Abstract
Immunological synapse formation between T cells and target cells can affect the functional outcome of TCR ligation by a given MHC/peptide complex. Although synapse formation is usually induced by TCR signaling, it is not clear whether other factors can affect the efficiency of synapse formation. Here we tested whether cytokines could influence synapse formation between murine CTL and target cells. We found that IL-12 enhanced synapse formation, whereas TGF-β decreased synapse formation. The enhanced synapse formation induced by IL-12 appeared to be functional, as IL-12-treated cells could respond to weak peptides, including self-peptides, to which the T cells were normally unresponsive. These responses correlated with expression of functionally higher avidity LFA-1 on IL-12-treated CTL. These findings have implications for the function of IL-12 in T cell-mediated autoimmunity.
Keywords: T cells, cytotoxic, autoimmunity, peptides, cytokines, T cell receptors
CTLs are critical for eliminating viral infections, intracellular pathogens, and potentially play an important role in tumor immunity. In addition to their protective role, CTL are also involved in pathogenic autoimmune responses. The primary signal used to determine which cells CTLs attack is engagement of the TCR with MHC class I/peptide complexes on potential target cells. The peptide bound to the MHC is critical to the CTL response. Although in general the TCR of a given T cell is thought to be specific for one optimal ligand, it is now clear that a single TCR can be stimulated by a broad range of different peptides (1-4). These various peptide-MHC complexes bind to the TCR with varying affinities and elicit a variety of different outcomes (5, 6). Based on the functional responses that they evoke, peptides are classified as superagonists, agonists, weak agonists, antagonists or null ligands for an individual TCR. Agonists induce the full functional repertoire of the T cell; weak agonists induce some, but not all T cell functions; antagonists do not induce T cell function, but inhibit agonist-induced responses; null peptides have no influence on T cell function.
While it was originally thought that mature T cells only recognize foreign antigens in an infected host, it is now becoming clear that T cells can react to some proportion of self-peptides in the periphery. For example, T cells require low-affinity interactions with self-peptide/MHC complexes to undergo positive selection in the thymus (reviewed in (7). Similarly, both naïve and activated (but not memory) T cells require a similar interaction to survive in the periphery (reviewed in 8). Further, it has recently been proposed that interactions with self-peptides are required to amplify signals when only small numbers of antigenic MHC/peptide complexes are present on the surface of APCs (9, 10). The recognition of self-peptides also underlies the pathogenesis of autoimmune disease and is the goal of many tumor immunotherapies.
Recent work in our laboratory suggests that formation of a mature immunological synapse can affect the sensitivity and specificity of a T cell response to a given peptide (11). A synapse is characterized by the concentration of TCRs and peptide-MHCs at the contact surface between a T cell and APC or target cell. In some cases, the synapse forms a characteristic bulls-eye pattern with the TCR and peptide-MHC concentrated at the center of the contact (referred to as the central supramolecular activation cluster or cSMAC) surrounded by a ring of LFA-1 and ICAM-1 molecules (the peripheral-SMAC or pSMAC) (12-14). The results from our previous work (11, 15) suggest that by concentrating peptide-MHC in the center of the contact, TCR occupancy is enhanced allowing for stronger signaling. We hypothesize that the concentration of weak ligands in the synapse could amplify signaling by a weak agonist and may convert an antagonist into a weak agonist.
Until recently, TCR stimulation was thought to be required for synapse formation (11-14). We demonstrated, however, that engagement of a surface receptor on CTL, namely NKG2D, can drive synapse formation (16). We postulated that NKG2D engagement might lower the threshold for synapse formation in an inflammatory situation, enhancing the sensitivity and specificity of an early immune response.
In the present study, we set out to determine whether cytokines could influence synapse formation. We found that the pro-inflammatory cytokine IL-12 could strongly enhance synapse and cSMAC formation between CTL and target cells, whereas the immunosuppressive cytokine TGF-β strongly inhibited synapse formation. Consistent with our hypothesis that concentration of weak agonists in the synapse could enhance signaling, we found that IL-12 treatment enhanced OT-1 CTL reactivity to a weak agonist and converted an antagonist peptide to a weak agonist. Surprisingly, we found that IL-12 could also stimulate killing of syngeneic target cells by both OT-1 and DUC18 CTL, suggesting that IL-12 induces CTL reactivity against self-peptide(s). Supporting this, we identified self-peptides that induced killing of target cells by IL-12-treated OT-1 or DUC18 CTL. These data suggest that the production of IL-12 during inflammation may function to enhance the sensitivity and broaden the specificity of T cells during the early phase of the immune response allowing for reactivity with self-antigens.
Materials and Methods
Mice
All mice were housed under specific pathogen-free conditions in the Washington University animal facilities in accordance with institutional guidelines. OT-1 TCR transgenic mice (C57BL/6) (17) were provided by H. Virgin (Washington University, St. Louis, MO). DUC18 TCR transgenic mice (BALB/c) (18) were bred in house. C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Cytokines and peptides
Recombinant mouse IL-12 (rmIL-12) and recombinant human TGF-β (rhTGF-β2) were purchased from BioSource International, Invitrogen (Carlsbad, CA). The following peptides were synthesized and purified as previously described (18): SIINFEKL; EIINFEKL; RGYNYEKL; SIIRFEKL; RTYTYEKL; ISFKFDHL; QYIHSANVL; RGYVYQGL; H-2Kd-binding peptides (19): KYQEVTNNL, SYLEMGHDI, VYNASNNEL, KYLTVKDYL, KYKDIYTEL, KYIHSANVL, KYMEDVTQI, KYKASENAI, AKPTGNVDIGSL, ESRVSDTGSAGLML, and IFIKPGADLSTGHDEL; FVQMMTAK, LYKESLTKL, GYVQSKEMI, TWNKLLTTI, HYFEDKENI, AYSIVIRQI, TYGLTPHYI, KYLSVQGAL, KYLSVQGQLF, MRYVASYLL, FGLLGLGSDGQPPVQK, GFGDLKTPAGLQVLND, NYGPMKGGSFGG, and NYGPMKGGSFGGRSSGSP. The following peptides were purchased from GenScript Corporation (Piscataway, NJ): PTYRFERL, AGYSFEKL, HTYDFEKL, RAKRYEKL, VGYMYETL.
Generation of CD3ζ-GFP CTL for synapse studies
The Plat E packaging cell line (20) was transfected with CD3ζ-GFP (provided by Mark Davis, Stanford University, Standford, CA) expressed in the pMX vector (21) using Lipofectamine 2000 (Invitrogen). Viral supernatant was harvested 48 hours later and put through a 0.45 μM filter. Total splenocytes from OT-1 TCR transgenic mice were cultured (5×106/ml) with SIINFEKL peptide (0.3μM/ml) and anti-CD3ε antibody (clone 2C11) (1μg/ml). Eighteen hours after stimulation, 2ml of the retroviral supernatant, along with Lipofectamine 2000 (1μl/ml), was spun onto the cells (2200 RPM, 20′, RT). After 4 hours at 37° C, this spinfection was repeated a second time. Live T cells were then harvested 3 days later using Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ) and cultured with 20ng/ml IL-12, 10ng/ml TGF-β2, or no cytokine for another 24 hours. The live cells harvested, either with or without cytokine treatment, were >95% CD8+Vα2+ (OT-1).
Formation and imaging of OT-1 CTL and target cell conjugates
RMA-S or EL-4 cells were cultured overnight with 10-6M SIINFEKL peptide, 10-6M EIINFEKL peptide, or no peptide in IMDM supplemented with 2% FCS. CD3ζ-GFP-expressing OT-1 CTL were mixed with RMA-S cells, or untransduced OT-1 CTL were mixed with EL-4 cells, in 100μl IMDM with 10% FCS in 1.5ml tubes, spun briefly, and incubated at 37°C for 30 minutes. The cells were then lightly resuspended in PBS with 2μM Mg++, 1μM Ca++ and 1% FCS. In the case of the EL-4 conjugates, the cells were stained with a TCRVα2-specific antibody (BD Biosciences). The cells were then flowed onto a parallel plate flow cell (Bioptechs, Tucson, AZ) at 37°C. Cells were imaged using a Zeiss LSM510 confocal system. All images were taken with the pinhole open. Using the Zeiss LSM510 software, conjugates were scored as synapse positive when there was at least a two-fold enrichment of CD3ζ-GFP at the cell-cell contact site compared with the rest of the T cell membrane. Conjugates with synapse formation in which the enrichment of CD3ζ-GFP was in a central area not larger than 1/3 the total contact area were scored as cSMAC positive.
In vitro generation of CTL
Following RBC lysis, OT-1 or DUC18 splenocytes (5×106/ml) were cultured in six well-plates (5-6ml/well) in IMDM supplemented with 10% FCS and 55μM 2-ME with 1μM SIINFEKL (OT-1) or QYIHSANVL (DUC18). After 4 days, the cells were harvested by purification with Ficoll-Paque PLUS and placed in fresh medium containing 0 or 20ng/ml rmIL-12. Twenty-four hours later the cells were harvested and used in experiments. The live cells harvested, either with or without cytokine treatment, were >95% CD8+Vα2+ (OT-1) or CD8+Vβ8.3+ (DUC18).
Cytotoxicity assays
When appropriate, target cells were cultured overnight in IMDM supplemented with 2% FCS with peptide. ConA blasts were generated by culturing C57BL/6J or BALB/c splenocytes (2×107/10ml) in complete IMDM supplemented with 2.5μg/ml ConA for 48 hours. Purified anti-H-2Kb antibody (clone B8-24-3) was used at 20μg/ml. CTL cytotoxicity was measured using a standard chromium release assay. 51Cr-labeled target cells were plated in 96-well U-bottom plates (10,000 cells/well) with CTL at varying E:T cell ratios. The plates were spun briefly and cultured for 4 h at 37°C. The supernatants were then collected and read on a MicroBeta counter (PerkinElmer, Waltham, MA), and the percent specific lysis was determined as: ((sample counts per minute − spontaneous counts per minute)/(maximum counts per minute − spontaneous counts per minute)) × 100.
Intracellular cytokine staining
OT-1 CTL (105/well) and RMA-S cells (105/well) were plated in a 96-well U-bottom plate and cultured at 37°C for 16 hours. Brefeldin A (Sigma Aldrich) was added the last 6 hours of culture. The cells were then stained with a biotinylated-TCRVα2 antibody (BD Biosciences) followed by SA-FITC (BD Biosciences), fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1% saponin in PBS, and stained with a PE-labeled IFN-γ-specific antibody (BD Biosciences). The cells were analyzed using a FACScan (BD Biosciences).
Granzyme B Elispot
The Mouse Granzyme B Elispot Development Module was purchased from R&D Systems (Minneapolis, MN) and the assay performed following the manufacturer's instructions. Briefly, 96-well filter plates (Millipore, Billerica, MA) were coated with capture antibody overnight, blocked with PBS contaning 1% BSA and 5% sucrose for 1 hour at RT, and washed three times with PBS and once with IMDM. Untreated and IL-12-treated OT-1 CTL (100/well) and RMA-S cells +/- peptide (1000/well) were added to the plates and incubated overnight at 37°C overnight. The plates were washed three times with 0.05% Tween in PBS, the detection antibody added, and incubated overnight at 4°C. The plates were then developed using the Elispot Blue Color Module (R&D Systems). The plates were then read on an Immunospot (Cellular Technology Limited, Cleveleand, OH) elispot reader.
LFA-1 measurements
LFA-1 surface expression
OT-1 CTLs were stained with a purified LFA-1-speicifc antibody (BD Biosciences) followed by a PE-labeled anti-rat IgG secondary (Jackson ImmunoResearch, West Grove, PA) or secondary antibody alone. The cells were then analyzed on a FACscan.
Plate Adhesion assay
96 well Immunolon HB plates (Thermo-Scientific, Waltham, MA) were coated with rmICAM-1-Fc (R&D Systems), or BSA (Sigma-Aldrich) as control, (2μg/ml) in 100μl PBS without Mg++ and Ca++ and left overnight at 4°C. The plate was blocked with 10mg/ml heat denatured BSA in PBS and incubated at 37°C for 2 hours. Following one wash with PBS, the CTL were plated at 2×106 cells/ml, 100μl/well in IMDM (no FCS). The plate was briefly centrifuged and incubated for 1 hour at 37°C. The plate was washed gently once with PBS and the cells in each well counted using a hemocytometer. When PMA was used, the cells were pre-incubated with 20ng/ml PMA for 15 minutes at 37°C. The percentage of cells that adhered to BSA-coated wells was subtracted as background.
Shear stress assay
35mm tissue culture plates (Corning Life Sciences, Corning, NY) were coated with rmICAM-1-Fc (2μg/ml in 1ml PBS) overnight at 4°C. These plates were used with a parallel plate flow chamber (Glycotech, Gaithersburg, MD) and PHD 2000 infusion pump (Harvard Apparatus, Holliston, MA). 2×106 CTL were flowed at a force of 1 dyn/cm2 for 2 minutes. The cells were visualized using the 10X lens of a light microscope and camera (AmScope, Chino, CA). The number of cells adhering to the plate was immediately determined in 5 different fields.
Supplementary Materials and Methods
Conjugate Assays
RMA-S or EL-4 cells were cultured overnight in IMDM+2% FCS with or without peptide. The RMA-S or EL-4 cells were then labeled with 1μM CellTrace calcein red-orange AM (Invitrogen) and CTL were labeled with 1μm CFSE (Invitrogen). RMA-S or EL-4 cells (5×105) and CTL (5×105) were mixed on ice in 1.5ml tubes. The cells were spun briefly, incubated for the given time at 37°C, and resuspended in cold 4% paraformaldehyde in PBS. The cells were then analyzed on a FACscan (BD Biosciences, Franklin Lakes, NJ).
TCR staining
CTL were mixed with unpulsed or RMA-S cells pulsed with SIINFEKL (10-6M) in 1.5ml tubes and spun briefly. Following incubation for 1 hour at 37°C, the cells were stained with a biotin-labeled TCRVα2-specific antibody (BD Biosciences) follwed by PE-SA. The cells were then analyzed on a FACscan.
Microarray studies
Total RNA was isolated from untreated and IL-12-treated OT-1 CTL (107 cells) using Trizol (Invitrogen) following the manufacturer's instructions. Further purification of the RNA was then performed using the RNeasy Mini Kit (Qiagen, Valencia, CA). The RNA was then analyzed by the Washington University Mutiplexed Gene Analysis Core Facility using the Affymetix mouse genome 430 2.0 array. The results were analyzed using dChip software (22) and were reported in the NCBI Gene Expression Omnibus (GEO) database (accession # GSE13173).
Results
Cytokines differentially affect CTL immune synapse formation
We hypothesized that the cytokine milieu surrounding CTL may affect the efficiency of synapse formation between CTL and target cells. We therefore tested whether two cytokines important for CTL function, IL-12 and TGF-β, could influence the efficiency of immunological synapse formation. We hypothesized that part of the differing effects of these cytokines on CTL function may be related to how they influence synapse formation. To test this hypothesis, we quantified synapse formation by transgenic OT-1 CTL treated with these two cytokines.
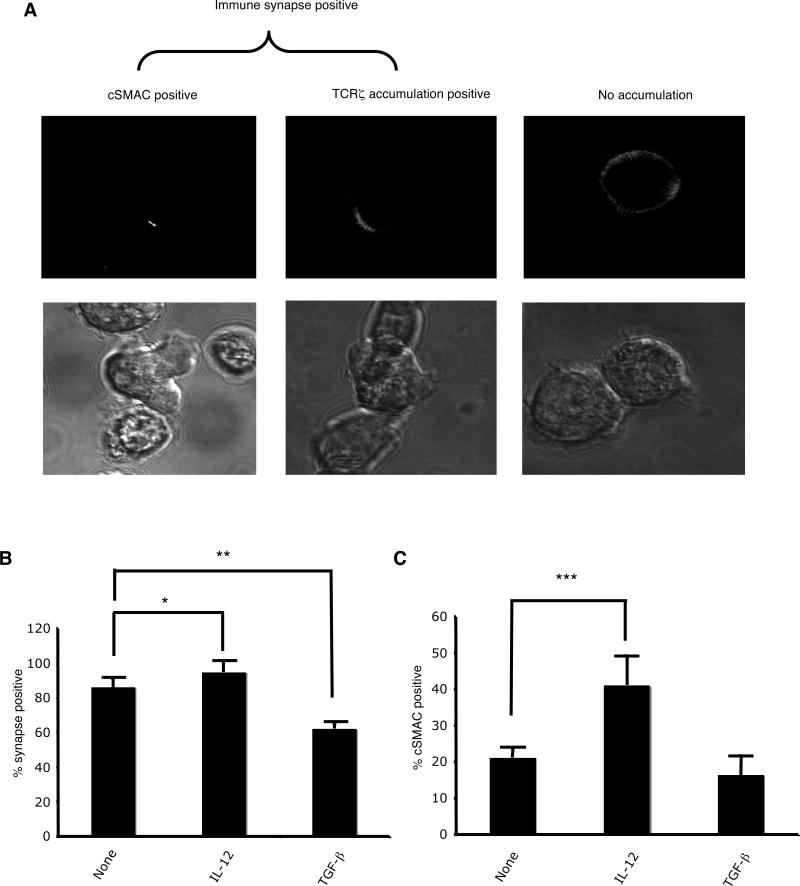
OT-1 CTL were transduced with CD3ζ-GFP, treated with cytokine for 24 hours, and allowed to form conjugates with histocompatible target cells (RMA-S). Live conjugates were then scored for synapse formation. Conjugates in which there was at least a two-fold enrichment of CD3ζ-GFP at the cell-cell contact site compared with the rest of the T cell membrane were scored as positive for synapse formation (Fig. 1A). In addition, conjugates with synpase formation in which the enrichment of CD3ζ-GFP was in a central area not larger than 1/3 the total contact area were scored as having synapses with a cSMAC.
FIGURE 1.
IL-12 enhances and TGF-β decreases antigen-driven synapse formation by OT-1 CTL. (A) Examples of synapse positive and cSMAC positive conjugates formed between OT-1 CTL and RMA-S cells pulsed with SIINFEKL peptide. (B) Percentage of synapse positive conjugates (+/- SD) formed between untreated, IL-12-treated, or TGF-β-treated OT-1 CTL and RMA-S/SIINFEKL (10-6M). No synapses were seen under any condition when conjugates were formed with RMA-S cells in the absence of exogenous peptide. (C) Percentage of synapse positive conjugates that were cSMAC positive formed between untreated, IL-12-treated, or TGF-β-treated OT-1 CTL and RMA-S/SIINFEKL (10-6M). These results are the average of five independent experiments. *p<0.02, **p<0.0003, ***p<0.03 in a two-sided student's T-test.
In the absence of exogenous cytokine, OT-1 CTL efficiently generated synapses with target cells pulsed with the antigenic peptide, SIINFEKL (Fig. 1B). About 85% of conjugates scored positive for synapse formation; 23% of the synapses had a clearly defined cSMAC (Fig. 1C). After treatment with IL-12, the CTL demonstrated a slightly increased efficiency of synapse formation (95%) with a significantly higher percentage of synapses that formed cSMACs (41%). In contrast, treatment with TGF-β significantly inhibited synapse formation (60%). These differences were not related to differences in the efficiency of conjugate formation, as pre-treatment with IL-12 or TGF-β had no effects on conjugate formation (Supplementary Fig. 1A). Nor were they due to a change in TCR expression (Supplementary Fig. 1C). Lastly, synapse formation was antigen-specific, as no synapses were detected in the absence of antigenic peptide (data not shown).
These results demonstrate that cytokines can affect both the quantity and quality of antigen-driven synapse formation. IL-12, a pro-inflammatory cytokine, significantly enhanced synapse and cSMAC formation. TGF-β, an immunosuppressive cytokine, significantly decreased synapse formation. These results lead to the hypothesis that the pro-inflammatory or immunosuppressive effects of these cytokines may be at least partially mediated via effects on synapse formation. To begin to address this question, we investigated how the enhanced synapse formation mediated by IL-12 affected CTL effector mechanisms.
IL-12-treated CTL lyse histocompatible target cells
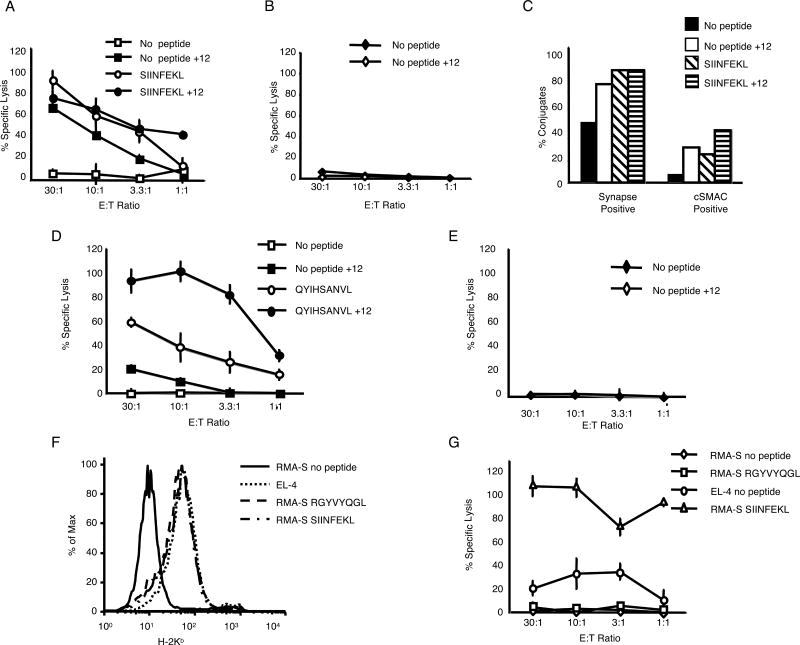
We measured the cytotoxic capabilities of untreated and IL-12-treated OT-1 CTL using the histocompatible cell line EL-4. To our surprise, we found that IL-12-treated CTL lysed these target cells in the absence of antigenic peptide (Fig. 2A). The lysis appeared to be MHC-restricted as the IL-12 treated CTL did not kill the histo-incompatible cell line P815 (Fig. 2B). Unlike RMA-S cells, EL-4 cells induced a significant level of synapse formation by the OT-1 CTL in the absence of exogenous peptide (Fig. 2C). A significant percentage of untreated CTL formed synapses with EL-4 (47%), with 15% of these forming a cSMAC. IL-12 increased the percentage of CTL that formed synapses (80%), as well as the percentage that formed cSMACs (30%). However, similar percentages of the two CTL formed conjugates with EL-4 cells (Supplementary Fig. 1B).
FIGURE 2.
IL-12-treated OT-1 CTL lyse histocompatible target cells in a peptide-dependent manner. (A and B) Untreated and IL-12-treated OT-1 CTL were tested for cytotoxicity (+/- SD) against the histocompatible cell line EL-4 (H-2b) (+/- 10-6M SIINFEKL) and the histo-incompatible cell line P815 (H-2Dd). These results are representative of multiple independent experiments. (C) Percentage of synapse positive and cSMAC positive conjugates formed between untreated or IL-12-treated CTL and unpulsed or SIINFEKL (10-6 M)-pulsed EL-4 cells. These results are representative of two independent experiments. (D and E) Untreated and IL-12-treated DUC18 CTL were tested for cytotoxicity (+/- SD) against the histocompatible cell line P815 (H-2Dd) (+/- 10-6M QYIHSANV) and the histo-incompatible cell line EL-4 (H-2b). (F) Surface expression of H-2Kb on unpulsed EL-4, RMA-S, and RMA-S pulsed with 10-6M peptides. (G) Lysis by IL-12-treated OT-1 CTL (+/- SD) of the surface H-2b high cell line EL-4, the surface H-2b low cell line RMA-S, or RMA-S pulsed with 10-6M SIINFEKL or RGYVYQGL (control peptide). These data are representative of multiple independent experiments.
To determine whether this effect was unique to the OT-1 cells, we tested another CD8+ TCR transgenic line from the DUC18 mice (BALB/c) that is specific for a mutated ERK2 peptide, QYIHSANVL, in the context of H-2Kd (18). Incubation of DUC18 CTL with IL-12 allowed the CTL to lyse the histocompatible P815 cell line in the absence of antigenic peptide, but not the histo-incompatible cell line EL-4 (Fig. 2D and E). Together, these data suggest that IL-12 treatment can lead to MHC-restricted, antigen-independent killing by CTL, most likely via self-peptide reactivity.
MHC class I-expression is not sufficient for lysis of histocompatible target cells by IL-12-treated CTL
To begin to test the hypothesis that the lysis of targets by IL-12-treated CTL was dependent on specific self-peptide(s) presented in class I MHC, we tested the ability of IL-12-treated OT-1 CTL to lyse the RMA-S cell line (23). Due to a deficiency in the peptide transporting TAP complex, these cells are unable to load peptide into class I MHC, resulting in very low MHC Class I expression on the surface of these cells. IL-12-treated OT-1 CTL were unable to kill RMA-S cells in the absence of exogenous peptide (Fig. 2F and G), suggesting that killing required MHC Class I surface expression.
To determine whether plasma membrane expression of syngeneic MHC Class I molecules was sufficient to induce killing by IL-12 treated CTL, regardless of the peptide presented, RMA-S cells were incubated with a peptide encoded by Vesicular Stomatitis Virus (VSV), RGYVYQGL (24). This peptide binds efficiently to H-2Kb and can upregulate MHC Class I expression on RMA-S cells (Fig 2F). While incubation with the VSV peptide up-regulated MHC Class I, it was not sufficient to induce killing of RMA-S cells by IL-12-treated OT-1 CTL, although these CTL were able to kill EL-4 cells and RMA-S cells pulsed with SIINFEKL (Figure 2G). Together, these results suggest that specific peptide/MHC Class I complex(s) on EL-4 cells was recognized by the IL-12-treated OT-1 CTL.
IL-12 treatment enhances peptide sensitivity of CTL
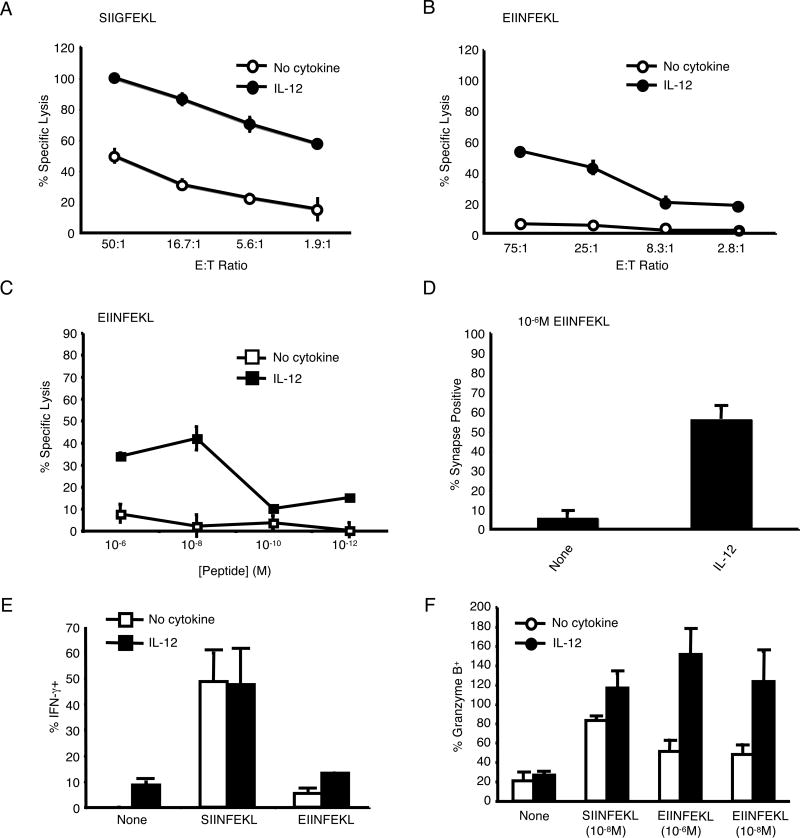
Our data led us to hypothesize that IL-12 enhanced responses of CTL to self-peptides for which they had weak affinity in the absence of exogenous cytokine. To begin to address this, we first determined whether IL-12 could increase CTL responses against normally weak peptides. To do this, we made use of a group of well-characterized altered peptide ligands (APLs) for the OT-1 TCR (25-28) (listed in Table I). We first tested the effects of IL-12 on the sensitivity of OT-1 CTL to an intermediate agonist peptide, SIIGFEKL. IL-12-treated OT-1 CTL were indeed much more efficient at lysing RMA-S cells pulsed with this peptide compared with untreated OT-1 CTL (Fig. 3A). These results demonstrate that IL-12 can increase the peptide sensitivity of CTL to weak agonists.
Table I. List of peptides tested for OT-1 CTL reactivity.
| Peptide Sequence | Source | Phenotype | KD (μM) | T1/2 (s) | Refs |
|---|---|---|---|---|---|
| SIINFEKL | Ovalbumin | Agonist | 6.2 | 33 | 25,26 |
| SIIGFEKL | APL of SIINFEKL | Intermediate agonist | 10 | 99 | 27 |
| EIINFEKL | APL of SIINFEKL | Weak agonist/antagonist/Induces positive selection | 20.8 | 10.3 | 25,26 |
| RGYNYEKL | APL of SIINFEKL | Antagonist/Induces positive selection | 22.9 | 18.7 | 25,26 |
| SIIRFEKL | APL of SIINFEKL | Antagonist/Induces positive selection | 48.9 | 5.4 | 25,26 |
| PTYRFERL | SH2 cont. inositol phosph. | Induces positive selection | ND* | ND | 28 |
| RTYTYEKL | β-catenin | Induces positive selection | ND | ND | 28 |
| AGYSFEKL | Map kinase 8 | Induces positive selection | ND | ND | 28 |
| HTYDFEKL | Ribonucleotide reductase | Induces positive selection | ND | ND | 28 |
| RAKRYEKL | Cell divisionPK 7 | Induces positive selection | ND | ND | 28 |
| ISFKFDHL | F-actin capping protein A | Induces positive selection | ND | ND | 28 |
ND: Not determined
FIGURE 3.
IL-12 enhances peptide sensitivity and decreases peptide-specificity of OT-1 CTL. (A) Untreated and IL-12-treated OT-1 CTL were tested for cytotoxicity against RMA-S cells pulsed with the intermediate agonist SIIGFEKL (10-6M) (+/-SD). Lysis of SIINFEKL at an E:T of 50:1 was 67% (No cytokine) and 78% (+IL-12). Lysis with control peptide at an E:T of 50:1 was 6% for both types of CTL. (B) Untreated and IL-12-treated CTL were tested for cytotoxicity against RMA-S cells pulsed with the weak agonist/antagonist EIINFEKL (10-6M) (+/- SD). Lysis of SIINFEKL at an E:T of 75:1 was 95% (No cytokine) and 100% (+IL-12). Lysis with control peptide at an E:T of 75:1 was 5% for both types of CTL. (C) Untreated and IL-12-treated OT-1 CTL were tested for cytotoxicity against RMA-S cells pulsed with various concentrations of EIINFEKL at an E:T ratio of 30:1 (+/- SD). Lysis of SIINFEKL at 10-6M was 65% (No cytokine) and 70% (+IL-12). Lysis with control peptide at 10-6M was 5% (No cytokine) and 15% (+IL-12). (D) The percentage (+/-SD of 3 independent experiments) of untreated and IL-12-treated OT-1 CTL that formed synapses with RMA-S cells pulsed with EIINFEKL (10-6M). No cSMACs were formed. (E) Percentage (+/- SD) of untreated and IL-12 treated OT-1 CTL that expressed IFN-γ as measured by intracellular staining. (F) The percentage (+/- SD) of untreated and IL-12-treated OT-1 CTL that produced granzyme B as measured by an ELISPOT assay.
We next tested the effect of IL-12 treatment on OT-1 CTL to the weak agonist/antagonist peptide EIINFEKL. While the EIINFEKL peptide was unable to stimulate cytolysis by untreated CTL, treatment with IL-12 allowed OT-1 CTL to efficiently lyse EIINFEKL-pulsed RMA-S cells (Fig. 3B and C). To ensure that this killing was not due to antigenic peptide carried over from the initial T cell stimulation, we confirmed these findings using CTL generated by stimulation with plate-bound anti-CD3 antibody (data not shown). In addition, by titrating the IL-12 concentration, we found that 1ng/ml of rmIL-12 was enough to induce a response from the CTL against this weak peptide (Supplementary Fig. 2). Corresponding with the lytic response, while IL-12 did not effect conjugate formation (data not shown), it greatly increased the percentage of OT-1 CTL that formed immune synapses with RMA-S cells pulsed with EIINFEKL (55% vs 5%, Fig. 3D). However, even with IL-12, the EIINFEKL peptide did not induce cSMAC formation or IFN-γ production (data not shown; Fig. 3E).
To determine whether IL-12 treatment induced more CTLs to kill or induced each CTL to lyse more EIINFEKL-pulsed target cells, we performed a granzyme B ELISPOT assay. IL-12 treatment resulted in a greater number of CTLs secreting lytic granules in response to RMA-S cells pulsed with EIINFEKL (Fig. 3F). This suggested that IL-12 treatment created more CTLs capable of responding to EIINFEKL. These results suggest that IL-12 can enhance the response of CTL to some weak peptides, with the capability of converting an antagonist peptide into a weak agonist peptide.
We next tested two strong antagonist peptides, RGYNYEKL and SIIRFEKL. Since these peptides are able to positively select OT-1 T cells in the thymus, the OT-1 TCR must have at least some functional affinity for these peptides (25, 26). However, treatment with IL-12 did not allow these antagonist peptides to induce activation of OT-1 CTL (data not shown). Taken together, these results suggest that IL-12 can enhance the response of CTL to some weak peptides, altering the specificity of the CTL response within a small range of affinity.
IL-12-treated CTL respond to normal self-peptides
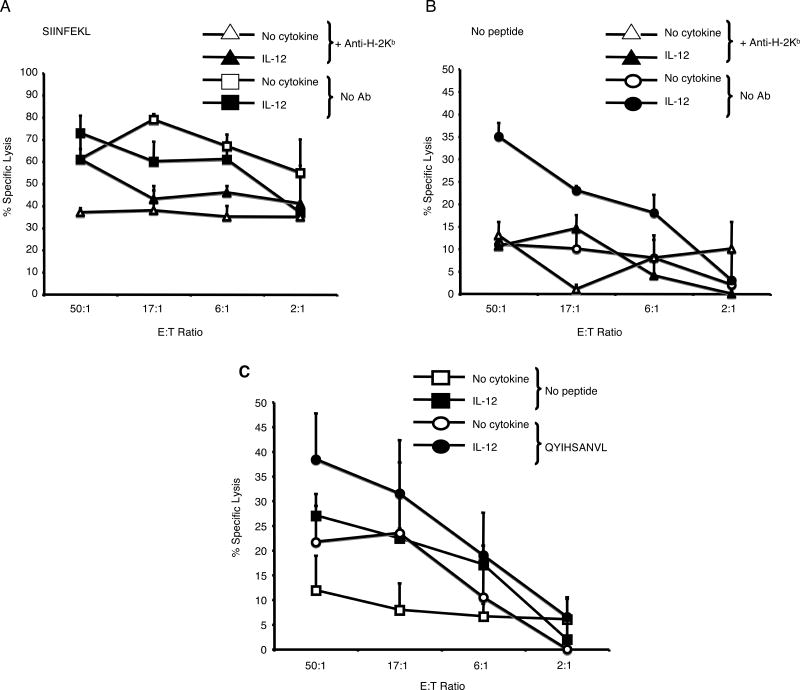
The ability of IL-12 to induce reactivity to EL-4 cells suggested that IL-12 might allow CTL to respond to a self-peptide. To directly test this, we tested whether IL-12-treated OT-1 and DUC18 CTL could lyse non-transformed, syngeneic splenocytes. Splenocytes were activated using Con A, so that they could be labeled with 51Cr, and tested for the ability to be killed by IL-12-treated OT-1 and DUC18 CTL. Whereas killing by untreated OT-1 and DUC18 CTL required antigenic peptide, IL-12-treated OT-1 and DUC18 CTL were able to lyse syngenic splenocytes in the absence of exogenous peptide (Fig. 4). We confirmed that the killing by the IL-12-treated OT-1 CTL was MHC-restricted using a blocking antibody against H-2Kb. These results suggest that treatment with IL-12 allows CTL to respond to self-peptide(s) present on syngeneic splenocytes.
FIGURE 4.
IL-12-treated CTL lyse syngeneic splenocytes. (A and B) Cytotoxicity (+/- SD) of untreated and IL-12-treated OT-1 CTL against ConA-blasted C57BL/6 splenocytes. (C) Cytotoxicity (+/- SD) of untreated and IL-12-treated DUC18 CTL against ConA-blasted Balb/c splenocytes +/- QYIHSANVL (10-6M).
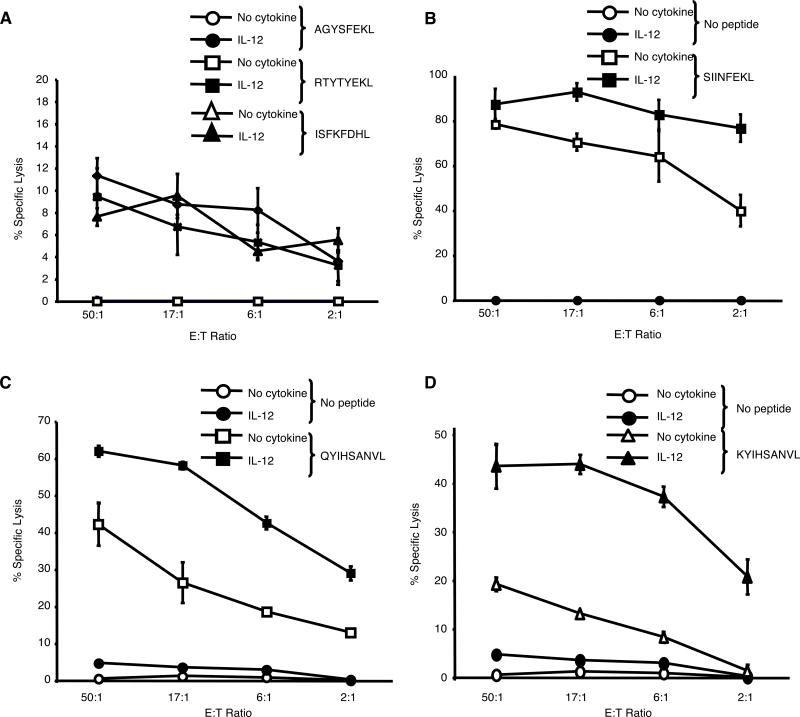
We next set out to identify self-peptides to which IL-12-treated OT-1 or DUC18 CTL were responsive to. With the OT-1 CTL, we tested self-peptides that are able to induce the positive selection of these T cells in the thymus (25-28) (Table I). IL-12-treated OT-1 CTL exhibited a low, but significant, level of lysis against RMA-S cells pulsed with 3 of the 6 peptides tested (Fig. 5A and B; data not shown). In contrast, the untreated CTL did not lyse RMA-S cells pulsed with any of these peptides.
FIGURE 5.
IL-12-treated CTL react to self-peptides. (A and B) Cytotoxicity (+/- SD) of untreated and IL-12-treated OT-1 CTL against RMA-S cells pulsed with the self-peptides AGYSFEKL, RYTYTEKL, or ISFKFDHL, or the antigenic peptide, SIINFEKL. (C and D) Cytotoxicity (+/- SD) of untreated and IL-12-treated DUC18 CTL against T2-Kd cells pulsed with the wild-type ERK2 peptide, KYIHSANVL, or the antigenic peptide, QYIHSANVL. These results are representative of two independent experiments.
We also tested IL-12-treated DUC18 CTL responses against a panel of H-2Kd-binding self-peptides using the T2-Kd cell line (TAP1/2 negative) (29) pulsed with a panel of endogenous peptides known to be presented on H-2Kd (19). Due to low MHC class I expression on these cells, there was little lysis by either CTL against T2-Kd cells in the absence of exogenous peptide. Additionally, no peptides in this panel of self-peptides were able to induce killing by IL-12-treated DUC18 CTL (data not shown). Since the DUC18 T cells are specific for a mutated ERK2 peptide with a single amino acid substitution which does not affect binding to H-2Kd (30), we next tested whether IL-12 treatment would allow these cells to respond to the corresponding wild-type ERK2 peptide, KYIHSANVL. Indeed, IL-12-treated DUC18 CTL efficiently lysed target cells pulsed with the wild-type ERK2 peptide, whereas untreated DUC18 CTL had only a low reactivity to these targets (Fig. 5C and D). These data suggest that the self-peptide recognized by the IL-12-treated DUC18 CTL is from the wild-type ERK2 peptide.
Together, these data suggest that IL-12 treatment allows CTL to recognize a very restricted subset of self-peptides. These are peptides for which the CTL has a low affinity in the absence of IL-12, possibly the same peptides involved in the positive selection of the cells in the thymus.
LFA-1 on IL-12-treated CTL is in a higher activation state
Interaction between the β2-integrin LFA-1 on T cells and ICAM-1 on APCs or target cells is required for T cell synapse formation (31). High levels of ICAM-1 alone are known to induce CTL to form ICAM-1 ring structures similar to pSMACs (32). Enhanced LFA-1/ICAM-1 interactions are also known to be capable of enhancing T cell responses to class I MHC/peptide (33). Therefore, we wondered whether differences in LFA-1 could explain the enhanced synapse formation and altered peptide specificity of IL-12-treated CTL.
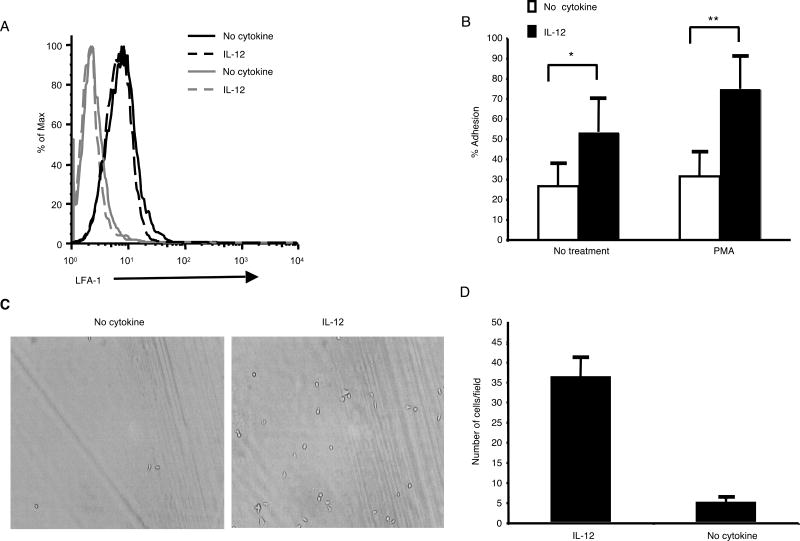
We first determined that the level of LFA-1 on the surface of untreated and IL-12-treated CTL was similar by staining with an LFA-1-specific antibody followed by flow cytometry (Fig. 6A). The function of LFA-1 is not only determined by expression level, but also by its activation state (reviewed in 34). Therefore, we performed two assays to compare the activation state of LFA-1 on untreated and IL-12-treated CTL. First, we tested the ability of the CTL to bind to plate-bound ICAM-1 in a plate adhesion assay (Fig. 6B). A greater percentage of IL-12-treated OT-1 CTL adhered to ICAM-1 in this assay compared with untreated OT-1 CTL. This difference was most apparent in the presence of the integrin activator PMA. We next tested the ability of the CTL to adhere to ICAM-1 under continuous flow conditions (1 dyn/cm2) using a parallel plate flow chamber. Under these more rigorous conditions, significantly more IL-12-treated CTLs were able to bind to ICAM-1 (Fig. 6C and D). These data demonstrated that IL-12 treatment enhanced the formation of a higher affinity LFA-1 on the CTL. This increased LFA-1 affinity is likely responsible for the increased synapse formation and peptide sensitivity of the IL-12-treated CTL.
FIGURE 6.
LFA-1 on IL-12-treated CTL is in a higher activation state. (A) Untreated and IL-12-treated OT-1 CTL were stained with an LFA-1-specific (black lines) or control (gray lines) antibody. (B) The percentage of untreated and IL-12-treated OT-1 CTL capable of binding to ICAM-1 in a plate adhesion assay. The values shown are the average of 6 wells at each condition +/- SD. These data are representative of multiple separate experiments. *p<0.01, **p<0.003 in a two-sided student's T-test. (C and D) The number of untreated and IL-12-treated OT-1 CTL that adhered to ICAM-1 under shear stress of 1 dyn/cm2 was determined. A representative field is pictured. The values shown are the average number of cells adhered in each of 5 different fields +/- SD. These data are representative of two separate experiments.
Discussion
Here we tested whether cytokines could affect the quantity and quality of immunological synapse formation by CTL in response to antigenic peptide. We found that IL-12 enhanced, whereas TGF-β decreased, synapse and cSMAC formation by OT-1 CTL. As both of these cytokines are present in inflammatory reactions in vivo, it seems likely that the effects that we saw are biologically relevant. IL-12 is predominantly produced by activated monocytes, macrophages and DCs, and acts primarily on activated CD4+ and CD8+ T cells and NK cells to induce cell-mediated immunity (35). On the other hand, TGF-β can be produced by regulatory T cells (Tregs), suppressing effector T cell function (36). Our data suggest that the differing effects of these two cytokines may be mediated via their effect on synapse formation. In further support of this, Esquerre et al. (37) recently reported that human Tregs can suppress Th function via TGF-β-mediated-inhibition of synapse formation between Th cells and DCs.
The enhancement of synapse formation by IL-12 correlated with enhanced LFA-1 avidity towards ICAM-1. LFA-1 interaction with ICAM-1 has a well-characterized role in both synapse formation and co-stimulation of both T and B cell responses (32, 38, 39). Dustin and co-workers previously reported that high densities of ICAM-1 could stimulate pSMAC formation by CTL in the absence of MHC (32), and recent studies demonstrate a role for LFA-1/ICAM-1 interaction in peptide sensitivity (40, 41). Thus, we were interested to test whether increased LFA-1 avidity might explain our phenotype. Our finding that IL-12 treatment resulted in enhanced LFA-1 avidity can explain both the enhanced synapse formation as well as the enhanced sensitivity to weak ligands.
While we were surprised that the higher activation state of LFA-1 on IL-12-treated CTL enhanced synapse formation without altering the efficiency of conjugate formation, this suggests that the resting avidity of LFA-1 on CTL is high enough to induce maximal conjugate formation, but that a higher avidity state is required to facilitate synapse formation. Such a disparity between conjugate and synapse formation requirements has been previously reported (37, 42). The cytokines may either alter the kinetics of synapse formation or the ability of the cells to form synapses entirely. Our current data does not allow us to discriminate between these two possibilities.
Studies suggest that generation of an immunological synapse can affect the sensitivity and/or specificity of a T cell response to a given peptide (40, 43). In particular, experiments with NKG2D, which we have shown also facilitates enhanced synapse and cSMAC formation (16), demonstrated a similar effect with enhanced recognition of weak peptides (43). Computational studies suggest that by concentrating TCRs and peptide/MHC complexes in the synapse facilitates signaling, as occupancy of the TCR is enhanced (11). This would be especially important when the number of antigenic peptide/MHC complexes is low.
The ability of IL-12 to facilitate synapse formation suggested that it would enhance signaling by weaker ligands for the TCR. We found that IL-12 treatment strongly amplified CTL responses of OT-1 cells to a weak agonist (SIIGFEKL) and converted an antagonist (EIINFEKL) into a weak agonist. This is consistent with our hypothesis that increased synapse formation leads to enhanced TCR sensitivity to peptides. It is interesting to speculate on the potentially contrasting roles for an antagonist peptide in the presence or absence of IL-12. In the absence of IL-12, the antagonist peptide might function to inhibit T cell responses; in the presence of IL-12 it could strongly enhance the response.
Although we have not demonstrated that the increased synapse formation seen with IL-12 directly leads to increased CTL killing, the enhanced synapse formation is indicative of stronger signaling. We propose that the increased LFA-1 avidity induced by IL-12 tightens the interaction between CTL and target cells, resulting in enhanced signaling in response to weak peptides. This would then lead to greater synapse formation resulting in even greater signaling.
Using two different CTLs, OT-1 and DUC18, we found that IL-12 treatment led to self-reactivity, suggesting that changes in the efficiency of synapse formation induced by IL-12 allowed for recognition of a self-peptide. This was shown by the fact that IL-12-treated OT-1 and DUC18 CTL could lyse syngeneic splenocytes and by the fact that OT-1 CTL could kill unpulsed EL-4 cells and DUC18 could kill unpulsed P815 cells. Antibody blocking experiments confirmed that the killing was MHC-dependent.
We identified putative self-peptides recognized by IL-12-treated OT-1 and DUC18 CTL using a trial and error approach, testing endogenous peptides known to bind H-2Kb (for OT-1) and H-2Kd (for DUC18). We reasoned that good candidates were those peptides that induce positive selection in the thymus. Positively selecting peptides are thought to have intermediate affinities, too low to trigger T cell activation but higher than the vast majority of non-stimulatory endogenous peptides. Three of the 6 endogenous peptides that we tested were able to stimulate IL-12-treated OT-1 CTL.
In the case of the DUC18 CTL, we reasoned that the self-peptide might be the peptide that corresponds to the sequence from the wild-type ERK2 peptide. The DUC18 TCR transgenic mouse contains a TCR cloned from the C18 cell line, a CTL line that specifically recognizes a mutated ERK2 peptide in the methyl-cholanthrene induced CMS5 tumor cell line. This mutated peptide, QYIHSANVL, contains a glutamine instead of a lysine in the first position of the peptide. Previous studies showed that the DUC18 CTL (18) as well as the original C18 cell line (30), recognize the mutated ERK2 peptide but not the wild-type. Since ERK2 is an abundant cellular protein that is ubiquitously expressed, we expect that the wild-type ERK2 peptide should also be ubiquitously present as a self-peptide. In this study we found that IL-12 treatment induced significant lysis of target cells pulsed with the wild-type ERK2 peptide by DUC18 CTL. Thus, it seems likely that the wild-type ERK2 peptide is at least one of the self-peptides recognized by the IL-12-treated DUC18 CTL.
Together, these data suggest that IL-12 increases the sensitivity of CTLs against a restricted set of self-peptides for which they normally have a low, but detectable affinity. These are likely self-peptides involved in the positive selection of the cells during thymic development and maintenance of the cells in the periphery.
While attempting to determine the mechanism by which IL-12 exerted its effects on CTL peptide reactivity, we explored whether there were other differences between untreated and IL-12-treated CTL in addition to LFA-1 avidity. We found no gross difference in the amount of tyrosine-phosphorylated proteins expressed by the two CTL prior to stimulation. We tested the possibility that changes in the expression of inhibitory receptors could explain changes in sensitivity. While we found that expression of the inhibitory receptor NKG2A was consistently lower on IL-12-treated OT-1 CTL, antibody-blocking experiments did not increase the sensitivity of OT-1 CTL to low affinity peptides. Blocking another inhibitory receptor, PD-1, also had no effect in our system. Since our target cell lines do not express any CD28 or NKG2D ligands, neither of these co-stimulatory molecules is involved. Lastly, there was no difference between untreated and IL-12-treated CTL in the expression of the microRNA miR-181a, the expression of which has been shown to correlate with TCR peptide sensitivity (Li et al., 2007). A microarray comparison of IL-12-treated and untreated CTL showed only twenty-three genes or ESTs that were differentially expressed (Supplementary Table I; NCBI Gene Expression Omnibus (GEO) database accession # GSE13173). No obvious candidate genes identified could readily explain the altered specificity of IL-12-treated CTL.
IL-12 is a key cytokine in Th1 differentiation, inducing the production of IFN-γ by T and NK cells. IL-12 enhances the generation of CTL by promoting transcription of cytolytic factors, including perforin and granzymes (44). In addition, IL-12 has been described as a third signal required for activated CD8+ T cell survival and for full CTL and memory cell differentiation (reviewed in 45). Because of all these effector functions, IL-12 is critical to the resistance of many intracellular pathogens (44), enhances anti-tumor immunity (46), and is involved in multiple autoimmune diseases (47). The importance of IL-12 in all of these diseases is generally believed to be due to its role in driving the generation of a Th1/Tc1 response. However, our data suggest that IL-12 may also be acting to enhance the affinity of CTL for peptides. Early in an immune reaction, this broadened specificity could be important in allowing a broader range of CTL to participate, buying time while more antigen-specific T cells are expanding. These responses would also be beneficial to anti-tumor immunity, which is usually aimed at normal self-antigens. Alternatively, if these self-reactive responses are not controlled, autoimmunity could develop.
In murine studies, IL-12 treatment can result in CD8+ T cell-mediated tumor rejection, and IL-12 has also been shown to increase the number of CD8+ T cells infiltrating tumor sites in both murine and human studies (48). Further, IL-12 can act as a vaccine adjuvant in both mice and humans, resulting in effective T cell responses against self-peptides (49-52). Our data suggest that one mechanism by which IL-12 may be acting as an anti-tumor agent is by allowing CTL to respond to self-peptides expressed on tumor cells for which the CTL have a low affinity in the absence of IL-12.
Our data leads to the hypothesis that the large amounts of IL-12 found in autoimmune sites could be leading to CTL responses against self-peptides. This is also a possible mechanism by which autoimmunity could be induced by a microbial infection, as has been suggested by several studies (53). Inflammatory sites induced by infection often contain large amounts of IL-12 and this could induce an autoimmune CTL response via normally low affinity self-peptides. Such a CTL response is probably usually kept in check by Tregs that suppress autoreactive T cell responses (reviewed in (36). However, if Tregs are absent or deficient, as is the case in many autoimmune diseases, IL-12 may increase CTL-mediated damage.
Supplementary Material
Similar ability to form conjugates and similar TCR level between untreated and cytokine-treated CTL. (A) Percentage (+/- SD) of untreated, IL-12-treated, and TGF-β-treated OT-1 CTL that formed conjugates with unpulsed RMA-S cells (left panels) or SIINFEKL (10-6M)-pulsed RMA-S cells (right panels). (B) Percentage (+/- SD) of untreated and IL-12-treated OT-1 CTL that formed conjugates with unpulsed EL-4 cells (left panels) or SIINFEKL (10-6M)-pulsed EL-4 cells (right panels). (C) Untreated and IL-12-treated CTL were stained with a TCRVα2-specific antibody before (black lines) or after (gray lines) co-culture with SIINFEKL (10-6M)-pulsed RMA-S cells for 1 hour.
Altered peptide specificity of OT-1 CTL occurs with lower IL-12 concentrations. OT-1 CTL were treated with various concentrations of IL-12 and tested for cytotoxicity against RMA-S cells pulsed with SIINFEKL (10-6M), EIINFEKL (10-6M) or no peptide.
Acknowledgments
We thank Kristin Hogquist and Matthew Mescher for helpful discussions.
Footnotes
This work was partially supported by the American Cancer Society (M.A.M.).
References
- 1.Hemmer B, Fleckenstein BT, Vergelli M, Jung G, McFarland H, Martin R, Wiesmuller KH. Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus SE, McFarland HF, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 3.Hemmer B, Pinilla C, Appel J, Pascal J, Houghten R, Martin R. The use of soluble synthetic peptide combinatorial libraries to determine antigen recognition of T cells. J Pept Res. 1998;52:338–345. doi: 10.1111/j.1399-3011.1998.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 4.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 5.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 6.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 7.Barton GM, Rudensky AY. Evaluating peptide repertoires within the context of thymocyte development. Semin Immunol. 1999;11:417–422. doi: 10.1006/smim.1999.0199. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan VS, Leskov IB, Chen JZ. Homeostasis of T cell diversity. Cell Mol Immunol. 2005;2:1–10. [PubMed] [Google Scholar]
- 9.Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 10.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 11.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw AS. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 12.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 13.Krummel MF, Davis MM. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol. 2002;14:66–74. doi: 10.1016/s0952-7915(01)00299-0. [DOI] [PubMed] [Google Scholar]
- 14.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 15.Cemerski S, Das J, Locasale J, Arnold P, Giurisato E, Markiewicz MA, Fremont D, Allen PM, Chakraborty AK, Shaw AS. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–355. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markiewicz MA, Carayannopoulos LN, Naidenko OV, Matsui K, Burack WR, Wise EL, Fremont DH, Allen PM, Yokoyama WM, Colonna M, Shaw AS. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J Immunol. 2005;175:2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- 17.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 18.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 19.Suri A, Walters JJ, Levisetti MG, Gross ML, Unanue ER. Identification of naturally processed peptides bound to the class I MHC molecule H-2Kd of normal and TAP-deficient cells. Eur J Immunol. 2006;36:544–557. doi: 10.1002/eji.200526235. [DOI] [PubMed] [Google Scholar]
- 20.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 21.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, Gorman DM, Nolan GP, Miyajima A, Kitamura T. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- 22.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend A, Ohlen C, Foster L, Bastin J, Ljunggren HG, Karre K. A mutant cell in which association of class I heavy and light chains is induced by viral peptides. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):299–308. doi: 10.1101/sqb.1989.054.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 25.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NR, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 26.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 27.Gascoigne NR, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. [DOI] [PubMed] [Google Scholar]
- 28.Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–142. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 29.Salter RD, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda H, Ohta N, Furukawa K, Miyazaki H, Wang L, Kuribayashi K, Old LJ, Shiku H. Mutated mitogen-activated protein kinase: a tumor rejection antigen of mouse sarcoma. Proc Natl Acad Sci U S A. 1997;94:6375–6379. doi: 10.1073/pnas.94.12.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Somersalo K, Anikeeva N, Sims TN, Thomas VK, Strong RK, Spies T, Lebedeva T, Sykulev Y, Dustin ML. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaglia JL, Greenfield EA, Mattoo A, Sharpe AH, Freeman GJ, Kuchroo VK. Intercellular adhesion molecule 1 is critical for activation of CD28-deficient T cells. J Immunol. 2000;165:6091–6098. doi: 10.4049/jimmunol.165.11.6091. [DOI] [PubMed] [Google Scholar]
- 34.Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 36.Zwar TD, van Driel IR, Gleeson PA. Guarding the immune system: suppression of autoimmunity by CD4+CD25+ immunoregulatory T cells. Immunol Cell Biol. 2006;84:487–501. doi: 10.1111/j.1440-1711.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 37.Esquerre M, Tauzin B, Guiraud M, Muller S, Saoudi A, Valitutti S. Human regulatory T cells inhibit polarization of T helper cells toward antigen-presenting cells via a TGF-beta-dependent mechanism. Proc Natl Acad Sci U S A. 2008;105:2550–2555. doi: 10.1073/pnas.0708350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bromley SK, Dustin ML. Stimulation of naive T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms. Immunology. 2002;106:289–298. doi: 10.1046/j.1365-2567.2002.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–599. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 40.Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase Ctheta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 42.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci U S A. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 45.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 46.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 47.Kang BY, Kim TS. Targeting cytokines of the interleukin-12 family in autoimmunity. Curr Med Chem. 2006;13:1149–1156. doi: 10.2174/092986706776360879. [DOI] [PubMed] [Google Scholar]
- 48.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 49.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 50.Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Improved efficacy of dendritic cell vaccines and successful immunization with tumor antigen peptide-pulsed peripheral blood mononuclear cells by coadministration of recombinant murine interleukin-12. Int J Cancer. 1999;80:324–333. doi: 10.1002/(sici)1097-0215(19990118)80:2<324::aid-ijc25>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 51.Cebon J, Jager E, Shackleton MJ, Gibbs P, Davis ID, Hopkins W, Gibbs S, Chen Q, Karbach J, Jackson H, MacGregor DP, Sturrock S, Vaughan H, Maraskovsky E, Neumann A, Hoffman E, Sherman ML, Knuth A. Two phase I studies of low dose recombinant human IL-12 with Melan-A and influenza peptides in subjects with advanced malignant melanoma. Cancer Immun. 2003;3:7. [PubMed] [Google Scholar]
- 52.Lee P, Wang F, Kuniyoshi J, Rubio V, Stuges T, Groshen S, Gee C, Lau R, Jeffery G, Margolin K, Marty V, Weber J. Effects of interleukin-12 on the immune response to a multipeptide vaccine for resected metastatic melanoma. J Clin Oncol. 2001;19:3836–3847. doi: 10.1200/JCO.2001.19.18.3836. [DOI] [PubMed] [Google Scholar]
- 53.Libbey JE, McCoy LL, Fujinami RS. Molecular mimicry in multiple sclerosis. Int Rev Neurobiol. 2007;79:127–147. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Similar ability to form conjugates and similar TCR level between untreated and cytokine-treated CTL. (A) Percentage (+/- SD) of untreated, IL-12-treated, and TGF-β-treated OT-1 CTL that formed conjugates with unpulsed RMA-S cells (left panels) or SIINFEKL (10-6M)-pulsed RMA-S cells (right panels). (B) Percentage (+/- SD) of untreated and IL-12-treated OT-1 CTL that formed conjugates with unpulsed EL-4 cells (left panels) or SIINFEKL (10-6M)-pulsed EL-4 cells (right panels). (C) Untreated and IL-12-treated CTL were stained with a TCRVα2-specific antibody before (black lines) or after (gray lines) co-culture with SIINFEKL (10-6M)-pulsed RMA-S cells for 1 hour.
Altered peptide specificity of OT-1 CTL occurs with lower IL-12 concentrations. OT-1 CTL were treated with various concentrations of IL-12 and tested for cytotoxicity against RMA-S cells pulsed with SIINFEKL (10-6M), EIINFEKL (10-6M) or no peptide.