Abstract
Background
Among patients with type 1 diabetes mellitus, intensive therapy (with the aim of achieving near-normal blood glucose and glycosylated hemoglobin concentrations) markedly reduces the risk of microvascular complications as compared with conventional therapy. To assess whether these benefits persist, we compared the effects of former intensive and conventional therapy on the occurrence and severity of retinopathy and nephropathy for four years after the end of the Diabetes Control and Complications Trial (DCCT).
Methods
At the end of the DCCT, the patients in the conventional-therapy group were offered intensive therapy, and the care of all patients was transferred to their own physicians. Retinopathy was evaluated on the basis of centrally graded fundus photographs in 1208 patients during the fourth year after the DCCT ended, and nephropathy was evaluated on the basis of urine specimens obtained from 1302 patients during the third or fourth year, approximately half of whom were from each treatment group.
Results
The difference in the median glycosylated hemoglobin values between the conventional-therapy and intensive-therapy groups during the 6.5 years of the DCCT (average, 9.1 percent and 7.2 percent, respectively) narrowed during follow-up (median during 4 years, 8.2 percent and 7.9 percent, respectively; P<0.001). Nevertheless, the proportion of patients who had worsening retinopathy, including proliferative retinopathy, macular edema, and the need for laser therapy, was lower in the intensive-therapy group than in the conventional-therapy group (odds reduction, 72 percent to 87 percent; P<0.001). The proportion of patients with an increase in urinary albumin excretion was significantly lower in the intensive-therapy group.
Conclusions
The reduction in the risk of progressive retinopathy and nephropathy resulting from intensive therapy in patients with type 1 diabetes persists for at least four years, despite increasing hyperglycemia.
THE Diabetes Control and Complications Trial1 (DCCT) was a multicenter clinical trial conducted between 1983 and 1993. It was designed to determine whether intensive therapy with the aim of maintaining blood glucose and glycosylated hemoglobin concentrations as close to the normal range as possible would prevent or delay long-term complications in patients with type 1 diabetes mellitus. The trial showed that during an average treatment period of 6.5 years, the risk of the development or progression of early microvascular complications of diabetes was substantially lower in the intensive-therapy group than in the conventional-therapy group. At the close of the trial in 1993, patients in the conventional-therapy group were offered intensive therapy and instructed in its use. All patients received subsequent care from their own physicians, and most were enrolled in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, a long-term observational study.2 One of the objectives of the EDIC study is to compare the long-term effects of the intensive or conventional therapy provided during the DCCT on the development of more advanced retinal and renal complications of diabetes. In this report, we describe the continued differences between the two original treatment groups in the incidence of these complications four years after the close of the DCCT.
METHODS
Patients
The 1441 patients enrolled in the DCCT between 1983 and 1989 were 13 to 39 years old, had had type 1 diabetes for 1 to 15 years, and were in generally good health. The primary-prevention cohort consisted of 726 patients who had no retinopathy and who had a urinary albumin excretion rate of less than 28 μg per minute (less than 40 mg per 24 hours); the duration of their diabetes ranged from one to five years. The secondary-intervention cohort consisted of 715 patients who had had diabetes for 1 to 15 years and who had minimal-to-moderate nonproliferative retinopathy and a urinary albumin excretion rate of less than 139 μg per minute (less than 200 mg per 24 hours). The patients in the primary-prevention and secondary-intervention cohorts were randomly assigned to receive either intensive therapy, with the goal of achieving blood glucose and glycosylated hemoglobin concentrations as close to the normal range as possible, or conventional therapy. Intensive therapy consisted of at least three daily injections of insulin or treatment with an insulin pump, with the dose adjusted frequently on the basis of self-monitored blood glucose values (at least four measurements per day), diet, and exercise. Conventional therapy consisted of one or two insulin injections per day with one urine or blood glucose test per day. The mean duration of follow-up was 6.5 years.
All surviving patients were evaluated at the close of the trial, between January and April 1993. In 1994, 1375 of the patients in the original cohort, including 688 patients in the former conventional-therapy group and 687 patients in the former intensive-therapy group, volunteered to participate in the EDIC study, which included annual follow-up examinations. During the EDIC study, all therapy was provided by the patients' own physicians.
Assessment of Retinopathy, Renal Function, and Glycemic Control
Retinopathy was assessed by fundus photography according to the DCCT-EDIC protocol in 369 patients during EDIC study year 1, 443 patients during year 2, 419 patients during year 3, and 1208 patients during year 4 (1997). All photographs were graded centrally according to the final Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale3 and DCCT methods4; the graders were unaware of the DCCT therapy assignment. The outcomes related to retinopathy included a progression of at least three steps in the grade of retinopathy from the level on enrollment in the DCCT, the presence of severe, nonproliferative diabetic retinopathy or worse, and the development of proliferative retinopathy. Patients who received panretinal scatter-photocoagulation (laser) therapy were thereafter counted as having worse retinopathy for all these outcomes. The presence of clinically significant macular edema was defined according to ETDRS criteria.5 Patients who underwent focal photocoagulation for macular edema were counted as having macular edema thereafter. The level of retinopathy at the end of the DCCT was classified as no retinopathy (ETDRS grade 10 in both eyes), microaneurysms only (grade 20 in either eye), mild nonproliferative diabetic retinopathy (grade 30 in either eye), moderate or greater nonproliferative diabetic retinopathy (grade 40 or more in either eye), and any previous laser therapy (focal or scatter). Visual acuity was assessed by ETDRS methods.6
Renal function was assessed in 649 patients during year 3 of the EDIC study and in 653 patients during year 4 by the measurement of urinary albumin excretion and creatinine clearance in a four-hour urine specimen.7 Urinary albumin excretion was expressed in micrograms per minute. Creatinine clearance was also estimated on the basis of the inverse of the serum creatinine concentration (with the equations of Cockcroft and Gault8), as follows: K×(104-age)×kg÷(72×serum creatinine), with K equal to 1 for men and 0.85 for women. Microalbuminuria was defined as a urinary albumin excretion rate of more than 28 μg per minute (40 mg per 24 hours), albuminuria as a urinary albumin excretion rate of more than 208 μg per minute (300 mg per 24 hours), and abnormal glomerular filtration as a creatinine clearance of less than 70 ml per minute per 1.73 m2 of body-surface area.
Glycosylated hemoglobin was measured annually in a central laboratory by high-performance liquid chromatography.9 The total mean glycosylated hemoglobin value was calculated as the time-weighted average during both the DCCT and the EDIC study.
Statistical Analysis
To test for differences between groups, Wilcoxon rank-sum tests were used for quantitative or ordinal data, and chi-square tests were used for categorical data.10 The Mantel-Haenszel method was used to calculate stratified, adjusted odds ratios,11 with test-based confidence limits. Logistic-regression analysis was used to assess the effects of covariates on the odds of a particular outcome with specific outcomes.11 The percent reduction in the odds of a particular outcome with intensive therapy as compared with conventional therapy was computed as (1-the odds ratio)×100. Group comparisons were adjusted for the level of severity of retinopathy at the end of the DCCT with the use of the Mantel-Haenszel method or logistic-regression analysis. For the logistic-regression analysis, P values were calculated with likelihood-ratio tests.
Proportional-hazards regression analysis was used to estimate the cumulative incidence of the progression of retinopathy during the EDIC study with the use of all photographs in all patients, including those obtained at one, two, and three years in some patients.12 All analyses were performed with SAS software.13
RESULTS
The level of retinopathy was evaluated in 1208 patients during year 4 of the EDIC study. The characteristics of these patients on enrollment in the DCCT and at its end are shown in Table 1. The characteristics of the patients at the end of the DCCT were the base-line characteristics for the EDIC study. The groups that had received intensive and conventional treatment did not differ significantly with respect to sex, age, duration of diabetes, or duration of follow-up in the DCCT. However, they did differ with respect to the level of retinopathy at the end of the DCCT and the need for photocoagulation therapy during the DCCT. These differences reflect the benefit of intensive therapy as compared with conventional therapy during the trial.
Table 1.
Characteristics of the 1208 Patients Enrolled in the EDIC Study Who Were Evaluated after Four Years of Follow-up.*
| Characteristic | DCCT Treatment Group | P Value |
|
|---|---|---|---|
| CONVENTIONAL (N=603) |
INTENSIVE (N=605) |
||
| At DCCT entry | |||
| Women (%) | 47 | 48 | 0.69 |
| Age (yr) | 27±7 | 27±7 | 0.13 |
| Duration of diabetes (yr) | 5.6±4.1 | 5.9±4.2 | 0.18 |
| Glycosylated hemoglobin (%) | 9.0±1.6 | 9.0±1.6 | 0.41 |
| At EDIC entry† | |||
| Age (yr) | 33±7 | 34±7 | 0.08 |
| Duration of diabetes (yr) | 11.7±4.8 | 12.1±4.9 | 0.11 |
| DCCT follow-up (yr) | 6.1±1.7 | 6.2±1.7 | 0.16 |
| Glycosylated hemoglobin (%) | 9.0±1.2 | 7.3±0.9 | <0.001 |
| Level of retinopathy (%) | <0.001 | ||
| None | 18 | 29 | |
| Microaneurysms only | 30 | 38 | |
| Mild nonproliferative retinopathy | 30 | 22 | |
| Moderate or severe nonproliferative retinopathy |
22 | 11 | |
| Photocoagulation during DCCT (%) | |||
| Scatter, for retinopathy | 4 | 2 | 0.018 |
| Focal, for macular edema | 5 | 2 | 0.038 |
| Nephropathy at EDIC year 3 or 4 (%)‡ | |||
| Albumin excretion >28 μg/min | 13 | 7 | 0.002 |
| Albumin excretion >208 μg/min | 3 | 2 | 0.14 |
| Creatinine clearance <70 ml/min/ 1.73 m2 |
1 | 2 | 0.63 |
| Treatment at EDIC year 4 (%) | |||
| Continuous subcutaneous insulin in- fusion (pump) or multiple daily injections |
75 | 95 | <0.001 |
| Self-monitoring of blood glucose ≥4 times per day |
40 | 45 | 0.064 |
Plus-minus values are means ±SD. EDIC denotes Epidemiology of Diabetes Interventions and Complications, and DCCT Diabetes Control and Complications Trial.
The base-line data in the EDIC study were the same as the data at the end of the DCCT.
The results for nephropathy include 1302 EDIC participants (649 from the former conventional-therapy group and 653 from the former intensive-therapy group).
Among the 1302 patients in whom renal function was evaluated during year 3 or 4 of the EDIC study, the proportion with microalbuminuria at the end of the DCCT was nearly twice as high in the group of patients who had received conventional therapy as in the group of patients who had received intensive therapy (Table 1). The prevalence of urinary albumin values above 208 μg per minute and creatinine clearance values under 70 ml per minute per 1.73 m2 was low and did not differ significantly between the treatment groups at the end of the DCCT.
During the 6.5 years of treatment in the DCCT, the patients in the intensive-therapy group used their assigned therapy (at least three insulin injections per day or continuous infusion of insulin with an external pump) 98 percent of the time, and the patients in the conventional-therapy group gave themselves one or two insulin injections per day 97 percent of the time. During year 4 of the EDIC study, 95 percent of the patients in the former intensive-therapy group continued treatment with multiple daily injections of insulin or an insulin infusion pump, as compared with 75 percent of the patients in the former conventional-therapy group (P<0.001). Less than half the patients in each group were performing self-monitoring of blood glucose four or more times per day.
At the time of enrollment in the DCCT, the mean glycosylated hemoglobin value in each group was about 9 percent (Table 1). The distribution of glycosylated hemoglobin values during the DCCT and during the EDIC study for the 1208 patients who had an eye evaluation during year 4 of the EDIC study is shown in Figure 1. Over the average of 6.5 years of follow-up in the DCCT, the median glycosylated hemoglobin value was 7.2 percent in the intensive-therapy group and 9.1 percent in the conventional-therapy group. By the end of year 1 in the EDIC study, the glycosylated hemoglobin values in the two groups had almost converged; the median value was 8.1 percent in the conventional-therapy group and 7.7 percent in the intensive-therapy group. Thereafter, the difference continued to narrow. During the four-year follow-up period in the EDIC study, the median glycosylated hemoglobin values were 8.2 percent in the conventional-therapy group and 7.9 percent in the intensive-therapy group (P<0.001). The correlation coefficient for the mean glycosylated hemoglobin value during the EDIC study and that during the DCCT was 0.58 in the conventional-therapy group and 0.67 in the intensive-therapy group.
Figure 1.
Distribution of Glycosylated Hemoglobin (Hemoglobin A1c) Values in the Conventional-Therapy and Intensive-Therapy Groups at the End of the Diabetes Control and Complications Trial (DCCT), in Each of the Four Years of the Epidemiology of Diabetes Interventions and Complications (EDIC) Study, and Averaged over the Four Years of the EDIC Study.
Data are for the 1208 patients who had an eye evaluation in year 4 of the EDIC study. The boxes represent the second and third quartiles of the distribution, the center lines the medians, and the plus signs the means.
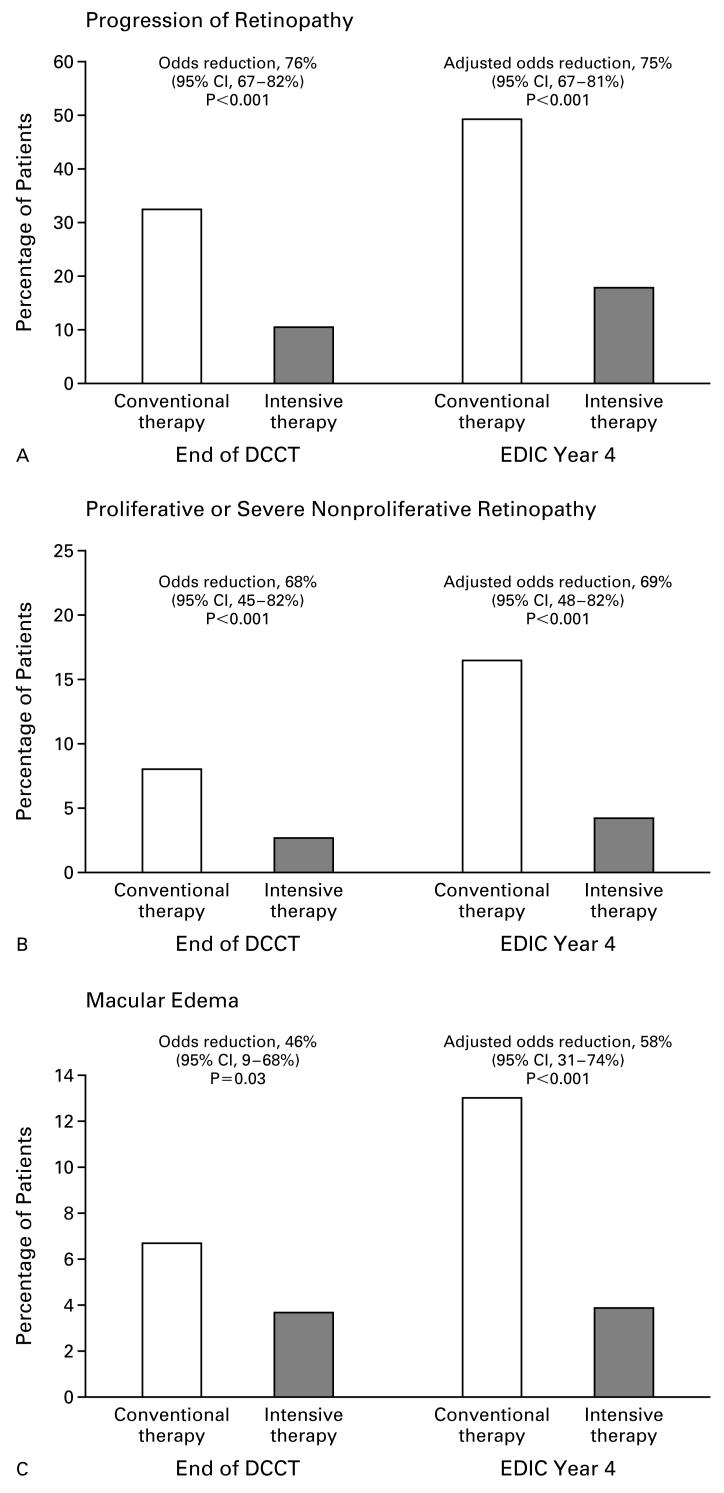
Ophthalmologic Outcomes
The rates of prevalence of various levels of retinopathy and of clinically important macular edema were significantly lower in the former intensive-therapy group than in the former conventional-therapy group during year 4 of the EDIC study, as was the case in the same 1208 patients at the end of the DCCT (Fig. 2). With respect to the principal DCCT outcome, the likelihood (odds) of an increase in retinopathy of three or more steps from base line was 76 percent lower in the intensive-therapy group than in the conventional-therapy group at the end of the DCCT. After four years of follow-up in the EDIC study, 49 percent of the patients in the conventional-therapy group had had a progression in retinopathy of three or more steps from the DCCT base line, as compared with 18 percent of the patients in the intensive-therapy group. Logistic-regression analysis with adjustment for the level of retinopathy at the end of the DCCT showed a 75 percent reduction in the likelihood of progression (P<0.001). For each outcome included in Figure 2, there was a significantly lower risk in the intensive-therapy group at the end of year 4 of the EDIC study, after adjustment for group differences at the end of the DCCT.
Figure 2.
Prevalence of More Severe Retinopathy as Compared with the Level of Retinopathy at Entry into the Diabetes Control and Complications Trial (DCCT), at the End of the DCCT, and after an Additional Four Years of Follow-up in the Epidemiology of Diabetes Interventions and Complications (EDIC) Study among 1208 Patients Evaluated at Year 4 of the EDIC Study.
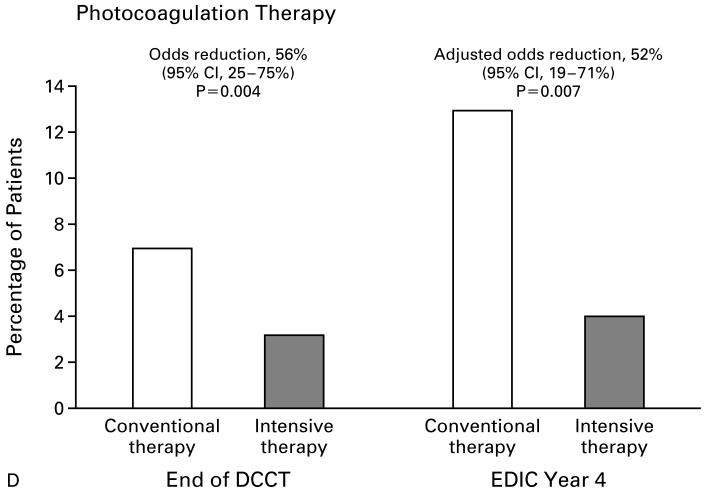
There were 603 patients in the conventional-therapy group and 605 in the intensive-therapy group. Patients who underwent scatter photocoagulation after entry into the DCCT were counted as having worsening retinopathy, and those who underwent focal photocoagulation were counted as having macular edema. Adjusted odds ratios were computed after stratification according to the level of retinopathy at the end of the DCCT, as shown in Table 1. The percent reduction in the likelihood of worsening retinopathy was computed as (1-OR)×100, where OR is the odds ratio for intensive therapy as compared with conventional therapy. Panel A shows the percentage of subjects with progression of retinopathy (three or more steps) after DCCT entry. Panel B shows the percentage of patients with development of proliferative or severe nonproliferative retinopathy. Panel C shows the percentage of patients with clinically significant macular edema. Panel D shows the percentage of patients who underwent photocoagulation (scatter or focal).
To describe better the persistence of the effect of therapy received in the DCCT during the subsequent four years of the EDIC study, we analyzed the incidence of further progression of retinopathy, defined as an increase of at least three steps from the level of retinopathy at the end of the DCCT (Table 2). Overall, 21 percent of the 581 patients in the conventional-therapy group had progression of retinopathy, as compared with 6 percent of the 596 patients in the intensive-therapy group, for an unadjusted reduction in the odds of this outcome of 75 percent. When the results were analyzed separately for each of the levels of retinopathy at the end of the DCCT, the incidence of progression was significantly lower in the intensive-therapy group. The adjusted reduction in the odds of progression of retinopathy of three or more steps, averaged over all levels of retinopathy at the end of the DCCT, was 72 percent (P<0.001).
Table 2.
Progression of Retinopathy between the End of the DCCT and after Four Years of the EDIC Study, According to the DCCT Treatment Group.*
| Level of Retinopathy at End of DCCT | NO. of PATIENTS† |
Progression of Retinopathy‡ |
Odds Reduction (95% CI)§ |
P Value¶ |
|---|---|---|---|---|
| percent | ||||
| All levels | 75 (64-83) | <0.001 | ||
| Conventional therapy | 581 | 21 | ||
| Intensive therapy | 596 | 6 | ||
| No retinopathy | 66 (26-84) | 0.006 | ||
| Conventional therapy | 109 | 16 | ||
| Intensive therapy | 173 | 6 | ||
| Microaneurysms only | 76 (49-88) | <0.001 | ||
| Conventional therapy | 184 | 14 | ||
| Intensive therapy | 233 | 4 | ||
| Mild nonproliferative retinopathy | 83 (60-93) | <0.001 | ||
| Conventional therapy | 178 | 19 | ||
| Intensive therapy | 132 | 4 | ||
| Moderate nonproliferative retinopathy or worse |
60 (18-80) | 0.012 | ||
| Conventional therapy | 110 | 42 | ||
| Intensive therapy | 58 | 22 | ||
| Adjusted ‖ | 72 (59-81) | <0.001 | ||
DCCT denotes Diabetes Control and Complications Trial, EDIC Epidemiology of Diabetes Intervention and Complications, and CI confidence interval.
Patients who underwent scatter photocoagulation during the DCCT were excluded from the analysis (22 patients in the conventional-therapy group and 9 in the intensive-therapy group). The numbers of patients in each group and stratum reflect the effect of the original DCCT therapy (e.g., more patients in the intensive-therapy group than in the conventional-therapy group were free of retinopathy at the end of the DCCT).
Progression was defined as an increase of at least three steps between the end of the DCCT and year 4 of the EDIC study. Patients who underwent scatter photocoagulation after the DCCT were counted as having progressive retinopathy.
The odds reduction is for intensive therapy as compared with conventional therapy.
P values were calculated by the likelihood-ratio test.
Logistic-regression analysis was performed with adjustment for the severity of retinopathy at the end of the DCCT according to the Early Treatment Diabetic Retinopathy Study categories.
An interval-censored life-table analysis (Fig. 3) that included assessments of the level of retinopathy in approximately 25 percent of the cohort at years 1, 2, and 3 of the EDIC study showed that the difference in cumulative incidence of progressive retinopathy between groups increased steadily each year. By year 4, the cumulative incidence in the intensive-therapy group was significantly (70 percent) lower than that in the conventional-therapy group (95 percent confidence interval, 58 percent to 78 percent; P<0.001).
Figure 3.
Cumulative Incidence of Further Progression of Retinopathy (an Increase of at Least Three Steps from the Level at the End of the Diabetes Control and Complications Trial [DCCT]) in the Former Conventional-Therapy and Intensive-Therapy Groups.
The data are based on regression analysis adjusted for the level of retinopathy at the end of the DCCT, whether patients received therapy as primary prevention or secondary intervention, and both the duration of diabetes and the glycosylated hemoglobin value on enrollment in the DCCT. Patients who underwent scatter photocoagulation during the DCCT were excluded from the analysis (22 in the conventional-therapy group and 9 in the intensive-therapy group). Bars denote 95 percent confidence intervals.
The incidence of worsening of retinopathy at four years in the EDIC study among patients who had been free of each outcome at the end of the DCCT is shown in Table 3. Severe nonproliferative retinopathy, or worse, was detected in 10 percent of the 556 patients in the conventional-therapy group and in 2 percent of the 589 patients in the intensive-therapy group, representing a 76 percent reduction in the odds of this outcome, after adjustment for the level of retinopathy at the end of the DCCT. Among the patients in the conventional-therapy group, 6 percent required laser therapy for the first time during the first four years of the EDIC study, as compared with only 1 percent of the patients in the intensive-therapy group (adjusted odds reduction, 77 percent). Among the patients in the conventional-therapy group, five had visual acuity that was worse than 20/100 in one eye, three of whom had visual acuity that was worse than 20/200 in one eye; none had visual acuity worse than 20/200 in both eyes. No patient in the intensive-therapy group had visual acuity that was worse than 20/100 in either eye.
Table 3.
Incidence of Worsening of Retinopathy between the End of the Dcct and After Four Years of the Edic Study.*
| Retinal Change† | NO. of Patients‡ |
Progression of Retinopathy |
Adjusted Odds Reduction (95% CI)§ |
P Value¶ |
|---|---|---|---|---|
| percent | ||||
| Severe nonproliferative retinopathy or worse | 76 (52-88) | <0.001 | ||
| Conventional therapy | 556 | 10 | ||
| Intensive therapy | 589 | 2 | ||
| Proliferative retinopathy | 74 (46-87) | <0.001 | ||
| Conventional therapy | 564 | 9 | ||
| Intensive therapy | 590 | 2 | ||
| Clinically significant macular edema | 77 (52-89) | <0.001 | ||
| Conventional therapy | 564 | 8 | ||
| Intensive therapy | 582 | 2 | ||
| Laser therapy (focal or scatter) | 77 (45-91) | 0.002 | ||
| Conventional therapy | 544 | 6 | ||
| Intensive therapy | 575 | 1 | ||
DCCT denotes Diabetes Control and Complications Trial, EDIC Epidemiology of Diabetes Intervention and Complications, and CI confidence interval.
Patients who underwent scatter photocoagulation after the DCCT were counted as having a progression of retinopathy; those who underwent focal photocoagulation were counted as having clinically significant macular edema.
The numbers of patients free of each specific type of worsening at the end of the DCCT are given.
The odds reduction is for former intensive therapy as compared with former conventional therapy on the basis of a logistic-regression analysis with adjustment for the level of severity of retinopathy at the end of the DCCT according to the Early Treatment Diabetic Retinopathy Study categories shown in Table 2, plus any previous laser therapy (focal or scatter).
P values were calculated by the likelihood-ratio test.
Renal Outcomes
During year 3 or 4 of the EDIC study, microalbuminuria was detected for the first time in 11 percent of 573 patients in the former conventional-therapy group, as compared with 5 percent of 601 patients in the former intensive-therapy group (Table 4), representing a 53 percent odds reduction. Likewise, the risk of new albuminuria was reduced by 86 percent in the intensive-therapy group, with similar reductions for patients with normal albumin excretion (no more than 28 μg per minute) and those with microalbuminuria (29 to 208 μg per minute) at the end of the DCCT. Very few patients in either group had a decrease in creatinine clearance, and the adjusted risk of a decrease was similar in the two groups.
Table 4.
Incidence of Worsening of Nephropathy between the End of the Dcct and After Four Years of the Edic Study.*
| Renal Complication during EDIC† | NO. of Patients‡ |
Worsening Nephropathy |
Adjusted Odds Reduction (95% CI)§ |
P Value¶ |
|---|---|---|---|---|
| percent | ||||
| Microalbuminuria (urinary albumin excretion rate >28 μg/min) |
53 (26-70) | 0.002 | ||
| Conventional therapy | 573 | 11 | ||
| Intensive therapy | 601 | 5 | ||
| Albuminuria (urinary albumin excretion rate >208 μg/min) |
86 (60-95) | <0.001 | ||
| All patients | ||||
| Conventional therapy | 637 | 5 | ||
| Intensive therapy | 639 | 1 | ||
| Urinary albumin excretion, ≤28 μg/min at end of DCCT |
92 (39-99) | <0.001 | ||
| Conventional therapy | 573 | 2 | ||
| Intensive therapy | 601 | 0 | ||
| Urinary albumin excretion, 29-208 μg/ min at end of DCCT |
80 (27-95) | 0.006 | ||
| Conventional therapy | 64 | 31 | ||
| Intensive therapy | 38 | 8 | ||
DCCT denotes Diabetes Control and Complications Trial, EDIC Epidemiology of Diabetes Intervention and Complications, and CI confidence interval.
Measurements were performed in year 3 or 4 of the EDIC study (in approximately 50 percent of patients each year).
The numbers of patients free of each specific type of worsening at the end of the DCCT are given.
The odds reduction is for former intensive therapy as compared with former conventional therapy on the basis of a logistic-regression analysis with adjustment for the albumin excretion rate at the end of the DCCT.
P values were calculated by the likelihood-ratio test.
Relation of Progression of Retinopathy to Hyperglycemia
Within each former therapy group, the likelihood of further progression of retinopathy during the EDIC study increased as the mean glycosylated hemoglobin values during the DCCT and the EDIC study increased, after adjustment for other factors, including the level of retinopathy at the end of the DCCT. In the conventional-therapy group, the risk of a progression of retinopathy was multiplied by 2.8 for every 1 percent increase in the glycosylated hemoglobin value during the DCCT and the EDIC study (95 percent confidence interval, 2.2 to 3.8; P<0.001). In the intensive-therapy group, the risk of a progression of retinopathy was multiplied by 2.6 for every 1 percent increase in the glycosylated hemoglobin value during the DCCT and the EDIC study (95 percent confidence interval, 1.7 to 3.9; P<0.001). No other variables, including blood pressure and serum lipid concentrations, had a substantial effect on these complications, perhaps because patients with hypertension or hyperlipidemia had been excluded from the DCCT.
DISCUSSION
During four years of follow-up in the EDIC study, the levels of glycemic control converged for the group of patients who had received intensive therapy and the group that had received conventional therapy during the DCCT. On the basis of previous epidemiologic assessments,14 the small difference in glycosylated hemoglobin values between the two treatment groups would be expected to reduce the benefit of intensive therapy that was observed during the DCCT. To the contrary, however, the frequencies of progressive retinopathy, microalbuminuria, and albuminuria remained markedly lower in the former intensive-therapy group than in the former conventional-therapy group. These lower frequencies were not merely a reflection of the differences between the two groups at the end of the DCCT (the beginning of the EDIC study), since the reductions in the risk of progressive retinopathy and of nephropathy persisted after adjustment for the differences in the frequency of complications between the two treatment groups at the end of the DCCT.
In the intensive-therapy group, the risks of progressive retinopathy and nephropathy remained low, despite an increase in the median glycosylated hemoglobin value from 7.2 percent during the DCCT to 7.9 percent during the EDIC study. Thus, after four additional years of follow-up, the rate of worsening of complications did not increase in the intensive-therapy group. In contrast, in the former conventional-therapy group, the risk of a progression of retinopathy during the first four years of the EDIC study remained elevated and about the same as during the first four years of the DCCT.15 The increased risk of progression of retinopathy persisted in the conventional-therapy group, despite a decrease in the median glycosylated hemoglobin value from 9.1 percent during the DCCT to 8.2 percent during the EDIC study.
When examined in relation to the glycosylated hemoglobin values, the likelihood of progressive retinopathy in both groups was strongly associated with the mean glycosylated hemoglobin value during the DCCT and the EDIC study combined. The value during the DCCT appeared to be the stronger determinant of the risk of progression. Similarly, in the Stockholm Diabetes Intervention Study, the prevalence of severe retinopathy after 7.5 years of follow-up was related to the mean glycosylated hemoglobin value during the first 5 years of follow-up.16
During the DCCT, the beneficial effects of intensive therapy on the onset and progression of retinopathy and nephropathy were not evident until after three or four years of therapy. In the current study, we found that the marked reduction in the risk of progressive retinopathy in the intensive-therapy group during the DCCT persisted for at least four years despite rising glycosylated hemoglobin values. These findings strongly suggest that intensive therapy that maintains near-normal glycosylated hemoglobin concentrations has a beneficial effect on the long-term complications of diabetes that persists long after the actual period of such therapy. However, the results of the DCCT and the EDIC study should not be interpreted to mean that intensive therapy needs to be administered for only a limited period of time.
The risk of microvascular complications does not appear to be affected in the short term by the prevailing level of hyperglycemia. Instead, these risks are associated with the effects of chronic hyperglycemia and appear to decrease slowly with a decrease in the level of hyperglycemia. In diabetic animals, the institution of normal glycemia after a prolonged period of severe hyperglycemia does not reverse the risk of microvascular complications quickly, if at all.17 One possible explanation for these slow changes is the slow accumulation, and subsequent slow degradation, of advanced glycation end products in tissues.18 In the DCCT, the patients in the intensive-therapy group had lower concentrations of these substances in their skin than did the patients in the conventional-therapy group.19
In addition to the finding that 6.5 years of intensive therapy markedly reduced the risk of progressive retinopathy over a subsequent period of 4 years, the DCCT previously demonstrated that intensive therapy was more effective when introduced during the first 5 years of diabetes as primary prevention than when introduced as secondary intervention after complications had begun to develop.1 Moreover, the effects of any level of hyperglycemia increased exponentially over time in the DCCT.14,20 In concert, these findings strongly support the implementation of intensive therapy as early as is safely possible and the maintenance of such therapy for as long as possible, with the expectation that a prolonged period of nearly normal blood glucose levels will result in an even greater reduction in the risk of complications in patients with type 1 diabetes.
Acknowledgments
Supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases and the General Clinical Research Centers Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
APPENDIX
The following investigators participated in the DCCT and the EDIC Research Group: Albert Einstein College of Medicine — H. Shamoon and H. Duffy; Case Western Reserve University — W. Dahms and L. Mayer; Cornell University Medical Center — D. Brillion and M. Lackaye; Henry Ford Health System — F. Whitehouse and D. Kruger; International Diabetes Center — R. Bergenstal and M. Johnson; Joslin Diabetes Center — A. Jacobson, J. Doyle, and D. Soroko; Massachusetts General Hospital — D. Nathan, S. Fritz, J. Godine, and C. McKitrick; Mayo Foundation — J. Service and G. Ziegler; Medical University of South Carolina — J. Col-well, D. Wood, R. Mayfield, T. Garvey, T. Lyons, J. Smith, and K. Her-mayer; Northwestern University — M. Molitch and B. Schaefer; University of California at San Diego — O. Kolterman and G. Lorenzi; University of Iowa — W. Sivitz and M. Bayless; University of Maryland School of Medicine — D. Counts, A. Kowarski (former), and D. Ostrowski; University of Michigan — D. Greene, C. Martin, and W. Herman; University of Minnesota — J. Bantle and B. Rogness; University of Missouri — D. Goldstein and S. Hitt; University of New Mexico — D. Schade and D. Hornbeck; University of Pennsylvania — S. Schwartz and B.J. Maschak-Carey; University of Pittsburgh — T. Orchard, N. Silvers, and T. Songer; University of South Florida — J. Malone and H. Wetz; University of Tennessee — A. Kitabchi, H. Lambeth, and M.B. Murphy; University of Texas Southwestern Medical Center — P. Raskin and S. Strowig; University of Toronto — B. Zinman and A. Barnie; University of Washington — J. Palmer and L. Van Ottingham; University of Western Ontario — J. Dupre and J. Harth; Vanderbilt University — M. May, R. Lorenz (former), and J. Lipps; Washington University, St. Louis — N. White, J. Santiago (deceased), and L. Levandoski; Yale University School of Medicine — W. Tamborlane and P. Gatcomb; Clinical Coordinating Center (Case Western Reserve University) — B. Dahms, P. Corcoran, and J. Quin; Data Coordinating Center (George Washington University, Biostatistics Center) — J. Lachin, P. Cleary, D. Kenny, J. Backlund, L. Diminick, A. Henry, and D. Lamas; National Institute of Diabetes and Digestive and Kidney Diseases Program Office — C. Cowie and R. Eastman; Central Fundus Photograph Reading Center (University of Wisconsin) — M. Davis, L. Hubbard, P. Geithman, J. Brickbauer, L. Kastorff, and M. Neider; Central Biochemistry Laboratory (University of Minnesota) — M. Steffes, J. Bucksa, and B. Chavers; External Advisory Committee — G. Weir (chair), C. Clark, R. D'Agnostino, M. Espeland, B. Klein, H. Jacobson, T. Manolio, L. Rand, D. Singer, and M. Stern; Study Chairs — S. Genuth and D. Nathan; Editor for DCCT/EDIC Publications — D. Nathan.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive therapy of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(Suppl):823–33. [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 6.Early Therapy Diabetic Retinopathy Study (ETDRS) manual of operations. National Technical Information Service; Springfield, Va.: 1985. (NTIS accession no. PB-85223006.) [Google Scholar]
- 7.Molitch ME, Steffers MW, Cleary PA, Nathan DM. Baseline analysis of renal function in the Diabetes Control and Complications Trial. Kidney Int. 1993;43:668–74. doi: 10.1038/ki.1993.96. [Erratum, Kidney Int 1993;43:1196.] [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 9.The DCCT Research Group Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem. 1987;33:2267–71. [PubMed] [Google Scholar]
- 10.Snedecor GW, Cochran WG. Statistical methods. 7th ed. Iowa State University Press; Ames: 1980. [Google Scholar]
- 11.Agresti A. Categorical data analysis. John Wiley; New York: 1990. pp. 80–91.pp. 235–6. [Google Scholar]
- 12.Odell PM, Anderson KM, D'Agostino RB. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics. 1992;48:951–9. [PubMed] [Google Scholar]
- 13.SAS/STAT user's guide, version 6. 4th ed. SAS Institute; Cary, N.C.: 1989. [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 15.Idem Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [Erratum, Arch Ophthalmol 1998;116:1469.] [DOI] [PubMed] [Google Scholar]
- 16.Reichard P. Are there any glycemic thresholds for the serious microvascular complications? J Diabetes Complications. 1995;9:25–30. doi: 10.1016/1056-8727(94)00008-c. [DOI] [PubMed] [Google Scholar]
- 17.Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–12. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- 18.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biological, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–51. [PubMed] [Google Scholar]
- 19.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. Diabetes. 1999;48:870–80. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complications Trial Research Group The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–98. [PubMed] [Google Scholar]