Abstract
Background
Heavy drinking can cause chronic hypertension, possibly due to effects on the autonomic nervous system. Catechol-O-methyltransferase (COMT) inactivates catecholamines, and a G to A substitution in codon 108 in the soluble COMT mRNA (or codon 158 in the membrane bound form) substitutes methionine for valine and alters enzyme activity.
Methods
We evaluated the association of COMT genotype at this locus with blood pressure in 839 alcohol dependent individuals before and during participation in an alcoholism treatment trial. Hierarchical linear models were used to account for within-subject correlation on repeated blood pressure measurements, and findings were adjusted for age, gender, ethnicity, alcohol use, body-mass index, current smoking, hypertension history, and study site.
Results
Relative to those with the val-val genotype, those with the met-met genotype had higher adjusted systolic (+4.9 mm Hg, p<0.01) and diastolic (+3.2 mm Hg, p<0.01) blood pressure at baseline. Those with the val-met genotype did not significantly differ from the val-val genotype. Changes in blood pressure between baseline and 4 weeks of alcohol treatment also differed by genotype. Relative to the val-val genotype, the met-met genotype had a greater reduction in adjusted systolic pressure (-3.9 mm Hg, p<0.01) and diastolic pressure (-2.8 mm Hg, p<0.01). Corresponding relative reductions for the val-met genotype were -2.2 mm Hg systolic (p=0.070) and -1.5 mm Hg diastolic (p<0.05).
Conclusion
Findings suggest that alcohol-induced blood pressure elevation may be related to the effects of catecholamines and their genetically determined inactivation.
Keywords: Catechol-O-methyltransferase, genotype, SNP, blood pressure, alcoholism
INTRODUCTION
Heavy drinking is a major preventable cause of high blood pressure, contributing to an estimated 5 to 15% of hypertension cases 1. It has been estimated that approximately 50% of heavy drinkers experience alcohol-induced blood pressure elevation 2. The mechanisms underlying the pressor effect of heavy drinking are unknown, with several pathways being suggested 3. Among these is an alcohol-induced alteration in sympathetic nervous activity, which has been shown to occur with acute alcohol exposure in non-heavy drinkers 4-6. However, it is not known if sustained sympathetic effects lead to chronic blood pressure elevation in regular heavy drinkers.
Catechol-O-methyltransferase (COMT) is one of the enzymes responsible for inactivating dopamine, norepinephrine, and epinephrine via a transmethylation reaction involving the coenzyme S-adenosyl-methionine, and exists in a soluble and membrane-bound form 7. The activity of this enzyme has been linked to a non-synonymous single nucleotide polymorphism (SNP) corresponding to position 158 of the membrane bound enzyme and position 108 of the soluble enzyme, involving substitution of valine for methionine 8. The enzyme has higher activity in persons with the val-val genotype, and associations of this genotype with physiological processes have been demonstrated 9-11.
Genotypic differences in the COMT enzyme may in theory contribute to individual differences in blood pressure regulation and hypertension risk in the general population, but the association of the val108met SNP with blood pressure has been weak in some prior studies 12, 13. While many physiologic processes contribute to blood pressure regulation, COMT would only be expected to affect a catecholaminergic regulatory pathway. If alcohol-induced chronic blood pressure elevation is caused by increased catecholaminergic activity, the met-met COMT genotype, with its correspondingly lower COMT activity, may confer risk for an alcohol-induced blood pressure increase, and as a result be associated with relatively greater reduction in blood pressure accompanying abstinence. To examine this hypothesis, we evaluated the relationship of COMT genotype to pre-treatment blood pressure among alcohol-dependent individuals, as well as subsequent changes in blood pressure occurring with treatment for alcohol dependence.
METHODS
Subjects and blood pressure measurement
Subjects for this study included participants in the COMBINE Study, a large, multicenter, randomized controlled trial designed to assess the efficacy of several pharmacologic and counseling treatments for alcohol dependence 14. Out of 1,383 subjects in COMBINE, 885 were typed for the val108met SNP, of whom 839 had information on blood pressure and the covariates included in this study. Subjects were followed during 16 weeks of active treatment and periodically thereafter. For this study, we included blood pressure measurements recorded at pre-treatment baseline and at weeks 4, 8, 12, and 16 during alcoholism treatment. During the COMBINE Study blood pressure was measured per each of the 11 sites usual routine. This was not standardized and blood pressure varied by center as described elsewhere 15. As a result, we adjusted analyses for study site.
Independent variable and genotyping
Our main independent variable was COMT genotype, which was included in the COMBINE Study due to COMT influences on pre-frontal cortex dopamine metabolism and potential interactions with alcoholism treatment 16. A common G to A (db SNP rs number 4680) transition in exon 4 of COMT results in the Val to Met substitution at amino acid 108 of soluble-COMT and 158 of membrane bound-COMT 17. Genotyping of COMBINE Study participants had been performed previously with 5’ nuclease (TaqMan®), a rapid and accurate method for high throughput genotyping of SNPs 18, 19. Genomic DNA was extracted from peripheral blood mononuclear cells. The 5’ nuclease genotyping assay (TaqMan®) combines polymerase chain reaction (PCR) amplification and sequence variant detection into a single step. Locus specific primers and fluorogenic allele-specific probes were designed and manufactured by Applied Biosystems (ABI) (Foster City, CA, USA). The identification number of the TaqMan® Drug Metabolism Genotyping Assay used is C_25746809_50.
The 5μl reaction mixture consisted of 2.5μl of Taqman Universal Master Mix (ABI), 0.125μl of 20X Assay Mix (ABI) (4μM detection probe for each allele, 18μM forward and reverse primer each), and 10 ng of genomic DNA diluted in 2.375μl of Tris EDTA (TE) pH 8.0 (Quality Biological, Inc; Gaithersburg, MD). Amplification was performed with an ABI Gene Amp® PCR System 9700 using 384-well plates and the following amplification profile: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 92°C for 15 s and 60°C for 1 min. After amplification, endpoint fluorescence intensity was measured directly in the reaction plates, by means of 7900 ABI Sequence Detector. Genotypes were determined using Sequence Detection System Software Version 2.0 (ABI). Four genotyping signal clusters were identified, representing Val108 and Met108 homozygotes, Val108/Met108 heterozygotes and no-DNA-template controls. Genotyping accuracy was determined by replicate genotyping of 169 DNA samples. The genotyping error rate was 0.59%. Genotyping completion rate was 96.72%. No significant deviation from Hardy-Weinberg equilibrium was found (P = 0.34). All genotyping was done by one of the authors (GO) at the Laboratory of Neurogenetics (David Goldman, Director) at the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Covariates
We adjusted the analyses for a number of factors other than COMT genotype that may influence blood pressure. These included age, gender, ethnicity (Hispanic, non-Hispanic black, non-Hispanic white, other ethnicity), % drinking days (i.e., the percent of days during which any alcohol was consumed in the 4 weeks preceding each blood pressure measurement), current smoking (yes or no), self-reported history of hypertension or currently taking blood pressure medication, body-mass index (kg/m2), and study site. We also utilized the number of drinks in the week preceding the baseline blood pressure measurement, and pulse at the time of baseline blood pressure measurement, to estimate effects of alcohol withdrawal.
Statistical methods
The baseline and trajectory of systolic and diastolic blood pressure were analyzed as a hierarchical linear model in which time (or observation number) was nested within subjects. Variables distinguishing particular observations were considered level 1 variables and those distinguishing different subjects were considered level 2 variables. Previous work examining the trajectory of blood pressure measurements across the trial revealed a discontinuous process across weeks such that a dramatic drop occurred between baseline and week 4 (during a time of initial abstinence for most subjects), followed by a more modest increase throughout the remainder of the study 15. This pattern was well described by a piecewise linear regression in which the intercept (estimated baseline), slope 1, and slope 2 were considered random variables (i.e. significant subject to subject variation was detected in both slopes). The hierarchical linear model accommodates such data well. Using these two-level hierarchical models, we tested the significance of COMT genotype as a predictor of pre-treatment blood pressure and subsequent changes in blood pressure between pre-treatment baseline to week 4, and between weeks 5 to 16. The unadjusted models included the intercept and time effects at level I. Level II included COMT genotype as a predictor of pre-treatment blood pressure and subsequent blood pressure changes. Adjusted models added % drinking days (which varied over time) at level I, and the other covariates as predictors of pre-treatment blood pressure at level II. Finally, since the pre-treatment pressure was related to blood pressure reduction at week 4 (correlation = -0.517, p<0.001), we estimated the significance of COMT genotype on changes in blood pressure with adjustment for pre-treatment blood pressure. This was accomplished by adding pre-treatment blood pressure as a level II predictor of blood pressure reduction at week 4.
RESULTS
Overall characteristics of the COMBINE group have been published 14. Characteristics of the 839 subjects included in this COMT analysis are listed in the table. Age and gender did not differ by COMT genotype, but the val-met group (76.6% non-Hispanic white) and met-met group (84.3% non-Hispanic white) were more likely to be non-Hispanic white relative to the val-val group (64.8%, p<0.001). This ethnic difference is consistent with findings from the International HapMap Project 20. All subjects had reliable summary measures of drinking (e.g., % drinking days) for the month preceding the initial blood pressure measurement. However, due to difficulty in matching the day of initial blood pressure assessment to a specific day on the alcohol consumption measure used for the COMBINE Study, we were able to reliably estimate very recent consumption in a total of 661 subjects (79% of the 839 subjects included in this analysis). In this subset, during a time of required abstinence/drinking reduction for study eligibility, the median number of drinks in the week preceding the baseline blood pressure was 38.4 for val-val subjects, 32.0 for val-met subjects, and 40.0 for met-met subjects (Kruskal-Wallis p=0.168). During that same week, 19.6% of val-val subjects, 22.2% of val-met subjects, and 16.9% of met-met subjects reported complete abstinence (chi-square p=0.409). Median pulse at the time of the pre-treatment blood pressure differed slightly be genotype (72 for the val-val group, 74 for val-met, and 76 for met-met; p<0.01).
Table.
Subject Characteristics by COMT Genotype
| COMT Genotype |
||||
|---|---|---|---|---|
| Characteristic* | Val-Val (n=253) | Val-Met (n=401) | Met-Met (n=185) | p-value† |
| Age | 44.2 (10.2) | 44.5 (10.5) | 43.9 (10.7) | 0.826 |
| % Male | 72.3 | 70.6 | 70.3 | 0.860 |
| Ethnicity (%) | ||||
| African American | 12.3 | 6.7 | 4.3 | <0.01# |
| Hispanic | 17.8 | 11.7 | 7.0 | <0.01‡ |
| non-Hispanic white | 64.8 | 76.6 | 84.3 | <0.001# |
| other ethnicity | 5.1 | 5.0 | 4.3 | 0.919 |
| Pre-treatment systolic pressure | 132.9 (18.0) | 133.5 (18.3) | 137.5 (18.1) | <0.05‡ |
| Pre-treatment diastolic pressure | 82.4 (10.9) | 83.0 (10.9) | 85.3 (11.8) | <0.05‡ |
| Pre-treatment % drinking days | 76.7 (23.6) | 75.9 (24.6) | 75.2 (26.0) | 0.809 |
| Body-mass index (kg/m2) | 27.2 (4.9) | 27.1 (4.9) | 27.2 (5.6) | 0.980 |
| % with history of hypertension | 24.5 | 28.4 | 27.6 | 0.538 |
| % on anti-hypertensive drug | 17.4 | 17.5 | 18.4 | 0.956 |
| % current smokers | 49.4 | 47.4 | 49.2 | 0.854 |
Interval-ratio variables are presented as means, with standard deviations enclosed in parentheses. Categorical variables are presented as percentages.
Overall comparisons made by ANOVA for continuous variables and chi-square for categorical variables.
Indicates met-met significantly different than val-val using Bonferroni’s correction for multiple comparisons.
Indicates met-met and val-met significantly different than val-val using Bonferroni’s correction for multiple comparisons. There were no comparisons where only the val-val and val-met groups differed significantly.
At baseline the overall mean systolic blood pressure was 134.2 mm Hg (SD 18.2), and the mean diastolic blood pressure was 83.3 mm Hg (SD 11.1). The average reduction in systolic blood pressure between baseline and week 4 for the entire sample was -5.4 mm Hg (p<0.001), with a total increase of 2.0 mm Hg between weeks 5 to 16 (p<0.001). The average reduction in diastolic blood pressure between baseline and week 4 was -3.9 mm Hg (p<0.001), with a total increase of 1.0 mm Hg between weeks 5 to 16 (p<0.01). There was significant variability between individuals, with improved model fit by treating the blood pressure changes as random variables (likelihood ratio test p<0.001 for both systolic and diastolic pressure).
In unadjusted analyses, COMT genotype predicted pre-treatment blood pressure and reduction in blood pressure between baseline and week 4 as hypothesized. These results are illustrated in the figures. Relative to the val-val genotype at baseline, the met-met genotype was associated with higher systolic (+ 4.7 mm Hg, p<0.01) and diastolic (+ 2.9 mm Hg, p<0.01) blood pressure. Findings for heterozygotes were not statistically different from the val-val group (+0.6 mm Hg, p=0.678 for systolic; + 0.6 mm Hg, p=0.495 for diastolic). Relative to the valine homozygotes at week 4, the methionine homozygotes experienced a greater reduction from baseline in systolic pressure (-4.1 mm Hg, p<0.05) and diastolic pressure (-2.3 mm Hg, p<0.05). Findings were intermediate for heterozygotes (-2.7 mm Hg, p<0.05 for systolic; -1.1 mm Hg, p=0.199 for diastolic). Overall there was an increase in systolic (slope=0.650, p<0.001) and diastolic (slope=0.334, p<0.01) blood pressure between weeks 5 and 16, but these did not differ significantly by COMT genotype (p>0.200 for all comparison of slopes between genotypes). We also tested for differences in blood pressure between the genotype groups at each 4 week time interval, and found that genotype was only related to baseline blood pressure and the change in blood pressure between baseline and week 4 (all p>0.1 for systolic and diastolic blood pressure comparisons at week 4, 8, 12, and 16). A subset analysis in persons reporting abstinence at the monthly intervals (479 at week 4, 446 at week 8, 479 at week 12, 471 at week 16) did not demonstrate the same increased blood pressure (for systolic slope=0.382, p=0.143; for diastolic slope=0.121, p=0.470). Furthermore, in comparison to the group as a whole, blood pressure reduction relative to baseline at each 4-week time interval was greater in reported abstainers, and in these reported abstainers BP reduction was greatest for those having the met-met genotype (data not shown). These findings suggest that the slight increase between weeks 5 and 16 in the overall samples was largely due to the resumption of drinking in some individuals.
The remaining covariates did not confound the association of COMT genotype with pre-treatment blood pressure or reduction in blood pressure between baseline and week 4. After adjustment, pre-treatment systolic and diastolic blood pressures were still significantly associated with the val108met polymorphism. Relative to the val-val group, the methionine homozygotes had the higher adjusted blood pressure (+4.9 mm Hg systolic, p<0.01; +3.2 mm Hg diastolic, p<0.01). Findings for the heterozygotes were not significantly different from valine homozygotes (+0.8 mm Hg systolic, p=0.539; +0.6 mm Hg diastolic, p=0.474). The change in blood pressure also followed a similar pattern compared to unadjusted analyses. For the met-met group the reduction from baseline to week 4 differed from the val-val group (-3.9 mm Hg systolic, p<0.1;-2.8 mm Hg diastolic, p<0.01). Heterozygotes were intermediate (-2.2 mm Hg systolic, p=0.070; -1.5 mm Hg diastolic, p<0.05). In the most conservative analysis, COMT genotype remained a statistically significant predictor of blood pressure reduction even after adjustment for pre-treatment blood pressure differences. Relative to the val-val group, the methionine homozygotes had a reduction of-3.5 mm Hg systolic (p<0.05), and a reduction of -2.4 mm Hg diastolic (p<0.01). Heterozygotes had a reduction of -2.2 mm Hg systolic (p=0.058), and a reduction of -1.4 mm Hg diastolic (p<0.05). Finally, in the 661 subjects with reliable drinking data in the week immediately prior to baseline blood pressure estimation, pulse and number of drinks in the week prior to BP measurement (i.e., our available indicators for potential alcohol withdrawal) did not interact with COMT genotype (p>0.2 for all comparisons) nor confound the association between genotype and blood pressure.
DISCUSSION
Blood pressure regulation in humans is a complex process that is influenced by chronic heavy drinking. Results of this observational analysis in a well-characterized alcohol treatment-seeking population demonstrated that COMT genotypes were differentially associated with pre-treatment blood pressure in alcohol dependent individuals, as well as the magnitude of blood pressure reduction occurring during the first month of alcoholism treatment. These findings support the hypothesis that frequent heavy drinking increases blood pressure partly through chronic activation of the sympathetic nervous system, the magnitude of which is under at least partial genetic control.
A recent study in 214 middle-aged Swedish males reported a similar cross-sectional association between COMT genotype and blood pressure 21. However, at least two larger population-based genetic association studies have evaluated the relationship between this COMT polymorphism and systemic blood pressure in humans without control for environmental influences. In a population-based cohort in Japan, the valine allele had a borderline association (p=0.07) with hypertension in men but not women 13. In a Norwegian population-based cohort, the val-val genotype was associated with higher systolic blood pressure 12. The observed contradictions may be related to relative differences in the physiology of blood pressure regulation between large population-based cohorts (where environmental variability leaves the gene as the main focus) and an alcohol dependent cohort (where a gene by environment interaction might be detected), particularly with regard to alcohol’s effect on sympathetic activity. Indeed, our results are consistent with a dominant catecholaminergic mechanism for alcohol-induced blood pressure changes, rendering blood pressure regulation particularly sensitive to the inactivation of catecholamines in heavy drinkers. The probability that a sustained sympathetic effect exists is bolstered by prior research suggesting higher circulating catecholamine concentrations in alcohol-dependent individuals 22, 23.
In order to induce sustained blood pressure elevation, it is probably necessary to alter renal excretion of sodium and water 24. In theory, chronic heavy drinking could decrease sodium excretion through activation of post-ganglionic sympathetic fibers to the kidneys or the adrenal gland, resulting in a sustained elevation in systemic blood pressure 25. Less efficient methylation of epinephrine and norepinephrine, as determined by COMT genotype, could enhance such an effect. In the absence of heavy consumption, natriuresis may return to a normal level, with an accompanying reduction in blood pressure. If present, drinking-induced enhancement of renal sympathetic activity could represent either a peripheral effect or central regulation of the autonomic nervous system.
Strengths of this study included genotyping in a large sample with repeated measurements of blood pressure and alcohol drinking. This allowed us to uniquely estimate the association of COMT genotype on baseline blood pressure and changes in blood pressure during alcoholism treatment. In this manner, we were able to explore how genetic differences in catecholamine metabolism might be a common intermediary process for alcohol-induced hypertension. However, some limitations merit consideration. Since blood pressure is known to increase during initial stages of alcohol withdrawal, this may have influenced the associations between COMT genotype and blood pressure. While we cannot entirely exclude this, alcohol involvement in the week preceding the pre-treatment blood pressure, and pulse at the time of initial blood pressure assessment, neither modified nor confounded the association of COMT genotype with blood pressure. Nevertheless, these methods were not sufficiently sensitive to fully evaluate the potential effects of alcohol withdrawal and to entirely eliminate the possibility that catecholamine metabolism during alcohol withdrawal interacted with COMT genotype. There was no association of COMT genotype with the slight increase in blood pressure between weeks 5 and 16, which was apparently related to the resumption of drinking in some individuals. It is possible that the overall small increase in blood pressure and the continuing abstinence in many subjects obscured any tendency for greater blood pressure elevation with the methionine genotype.
Other factors may have resulted in an underestimation of the relationship between COMT genotype and blood pressure. Specifically, in the week preceding the pre-treatment blood pressure assessment, about 1 in 5 subjects reported abstinence, and it is possible that blood pressure in such individuals was already recovering from the effects of chronic heavy drinking. In addition, non-standardized blood pressure assessment was only partially accounted for by adjustment for study site, and measurement error would tend to minimize the significance of blood pressure associations with COMT genotype.
Alcohol consumption in excess of 2 to 3 drinks per day is an important secondary cause of hypertension. Our results suggest that susceptibility to alcohol-induced hypertension could be at least partly mediated by reduced activity of COMT, with the low activity genotype also predicting a greater reduction in blood pressure occurring with reduced drinking or abstinence. If confirmed, these findings add a genetic link between an important health consequence and heavy drinking, explain some of the observed variation in susceptibility to alcohol induced blood pressure elevation, and suggest novel detection and treatment methods for hypertensive heavy drinkers who are unable to substantially reduce their alcohol use.
Figure 1.
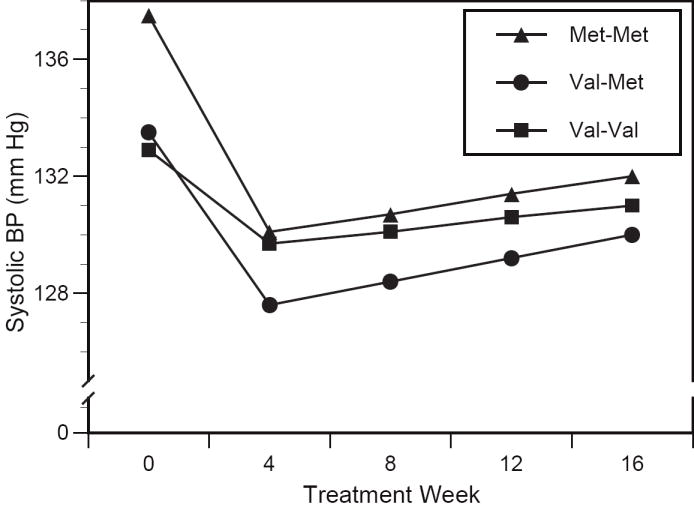
Genotype-specific changes in systolic blood pressure. Relative to val-val, the magnitude of blood pressure reduction by week 4 was greater in the val-met group (p<0.05) and met-met group (p<0.05). Changes between weeks 4 and 16 did not significantly differ by genotype.
Figure 2.
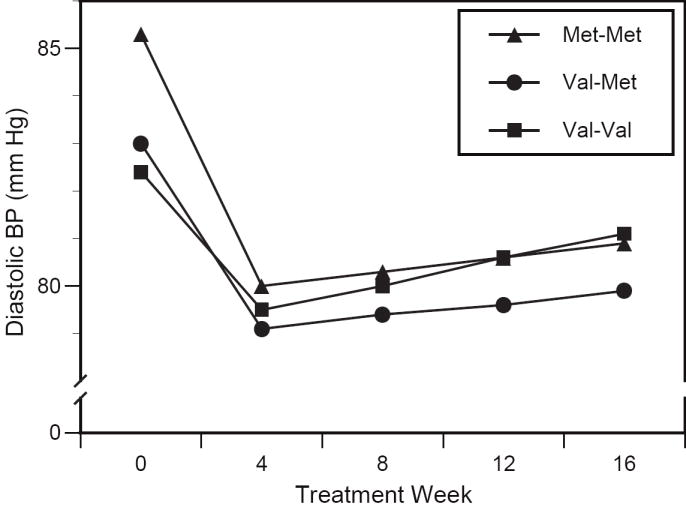
Genotype-specific changes in diastolic blood pressure. Relative to val-val, the magnitude of blood pressure reduction by week 4 did not differ statistically in the val-met group (p=0.199), and was greater in the met-met group (p<0.05). Changes between weeks 4 and 16 did not significantly differ by genotype.
Acknowledgments
FUNDING SOURCE
Dr. Stewart was supported by a Career Development Award from NIAAA (K23AA014188). Dr. Anton is supported in part by a Career Development Award from NIAAA (K05AA017435). Data presented in this report were collected as part of the multisite COMBINE trial sponsored by the National Institute on Alcohol Abuse and Alcoholism, in collaboration with the Combine Study Research Group. A full listing of the staff of the COMBINE study can be found at http://www.cscc.unc.edu/combine/.
Footnotes
DISCLOSURE
The authors have no conflict of interest to declare.
References
- 1.Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, Sempos CT. Alcohol as a risk factor for global burden of disease. Eur Addict Res. 2003;9:157–164. doi: 10.1159/000072222. [DOI] [PubMed] [Google Scholar]
- 2.Estruch R, Coca A, Rodicio JL. High blood pressure, alcohol, and cardiovascular risk. J Hypertens. 2005;23:226–229. doi: 10.1097/00004872-200501000-00039. [DOI] [PubMed] [Google Scholar]
- 3.Zakhari S. Alcohol and the cardiovascular system. Molecular mechanisms for beneficial and harmful actions. Alcohol Health Res World. 1997;21:21–29. [PMC free article] [PubMed] [Google Scholar]
- 4.Randin D, Vollenweider P, Tappy L, Jéquier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. N Engl J Med. 1995;332:1733–1738. doi: 10.1056/NEJM199506293322601. [DOI] [PubMed] [Google Scholar]
- 5.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. J Hypertens. 1989;7(Suppl):S20–21. doi: 10.1097/00004872-198900076-00007. [DOI] [PubMed] [Google Scholar]
- 6.van de Borne P, Mark AL, Montano N, Mion D, Somers VK. Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension. 1997;29:1278–1283. doi: 10.1161/01.hyp.29.6.1278. [DOI] [PubMed] [Google Scholar]
- 7.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 8.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound Catechol-O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 9.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Yanjun X, Koeppe RA, Stohler CS, Goldman D. COMT val158met affects μ-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Lindberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 11.Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with the val/val genotype in the Catechol-O-methyltransferase gene. Am J Hypertens. 2007;20:21–26. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Kamide K, Kokubo Y, Yang J, Matayoshi T, Inamoto N, Takiuchi S, Horio T, Miwa Y, Yoshii M, Tomoike H, Tanaka C, Banno M, Okuda T, Kawano Y, Miyata T. Association of genetic polymorphisms of ACADSB and COMT with human hypertension. J Hypertens. 2007;25:103–110. doi: 10.1097/HJH.0b013e3280103a40. [DOI] [PubMed] [Google Scholar]
- 14.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A for the COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 15.Stewart SH, Latham PK, Miller PM, Randall P, Anton RF. Blood pressure reduction during treatment for alcohol dependence: Results from the COMBINE Study. Addiction. 2008;103:1622–1628. doi: 10.1111/j.1360-0443.2008.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nature Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 17.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ. Allelic discrimination using fluorogenic probes and the 5’ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Shi MM, Myrand SP, Bleavins MR, de la Iglesia FA. High throughput genotyping for the detection of a single nucleotide polymorphism in NAD(P)H quinone oxidoreductase (DT diaphorase) using TagMan probes. Mol Pathol. 1999;52:295–299. doi: 10.1136/mp.52.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 21.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol-O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–711. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Parlesak A, Reisenauer C, Biermann J, Ratge D, Bode JC, Bode C. Reversibility of increased formation of catecholamines in patients with alcoholic liver disease. Scand J Gastroenterol. 2004;39:60–66. doi: 10.1080/00365520310007738. [DOI] [PubMed] [Google Scholar]
- 23.Patkar AA, Marsden CA, Naik PC, Kendall DA, Gopalakrishnan R, Vergare MJ, Weinstein SP. Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol-dependent individuals. Am J Addict. 2004;13:225–235. doi: 10.1080/10550490490459898. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE, Granger JP, Hall ME, Jones DW. Pathophysiology of hypertension. In: Walsh RA, Simon DI, Hoit BD, Fang JC, Costa M, editors. Hurst’s the Heart. 12. McGraw-Hill Companies; 2008. [Google Scholar]
- 25.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]


