not to drink alcohol while taking acetaminophen and counsel the patient on the potential for increased toxic effects with chronic use and concurrent chronic alcohol and tobacco consumption.
Pursue a residency program/fellowship in clinical toxicology.
Become an active member of the American Academy of Clinical Toxicology and pursue ABAT credentialing.
Student perceptions based on course evaluations over the last 11 academic years have been consistent. Ninety percent of the students agreed that the course content and assessments were consistent with the syllabus and that the course encouraged refinement of critical thinking. Students' overall attitude towards the course content was positive, with more than 90% indicating that the course content was organized and helpful in understanding the material. One hundred percent of the students agreed that the course encouraged them to strive to expand their knowledge of the subject matter. Students' subjective comments support the above data with comments: “Course provides valuable and necessary information for clinical practice.” “The lessons are organized with an easy to follow format for each poisoning.” “Provides important case scenarios with real life overdose situations.” “Helped me organize my understanding and approach to poisoning.”
DISCUSSION
The new standards for the Accreditation Council for Pharmacy Education (ACPE), guideline 10.2.26 state that the pharmacy curriculum should “strive for availability of sufficient elective courses and pharmacy practice experiences to allow students to pursue special interests.” Therefore, the curriculum must provide such opportunities for students to explore specific disciplines and areas of practice. It was not an easy decision when faculty members voted in 2001 to no longer require the PHA457 course and to have the content covered in pharmacology. However, out of a strong belief that pharmacy students should be exposed to this important area of clinical practice, the course was offered as an elective. In our School, students are required to take 10 elective hours; however, they can apply 5 credit hours from the prepharmacy coursework if they completed hours above the required prepharmacy credits. Thus, the majority of students only need to fill 5 additional elective credit hours. That coupled with the availability of several excellent electives including the popular immunization and diabetes certificate courses, makes recruiting students for PHA457 an ongoing challenge. Efforts to increase enrollment in the course are continuous, including e-mailing students during registration periods and before the semester starts, and by briefly introducing/previewing the course in other required courses. In addition, students who take the course are encouraged to share their experience with their peers. Further, an effort is underway to integrate clinical toxicology into pharmacology in the newly revised curriculum so that all students get exposure to this important area of clinical practice. Since the course was introduced in fall 1996, 2 students have elected to pursue and complete a clinical residency in a PCC and have been practicing at an academic institution under that capacity. In addition, several students have also elected to take a PCC elective clerkship in their P4 year, including 2 of the 9 students who completed the course last year.
Although both subjective observations and objective data show a positive response by the students, several course modifications were made over the years that have improved on the student experience including modifications to the organization of the lesson handouts, adding more discussion questions in the lesson handout, providing online review sessions through our conferencing server, and prompting students to interact online by constant e-mail messages and reminders. In addition, based on student input for the last 2 years, and starting fall 2008, students will be required to arrange for and provide a reflection on a 2-day experience in either a PCC (if one is within a 30-minute driving distance), emergency room, intensive care unit, or community pharmacy setting where students can observe and experience poisoning treatment and prevention. This was an option for students when the course was required (1996-2001) and several of the students chose to visit our local PCC.
SUMMARY
Based on 6 years' experience teaching the required clinical toxicology course, conducting an extensive review of major clinical toxicology textbooks and the primary literature, and utilizing the local PCC as a resource, the required course in clinical toxicology was reorganized/redesigned/reformatted as an elective online course. The offering of the course has been a rewarding experience for the students and the instructor. The standardized approach to looking at poisoning helped the students to appreciate the discipline more and value the knowledge gained, and for some students, motivated them to pursue further training in this area of pharmacy. Providing students with opportunities that meet their special interests can open new career opportunities for them and enhance the role future pharmacists can play in the community and as active members of the health care team.
Appendix 1. Acetaminophen Case Study
NZA is a 37 year-old female, known alcoholic, was in an auto accident for which she was treated and released at a local emergency department. Over the ensuing 48 hours, she ingested approximately 35 extra strength acetaminophen along with some alcohol. She presented to her local hospital on day three following the accident with a complaint of persistent vomiting. She was alert and oriented with jaundice noted on initial exam. Labs drawn on admission showed AST 3646 U/L (N 0-46 U/L), ALT 1515 U/L (N 0-56 U/L), and Creatinine 2.0 mg/dl (N 0.6-1.1 mg/dl). The Poison Center was contacted at this time concerning N-acetylcysteine recommendations. A loading dose was given and the patient was transferred to a nearby tertiary care facility and liver transplant center.
Admission vital signs at the liver center on day 3 were T 33.4 C, P 97, R 39, and BP 115/68. Admissions lab were as follows: WBC 15,800 (N 4,000-11,000) with 75% segs/ 16% bands/ 9% lymphs, Hgb 10.3 g/dl (N 11.5-15.5 g/dl), platelets 129,000 (N 150,000-450,000), PT 30.3 sec (N 11.2-13.8 sec), PTT 42.5 sec (N 22.0-37 sec), CO2 8 mEq/L (N 22-30 mEq/L), glucose 276 mg/dl (N 60-110 mg/dl), AST 6060 U/L (N 0-46 U/L), ALT 3738 U/L (N 0-56 U/L), GGT 131 U/L (N 23-208 U/L), and ABG's with pH 7.17 (N 7.35-7.45), pCO2 16 mmHg (N 35-45 mmHg), pO2 72 mmHg (N 75-100 mmHg). The serum acetaminophen level was 12.9 mcg/ml and a drug screen was negative for PCP, benzodiazepines, cocaine, amphetamines, cannabinoids, opiates, barbiturates, and tricyclics. The patient was placed in the ICU and continued on n-acetylcysteine.
The patient developed renal failure on day four with markedly decreased urine output and multiple casts and renal cells noted on urinalysis. Her lab values also peaked with PT 39.7 sec, PTT 43.7 sec, AST 6743 U/L, and ALT 4350 U/L. The patient required hemodialysis and blood transfusions. This was repeated on day five but the patient continued to deteriorate. She required intubation and ventilation for increasing encephalopathy. An intracranial pressure monitor was placed. Liver transplant was discussed with the family, but the patient was not considered a candidate due to her past history of unremitting alcoholism. On day six, the patient developed severe hypotension and acidosis unresponsive to dobutamine and levophed. Patchy infiltrates were noted on chest x-ray consistent with ARDS. She coded late in the morning on day six and died. Death was attributed to her acetaminophen overdose superimposed on her chronic alcoholism. Autopsy findings were consistent with fulminate hepatic failure, severe liver necrosis and ARDS.
Appendix 2. Examples of the Problem-Solving Questions Utilized in Examinations
ACETAMINOPHEN
Question 1.
A 28 year old woman (A.A.) is admitted to the ER after her boyfriend suspected she ingested approximately 30 extra strength Tylenol around 3 hours ago. The patient will neither confirm nor deny the ingestion. A stat acetaminophen level was ordered and the patient is observed for another hour after which another blood sample is obtained. The toxicology screen comes back negative for everything but acetaminophen which has a blood level of 150mcg/ml at 4 hours post exposure. The patient is started on oral 20% N-acetylcysteine (NAC) and is given the loading dose of 140 mg/kg plus 70 mg/kg maintenance doses every eight hours, mixed with a soft drink to make a 5% solution. A total of 5 maintenance doses of NAC are administered, all but the last one is well tolerated. Following the fifth maintenance dose the patient vomited and the maintenance doses were stopped due to intolerance of the antidote. Aside from the acetaminophen level in the blood and clotting time, no other laboratory tests were performed. Patient was released 36 hours after admission. For the past year, A.A. has been on phenobarbital for control of tonic-clonic seizures.
Evaluate the treatment provided to AA and what was done correctly or incorrectly.
Other than any problem with the treatment initiated on A.A. as described in the case above, list and explain one specific factor related to A.A. that might worsen the outcome of this poisoning in A.A.
If A.A. ingested an extended release formulation of acetaminophen, give a one sentence recommendation that you would share with the attending E.R. physician.
Question 2.
For the assigned paper on acetaminophen overdose, provide two factors that might Have contributed to the negative outcome (death) of G.M.
Question 3.
Briefly discuss two specific epidemiological findings about acetaminophen poisoning and identify how you as a community pharmacist will incorporate these findings into a prevention educational program.
ASPIRIN
Question 4.
R.A. is a 5 year old boy who ingested approximately 20 adult strength aspirin tablets 2 hours ago. The patient has no signs of GI distress, is clear and coherent, and besides the general feeling of being hot, has no physical complaints. Blood gases are PCO2 = 35 mm Hg (35-45); PO2 = 97 mm Hg (75-100). Blood pH is 7.25 (7.35-7.45). Life support has been established.
What treatment would you initiate to prevent further toxicity and to treat current manifestations of aspirin toxicity in R.A.?
Syrup of ipecac 15 ml followed by 6-8 ounces of water
Correct metabolic acidosis
Cool the patient by sponging with cold or tepid water
All of the above
a and c
The following are metabolic effects of aspirin that would worsen the toxicity in R.A. EXCEPT:
Uncoupling of oxidative phosphorylation
Increased glucose demand
Direct effect on the respiratory center
Inhibition of Krebs cycle enzymes
Inhibition of amino acid metabolism
X.Y. a 10 y/o who has ingested 20 adult strength aspirin 13 hours ago and is presenting with nausea, mild hyperpnea and is complaining of ringing in the ears. His blood gases are PCO2 = 15 mm Hg; PO2 = 85 mm Hg. Blood pH is 7.40. Based on X.Y.'s presentation, and without knowing the blood salicylate levels for R.A.(above) and X.Y., can you predict the patient with the greatest risk for developing serious CNS toxicity. Please discuss your answer.
REFERENCES
- 1.Gossel TA, Bricker DJ. Basic principles of toxicology. In: Gossel TA, Bricker DJ, editors. Principles of Clinical Toxicology. New York, NY: Raven Press; 2002. pp. 1–19. [Google Scholar]
- 2.Eaton DL, Klaassen CD. Principles of toxicology. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill; 1996. pp. 13–33. [Google Scholar]
- 3.Sun HH, Hailemeskel B, Crumel CD, Kalejaiye BO. Mock pharmacy and therapeutics committee in drug information and clinical toxicology course [abstract]. International Pharmaceutical Abstracts. Abstract 36-13146.
- 4.Crumel CD, Hailemeskel B, Kalejaiye BO, Sun HH. Computer-assisted instruction as a teaching tool for drug information and clinical toxicology [abstract]. International Pharmaceutical Abstracts. Abstract 36-13146.
- 5.Hayes BE. Learning module in clinical toxicology [abstract]. AACP Annual Meeting Abstracts. Am J Pharm Educ. 1997;61(Supplement):75S. [Google Scholar]
- 6.Linowiecki KA. Assessment of problem solving skills and knowledge imparted by a patient case-formatted course in clinical toxicology [abstract]. AACP Annual Meeting Abstracts. Am J Pharm Educ. 1997;61(Supplement):75S. [Google Scholar]
- 7.Schauben JL. Clinical toxicology services in a teaching hospital. Fla J Hosp Pharm. 1990;10:43. [Google Scholar]
- 8.Czajka PA. Evaluation of a toxicologic controversy in a clinical toxicology course. Vet Hum Toxicol. 1981;23:108–9. [PubMed] [Google Scholar]
- 9.Lovejoy FH, Edlin AL, Goldman P. Utilization of the poison center for the teaching of clinical toxicology to medical and pharmacy students, housestaff, and health care professionals. Clin Toxicol. 1979;15:393–400. doi: 10.3109/15563657908989892. [DOI] [PubMed] [Google Scholar]
- 10.Malone PM, Glynn GF, Stohs SJ. The development and structure of a distance-based entry level doctor of pharmacy pathway at Creighton University Medical Center. Am J Pharm Educ. 2004;68((2)) Article 46. [Google Scholar]
- 11.Krathwohl DR, Bloom BS, Masia BB. Taxonomy of Educational Objectives: Book 2 Affective Domain. New York, NY: Longman; 1964. [Google Scholar]
- 12.Alsharif NZ, Galt KA, Mehanna A, Chapman R, Ogunbandeniyi AM. Instructional model to teach clinically relevant medicinal chemistry. Am J Pharm Educ. 2006;70((4)) doi: 10.5688/aj700491. Article 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon MW, Borron SW, Burns M. Haddad and Winchester's Clinical Management of Poisoning and Drug Overdose. 4th ed. Philadelphia, PA: W.B. Saunders; 2007. [Google Scholar]
- 14.Goldfrank LR, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS. Goldfrank's Toxicologic Emergencies. 8th ed. Stamford, Conn: McGraw Hill; 2006. [Google Scholar]
- 15.Ford M, Delaney KA, Ling L, Erickson T. Clinical Toxicology. 1st ed. Philadelphia, PA: W.B. Saunders; 2000. [Google Scholar]
- 16.Ellenhorn M, Schonwald SMD, Ordog G, Wasserberger J. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 17.Larsen LC, Fuller SH. Management of acetaminophen toxicity. Am Fam Phys. 1996;70:185–90. [PubMed] [Google Scholar]
- 18.Smilkstein MJ. Acetaminophen. In: Goldfrank LR, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS, editors. Goldfrank's Toxicologic Emergencies. 8th ed. Stamford, Conn: McGraw Hill; 2006. pp. 541–68. [Google Scholar]
- 19.Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS) Clin Toxicol. 2007;45:815–917. doi: 10.1080/15563650701754763. [DOI] [PubMed] [Google Scholar]
- 20.Schiodt FV, Lee WM, Bondesen S, Ott P, Christensen E. Influence of acute and chronic alcohol intake on the clinical course and outcome in acetaminophen overdose. Alimentary Pharmacol Ther. 2002;16:707–15. doi: 10.1046/j.1365-2036.2002.01224.x. [DOI] [PubMed] [Google Scholar]
- 21.Lane JE, Belson MG, Brown DK, Scheetz A. Chronic acetaminophen toxicity: a case report and review of the literature. J Emerg Med. 2002;23:253–6. doi: 10.1016/s0736-4679(02)00526-7. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt LE, Dalhoff K, Poulsen HE. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology. 2002;35:876–82. doi: 10.1053/jhep.2002.32148. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt LE, Dalhoff K. The impact of current tobacco use on the outcome of paracetamol poisoning. Alimentary Pharmacology and Therapeutics. 2003;18:979–85. doi: 10.1046/j.1365-2036.2003.01789.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldfrank LR, Flomenbaum NE, Lewin NA, Weisman RS, Howland M, Hoffman RS. Principles of Managing the Poisoned or Overdosed Patient: An Overview. In: Goldfrank LR, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS, editors. Goldfrank's Toxicologic Emergencies. 8th ed. Stamford, Conn: McGraw Hill; 2006. pp. 541–68. [Google Scholar]
- 25.Riggs BS, Bronstein AC, Kulig K, Archer PG, Rumack BH. Acute acetaminophen overdose during preganancy. Obstet Gynecol. 1989;74((2)):247–53. [PubMed] [Google Scholar]
- 26.Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Accreditation Council for Pharmacy Education. Available at: http://www.acpe-accredit.org/standards/standards1.asp. Accessed October 12, 2008.