Summary
Signal transduction through G protein-coupled receptors (GPCRs) is regulated by receptor desensitization and internalization that follow agonist stimulation. Nitric oxide (NO) can influence these processes, but the cellular source of NO bioactivity and the effects of NO on GPCR-mediated signal transduction are incompletely understood. Here we show in cells and mice that β-arrestin 2, a central element in GPCR trafficking, interacts with and is S-nitrosylated at a single cysteine by endothelial NO synthase (eNOS) and that S-nitrosylation of β-arrestin 2 is promoted by endogenous S-nitrosogluthathione. S-nitrosylation following agonist stimulation of the β-adrenergic receptor, a prototypical GPCR, dissociates eNOS from β-arrestin 2 and promotes binding of β-arrestin 2 to clathrin heavy chain/β-adaptin, thereby accelerating receptor internalization. The agonist-and NO-dependent shift in the affiliations of β-arrestin 2 is followed by denitrosylation. Thus, β-arrestin subserves functional coupling of eNOS and GPCRs, and dynamic S-nitrosylation/denitrosylation of β-arrestin 2 regulates stimulus-induced GPCR trafficking.
Introduction
G protein-coupled receptors (GPCRs), characterized structurally by seven transmembrane spans, comprise the largest family of ligand-activated plasma membrane receptors and transduce a broad range of cellular signals, in large part through the activation of heterotrimeric guanosine triphosphate-binding proteins (G-proteins) (Drake et al., 2006; Pierce et al., 2002; Reiter and Lefkowitz, 2006). Transduction through most or all GPCRs is regulated by agonist-induced desensitization, in which receptors are functionally uncoupled from G-protein activation, and by internalization, in which receptors undergo endocytosis followed by recycling or degradation (Drake et al., 2006; Reiter and Lefkowitz, 2006). The scaffolding protein β-arrestin (β-arrestin 1 and β-arrestin 2) serves as an essential element in those processes. β-arrestin is recruited to and binds the activated conformation of GPCRs, where it sterically inhibits coupling of GPCRs and G-proteins, and serves as an adaptor to link GPCRs to nascent clathrin-based endocytotic vesicles (Drake et al., 2006; Reiter and Lefkowitz, 2006), whose scission from the plasma membrane is controlled by the large GTPase dynamin (Sweitzer and Hinshaw, 1998).
The ligand-induced association of β-arrestin and the β-adrenergic receptor (β-AR), a prototypic GPCR, is dependent upon receptor phosphorylation by the G protein-coupled receptor kinases (GRKs) (Drake et al., 2006; Reiter and Lefkowitz, 2006), and GPCR desensitization and internalization are also regulated by additional post-translational modifications of both GPCRs and β-arrestin, including ubiquitination (Shenoy et al., 2001) and phosphorylation (Lin et al., 2002; Lin et al., 1997; Lin et al., 1999). It is important to note, however, that whereas regulation of the interaction between β-arrestin and GPCR by agonist-stimulated posttranslational modification has been relatively well-characterized, far less is known about regulation of the interaction of β-arrestin with the molecular constituents of the endocytotic machinery (in particular, clathrin and AP2).
It is well-established that numerous GPCRs are coupled functionally to endothelial nitric oxide (NO) synthase (eNOS, NOS3) (Dudzinski et al., 2006), and accumulating evidence (Adam et al., 1999; Kokkola et al., 2005; Leclerc et al., 2006; Nozik-Grayck et al., 2006; Wang et al., 2006) suggests that NO may provide a newly appreciated regulatory influence on desensitization and internalization by modulating the function of GPCR-related proteins through S-nitrosylation (Hess et al., 2005). In particular, it has been shown recently that activation of the β-AR results in dynamic S-nitrosylation of both GRK2 (Whalen et al., 2007) and dynamin (Wang et al., 2006), with consequent effects on receptor internalization. It is of note, however, that the effects of S-nitrosylation on proximal (GRK2) and distal (dynamin) elements of the internalization pathway are antipathetic, and mechanisms by which eNOS or NO might coordinately regulate internalization are not known. Moreover, β-arrestin-regulated GPCR trafficking, as exemplified in the case of the β-AR, is directed primarily through clathrin-mediated rather than caveolar-based pathways (Drake et al., 2006; Reiter and Lefkowitz, 2006), whereas eNOS is thought to reside primarily in caveolae through association with the scaffolding protein caveolin (Dudzinski et al., 2006). It is unclear, therefore, how NO might precisely target functionally related elements to regulate GPCR internalization (Whalen et al., 2007). Here we demonstrate that an important locus of action of NO in the control of clathrin-based receptor trafficking is in fact β-arrestin itself.
Results
S-nitrosylation of β-arrestin 2 by S-nitroso-cysteine and by eNOS
Following treatment of human embryonic kidney (HEK) cells transiently overexpressing rodent β-arrestin 2 with S-nitroso-cysteine (SNO-Cys), a cell permeable S-nitrosylating compound (and a precursor of intracellular S-nitroso-glutathione (GSNO))(Sandmann et al., 2005), S-nitrosylated β-arrestin 2 (SNO-β-arrestin 2) was detected in cell lysates with the biotin-switch assay (Jaffrey and Snyder, 2001) (Fig. 1A). We then asked whether β-arrestin 2 can be S-nitrosylated by endogenously generated NO and whether β-arrestin 1 is also susceptible to S-nitrosylation. In HEK cells stably overexpressing eNOS (HEK-eNOS) and transiently overexpressing β-arrestin 2 or β-arrestin 1, substantial S-nitrosylation of β-arrestin 2 was detected, which could be augmented modestly (~2-fold) by the addition of exogenous SNO-Cys (Suppl Fig. 1A, B), whereas modification of β-arrestin 1 was negligible (SNO-β-arrestin 1 levels are ~6% of SNO-β-arrestin 2 levels) (Fig 1B).
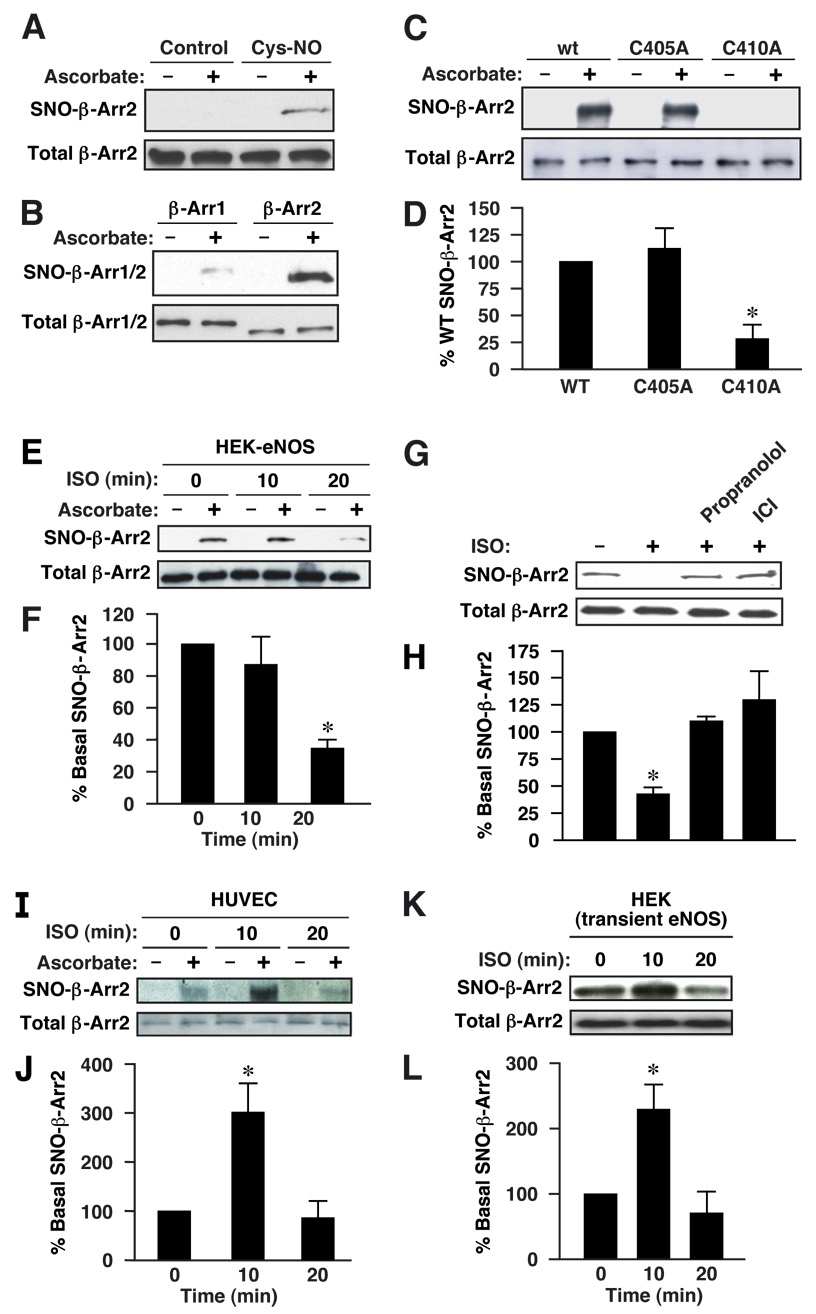
Figure 1. Cellular S-nitrosylation of β-arrestin 2.
(A) HEK cells transfected with FLAG-tagged β-arrestin 2 were treated with Cys-NO (50 µM), and lysates were analyzed by the biotin-switch assay, in which ascorbate-dependent labeling demonstrates the presence of S-nitrosylated Cys residues. (B) HEK-eNOS cells were transfected with FLAG-tagged β-arrestin 1 or 2, and lysates were analyzed by biotin-switch. (C) Lysates derived from HEK-eNOS cells overexpressing FLAG-tagged wild-type, C405A or C410A β-arrestin 2 were analyzed by biotin-switch. (D) The quantity of S-nitrosylated mutant β-arrestin 2 (C405A or C410A), as measured by scanning densitometry, is expressed as a percentage of wild type β-arrestin 2, normalized with respect to total β-arrestin 2. Data are means ± SE (n = 4); *P < 0. 01 re. control. (E) HEK-eNOS cells transiently transfected with FLAG-tagged β-arrestin 2 were treated with the β2AR agonist isoproterenol (ISO; 10 µM) for the indicated times and lysates were analyzed by biotinswitch. (F) The quantity of S-nitrosylated β-arrestin 2 (assessed by scanning densitometry) is expressed as a percentage of control values (0 min), normalized with respect to total β-arrestin 2. (G) HEK-eNOS cells transfected with FLAG-tagged β-arrestin 2 were treated with propranolol (10 µM) or ICI 118,551 (10 µM) for 10 min and stimulated with ISO (10 µM) for 20 min, and lysates were analyzed by biotin-switch. (H) A histogram shows the results of semiquantitative analysis as in (F). Data are means ± SE (n = 3); *P < 0. 01 re. control. (I) Human umbilical vein endothelial cells (HUVECs) were treated with ISO (10 µM) for the indicated times and lysates were analyzed by biotin-switch (endogenous β-arrestin 2 was 2 detected using a specific rabbit polyclonal 32 antibody (A2CT)). (J) A histogram shows the results of semiquantitative analysis as in (F). (K) Wild-type HEK cells transiently transfected with eNOS and FLAG-tagged β-arrestin 2 were treated with ISO (10 µM) for the indicated times and lysates were analyzed by biotin-switch. (L) A histogram shows the results of semiquantitative analysis as in (F). For (F), (I) and (L) data are means ± SE (n = 4); *P < 0. 05 re. control.
We next carried out a mutational analysis to localize the site(s) of S-nitrosylation within β-arrestin 2. Rodent β-arrestin 2 possesses ten Cys residues, three of which are not shared in the sequence of β-arrestin 1: Cys17 (numbering according to the rat sequence), Cys405 and Cys410 (C-terminal). In β-arrestin 1 the phosphorylation state of a C-terminus Ser residue (not present in β-arrestin 2) influences GPCR internalization (Lin et al., 2002; Lin et al., 1997; Lin et al., 1999); Cys410 is similarly placed in β-arrestin 2. Moreover, Cys410 (Cys409 in the human sequence) but not Cys405 is conserved in mammalian β-arrestin 2. C405A and C410A mutants of rat β-arrestin 2 were generated and expressed in HEK-eNOS cells. The C410A mutation virtually eliminated S-nitrosylation by eNOS, whereas S-nitrosylation of the C405A form was indistinguishable from wild-type (Fig. 1C, D). In addition, stimulation of HEK-eNOS cells with the β2-AR agonist isoproterenol resulted in marked denitrosylation of β-arrestin 2 by 20 min (Fig. 1E, F), which could be blocked with the β-antagonist, propranolol, or the inverse agonist ICI 118,551 (Fig. 1G, H), whereas high basal levels of S-nitrosylation were unchanged by isoproterenol, antagonist or inverse agonist at earlier time points (Suppl. Fig. 2A–D). These data suggest that eNOS-dependent S-nitrosylation of C410 can be regulated by GPCR stimulation.
We next examined whether β-arrestin 2 is S-nitrosylated in a more physiological context. Exposure of human umbilical vein endothelial cells (HUVECs) and HEK cells transiently over-expressing eNOS to isoproterenol resulted in the S-nitrosylation of endogenous β-arrestin 2 (Fig. 1I–L). Robust S-nitrosylation after 10 min of stimulation declined to very low, basal levels by 20 min, even in the maintained presence of isoproterenol (Fig. 1I-L). Therefore, in both endothelial cells, and HEK cells transiently overexpressing eNOS (i.e. cells exhibiting low basal levels of β-arrestin S-nitrosylation), we observed agonist-induced S-nitrosylation of β-arrestin 2, followed by denitrosylation.
Interaction of β-arrestin 2 and eNOS
The substrate specificity of protein S-nitrosylation is thought to be conferred in part by protein-protein interactions that place NOSs in close contiguity with target proteins (Hess et al., 2005). eNOS and β-arrestin 2 co-immunoprecipitated from extracts of HEK-eNOS cells transiently overexpressing β-arrestin 2 (Fig. 2A, B), and in vitro binding assays using purified β-arrestin 2 and recombinant eNOS confirmed an interaction (Fig. 2C–G); binding isotherms revealed apparent Kd’s of 50–100nM (Fig. 2D–G), with an eNOS: β-arrestin 2 stoichiometry of 1:1 at saturation (Fig. 2F, G), suggesting a direct interaction.
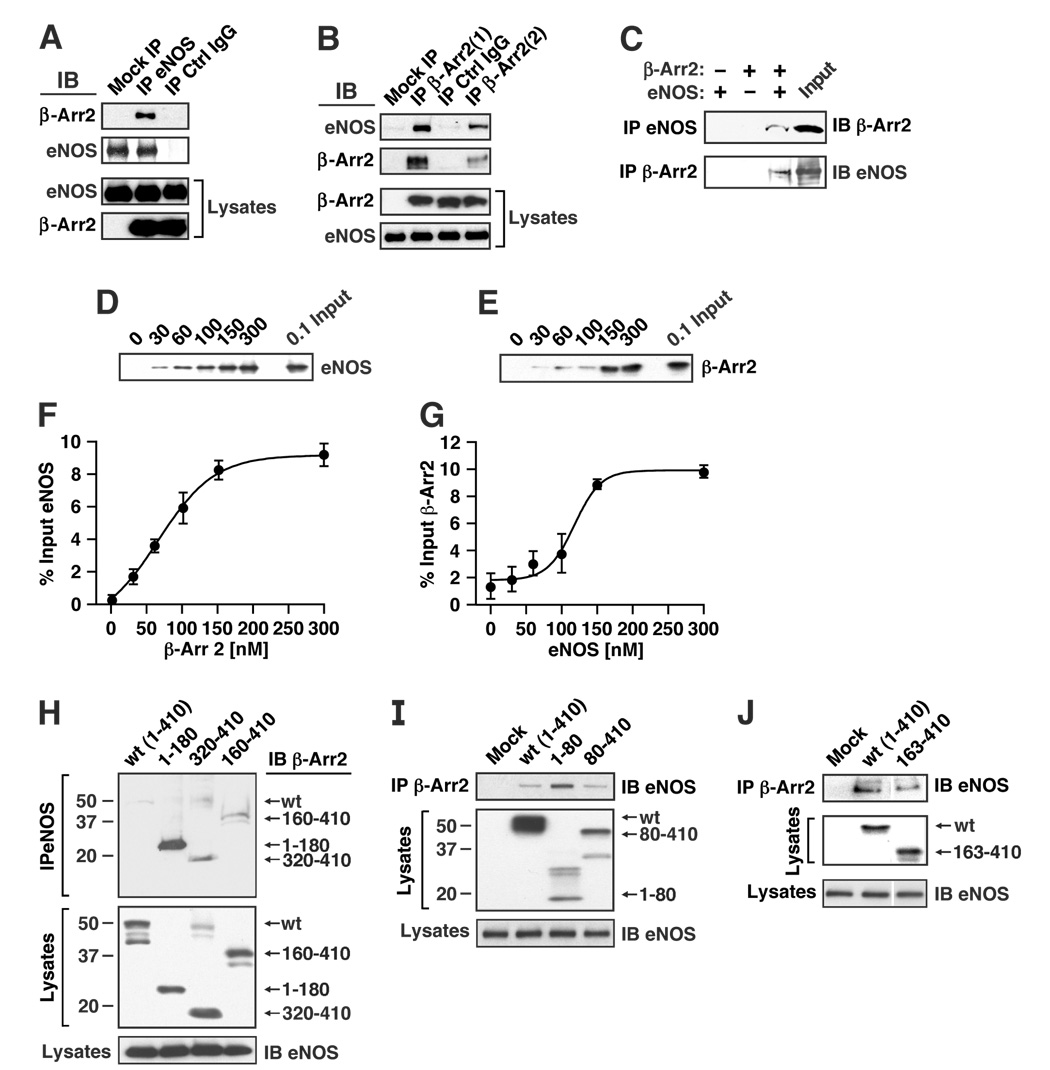
Figure 2. Interaction of β-arrestin 2 and eNOS in situ and in vitro.
(A, B) HEK-eNOS cells were transfected with empty vector or FLAG-tagged β-arrestin 2, and lysates were immunoprecipitated with (A) anti-eNOS antibody or (B) anti-FLAG antibody (either agarose-coupled anti-FLAG antibody (β-Arr 2(1)) or uncoupled anti-FLAG antibody followed by protein-G agarose (β-Arr 2(2)). Immunoprecipitates (IPs) were immunoblotted with anti-eNOS or anti-FLAG antibody. Mock = empty vector. (C) Purified eNOS and FLAG-tagged β-arrestin 2 were incubated at 4°C for 3 hr, then immunoprecipitated with anti-FLAG or anti-eNOS antibody. IPs were immunoblotted with anti-eNOS or anti-FLAG antibody (β-Arr2). (D) FLAG-tagged β-arrestin 2 (150 nM) and increasing concentrations of purified eNOS were incubated at 4°C for 3 hr, then immunoprecipitated with anti-FLAG antibody. IPs were immunoblotted with anti-eNOS. (E) Purified eNOS (150 nM) and increasing concentrations of FLAG-tagged β-arrestin 2 were incubated at 4°C for 3 hr, then immunoprecipitated with anti-eNOS antibody. IPs were immunoblotted with anti-FLAG antibody. (F) and (G) Graphs show the results of semiquantitative analysis in which the quantity of eNOS bound to β-arrestin 2 (F) and β-arrestin 2 bound to eNOS (G) are expressed as a percentage of input (n=3 in F and G). Data were fitted by sigmoidal functions; r2= 0.996 and 0.956 for F and G respectively. (H) HEK-eNOS cells were transfected with FLAG-tagged wild-type (full-length) β-arrestin 2 (wt: amino acids 1–410 of rat β-arrestin 2 open reading frame) or truncated β-arrestin 2 (amino acids 1–180, 320–410 or 160–410), and lysates were immunoprecipitated with anti-eNOS antibody. IPs and lysates were immunoblotted with anti-FLAG (β-Arr2) or anti-eNOS antibody. Molecular mass (kDa) is indicated at the left. (I) and (J) HEK-eNOS cells were transfected with FLAG-tagged wild-type β-arrestin 2 or truncated β-arrestin 2 (amino acids 1–80 or 80–410 (I), or 163–410 (J)), and lysates were immunoprecipitated with anti-FLAG antibody (β-Arr2). IPs and lysates were immunoblotted with anti-FLAG (β-Arr2) or anti-eNOS antibody.
To map the eNOS-binding region of β-arrestin 2, several truncated forms of β-arrestin 2 were generated and transfected into HEK-eNOS cells (Fig. 2H–J). The truncated forms of β-arrestin 2 containing the C-terminal amino acids 160–410 or 320–410 interacted with eNOS with efficiency equal to or slightly greater than wild-type β-arrestin 2 by co-immunoprecipitation, whereas the interaction of eNOS with the N-terminal (1–180) residues or N-terminus (1–80) was substantially greater than wild type (Fig. 2H–J). It is of note that the N-and C-terminal domains of β-arrestin 2 are in juxtaposition within the resting protein conformation (Xiao et al., 2004). Collectively, these and the above results support the likelihood that eNOS interacts with the N-terminus of β-arrestin 2, but that the C-terminal domain may also be involved.
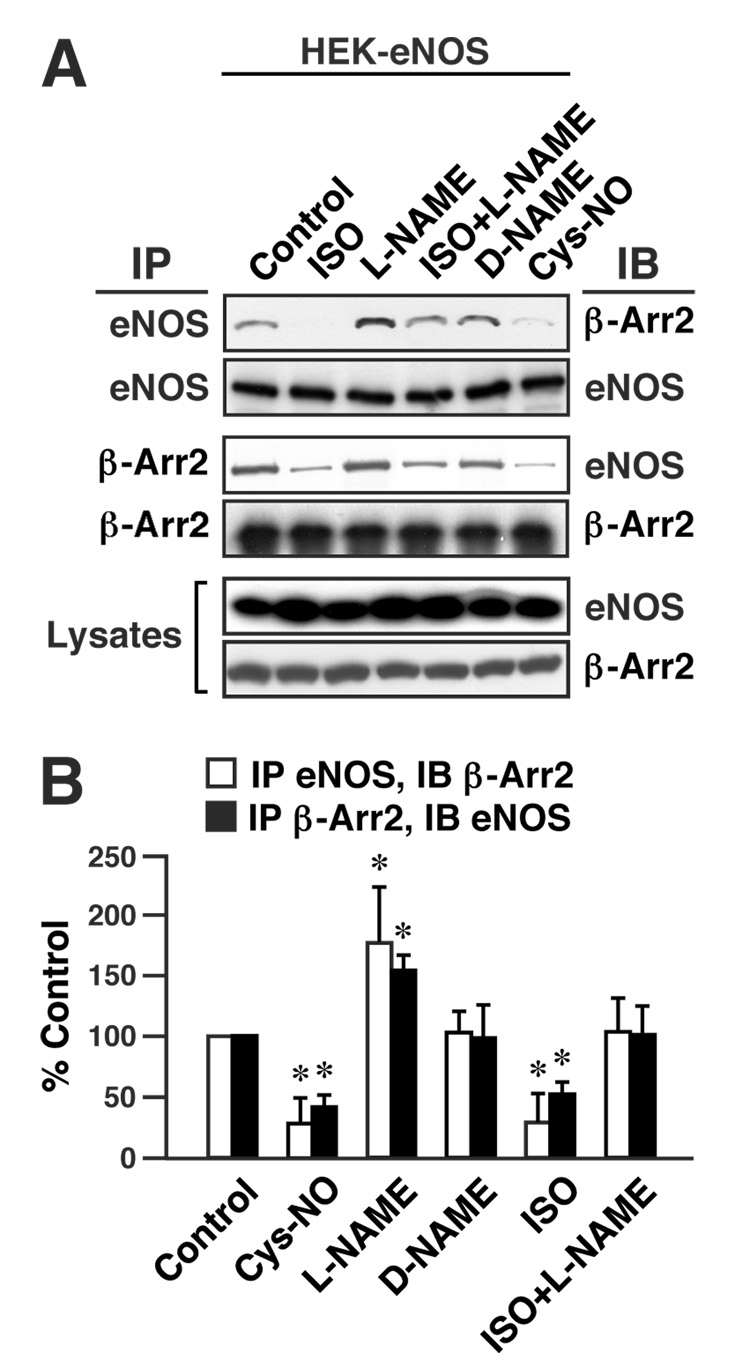
One mechanism through which S-nitrosylation modulates or mediates cellular signal transduction is the regulation of protein-protein interactions (Hara et al., 2005; Hess et al., 2005; Matsumoto et al., 2003; Rizzo and Piston, 2003). We examined the role of S-nitrosylation in regulating the interaction of β-arrestin 2 and eNOS. As illustrated in Fig. 3, the extent of co-immunoprecipitation of β-arrestin 2 and eNOS from extracts of HEK-eNOS cells transfected with β-arrestin 2 (as in Fig. 2A, B) was decreased significantly by prior treatment of intact cells with SNO-Cys (Fig. 3A, B), under conditions that result in S-nitrosylation of β-arrestin 2 (as in Fig. 1A and Suppl. Fig. 1A, B). Further, inhibiting NO production by eNOS with L-nitro-arginine methyl ester (L-NAME) significantly increased the efficacy of co-immunoprecipitation (whereas the inactive isomer D-NAME had no effect) (Fig. 3A, B). Conversely, activation of β-ARs by isoproterenol significantly decreased the extent of interaction between β-arrestin 2 and eNOS, and this decrease was inhibited with L-NAME (Fig. 3A, B). Taken together, these results indicate that S-nitrosylation of β-arrestin 2 consequent upon β 2AR stimulation inhibits the interaction of β-arrestin 2 and eNOS.
Figure 3. S-nitrosylation inhibits the interaction of β-arrestin 2 and eNOS.
(A) HEK-eNOS cells transfected with FLAG-tagged β-arrestin 2 were exposed to L-NAME (3mM) or D-NAME (3mM) for 48 h or untreated, and L-NAME-treated and untreated cells were exposed to isoproterenol (ISO; 10 µM) for 10 min or to Cys-NO (50 µM) for 20 min. Lysates were immunoprecipitated with anti-eNOS or anti-FLAG antibody, and IPs and lysates were immunoblotted with anti-FLAG antibody (β-Arr2) or anti-eNOS antibody. (B) The amounts of β-arrestin 2 bound to eNOS and eNOS bound to β-arrestin 2 are represented as percentages of control values, normalized with respect to the immunoprecipitated levels of eNOS and β-arrestin 2 respectively. Data are means ± SE (n = 3); *P < 0. 01 re. control.
Subcellular fractionation (HEK-eNOS cells and HUVECs) verified the predicted predominant cellular localizations of β-arrestin 2 (cytosol) and eNOS (membrane) (Suppl. Fig. 3A, B), but showed further that each protein co-immunoprecipitated with the other from either fraction (Suppl. Fig. 3A, B). Thus, eNOS and β-arrestin 2 evidently can interact at both membranes and cytosol under basal conditions.
S-nitrosylation of β-arrestin 2 coordinately regulates protein-protein interactions
The routing of GPCRs into the clathrin-based endocytotic pathway is mediated by direct interactions of receptor-bound β-arrestin 2 with the clathrin heavy chain (HC) (Goodman et al., 1996) and with the clathrin-interacting β2 subunit of the heterotetrameric AP2 adaptor protein (β-adaptin) (Laporte et al., 1999). Therefore, we examined the effects of eNOS-derived NO on the interaction of β-arrestin 2 with endogenous clathrin HC and β-adaptin in HEK-eNOS cells and in wild-type HEK cells. Wild type HEK cells are known to express lower levels of NOS ((Lin et al., 2004) and Suppl. Fig. 4A, B) and are thus more amenable to eNOS knockdown with siRNA.
In HEK-eNOS cells, each form of β-arrestin 2 (wild type, C405A and C410A) co-immunoprecipitated with both clathrin HC and β-adaptin (Fig. 4A). L-NAME greatly reduced the extent of interaction between clathrin HC/β-adaptin and both wild-type and C405A β-arrestin 2, but had no effect on the interaction with C410A β-arrestin 2 (Fig. 4A, B). Consistent with these results, silencing of eNOS with siRNA in wild-type HEK cells significantly reduced the extent of interaction of clathrin/AP-2 with wild-type β-arrestin 2, but had no effect on the interaction of clathrin/AP-2 with C410A β-arrestin 2 (Fig. 4C, D and Suppl. Fig. 4B). These results indicate that S-nitrosylation of Cys410 potentiates the interaction of β-arrestin 2 with clathrin HC/β-adaptin, in striking contrast to the inhibitory effect of S-nitrosylation on the interaction between β-arrestin 2 and eNOS.
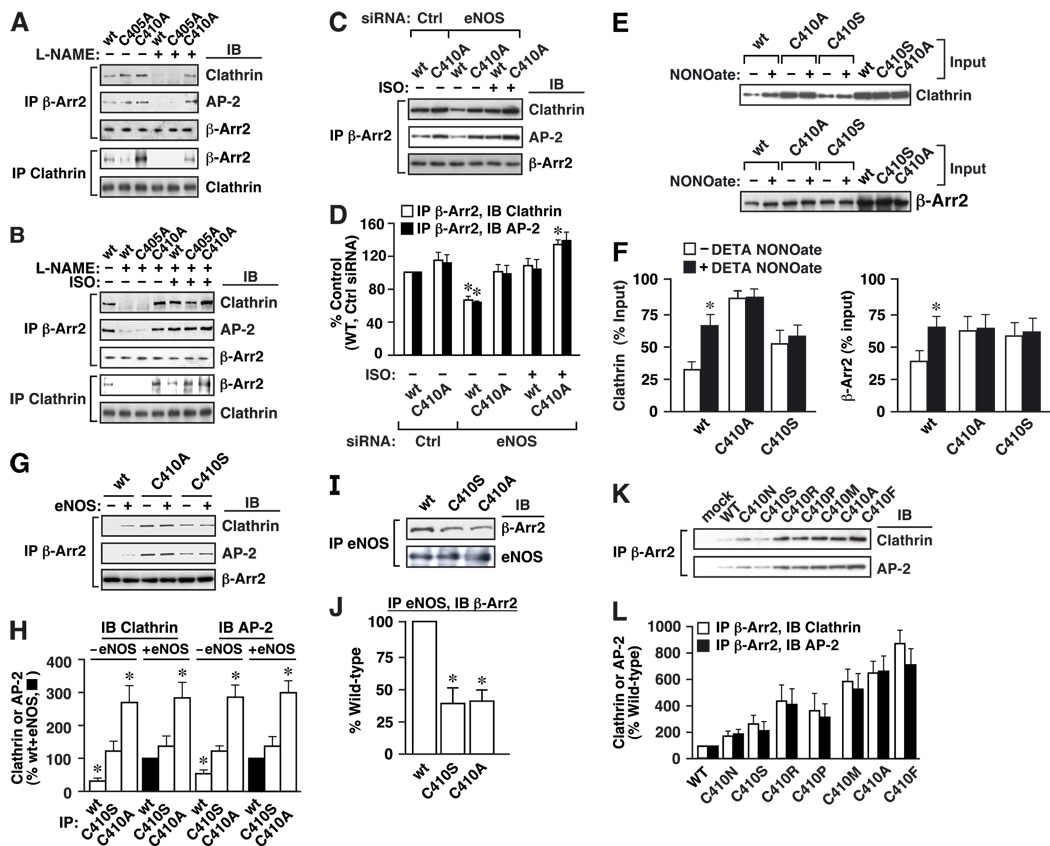
Figure 4. S-nitrosylation facilitates the interaction of β-arrestin 2 and clathrin/AP-2.
Expression controls for A–C, G and I are shown in Suppl. Fig. 5. (A, B) HEK-eNOS cells overexpressing FLAG-tagged wild-type, C405A or C410A β-arrestin 2 were untreated or exposed to L-NAME (3 mM) for 48 h (A, B), and subsequently untreated or treated with isoproterenol (ISO; 10 µM) for 10 min (B). Lysates were immunoprecipitated with anti-FLAG antibody (β-Arr2) and IPs and lysates were immunoblotted with anti-clathrin HC (Clathrin), anti-β-adaptin (AP-2) or anti-FLAG antibody (β-Arr2). Note increased efficacy of clathrin and β-adaptin binding by the C410A mutant form. (C) HEK cells were transfected with control or eNOS siRNA (50 µM), clathrin, β-adaptin (AP-2) and FLAG-tagged wild-type or C410A β-arrestin 2, and subsequently left untreated or treated with isoproterenol (ISO; 10 µM) for 10 min. Lysates were immunoprecipitated with the anti-FLAG antibody, and IPs were immunoblotted with anti-clathrin HC (Clathrin), anti-β-adaptin (AP-2) or anti-FLAG (β-Arr2). (D) Amounts of clathrin HC/β-adaptin co-immunoprecipitating with wild-type or C410A β-arrestin 2 are expressed relative to the amounts co-imunoprecipitating with wild-type β-arrestin 2 in cells transfected with control siRNA. Data are means ± SE (n = 3); *P < 0. 05, eNOS siRNA vs. control siRNA. (E) Effect of the NO-donor DETA-NONOate (50 µM) on in vitro binding (4° C for 12 hr) of clathrin and β-arrestin 2 (wild type, C410A and C410S). Immunoprecipitation was with anti-β-arrestin 2 or anti-clathrin antibodies, and immunoprecipitates were immunoblotted using anti-clathrin or anti-β-arrestin antibody, respectively. (F) Amounts of clathrin bound to β-arrestin 2 (at left), or β-arrestin 2 bound to clathrin (at right), are represented as percentage of input. Data are means ± SE (n = 3); *P < 0. 01 re. – DETA-NONOate vs. + DETA-NONOate. (G) Lysates from HEK cells overexpressing FLAG-tagged wild-type, C410A or C410S β-arrestin and transiently transfected with eNOS or with empty control vector were immunoprecipitated with anti-FLAG antibody, and IPs were immunoblotted with antibodies to clathrin HC (Clathrin), β-adaptin (AP-2) or FLAG (β-Arr2). (H) Amounts of clathrin HC/β-adaptin co-immunoprecipitating with the various forms of β-arrestin 2 in the presence or absence of eNOS are expressed relative to the amounts co-imunoprecipitating with wild-type β-arrestin 2 in the presence of eNOS. Data are means ± SE (n = 3); *P < 0. 01 re. wild-type + eNOS. (I) Lysates of HEK-eNOS cells overexpressing wild-type, C410A or C410S β-arrestin 2 were immunoprecipitated with anti-eNOS antibody, and IPs were immunoblotted with antibodies to FLAG (β-Arr2) and eNOS. (J) Amounts of the different forms of β-arrestin 2 bound to eNOS are shown as a percentage of the amount associated with wild-type β-arrestin 2, normalized with respect to the levels of eNOS and β-arrestin 2 in the lysates. Data are means ± SE (n = 3); *P < 0. 01 re. wild-type. (K) Lysates from HEK cells overexpressing FLAG-tagged wild-type β-arrestin 2 or C410 mutants (C→N, S, R, P, M, A or F) were immunoprecipitated with anti-FLAG antibody and IPs were immunoblotted with anti-clathrin HC (Clathrin) or anti-β-adaptin (AP-2) antibodies. (L) Amounts of clathrin HC/β-adaptin co-immunoprecipitating with wild-type or mutant β-arrestins are expressed relative to the amounts co-imunoprecipitating with wild-type β-arrestin 2. Data are means ± SE (n = 3).
Clathrin HC and β-adaptin can interact directly (Shih et al., 1995), and our results do not distinguish between the possibilities that S-nitrosylation of β-arrestin 2 alters its binding to clathrin HC, β-adaptin or both. To further address this question, we performed in vitro binding assays using purified forms of β-arrestin 2 and recombinant clathrin, which showed the interaction to be both direct and potentiated by NO (Fig. 4E, F and Suppl. Fig. 6). In contrast, NO had no effect on the interaction of C410A or C410S mutant arrestins with clathrin (Fig. 4E, F). Similar studies with β-adaptin were inconclusive (not shown). We observed consistently that, even in the absence of NO (following L-NAME treatment of HEK-eNOS cells or following eNOS knockdown with siRNA in wild-type HEK cells), basal binding of the C410A β-arrestin 2 mutant to clathrin HC/β-adaptin was more efficient than binding by either wild-type β-arrestin 2 or the C405A mutant (Fig. 4A–D). Furthermore, although isoproterenol potentiated binding of all three forms of β-arrestin 2 (WT, C405A and C410A) to clathrin HC/β-adaptin in L-NAME-treated HEK-eNOS cells or following eNOS knock-down in wild-type HEK cells, binding of C410A β-arrestin 2 remained notably more robust (Fig. 4B–D). These observations point to a significant role for the Cys410 sulfhydryl side chain in determining the properties of the β-arrestin 2 binding interface, consistent with the regulation of protein-protein interactions by S-nitrosylation of Cys410.
These findings were confirmed and extended by comparing the binding of wild-type, C410A and C410S β-arrestin 2 to both clathrin HC/β-adaptin (Fig. 4G, H) and eNOS (Fig. 4I, J). In HEK cells overexpressing β-arrestin 2, transient transfection with eNOS resulted in an enhancement of clathrin HC and β-adaptin binding to wild-type β-arrestin 2 (Fig. 4G, H). In contrast, eNOS had no effect on the binding of clathrin HC and β-adaptin to C410S or C410A β-arrestin 2, strongly supporting the critical role of Cys410 in mediating the effects of S-nitrosylation on β-arrestin 2 (Fig. 4G, H). It was also appreciated that C410A (and to a lesser extent C410S) β-arrestin 2 bound more clathrin HC/β-adaptin than wild-type β-arrestin 2, in the presence or absence of eNOS (Fig. 4G, H). Conversely, in HEK-eNOS cells, binding of eNOS by β-arrestin 2 (which is inhibited by S-nitrosylation of β-arrestin 2) was substantially less efficient for both C410S and C410A β-arrestin 2 vs. wild-type β-arrestin 2 (Fig. 4I, J). Thus, C410S and C410A mutations enhance to differing extents the binding of β-arrestin 2 to clathrin/AP-2 and suppress the binding of β-arrestin 2 to eNOS, as does S-nitrosylation of Cys410. Further, both the Cys410A and C410S mutations markedly inhibited the NO-dependent shift in the affiliations of β-arrestin 2 with both clathrin/AP2 and eNOS.
Comparative analysis of the effects of an additional series of Cys410 mutations on basal binding of clathrin/AP-2 confirmed the role of Cys410 in regulating β-arrestin 2 protein-protein interactions: all mutations examined enhanced basal binding (Fig. 4K, L). It is of note, however, that the degree of enhancement was substantially greater with mutations, including C410A, which increased side-chain hydrophobicity, whereas C410 S and C410 N had only a small effect (acidic C410 substitutions did not express). Because S-nitrosylation will increase local hydrophobicity, substitution with relatively hydrophobic residues may exert a SNO-mimetic effect, consistent with previous reports in which Cys→Ala mutation replicated effects of S-nitrosylation on the function and/or protein-protein interactions of hemoglobin (Mawjood et al., 2000; McMahon et al., 2000; Nagai et al., 1985), N-ethylmaleimide sensitive factor (Huang et al., 2005) and glyceraldehyde-3-phosphate dehydrogenase (Hara et al., 2003).
S-nitrosylation of β-arrestin 2 regulates β2-AR internalization
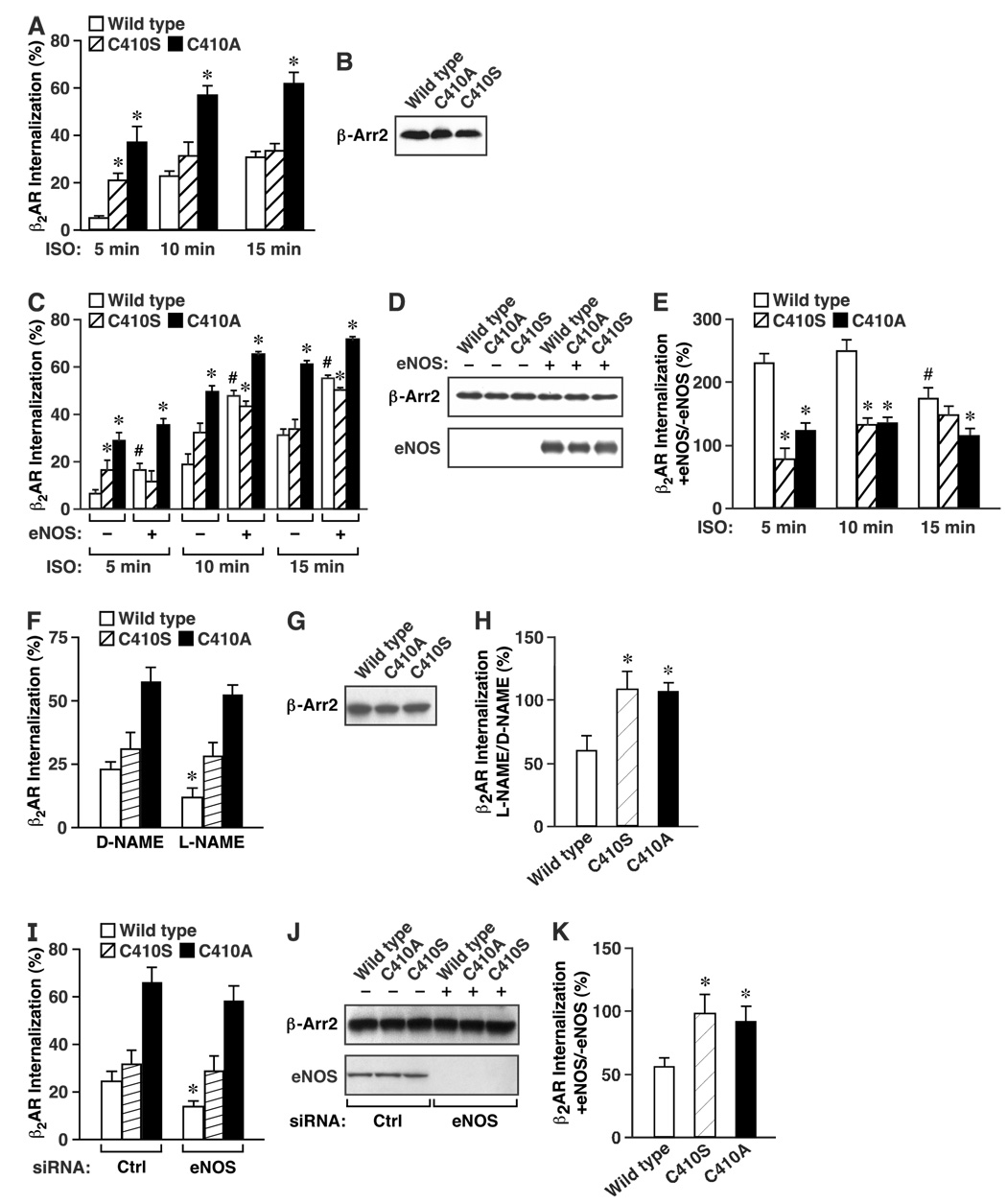
To assess the functional significance of β-arrestin 2 S-nitrosylation, we examined β2AR internalization induced by isoproterenol stimulation of HEK cells stably expressing the β2-AR (HEK-β2AR) and transiently overexpressing wild-type, C410S or C410A β-arrestin 2 (Fig. 5) as well as C410N or C410R β-arrestin 2 (Suppl. Fig. 7 A–C).
Figure 5. S-nitrosylation facilitates ligand-induced β2AR internalization.
(A) HEK cells overexpressing the β2-AR (HEK-β2AR cells) were transfected with wild-type, C410A or C410S β-arrestin 2 and internalization of β2AR was quantified by flow cytometry following stimulation by isoproterenol (ISO, 10 µM) for the indicated times. Data are means ± SE (n = 6); *P < 0. 05 re. wild-type at each time point. (B) Western blot of wild-type, C410A or C410S β-arrestin 2, showing comparable expression of each form. (C) HEK-β2AR cells were transfected with wild-type, C410A or C410S β-arrestin 2 and internalization of β2AR was quantified by flow cytometry following stimulation by isoproterenol (ISO, 10 µM) for the indicated times in the presence or absence of transiently overexpressed eNOS. Data are means ± SE (n = 6); *P < 0. 05 re. wild-type and # indicates P < 0. 01 re. −eNOS vs. +eNOS at each time point. (D) Western blot of wild-type, C410A or C410S β-arrestin 2, and of eNOS, showing comparable expression across groups. (E) A histogram summarizing the results shown in (C). Data are means ± SE (n = 6); *P < 0. 05 re. wild-type and # indicates P < 0. 01 re. 5 min. (F) HEK-β2AR cells were transfected with wild-type, C410A or C410S β-arrestin 2 and internalization of β2AR was quantified by flow cytometry following stimulation by isoproterenol (ISO, 10 µM) for 10 minutes in the presence or absence of L-NAME or D-NAME (3mM, 48hr pretreatment). (G) Western blot of wild-type, C410A or C410S β-arrestin 2, showing comparable expression of each form. (H) A histogram summarizing the results shown in (F). Data are means ± SE (n = 8); *P < 0. 05 re. wild-type. (I) HEK-β2AR cells were transfected with wild-type, C410A or C410S β-arrestin 2 and either control or eNOS siRNA, and β2AR internalization was quantified by flow cytometry following stimulation by isoproterenol (ISO, 10 µM) for 10 minutes. (J) Western blot of eNOS and β-arrestin 2, showing effective siRNA-mediated silencing of eNOS expression, and comparable expression of wild-type, C410A or C410S β-arrestin 2. (K) A histogram summarizing the results shown in (I). Data are means ± SE (n = 8); *P < 0. 05 re. wild-type.
Consistent with enhanced clathrin HC/β-adaptin binding, C410A and to lesser extent C410S, C410N and C410R β-arrestin 2 (Fig. 4K, L), accelerated β2AR internalization: internalization reached plateau values by 15 min following agonist stimulation in cells expressing wild-type β-arrestin 2 but was complete by 10 min in cells expressing the mutant forms (Fig. 5A, B). In addition, the proportion of β2AR internalized was significantly greater at all time points in cells expressing the C410A mutant (Fig. 5A), consistent with the significantly enhanced binding to clathrin HC/β-adaptin of that mutant form in comparison to either wild-type or C410S β-arrestin 2 (Fig. 4K, L). Co-expression of eNOS with wild-type β-arrestin 2 resulted in a two-to three-fold enhancement of isoproterenol-induced β2AR internalization (Fig. 5C, D and Suppl. Fig. 7 A, B), whereas co-expression of eNOS with mutant forms of β-arrestin 2 reduced markedly the NO-mediated enhancement of internalization (Fig. 5E and Suppl. Fig. 7C). More specifically, although co-expression of eNOS with C410 mutant β-arrestins enhanced β2AR internalization (consistent with known enhancing effects of eNOS exerted downstream of β-arrestin 2 (Wang et al., 2006)), enhancement by eNOS was much less pronounced than with wild-type β-arrestin 2 (Fig. 5C–E and Suppl. Fig. 7 A–C). Conversely, eNOS inhibition with L-NAME, but not D-NAME, decreased β2AR internalization in wild-type but not C410A or C410S transfected HEK-β2AR cells (Fig. 5F–H). Moreover, siRNA-mediated silencing of eNOS had a very similar effect (Fig. 5I–K).
Confirmatory results were obtained using HEK cells stably overexpressing the class B AT1A angiotensin receptor (AT1AR), in which eNOS enhanced angiotensin-induced AT1AR internalization in cells expressing wild-type β-arrestin 2, but not the C410S or C410A mutants (Suppl. Fig. 8A–C). In addition, the facilitation of β2AR internalization by eNOS in cells expressing wild-type β-arrestin 2, but not C410S or C410A β-arrestin 2, decreased significantly by 15 min following agonist stimulation (Fig. 5E), and thus followed a time course consistent with agonist-induced S-nitrosylation/denitrosylation of β-arrestin 2 (Fig. 1I–L).
S-nitrosylation regulates protein-protein interactions of β-arrestin 2 in vivo
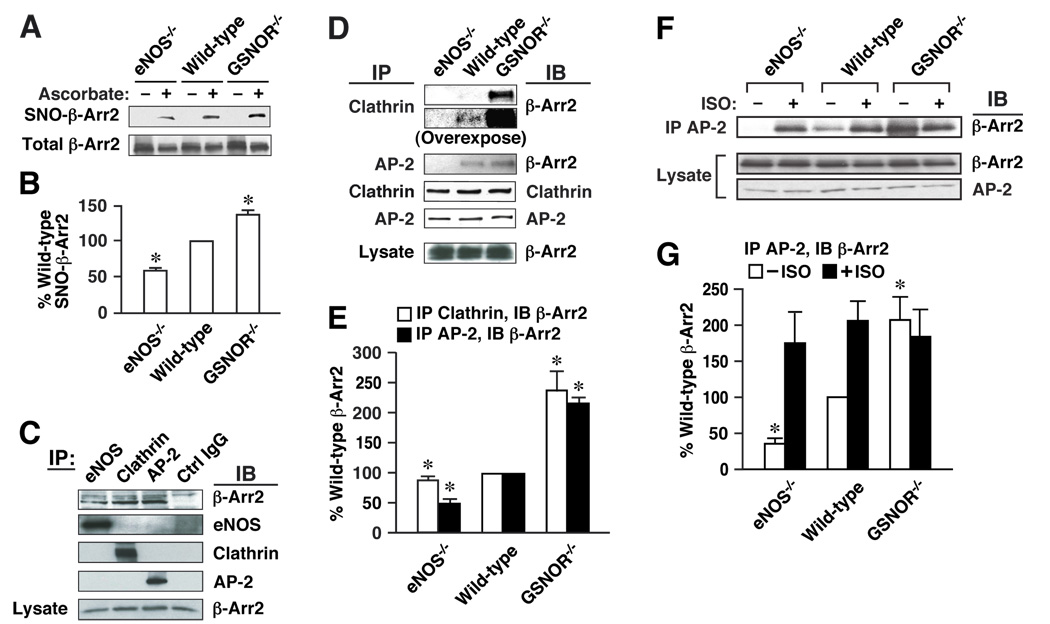
To verify that the regulation of β-arrestin 2 by S-nitrosylation we observed in cultured cells would also be exerted in situ, we utilized transgenic mice with specific alterations in the enzymatic mechanisms of NO production or processing. We examined the co-immunoprecipitation of β-arrestin 2 with clathrin HC/β-adaptin in tissue extracts from wild-type mice and from mice lacking eNOS (eNOS−/−) or GSNO reductase (GSNOR−/−). Genetic elimination of GSNOR is coupled to an increase in SNO-protein that is mediated by GSNO (Liu et al., 2004).
Analysis by biotin-switch revealed the presence of S-nitrosylated β-arrestin 2 in extracts of both liver (not shown) and spleen of wild-type mice (Fig. 6A), and demonstrated that the level of SNO-β-arrestin 2 was significantly decreased in extracts of spleen from eNOS−/− animals and significantly increased in extracts of GSNOR−/− spleen (Fig. 6A, B). The remaining SNO-β-arrestin 2 observed in the eNOS−/− mice was most likely generated by other NOS isoforms. Co-immunoprecipitation showed that β-arrestin 2 associated with eNOS and with clathrin HC/β-adaptin in spleen extracts from wild-type mice (Fig. 6C), and that these associations were significantly enhanced in extracts from GSNOR−/− spleen and significantly suppressed in samples from eNOS−/− animals (Fig. 6D, E). Thus, the basal association of β-arrestin 2 and HC/β-adaptin was commensurate with endogenous levels of S-nitrosothiols in these mice. These data show clearly that endogenous enzymatic activities governing protein S-nitrosylation regulate the protein affiliations and function of β-arrestin 2 in situ.
Figure 6. S-nitrosylation regulates protein-protein interactions of β-arrestin 2 in vivo.
(A) Extracts of spleen from wild-type (C57BL/6), GSNOR−/− or eNOS−/− mice were assayed by biotin-switch for SNO-β-arrestin 2 (ascorbate-dependent labeling). (B) Amount of SNO-β-arrestin 2 is represented as a percentage of SNO-β-arrestin 2 in wild-type spleen and normalized with respect to the level of total β-arrestin 2. Data are means ± SE (n = 3); *P < 0. 05 re. wild-type. (C) Extracts of spleen from wild-type mice were immunoprecipitated with antibodies to eNOS, clathrin HC (Clathrin), β-adaptin (AP-2) or non-specific IgG. IPs and extracts were immunoblotted with antibodies to β-arrestin 2, eNOS, clathrin HC (Clathrin) or β-adaptin (AP-2). (D) Extracts of spleen from wild-type, GSNOR−/− or eNOS−/− mice were immunoprecipitated with zantibodies to clathrin HC (Clathrin) or β-adaptin (AP-2), and IPs and extracts were immunoblotted with antibodies to β-arrestin 2, clathrin HC or β-adaptin. The immunoblot for β-Arr2 following IP for clathrin is shown at two different film exposure times to document coimmunoprecipitation in both wild-type and GSNOR−/− mice. (E) Amount of β-arrestin 2 bound to clathrin HC/β-adaptin in samples derived from GSNOR−/− or eNOS−/− mice is represented relative to wild-type mice and normalized with respect to the levels of clathrin HC/β-adaptin in the IPs or β-arrestin 2 in extracts. Data are means ± SE (n = 3 in each group); *P < 0. 05 re. wild-type. (F) Extracts of spleen from wild-type, GSNOR−/− eNOS−/− treated with isoproterenol (5 mg/kg i.p., 10 min.) or untreated were immunoprecipitated with anti-β-adaptin antibody, and IPs and extracts were immunoblotted with antibodies to β-arrestin 2 or β-adaptin (AP-2). (G) Amount of β-arrestin 2 bound to β-adaptin in samples derived from wild-type, GSNOR−/− eNOS−/− with and without isoproterenol treatment is represented relative to untreated wildt-ype mice and normalized with respect to the levels of β-arrestin 2 in extracts. Data are means ± SE (n = 4 in each group); *P < 0. 05 re. untreated wild-type.
We further examined the effect of β-AR stimulation with isoproterenol on the association AP-2 and β-arrestin 2 in spleen extracts from wild-type, eNOS−/− and GSNOR−/− mice (Fig. 6F, G). Consistent with the results in Fig. 6A (and similar to the results in Fig. 6D, E), eNOS−/− mice showed little to no basal association between AP-2 and β-arrestin 2 (vs. wild-type), whereas the GSNOR−/− mice showed increased association of these proteins. Stimulation with high pharmacological doses of isoproterenol (IP injection) leads to a significant increase in the association between AP-2 and β-arrestin 2 in the eNOS−/− and wild-type mice, but did not increase the association of these proteins in the GSNOR−/− mice, suggesting that maximum association had been achieved (Fig. 6F, G). These data indicate that GSNO, derived from eNOS and regulated by GSNOR (Liu et al., 2004), serves as a principal source of NO groups in the S-nitrosylation of β-arrestin 2 (Fig. 6G), and strongly support an agonist-and SNO-dependent shift in the protein affiliations of β-arrestin 2 in vivo.
Discussion
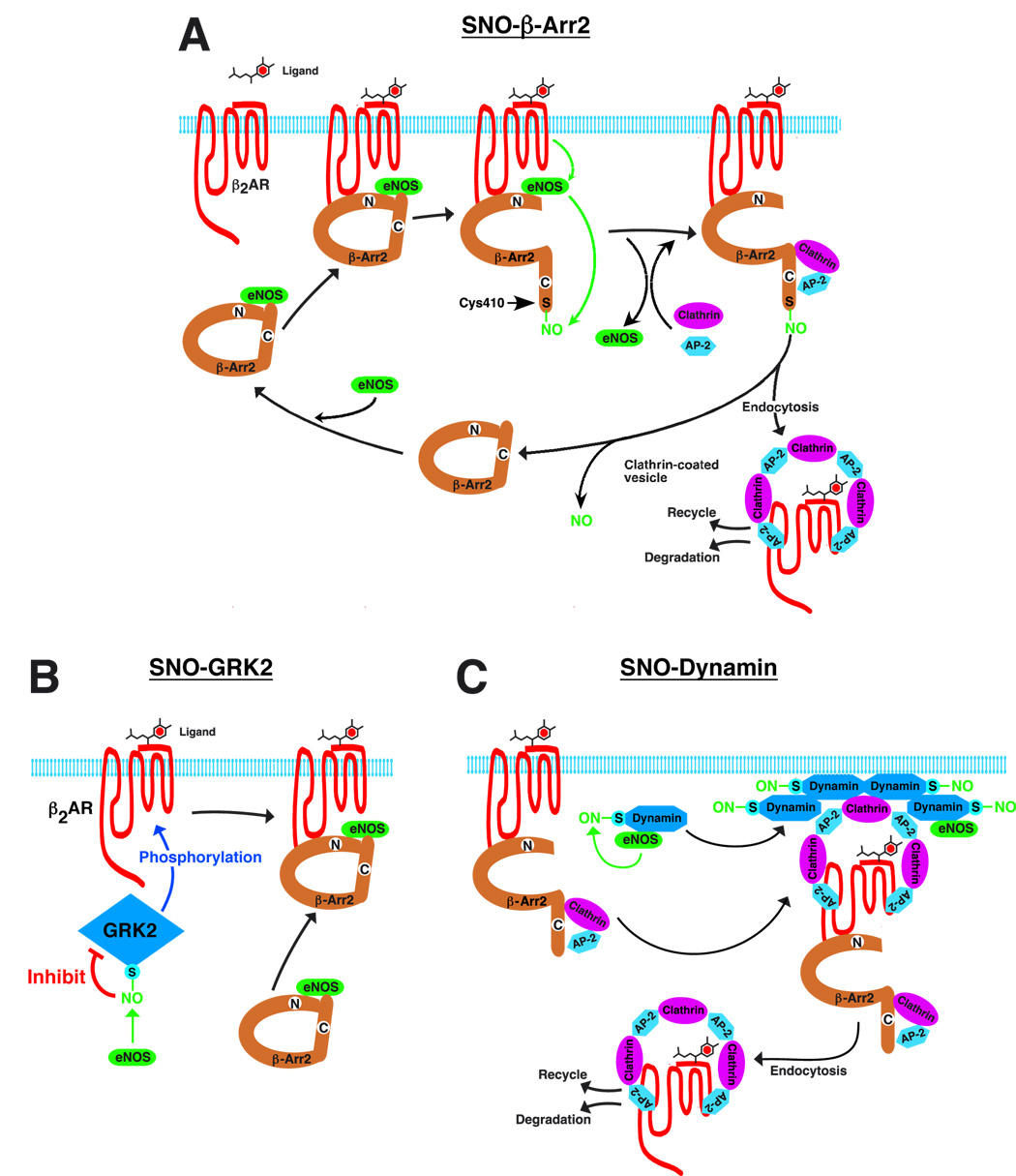
The findings reported here demonstrate that S-nitrosylation of β-arrestin 2 regulates protein-protein interactions that mediate agonist-induced trafficking of the β2-AR (Fig. 7A), and it seems likely that S-nitrosylation will play a similar role in regulating the β-arrestin-dependent trafficking of numerous GPCRs (including the AT1AR (Suppl. Fig. 7A–C)) that are functionally coupled to eNOS (Dudzinski et al., 2006). It is generally held that the functional association of GPCRs with eNOS reflects principally the direct interaction between caveolin and eNOS that co-localizes GPCRs and eNOS to caveolar membrane specializations (Dudzinski et al., 2006), and that receptor-mediated eNOS activation serves principally to generate NO-based signals that are conveyed to relatively distal downstream effectors (e.g. guanylate cyclase, to elicit vasodilation). While our findings in no way diminish the importance of the caveolar/eNOS connection, they substantially enlarge this conceptual scheme by demonstrating that β-arrestin 2 serves as a previously undescribed scaffold for eNOS: functional coupling of eNOS and GPCRs (through β-arrestin 2) enables S-nitrosylation of GPCR-associated elements following receptor activation (Fig. 7A). Accordingly, GPCR-mediated, NO-dependent responses may reflect either agonist-induced signaling by NO (classical NO paradigm) or, alternatively, the regulation by NO/SNO of GPCR-elicited signaling that is independent of NO as a second messenger.
Figure 7. A schematic summary of the regulation of agonist-induced β2-AR trafficking by S-nitrosylation/denitrosylation of β-arrestin 2, GRK2 and dynamin.
(A) β-arrestin 2 (β-Arr 2) serves as a scaffold to functionally co-localize eNOS and β-ARs (as well as other G-protein-coupled receptors, GPCRs). Ligand (isoproterenol) stimulation results in activation of eNOS and S-nitrosylation of β-arrestin 2. S-nitrosylation of β-arrestin 2 promotes its dissociation from eNOS and association with clathrin HC/β-adaptin, which facilitates routing of the β2-AR into the clathrin-based endocytotic pathway, and β-arrestin 2 is subsequently denitrosylated. (B) Ligand-coupled inhibition of GPCR kinase 2 (GRK2) by S-nitrosylation suppresses agonist-stimulated β-AR phosphorylation, β-arrestin recruitment, and receptor desensitization and down-regulation. (C) Following GPCR activation, eNOS-mediated S-nitrosylation of dynamin promotes multimerization and GTPase activity, which facilitates scission of endocytotic vesicles and receptor internalization.
S-nitrosylation is the only known facilitatory modification of β-arrestins (phosphorylation of β-arrestin 1 or β-arrestin 2 inhibits clathrin-mediated β2AR internalization (Lin et al., 2002; Lin et al., 1997; Lin et al., 1999)), and the selective S-nitrosylation of β-arrestin 2 vs. β-arrestin 1 provides for differential regulation of these widely-distributed isoforms. It is of note that stimulus-coupled dephosphorylation of Ser412 in β-arrestin 1 is prerequisite for clathrin binding and receptor endocytosis (Lin et al., 1997; Lin et al., 1999); β-arrestin 2 does not contain an equivalent C-terminal Ser, but does contain a highly conserved Cys residue at position 410. The dephosphorylation of Ser412 of β-arrestin 1 promotes clathrin binding, and this effect is recapitulated by S-nitrosylation (or Cys→Ser mutation) of Cys410 within β-arrestin 2. These data, as well as other recent work (see Fig. 7B (Whalen et al., 2007) and Fig 7C (Wang et al., 2006)), support the emerging concept that S-nitrosylation may operate together with phosphorylation to regulate GPCR function.
Ligand-induced S-nitrosylation inhibits binding of β-arrestin 2 to eNOS and conjointly facilitates binding to clathrin HC/β-adaptin, and β-arrestin 2 is subsequently denitrosylated (Fig. 7A). The β2AR is a member of the class A-family of GPCRs, defined in part by the fact that β-arrestin 2 dissociates from complexed clathrin HC/β-adaptin/GPCR prior to the formation of endocytotic vesicles (Lefkowitz and Shenoy, 2005). Thus, to the extent that denitrosylation precedes internalization, it may facilitate this step as well as re-association with eNOS, and thus β-arrestin 2 cycling (Fig. 7A). In contrast, class B-family of GPCRs, such as AT1AR, internalize together with clathrin/β-arrestin 2 and recycle very slowly. Although β-arrestin 2 S-nitrosylation facilitates internalization of AT1AR, one anticipates a smaller role for dentirosylation in receptor recycling. It will be interesting to determine if S-nitrosylation/denitrosylation may influence the class characteristics of receptors by altering their affinity for β-arrestin 2, and whether S-nitrosylation differentially regulates signaling that is differentially dependent upon β-arrestin isotypes (Lefkowitz et al., 2006), exemplified by the specific role of β-arrestin 2 in G-protein-independent activation of ERK following angiotensin II stimulation (Lefkowitz and Shenoy, 2005; Rajagopal et al., 2005).
Other recent findings indicate that S-nitrosylation regulates the function of both dynamin (Kang-Decker et al., 2007; Wang et al., 2006) and GRK2 (Whalen et al., 2007), which serve as additional, critical components of GPCR trafficking. S-nitrosylation of dynamin enhances self-assembly, relocation to the plasma membrane and GTPase activity (Wang et al., 2006) (all of which are required for scission of clathrin-coated, endocytotic vesicles), whereas S-nitrosylation of GRK2 inhibits kinase function and thus the phosphorylation-dependent interaction of β-arrestin and GPCR that routes receptors into clathrin-based endocytosis (Whalen et al., 2007). These disparate effects appear to be mediated by discrete pools of eNOS, which associate with both dynamin and β-arrestin but require the participation of additional elements to target GRK2 (Liu et al., 2005; Wang et al., 2006; Whalen et al., 2007). It is further suggested by our data that, although the influence of these various pools of eNOS/SNOs may vary with different receptors (Fig. 5E vs. Suppl. Fig. 7C), β-AR stimulation clearly results in coordinate regulation by eNOS of all three elements (GRK, β-arrestin and dynamin), which thereby facilitates both G protein signaling (Whalen et al., 2007) and receptor trafficking ((Wang et al., 2006) and this work). Taken together, these findings provide strong instantiation of the concept that co-ordinated changes in the S-nitrosylation of multiple, functionally interrelated components subserves dynamic regulation by NO of cellular signal transduction (Hess et al., 2005)(Fig. 7).
The possibility of NO/SNO regulating signal transduction through GPCRs provides a point of departure to assess dysregulated S-nitrosylation as a potential factor in multiple pathophysiologies that involve altered GPCR trafficking and function, including hypertension (Spieker et al., 2006), heart failure (Saraiva and Hare, 2006), diabetes (Rask-Madsen and King, 2007), and asthma (Gaston et al., 2006). Recent evidence in support of this connection can be found in both animal models ((Murphy and Steenbergen, 2007) (Que et al., 2005; Whalen et al., 2007)) and human studies (Chaudhry et al., 2007).
Experimental Procedures
Additional information regarding experimental materials, plasmids, mutants, cell culture, transfection methods, siRNA, immunoprecipitation and statistics can be found in Supplemental Materials.
In vitro binding
Recombinant bovine eNOS was prepared as in (Martasek et al., 1996). β-arrestin 2 (wild type, C410A and C410S) was purified from wild-type HEK cells overexpressing β-arrestin 2 possessing a FLAG tag using anti-FLAG agarose beads. Purified bovine clathrin was purchased from Sigma. Binding of eNOS to β-arrestin 2 was detected using an in vitro binding assay with some modifications (Cao et al., 2001). In brief, recombinant eNOS (150 nM) was incubated for 2 hours at 4°C with increasing concentrations of β-arrestin 2 (0–300 nM) in binding buffer (50mM Tris-HCl, 0. 1 mM EGTA, 0. 1 mM EDTA and protease inhibitor cocktail), and vice versa (i. e. 150 nM of purified β-arrestin 2 was incubated with increasing concentrations of eNOS). Binding between clathrin and wild-type or mutant β-arrestin 2 was detected using purified β-arrestin 2 (wild-type, C410A and C410S)(300 nM) incubated (overnight at 4°C) with purified clathrin (300 nM) in the presence and absence of 50 µM DETA NONOate (Cayman Chemical). Co-immunoprecipitation was performed using anti-eNOS, anti-β-arrestin 2 or anti-clathrin antibodies, and proteins were detected by immunoblotting.
Biotin-switch assay
S-nitrosylated β-arrestin 2 was detected using the biotin-switch assay (Jaffrey and Snyder, 2001) with some modifications. Briefly, lysates (250 µl diluted with 750 µl of HEN buffer containing 250 mM Hepes, 1mM EDTA, 0. 1 mM neocuproine, pH 7. 7) were incubated with SDS (1% final concentration) and methyl methanethiosulfonate (Sigma-Aldrich; St. Louis, MO) at 50°C for 20 min. Proteins were precipitated with acetone, washed 3 times and mixed with 0.2 mM biotin-HPDP (Pierce; Rockford, IL) with or without 2.5 mM ascorbate at ambient temperature for 1 h. Biotinylated proteins were purified using streptavidin-agarose beads (Sigma-Aldrich, St. Louis, MO), separated by SDS-PAGE and immunoblotted. (Note: HEK cells plated in 10 cm dishes were lysed 48 hr. following transfections with eNOS (5 µg), β-arrestin 1 (5 µg) or β-arrestin (2 µg)).
Cell fractionation
HEK-eNOS cells or HUVECs were separtated into cytosolic and membrane fractions using Qproteome Cell Compartment Kit (Qiagen, CA) according to manufacturer’s instructions.
Receptor internalization
Agonist-induced internalization of Flag-β2AR was assessed by flow cytometry. HEK-β2AR cells were transiently transfected with 5 µg of eNOS and/or 10 µg of β-arrestin 2 (wild type or mutants as indicated). Twelve hours after transfection, cells were detached using trypsin and seeded in 6-well dishes. At 48 hr, cells were washed once with PBS, incubated with Dulbecco's modified Eagle's medium containing 0.1% bovine serum albumin (BSA) for 2 hours, and stimulated with freshly prepared 10 µM isoproterenol. All subsequent steps were performed on ice. Following two washes with ice-cold PBS, cells were incubated with M2 anti-Flag antibody (1:1000) for 1 h, washed, and then incubated with FITC-conjugated anti-mouse antibody (1:400) for 1 h. Receptor internalization was defined as the fraction of total cell surface receptors that were removed from the plasma membrane following agonist treatment (and thus not accessible to extracellular antibody). For internalization assays using L-NAME and D-NAME, HEK-β2AR cells were transiently transfected with 10 µg of β-arrestin 2 and preincubated with 3mM L-NAME or D-NAME for 24 hours.
Mice
eNOS−/− mice were obtained from Jackson laboratory (Bar Harbor, ME). GSNOR−/− mice were generated previously (Liu et al., 2004). Extracts of liver and spleen were prepared from male C57BL/6 (wild-type) and mutant mice of 7–10 weeks of age. Specifically, control animals and animals receiving a single injection of isoproterenol (5 mg/kg i. p. were sacrificed (10 min post i.p. injection), and liver and spleen were removed. Organs were disrupted using a Dounce homogenizer, homogenates were clarified via centrifugation and the supernatant was subject to protein determination. Lysates were then subject to biotin switch, immunoprecipitation and immunoblot analysis.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (P01-HL075443, RO1-HL16037, RO1-HL70631, U19-ES012496), the Sandler Program for Asthma Research and the Howard Hughes Medical Institute. We thank S. Gross and P. Lane for purified eNOS and Yehia Daaka for truncated β-arrestin constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam L, Bouvier M, Jones TL. Nitric oxide modulates β(2)-adrenergic receptor palmitoylation and signaling. J Biol Chem. 1999;274:26337–26343. doi: 10.1074/jbc.274.37.26337. [DOI] [PubMed] [Google Scholar]
- Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem. 2001;276:14249–14256. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- Chaudhry S, Que LG, Liu L, Eng C, Nazario S, Casal J, Torrez A, Gomez I, Salas J, Chapela R et al. S-nitrosoglutathione reductase and β-adrenergic receptor gene gene interaction is associated with asthma in latinos. American Journal of Respiratory and Critical Care Medicine. 2007;175 A968 Abstract. [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001 doi: 10.1126/stke.2001.86.pl1. PL1. [DOI] [PubMed] [Google Scholar]
- Kang-Decker N, Cao S, Chatterjee S, Yao J, Egan LJ, Semela D, Mukhopadhyay D, Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- Kokkola T, Savinainen JR, Monkkonen KS, Retamal MD, Laitinen JT. S-nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner. BMC Cell Biol. 2005;6(21) doi: 10.1186/1471-2121-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The β2-adrenergic receptor/β-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc PC, Lanctot PM, Auger-Messier M, Escher E, Leduc R, Guillemette G. S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin II. Br J Pharmacol. 2006;148:306–313. doi: 10.1038/sj.bjp.0706725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lin FT, Chen W, Shenoy S, Cong M, Exum ST, Lefkowitz RJ. Phosphorylation of β-arrestin2 regulates its function in internalization of beta(2)-adrenergic receptors. Biochemistry. 2002;41:10692–10699. doi: 10.1021/bi025705n. [DOI] [PubMed] [Google Scholar]
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. Clathrin-mediated endocytosis of the β-adrenergic receptor is regulated by phosphorylation/dephosphorylation of β-arrestin1. J Biol Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. Feedback regulation of β-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem. 1999;274:15971–15974. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- Lin YF, Raab-Graham K, Jan YN, Jan LY. NO stimulation of ATP-sensitive potassium channels: Involvement of Ras/mitogen-activated protein kinase pathway and contribution to neuroprotection. Proc Natl Acad Sci U S A. 2004;101:7799–7804. doi: 10.1073/pnas.0402496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- Martasek P, Liu Q, Liu J, Roman LJ, Gross SS, Sessa WC, Masters BS. Characterization of bovine endothelial nitric oxide synthase expressed in E. coli. Biochem Biophys Res Commun. 1996;219:359–365. doi: 10.1006/bbrc.1996.0238. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Comatas KE, Liu L, Stamler JS. Screening for nitric oxide-dependent protein-protein interactions. Science. 2003;301:657–661. doi: 10.1126/science.1079319. [DOI] [PubMed] [Google Scholar]
- Mawjood AH, Miyazaki G, Kaneko R, Wada Y, Imai K. Site-directed mutagenesis in hemoglobin: test of functional homology of the F9 amino acid residues of hemoglobin alpha and beta chains. Protein Eng. 2000;13:113–120. doi: 10.1093/protein/13.2.113. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007 doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- Nagai K, Perutz MF, Poyart C. Oxygen binding properties of human mutant hemoglobins synthesized in Escherichia coli. Proc Natl Acad Sci U S A. 1985;82:7252–7255. doi: 10.1073/pnas.82.21.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by β-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- Nozik-Grayck E, Whalen EJ, Stamler JS, McMahon TJ, Chitano P, Piantadosi CA. S-nitrosoglutathione inhibits α1-adrenergic receptor-mediated vasoconstriction and ligand binding in pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2006;290:L136–L143. doi: 10.1152/ajplung.00230.2005. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen LR, Ferro A. Beta-adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci. 2006;63:1070–1083. doi: 10.1007/s00018-005-5451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115:2971–2974. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Piston DW. Regulation of β cell glucokinase by S-nitrosylation and association with nitric oxide synthase. J Cell Biol. 2003;161:243–248. doi: 10.1083/jcb.200301063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann J, Schwedhelm KS, Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: Implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Saraiva RM, Hare JM. Nitric oxide signaling in the cardiovascular system: implications for heart failure. Curr Opin Cardiol. 2006;21:221–228. doi: 10.1097/01.hco.0000221584.56372.dc. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β 2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T. A clathrin-binding site in the hinge of the β 2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Flammer AJ, Luscher TF. The vascular endothelium in hypertension. Handb Exp Pharmacol. 2006:249–283. doi: 10.1007/3-540-36028-x_8. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, et al. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in β-arrestin 2. J Biol Chem. 2004;279:55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.